Abstract
SUMMARY: Conventional and diffusion tensor MR imaging studies in twins sustaining severe pediatric traumatic brain injury identified reduction in fractional anisotropy (FA) in all regions of the corpus callosum, particularly the posterior body, rostral body, and genu, relative to healthy cotwins. FA from the rostrum, genu, anterior body, posterior body, and isthmus were correlated with measures of reading speed and comprehension; verbal working memory and math fact retrieval scores were correlated only with the rostral body FA.
Recent studies confirmed that diffusion tensor (DT) MR imaging is sensitive to disturbance of white matter after traumatic brain injury (TBI), after which diffusion anisotropy is reduced in the internal capsule and corpus callosum, even in patients with normal findings on conventional MR imaging.1,2 Given the disruption of callosal fibers by TBI, analysis of changes in anisotropy may illuminate the degree of microstructural injury in callosal regions and highlight functional relationships with cognitive abilities. We examined fractional anisotropy (FA) obtained from well-defined callosal regions in twins sustaining severe TBI in comparison with their cotwins and examined correlations of FA with neuropsychological scores.
Case Series
DT MR imaging and neuropsychological studies were completed 3 years postinjury in 2 sets of twins who were disconcordant for a severe TBI sustained during childhood. Demographic and injury information is provided in Table 1. Using twins provided excellent control of genetic, socioeconomic, familial, age, and experiential variables that may impact brain development. All 4 boys were diagnosed with attention deficit hyperactivity disorder (ADHD) before the injury of twin A of each pair. Written informed consent to participate was obtained from the study participants and their guardians. Study procedures were approved by the institutional review board.
Demographic and injury characteristics for identical and fraternal twins
| Age at TBI (y) | Age at Testing and MR Imaging (y) | Glasgow Coma Scale | Coma Duration | Acute CT Findings | |
|---|---|---|---|---|---|
| Identical twin A | 10.9 | 14.1 | 3 | 30 days | Right frontal subdural hematoma, bilateral temporal contusions, shear hemorrhage at the right frontal vertex |
| Fraternal twin A | 8.2 | 11.9 | 3 | 1.5 days | Small right temporal contusion, multiple left parietal hemorrhagic shear injuries |
Note:— TBI indicates traumatic brain injury.
MR Image Acquisition and Analysis
Entire brain data were acquired on a GE 1.5T CNV4 scanner (GE Healthcare, Milwaukee, Wis) with optimized DT imaging protocol. The data acquisition, processing, and visualization are described elsewhere.3–5
DT imaging measures of callosal subregions were obtained from a midline sagittal section by using a DT MR imaging–guided subdivision of the corpus callosum.5,6 For region of interest analyses, FA of the corpus callosum was obtained from a 3 × 3 voxel central homogeneous area representative of each subdivision to mimic histologic sampling. To increase the accuracy in region-of-interest placement, we overlaid the FA-modulated principal eigenvector on the T2-weighted images to identify and avoid CSF contribution to the region of interest.
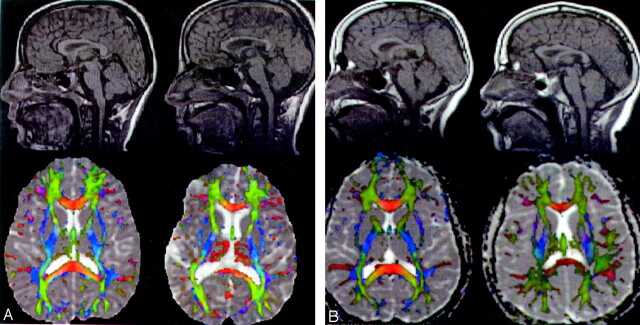
For the identical and fraternal twins, Fig 1 reveals a representative midsagittal section from the interpolated DT imaging set, clearly showing the entire corpus callosum from a full brain dataset. The figure shows the spoiled gradient-echo map and the principal eigenvector orientation map modulated by FA and overlaid on the mean diffusivity map. Red indicates right/left orientation (callosal fibers), blue indicates inferior/superior orientation (projection fibers), and green indicates anterior/posterior orientation (association fibers). The loss of fibers in the genu, rostral body, posterior body, and isthmus of the corpus callosum in the children with TBI relative to their twins, can clearly be recognized on the FA maps.
Fig 1.
Comparison of conventional and DT MR imaging findings in identical (A) and fraternal (B) twins with severe TBI (right) and cotwins (left). Note the posttraumatic diminution of white matter tracts, ventriculomegaly, and significant thinning of callosal regions, particularly the genu, rostral body, and posterior body.
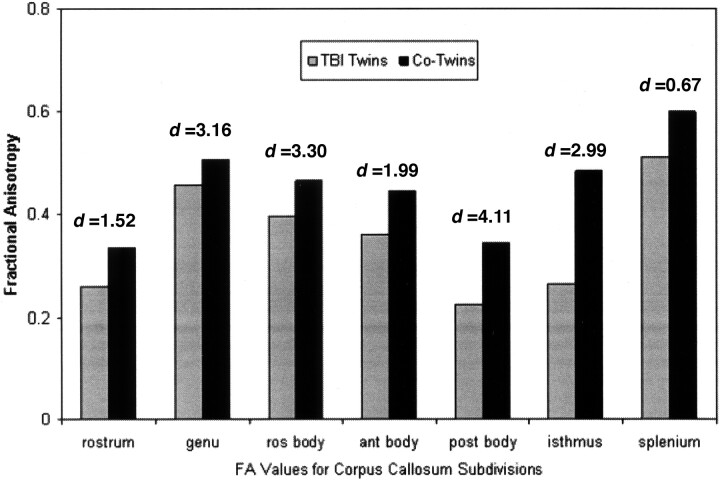
As depicted in Fig 2, FA from each callosal subregion was consistently lower in the twins with TBI relative to their cotwins. FA was smallest in the rostrum and largest in the splenium. This finding is similar to that of previous studies of callosal anisotropy.5,7 The effect size of regional callosal FA for the TBI twins relative to their cotwins was examined by using Cohen’s d.8 Effect sizes range from small (0.2–0.4) to medium (0.5–0.8) to large (>0.8). Effect sizes for group FA differences were medium to extremely large and ranged from 0.67–4.11 across callosal regions (Fig 2). FA values indicated prominent reduction in tissue integrity in the genu and isthmus as well as in the rostral, anterior, and posterior bodies in the twins sustaining TBI relative to their cotwins.
Fig 2.
Graph shows that FA values for each callosal region are reduced in the twins with TBI relative to their cotwins. Effect sizes (Cohen’s d) indicated above each region range from medium for the splenium to extremely large for the genu, rostral (ros) body, posterior (post) body, and isthmus. Ant indicates anterior.
Relationship of Integrity of Callosal Subregions to Neuropsychological Outcomes
We used Spearman rank order correlation coefficients to characterize brain-behavior relationships between regional callosal FA and neuropsychological measures. Callosal FA values had significant and specific patterns of correlation with neuropsychological measures of reading,9,10 calculation,9 math fact retrieval,11 and working memory.12
Reading.
FA of the rostrum, genu, anterior body, posterior body, and isthmus was significantly correlated with reading fluency scores (all, r = 1.00, P < .0001) and was marginally correlated with reading comprehension scores (all, r = 0.949, P =.051).
Math.
Math scores were specifically correlated with rostral body FA. Reaction times for the judgment of accuracy of math facts correlated significantly with rostral body FA (r = −1.00, P < .0001). The accuracy of written calculation problems was not significantly related to any regional FA values.
Working Memory.
Rostral body FA values were significantly correlated with the verbal working memory span score (r = 1.00, P < .0001). The spatial span score was not significantly related to callosal FA values.
Discussion
DT MR imaging, which allows assessment of tissue microstructure in vivo, has increased sensitivity to detect traumatic axonal injury and may better characterize the extent and nature of axonal injury after TBI than conventional MR imaging.13 DT MR imaging may serve as a biomarker of the extent of axonal damage after pediatric TBI. In the present cases, DT MR imaging obtained in twins 3 years after sustaining severe TBI revealed a reduction in FA across all 7 callosal subregions. The reduction in FA is consistent with pathologic and clinical studies noting posttraumatic thinning of the corpus callosum after severe TBI.14 The greatest reduction in FA occurred in the posterior body, rostral body, and genu. These findings are similar to those based on morphometric evaluation of the corpus callosum after TBI in children, which identified atrophy in the posterior body and genu as well as in the splenium.15
The diffusion anisotropy of different callosal regions may reflect the integrity of white matter that is functionally connected to different cerebral regions subserving cognitive abilities. This Case Series is the first to identify relations of FA with neuropsychological functions in children with severe TBI. Regional callosal FA values showed a striking relationship with specific neuropsychological processes. Reading speed and comprehension correlated with integrity of the rostrum, genu, anterior and posterior bodies, and the isthmus. This finding is consistent with imaging studies that highlight the large network of left occipital, parietal, temporal, and prefrontal regions involved in decoding and comprehending words and text.16,17 Reaction time for judgment of whether addition problems were correct or incorrect correlated with the rostral body FA. This finding is consistent with functional MR imaging studies identifying activation in inferior frontal regions that subserve linguistic and working memory functions on tasks involving the application of calculation rules and identification of incorrect problem solutions.11 The rostral body FA was also related to verbal working memory. This finding is consistent with reduction in size of the rostral body noted in children with ADHD,18 which may indicate alterations in frontostriatal pathways associated with performance on various measures of executive function. None of the neuropsychological measures was significantly related to splenium FA.
These preliminary findings suggest that DT imaging has excellent potential to quantify the degree of disruption of white matter in different cerebral regions and to enhance examination of brain-behavior relations. Strengths of this series include the high signal-to-noise ratio (∼40) by using full-brain optimal and rotationally invariant tensor encoding. In this preliminary study, examining FA in twins disconcordant for severe TBI revealed reduced FA in all callosal regions in the twins with severe TBI relative to their cotwins and revealed the relationship of FA from different callosal regions with performance on neuropsychological tasks. Using twins increased the power to identify posttraumatic effects by controlling for age and familial and environmental factors. Although this study identified interesting relationships of callosal FA with neuropsychological performance, generalization of findings awaits replication with a larger sample of youth with TBI.
Footnotes
Preparation of this article was supported in part by National Institutes of Health grants R01 NS 29462 and R01 NS046308 and by the Dunn Research RCH Foundation.
Presented in part at the annual meeting of the International Society of Magnetic Resonance in Medicine, Kyoto, Japan (abstracts 1350, 338), May 2004 and at the annual meeting of the International Neuropsychological Society, Baltimore, Md, February 2004.
References
- 1.Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol 2004;25:370–76 [PMC free article] [PubMed] [Google Scholar]
- 2.Rugg-Gunn FJ, Symms MR, Barker GJ, et al. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. J Neurol Neurosurg Psychiatry 2001;70:530–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan K, Parker DL, Alexander AL. Comparison of optimization procedures for diffusion-tensor encoding directions. J Magn Reson Imaging 2001;13:769–80 [DOI] [PubMed] [Google Scholar]
- 4.Hasan K, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: theoretical analysis and validation. Magn Reson Med 2003;50:589–98 [DOI] [PubMed] [Google Scholar]
- 5.Hasan K, Kanabar B, Santos RM, et al. Age Dependence of the Fractional Anisotropy of Genu and Splenium of Human Corpus Callosum Using Optimized DT-MRI: Proceedings of the 12th International Society of Magnetic Resonance in Medicine, 2004. Kyoto, Japan: ISMRM; 338
- 6.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain 1989;112:799–835 [DOI] [PubMed] [Google Scholar]
- 7.Chepuri NB, Yen Y, Burdette JH, et al. Diffusion anisotropy in the corpus callosum. AJNR Am J Neuroradiol 2002;23:803–08 [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates;1998
- 9.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, Ill: Riverside Publishing;2001
- 10.Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency. Austin, Tex: Pro-Ed;1999
- 11.Menon V, Mackenzie K, Rivera SM, et al. Prefrontal cortex involvement in processing incorrect arithmetic equations: evidence from event-related fMRI. Human Brain Map 2002;16:119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verb Learn Verb Behav 1980;19:450–66 [Google Scholar]
- 13.Arfanakis K, Haughton VM, Carew JD, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol 2002;53:794–802 [PMC free article] [PubMed] [Google Scholar]
- 14.Strich S. Lesions in the cerebral hemispheres after blunt head injury. In: Sevitt S, Stoner HB, eds. The Pathology of Trauma. London, UK; BMA House;1970. :166–71 [DOI] [PMC free article] [PubMed]
- 15.Levin HS, Benavidez D, Verger-Maestre K, et al. Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology 2000;54:647–53 [DOI] [PubMed] [Google Scholar]
- 16.Constable RT, Pugh KR, Berroya E, et al. Sentence complexity and input modality effects in sentence comprehension: an fMRI study. Neuroimage 2004;22:11–21 [DOI] [PubMed] [Google Scholar]
- 17.Simos PG, Breier JI, Fletcher JM, et al. Brain mechanisms for reading words and pseudowords: an integrated approach. Cereb Cortex 2002;12:297–305 [DOI] [PubMed] [Google Scholar]
- 18.Baumgardner TL, Singer HS, Denckla MB, et al. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology 1996;47:477–82 [DOI] [PubMed] [Google Scholar]