Abstract
Recent experimentation has shown that cognitive aptitude measures are predicted by tests of the scope of an individual’s attention or capacity in simple working-memory tasks, and also by the ability to control attention. However, these experiments do not indicate how separate or related the scope and control of attention are. An experiment with 52 children 10 to 11 years old and 52 college students included measures of the scope and control of attention as well as verbal and nonverbal aptitude measures. The children showed little evidence of using sophisticated attentional control, but the scope of attention predicted intelligence in that group. In adults, the scope and control of attention both varied among individuals, and they accounted for considerable individual variance in intelligence. About 1/3 that variance was shared between scope and control, the rest being unique to one or the other. Scope and control of attention appear to be related but distinct contributors to intelligence.
Scope of Attention, Control of Attention, and Intelligence in Children and Adults
In this paper, we use the classic selective listening procedure of Cherry (1953), along with tests of working memory and intelligence, to examine how the scope of attention (a storage function) and the control of attention (a processing function) are related during childhood development.
One of the fundamental distinctions in the field of information processing is that between storage and processing. This is certainly true in the case of working memory, which preserves a small amount of information in a readily accessible state for a limited time, allowing use of the information in an ongoing cognitive task (Baddeley & Hitch, 1974; Miyake & Shah, 1999). Miller’s (1956) seminal study discussed limits in the number of units that can be stored at one time for immediate recall, as well as the process of forming larger units so that more information can be included in the same number of units in working memory. Subsequent work further emphasized the importance of processes that regulate storage, such as control processes (Atkinson & Shiffrin, 1968) and central executive processes (Baddeley, 1986; Baddeley & Logie, 1999). In the investigation of individual differences in working memory, many studies have suggested that one can capture substantial individual differences in a wide variety of intellectual aptitude measures using tasks that combine storage with some type of processing (Case, Kurland, & Goldberg, 1982; Conway, Cowan, Bunting, Therriault, & Minkoff, 2002; Daneman & Carpenter, 1980; Daneman & Merikle, 1996; Engle, Tuholski, Laughlin, & Conway, 1999; Turner & Engle, 1989).
The distinction between storage and processing is important to consider within recent research on the role of attention in working memory. Emphasizing storage, Cowan (2001) reviewed a wide variety of evidence suggesting that people can retain in a core faculty within working memory up to about 4 separate chunks of information, with the amount increasing with age and varying among individuals. It was suggested that the faculty that is limited in this way is the focus or scope of attention. Cowan proposed that the magical number seven of Miller (1956) results from a process in which rehearsal and grouping of presented items expands the number that can be recalled, so that one must limit rehearsal and grouping in order to observe the core capacity limit. Cowan et al. (2005) showed that the core limit in children and adults predicts performance on verbal and nonverbal intelligence subtests, picking up the same common variance as the tests that combine storage and processing. Lépine, Barrouillet, and Camos (2005) similarly found that a simple primary task that was paced (reading successive letters) to prevent rehearsal and grouping of numbers to be remembered, resulted in capacity estimates that correlated well with scholastic aptitudes in a sample of sixth-grade children. These findings can be explained on the basis of a working-memory capacity limit that depends on how much information can be attended at one time. The nature or difficulty of the distracting task is not critical, provided that rehearsal and grouping are blocked (see also Conlin, Gathercole, & Adams, 2005; Cowan et al., 2005; Friedman & Miyake, 2004).
There are also other, slightly different ways of conceiving of the processing system that are still fundamentally compatible with the present research aim inasmuch as they allow that attention may be used for working memory storage. The working memory capacity limit may be the amount that can reside in the fringe, rather than the focus proper, of attention, as suggested by Oberauer, 2002; or it could be only one of several, independent types of attention with separate capacity limits as suggested by Woodman, Vogel, & Luck, 2001. It seems likely that attention also would be required for storage in the episodic buffer proposed by Baddeley (2000). That buffer was said to be capacity-limited (Baddeley, 2001) and responsible for retaining features in working memory that are abstract rather than modality- or code-specific, and for retaining links between different kinds of features. In contrast, other types of storage in this model, comprising phonological and visuospatial storage, are assumed not to require attention for memory maintenance (Baddeley, 1986; Baddeley & Logie, 1999).
In the tasks used by Cowan et al. (2005) to measure the scope of attention, information was presented in a visual array with too much information to be rehearsed or grouped, or in an auditory sequence too rapid to be rehearsed or grouped. Therefore, when the stimulus presentation ended, participants presumably had to transfer items individually from a sensory memory (or a set of activated features in memory) into the focus of attention to be recalled or retained during a retention interval. Even though sensory memory can include a rich set of features for many items, the capacity of the focus of attention is limited, and it apparently limits short-term recall to about 4 items in adults in such cases, an amount that develops in childhood and also differs somewhat among individuals. These measures of capacity correlated with the more typical, storage-and-processing tasks and also with intellectual aptitudes, with all three constructs sharing considerable variance.
In children too young to rehearse or group items, even the items within a simple digit span test presumably must be retained with the use of attention to store some of the items. For that reason, we suggest, even digit span had a high correlation with intellectual aptitudes for second- and fourth-grade children, though not for sixth-grade children or adults (Cowan et al., 2005; cf. Hutton & Towse, 2001).
Other studies have instead emphasized that what is important in working memory, accounting for individual differences in intellectual aptitudes, is some type of processing that involves the control of attention. Studies relating working memory to attention have included findings of the inhibition of proactive interference from previous trials (Gray, Chabris, & Braver, 2003; Lustig, May, & Hasher, 2001), and findings in which attention is needed to maintain a goal that is difficult in light of prepotent but incorrect responses. The latter include the goal of naming the color of ink of a word spelling out a different color (Stroop, 1935; Kane & Engle, 2003), the goal of making eye movements away from, rather than toward, an object appearing on the screen, an “antisaccade” movement (Kane, Bleckley, Conway, & Engle, 2001; Unsworth, Schrock, & Engle, 2004), the goal of focusing attention on a ring rather than a disc in space (Bleckley, Durso, Crutchfield, Engle, & Khanna, 2003), and the goal of concentrating attention on a message presented to one ear, so effectively that one does not even notice one’s own name in a different message presented to the other ear (Conway, Cowan, & Bunting, 2001). In most cases, it has been the correlation between the control of attention and working-memory tasks that was measured.
Despite the theoretical thrust of the controlled-attention view, there actually have been very few studies that have obtained correlations between attention-related processes and tests of intellectual aptitudes. Schweizer and Moosbrugger (2004) did so with complex attention tasks that may have a vigilance component (i.e., sustained attention across time). They did find a relation between attention and intelligence, as well as a working-memory component partly independent of the attention component. Whether the attention or the working-memory component accounted for more variance in intelligence depended on which intelligence test was used. Gray et al. (2003) obtained neuroimaging data indicating that high fluid intelligence scores were related to activity in lateral prefrontal and parietal regions. The discriminating neural activity was obtained in an n-back task, specifically on high-interference trials in which familiar lures were presented. According to some findings, it appears that the correlation between aptitudes, working memory, and attentional control is not ubiquitous; it may include the processes of updating attention, but not the inhibition of irrelevant stimuli or shifting of attention (Friedman et al., in press; Oberauer, Lange, & Engle, 2004).
One novel development of the present study is to examine the relation between intelligence and attention using a measure of attention derived from a classic procedure from cognitive psychology; in particular, a version of the selective-attention procedure (Cherry, 1953; Conway et al., 2001; Moray, 1959; Wood & Cowan, 1995). It is closely related to Conway et al. (2001), in which it was observed that high-span subjects are less likely to notice their names when presented in a spoken message to be ignored. The difficulty with that task is that it yields only a single name-recognition trial per participant, after which the subject does not remain naive. In our task, subjects received a spoken string of digits and a visual string of letters concurrently. In one condition, there was a task involving monitoring the visual stream, whereas in another condition it was the acoustic stream that was to be monitored. This was followed by a cue to recall the stream that had been monitored or the other stream. Even though subjects were aware that either stream could be tested, the notion is that carrying out the monitoring task efficiently requires that more attention be devoted to the monitored channel than to the other channel, and presents an opportunity to encode the attended items in a way that assists in recall. Therefore we used, as a measure of the control of attention, the benefit for recall when a channel was monitored as opposed to not monitored in the dual-task situation.
It is also unclear from past studies whether measures taken to reflect the control of attention (e.g., Conway et al., 2001) and those taken to reflect the scope or capacity of attention (Cowan et al., 2005) are related or independent. Some investigators consider storage and processing to be independent in situations in which storage apparently does not depend on attention or central executive processing (e.g., Cocchini, Logie, Della Sala, MacPherson, & Baddeley, 2002; Duff & Logie, 2001; Oberauer, Demmrich, Mayr, & Kliegl, 2001). However, it is possible to consider something slightly different, a view in which there are storage and control aspects of attention that are themselves separate. More research is needed to clarify this question. In many studies, it is left unspecified. For example, it has been found that the updating of working memory correlates with intellectual aptitude (Friedman et al., in press) but updating theoretically could involve both a storage component and a processing component, and it is not clear how much each of these is critical for individual differences in task performance. According to the theoretical view of Cowan (1995, 1999), there should be some separation of attention-demanding processing and storage functions, even though they are related. The parietal areas of the brain should be more heavily implicated as important for the focus of attention or attentive storage of information, whereas the frontal areas should be more heavily implicated in the control of attention. Postle, Berger, and D’Esposito, M. (1999) indeed found frontal regions of the brain to be more involved in the control of attention and posterior regions to be more involved in working-memory storage (see also Posner & Peterson, 1990; Postle, Druzgal, & D’Esposito, 2003; Todd & Marois, 2004; Vogel & Machizawa, 2004). Nevertheless, given how heavily integrated the frontal and parietal areas are, it remains quite possible that individuals who are relatively good at processing using controlled attention could be the same individuals who are relatively good at storing information in the focus of attention.
We asked about the relation between a task taken to reflect attentional control (described above) and a task taken to reflect the capacity or scope of attention. To examine the latter, we present a measure that has been widely researched in recent literature, a two-array comparison procedure developed by Luck and Vogel (1997). On each trial, an array of colored squares is presented and is followed by a second array that is identical to the first or differs in the color of just one square. The task is to indicate whether there has been a change. Very distinctive colors are used and there is a brief delay between arrays, so the task is one that depends on the ability to remember the first array in order to compare it to the second array. In the version of the task that we used, a cue surrounds one square in the second array, the instructions being that if any square has changed, it is the encircled square. That instruction limits the task to a single decision per trial. The fact that the squares are delivered in a simultaneous array that soon disappears makes it difficult to rehearse the items; this point is supported by evidence that array comparisons are disrupted by overt response retrieval placed in the delay between the two arrays, presumably usurping attention, but are not much disrupted simply by a silent memory load or articulatory suppression between the two visual arrays (Cocchini et al., 2002; Morey & Cowan, 2004, 2005; Stevanovski & Jolicoeur, 2003). Presumably, silent maintenance of a verbal memory load can rely on a phonological storage and rehearsal process (Baddeley, 1986; Gathercole & Baddeley, 1989), unlike a spoken load. Further supporting an attentional account of visual array memory, visual array comparisons are most strongly interrupted on trials in which the response to the recited verbal load is incorrect; attempts to correct the error presumably drain attention from maintenance of the visual array (Morey & Cowan, 2004).
We examined both age group differences and individual differences within an age group, in fourth-grade children and college students. Developmental study can help in understanding working memory both by supplying a wide range of variance in ability and by allowing an examination of the consequences of strategic differences between children and adults.
A comparison of children and adults possibly could provide an amplified examination of differences that have been observed using individual differences in adults. For example, one of the best arguments for the involvement of the control of attention in working memory is that individuals who have high working-memory spans are much more affected by dividing attention during a memory task than are individuals with a low span (Kane & Engle, 2000; Rosen & Engle, 1997). The theoretical explanation is that high-span individuals are more likely to carry out the memory task using control of attention, whereas low-span individuals tend to use only automatic processes that are not much interrupted by dividing attention. If that explanation is valid, the prediction is that children should be much less affected by diverting attention during a memory task than adults are (for reviews indicating poorer control of attention in children than in adults, see Doyle, 1973; Gomes, Molholm, Christodoulou, Ritter, & Cowan, 2000; Guttentag, 1997; Lane & Pearson, 1982; Plude, Enns, & Brodeur, 1994). We used the youngest children who could reliably carry out the dual-task procedure in pilot work; these 10- and 11-year-olds were still young enough that both their control of attention and their use of rehearsal strategies (e.g., Bjorklund & Douglas, 1997; Ornstein & Naus, 1978) were expected to be markedly inferior to those of adults.
There are many differences between processes in the visual and auditory modalities. There appear to be differences in the typical rate at which items can be processed, and a greater facility with spatial arrays in the visual modality and temporal sequences in audition (Cowan, 1988, 1995; Penney, 1989). For this reason, as we discovered in pilot work, it is not easy to develop tasks in which the role of the distribution of attention across modalities can be examined (cf. Jolicoeur, 1999; Tombu, & Jolicoeur, 2003). In order to achieve effective manipulations of attention in both children and adults, it proved necessary for us to use more stringent requirements for visual attention than for auditory attention, perhaps because speech perception is a better-learned, more automatic skill than reading.
Finally, for an efficient comparison of working-memory and attention tasks with intelligence, two measures of intelligence were excerpted from the fourth edition of the Stanford-Binet Intelligence Scale (Thorndike, Hagen, & Sattler, 1986). In a pattern-analysis task, a complex angular shape was to be reconstructed by arranging blocks with simpler triangular shapes on them. In a vocabulary task, the meanings of various words were to be explained. The Stanford-Binet was the most standard test that we could use, as we were asked by the school system to avoid using the Wechsler tests (which were being used as a screening device for the gifted-student program). The two subtests were chosen because of the following properties: (1) within a factor analysis across ages (Thorndike et al., p. 54), these subtests had relatively high loadings on a g factor reflecting the commonality among all subtests (vocabulary, .76; pattern analysis, .67); (2) vocabulary was a clear example of a verbal, crystallized intelligence subtest, with the highest loading of any subtest on a verbal factor (.47) and low loadings on other specific factors; and (3) conversely, pattern analysis was a clear example of a nonverbal, fluid intelligence subtest, with the highest loading of any subtest on an abstract/visual factor (.65) and low loadings on other specific factors.
Four general predictions can be articulated. (1) The most important experimental prediction of the study, extrapolating from Rosen and Engle (1997) and Kane and Engle (2000) and, is that diverting attention should affect memory much more in adults than in children. (2) Additionally, the visual-array task should reveal growth with age in the scope of attention as measured in the array comparison task, replicating a previous study (Cowan et al., 2005). (3) It is also possible to make predictions about within-age correlations between tasks. If at least part of the variance in aptitudes is shared between the attentional-control task (selective attention) and scope-of-attention (visual arrays) task, that will provide validity for the notion of a common attentional resource that is an important contributor to intelligence. It is of considerable interest whether two very different tasks that have been theoretically associated with different aspects of attention (processing versus storage aspects) will prove to be complementary or redundant in the variance accounted for. (4) Finally, there should be at least two age group differences in these correlations. First, if the control of attention is more important for performance in high-span participants (e.g., Kane & Engle, 2000), then individual differences in the control of attention could play a smaller role in children than in adults because children have lower spans. Second, if simple list-memory measures provide an index of the scope of attention in children too young to rehearse (Cowan et al., 2005) then these measures should correlate with intelligence in the children, who can be considered to be in the upper end of the non-rehearsing range (e.g., Ornstein & Naus, 1978).
Method
Participants
The 104 participants included 52 children (25 female, 27 male) with a mean age of 10 years and 10 months (SD = 5.18 months), who were recruited from the Columbia Missouri Public School District and received $20 and a book for their participation, and 52 University of Missouri-Columbia undergraduates (38 female, 14 male) with a mean age of 20 years and 6 months (SD = 41.41 months), who received course credit in return for their participation.
Design
Given our interest in comparisons between individuals within a group, procedures were administered in a standard order, including (1) an auditory digit span test, (2) a visual letter span test, (3) a dual task to measure capacity, in which auditory digits and visual letters were presented together, (4) a visual array task to measure capacity, (5) a vocabulary test, and (6) a pattern analysis test. Procedures 1 and 2 were used to adjust the difficulty levels for Procedure 3 on an individual basis. Procedures 3 and 4 provided measures of working-memory capacity, whereas Procedures 5 and 6 provided evidence on intellectual abilities.
Apparatus, Stimuli, and Procedure
The study took about 1.5 hours per participant. The first four tasks were computer-administered and the final two tasks were administered according to the instructions for the Stanford-Binet test (Thorndike et al., 1986).
Auditory digit span task
Every participant received the same sequence of digit lists, beginning with six 3-digit lists and proceeding to a test that included three lists per length, starting with 3-digit lists and increasing by one item at a time to a maximum of 9-digit lists. However, the test ended if all three lists of a particular length were recalled incorrectly. For each list, the stimuli making up the standard sequence had been drawn randomly, without replacement, from the set of digits 1 through 9.
At the beginning of each trial a white fixation cross appeared in the center of a black screen. After 750 ms, the fixation cross disappeared and playback of the digit list began. Digitized recordings of the stimuli were presented in a male voice at a fast rate of 500 ms per item (i.e., 2 items per second). All digits were within a range of 65–70 dB(A), as measured with a sound level meter and accompanying earphone coupler, and were presented through TDH-39 audiological headphones. After each list, a series of blank line segments arranged in a row, with one segment per list item, served as a recall cue. As the participant typed the response, the recalled numbers, displayed in a 30-point font, filled in the space previously occupied by the blank line segments. Participants were instructed to use the keyboard’s number keypad to type the digits in the presented order. They were asked to try not to make mistakes, and were given an opportunity to review and correct the answers before pressing a key to indicate that these answers were final. [Cowan, Nugent, Elliott, Ponomarev, and Saults (1999) previously showed that the age difference in digit span within this age range is no larger when a keyboard response is used than when a spoken response is used.] A participant’s digit span was taken to be equal to the length of the longest digit list successfully recalled.
Visual letter span task
The letters A, E, C, F, R, H, I, O, and L were used as stimuli. (There were thus the same number of choices as in the auditory case, and these letters were selected because they are easily discriminable.) The keys corresponding to these letters were marked with colorful, reflective stickers. These stimuli were presented in thirty-point font (15 mm tall), at a rate of one letter per second, each letter replacing the previous one in the center of the screen. In all other ways the visual letter span tasks was administered and scored in the same fashion as the auditory digit span task.
The use of different materials for visual and auditory spans made it impossible to compare the modalities directly. However, comparing them was not a goal of the present study, whereas making the task instructions simple and easy to follow, segregating stimuli in the two modalities, and eliminating intra-group variations in the presentation method (to allow the most powerful examination of individual differences in ability) were the central aims.
Dual-modality memory task
This task included three different types of trial block, distinguished by the stimulus arrangement and the task instructions. (1) In the first type of trial block (auditory-only), participants were to listen carefully to a digit list on each trial. Only spoken digits were presented, and were to be recalled in order using the keypad. (2) In the second type of trial block (attend auditory), participants were to listen carefully to the digit list on each trial and ignore a list of letters that was visually presented one at a time. Following each pair of lists, however, a cue appeared (a picture of an eye or an ear), indicating whether the list of spoken digits or printed letters should be recalled in order using the keypad. Half of the time, the list in the to-be-ignored channel was the one to be recalled. (3) In a third type of trial block (attend visual), the presentation was the same as in #2 but participants were to ignore the spoken digits and detect vowels within the stream of printed letters, pressing the “1” key every time a vowel occurred. Following the lists, as in #2, a cue appeared, indicating which modality should be recalled in order using the keypad.
Despite the fact that trial types were blocked by the modality to be attended during the list presentations, each trial also began with the image of an eye or an ear to remind the participant of the attentional instructions. Thus, on half the trials the eye or ear icon presented before the lists as an attention cue did not match the icon presented after the lists as a recall cue. For an example corresponding to a trial within Block Type 2 or 3, following the attention cue, see Figure 1.
Figure 1.
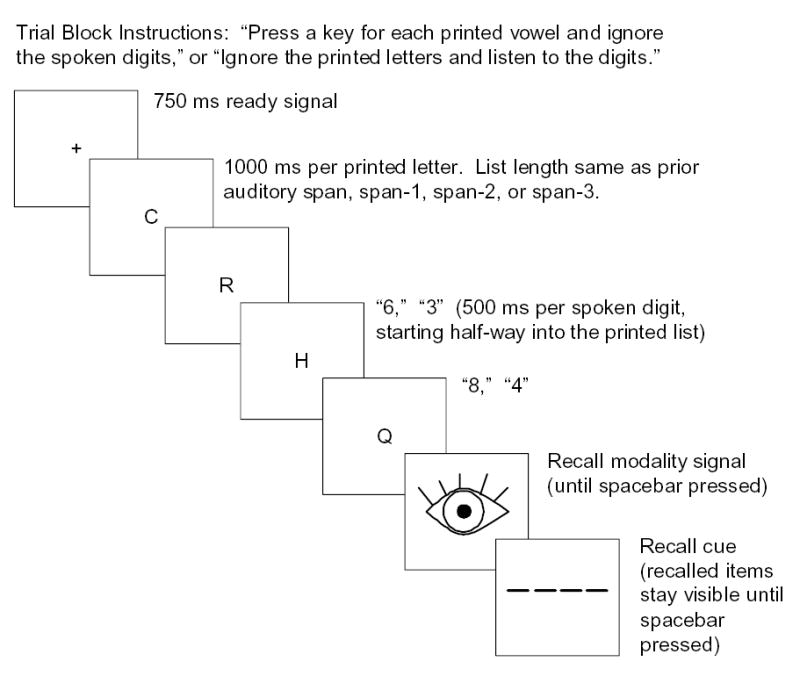
Illustration of a 4-item trial in the dual-modality task. List lengths were adjusted in relation to the auditory span task.
When printed letters were presented (Block Types 2 and 3), they were presented with the same timing as in the visual letter span task, at a 1-item-per-second rate. The auditory stimuli were presented at a faster, 2-item-per- second rate, beginning half-way through the visual sequence so that both visual and auditory sequences, which always included identical numbers of stimuli, ended simultaneously. When the printed letters were omitted (Block Type 1), the spoken digits were presented, again at a 2 item per second rate, after the same delay as if the letters had been presented. The recall cues were 7.3-cm × 11.3-cm, monochromatic pictures of either an eye or an ear, displayed in a white frame against a black background. These recall cues were displayed immediately after the presentation of the last digit and letter, and remained on the screen until the participant pressed the spacebar. For trials presenting both visual and auditory stimuli, the occurrence of either an eye or an ear recall cue was randomly determined, with the constraint that an equal number of each cue type be presented for each task at each list length. After the spacebar was pressed, a recall screen appeared that was identical to that used in the auditory digit and visual letter span tasks. After filling in all the blank lines the word ‘CORRECT’ flashed on the screen. If the participant was satisfied with his or her answer the ‘Y’ key was to be pressed; otherwise the ‘N’ key, after which the responses could be re-entered. The SPACEBAR was to be pressed to continue.
There were 3 practice trials for each type of trial block, presented in the order: Block Type 1, 2, and then 3. Blocks of 16 test trials were then run in the order: Block Type 1, 2, 3, 3, 2, and 1, for a total of 96 trials. In each block, list lengths were adjusted in relation to the longest auditory list length correctly repeated in the auditory digit span task, termed the “span.” Shorter lengths were defined relative to span. For example, “span-2” refers to a list length two items shorter than span. The list lengths were changed within a block in the following fixed order, with a list length defined across runs of 2 trials: span, span-1, span-2, span-3, span-3, span-2, span-1, and span.
Visual arrays task
The visual arrays task was modeled after an experiment in Luck and Vogel (1997). One array of 4, 6, 8, or 10 colored squares was presented, followed by a second array that was identical or that differed from the first only in the color of one square. One square in the second array had a circle around it to indicate that, if any square had changed color, it was that one. This experimental procedure was considered the most appropriate for judging storage capacity because only one decision had to be made (on the cued square), limiting interference. Trials of all array sizes (4, 6, 8, and 10 items) were randomized together in the experiment.
On each trial, which was initiated by the subject, a fixation cross was presented for 1 sec and was followed by a presentation of the first array of squares for 250 msec. At an estimated viewing distance of 50 cm, the array fell within 9.8 degrees horizontal × 7.3 degrees vertical visual angle of view. Squares were placed randomly except that the minimum separation between squares (center to center) and between any square and the fixation point was 2.0 degrees. Each square was 0.74 × 0.74 degrees in visual angle. The square colors were red, blue, violet, green, yellow, black, and white, and each square was assigned a color randomly with replacement (i.e., there was no restriction against the same color appearing more than once in an array, a method that minimizes the ability to answer correctly on the basis of guessing). There was a 1-sec blank interval with a light gray screen between the offset of the first array and the onset of the second array. The target square in the second array, the one that was to be judged, was surrounded by a black circle 1.48 degrees of visual angle in diameter. The subject answered by pressing one computer key to indicate that the color changed between arrays (the “/” key) or another key to indicate that it did not change (the “z” key). If it changed, any post-change color was possible, whether or not that color already was present elsewhere in the array. The inter-trial screen was light gray. There were 64 trials at each array size, or 256 trials in all.
Aptitude tests
In order to examine subjects’ aptitude, two subtests of the Stanford-Binet Intelligence Scale (Thorndike et al., 1986) were used. In the vocabulary subtest, words are presented in written form and also pronounced aloud by the experimenter, and the subject’s task is to explain the meaning of each word. In the pattern analysis test, designs are constructed by the experimenter from a set of black and white blocks according to test instructions and the subject’s task is to reproduce each pattern with a duplicate set of blocks before a time limit that depends on the level of the task. The test begins at a different point for each age group but is designed to assess the absolute performance level in any case. Thus, a rare subject who did not perform up to the age-specific entry level of the test would then move backward to easier levels of the test rather than progressing to harder levels.
Calculation of Capacity Scores
Measures were designed to express performance in terms of capacity. For list-recall tasks (attended and unattended spoken and printed lists), given that a small set of items was used, the score on each trial was taken as the number of items recalled in the correct serial positions, as in previous work (e.g., Cowan et al., 1999). For the visual arrays task, two formulae were tried out. One of these was described by Pashler (1988). His formula is based on the idea that k out of N items in the first array are transferred into working memory. If a changed item happens to be in working memory (with probability k/N) then the change will be noticed. If no change is noticed, the subject will guess that a change has occurred with probability g. Therefore, the capacity formula is k = N*(H-FA)/(1-FA), where H = the proportion of hits (proportion of “change” responses on change trials) and FA = the proportion of false alarms (proportion of “change” responses when in fact no change occurred). The other formula is a modification of Pashler’s formula that seems more suitable to the present version of the task in which a single item within the second array is cued. It is based on the assumption that the correct answer will be available if the cued item is in working memory, whether or not there is a change in color. This occurs on k/N of the trials in which there was a change and, unlike Pashler (1988), on k/N of the trials in which there was no change. If a change is not detected, the subject will guess that a change has occurred with probability g. This leads to the formula k = N*(H-FA), as was shown previously (Cowan, 2001; Appendix A of Cowan et al., 2005). Theoretically, these measures should increase with stimulus set size up to the capacity, after which they should reach an asymptotic level. It is only at this asymptotic level that the capacity is indicated by the measure. The measure that reaches a more stable asymptote thus is probably to be preferred.
Results
We first report experimental effects with an emphasis on age group differences, and then report correlation and regression analyses.
Age Effects
Means and standard deviations for all measures appear in Table 1. For the list-recall tasks, the number correct averaged across list lengths is reported in this table (because this measure is relatively constant across list lengths) whereas, for purposes of examining the data in more detail, a proportion correct measure will be used. Measures that produced a significant age effect in a t test are marked with an asterisk to the right of the adult scores. It is clear that most measures produced significant age effects.
Table 1.
Means (and SDs) for all Tasks in Each Age Group
| Measure | Children | Adults |
|---|---|---|
| Auditory Span | ||
| maximum correct | 6.33 (1.12) | 7.13 (1.09) * |
| average number correct | 4.03 (0.57) | 4.51 (0.49) * |
| Visual Span | ||
| maximum correct | 5.10 (0.98) | 6.31 (0.98) * |
| average number correct | 3.22 (0.57) | 4.07 (0.56) * |
| Dual-Modality Task | ||
| Mean Number of Items Correct | ||
| Attended Auditory | 3.59 (0.88) | 4.26 (0.89) * |
| Attended Visual | 1.68 (0.71) | 3.00 (1.01) * |
| Ignored Auditory | 3.35 (0.88) | 3.36 (0.66) n.s. |
| Ignored Visual | 1.69 (0.65) | 2.26 (0.67) * |
| Auditory Alone | 3.98 (0.84) | 4.76 (0.80) * |
| Vowel detection d’ | 1.93 (0.90) | 2.94 (0.94) * |
| Visual Array Capacity | 2.41 (1.30) | 3.59 (0.97) * |
| Stanford-Binet Intelligence Scale | ||
| Vocabulary | ||
| Raw | 27.02 (3.12) | 37.44 (3.85) * |
| Age-adjusted | 53.37 (6.03) | 53.37 (6.70) n.s. |
| Pattern Analysis | ||
| Raw | 34.12 (5.75) | 39.60 (3.52) * |
| Age-adjusted | 55.69 (7.31) | 53.71 (4.33) n.s. |
Note. Visual Array Capacity refers to the measure of Cowan (2001).
Age difference t test, p < .05, 2-tailed.
Auditory and visual span tasks
Two measures of spans are reported in Table 1. The first measure, maximum number of items correct, yielded the integer span that was necessary to adjust the list lengths in the list-recall tasks. However, for the sake of correlational analyses, a more sensitive measure was needed. The measure selected was the number of items correctly recalled per list, averaged across lists of all lengths. For both measures, the auditory and visual span results are both within a range that is typical for each age group, and they are significantly higher for adults.
List-recall tasks
Further analyses were undertaken to gain a better understanding of performance in the attended- and ignored-list tasks. The mean proportions of items in the list correctly recalled in each condition are shown in Figure 2 for auditory memory and Figure 3 for visual memory, separately for each relative list length (span-3, span-2, span-1, and span) in each figure. These figures suggest that, whereas adults recalled attended lists considerably better than ignored lists (bottom panel of each figure), that was not the case for children (top panel of each figure). The absence of an age effect for ignored auditory stimuli, unlike the other conditions (see Table 1), can be explained on the grounds that these stimuli were truly ignored by adults but received more attention by children, who may have been unable to ignore them.
Figure 2.

Mean proportion of correct items recalled per list for attended and ignored spoken digits, and for an auditory-only control condition, for each relative list length (x axis). Top panel, children; bottom panel, adults. Error bars are standard errors.
Figure 3.

Mean proportion of correct items recalled per list for attended and ignored printed letters, for each relative list length (x axis). Top panel, children; bottom panel, adults. Error bars are standard errors.
To verify this pattern of results, a 2 × 2 × 2 × 4 Analysis of Variance (ANOVA) was carried out on the proportion correct scores in the bimodal trials, with age group between subjects and with three within-subject factors: the modality cued for immediate recall (auditory or visual), its attentional status (i.e., whether the cued modality was to be attended or ignored during the list presentation), and the list length (Span-3, Span-2, Span-1, and Span length). Auditory-only trials were omitted from this analysis. For the sake of simplicity, only effects that include the age group factor will be reported. A measure of the proportion of variance accounted for that we report, ηp2, is calculated as SSeffect/(SSeffect + SSerror).
There was no main effect of age group, which is as expected given that list lengths were adjusted on the basis of individual spans. However, age group interacted with the modality to be recalled, F(1, 102)=34.66, p<.001, ηp2 = .25. Children performed much better on spoken lists (M = .77, SEM = .02) than on printed lists (M = .40, SEM = .02), whereas in adults the difference between spoken lists (M = .72, SEM = .02) and printed lists (M = .51, SEM = .02) was comparatively smaller. There was a small three-way interaction of Age Group × Modality × List Length, F(3, 306) = 4.43, p < .01, ηp2 = .04. The age difference in proportion correct for printed lists, from the shortest list length to the longest, respectively, was .06, .11, .16, and .10, favoring the adults; whereas, for spoken lists, for which performance was higher overall (cf. Figures 2 & 3), the differences were−.06,−.07,−.08, and .01, respectively, generally favoring the children.
Most importantly, age group also interacted with attentional status, F(1, 102) = 25.05, p < .001, ηp2 = .20. As Figures 2 and 3 illustrate, regardless of the list length, children’s memory was about the same for attended (M = .59, SEM = .02) and ignored (M = .57, SEM = .02) lists, whereas adults’ memory was much better for attended (M = .68, SEM = .02) than for ignored (M = .54, SEM = .02) lists. Newman-Keuls tests verified that the effect of attention was significant in the adults, but not in the children.
This result theoretically could be contaminated by differences between children and adults in response strategy in a dual-task situation. Children could choose to attend to both modalities equally instead of allocating more attention to the modality of the primary processing task. Such a difference in strategy could explain the poorer performance in the vowel-detection task in children than in adults (Table 1). However, this possibility can be checked by omitting adults with the best vowel-detection performance and children with the poorest such performance until arriving at subsamples matched in vowel-detection performance (d’). This resulted in N=30 per age group, with a vowel-detection d’ of 2.47 (SD = 0.62) for children and 2.46 (SD = 0.63) for adults. In that subsample, there were still the same significant effects in the proportion correct, most importantly including an interaction of group × attentional status, F(1, 58) = 6.29, p < .02, ηp2 = .10. This interaction is shown in Figure 4. Better memory of items in the attended modality was still a quality that applied to the adults much more strongly than it applied to the children. In fact, Newman-Keuls tests confirmed that it was significant for adults, but not for children.
Figure 4.
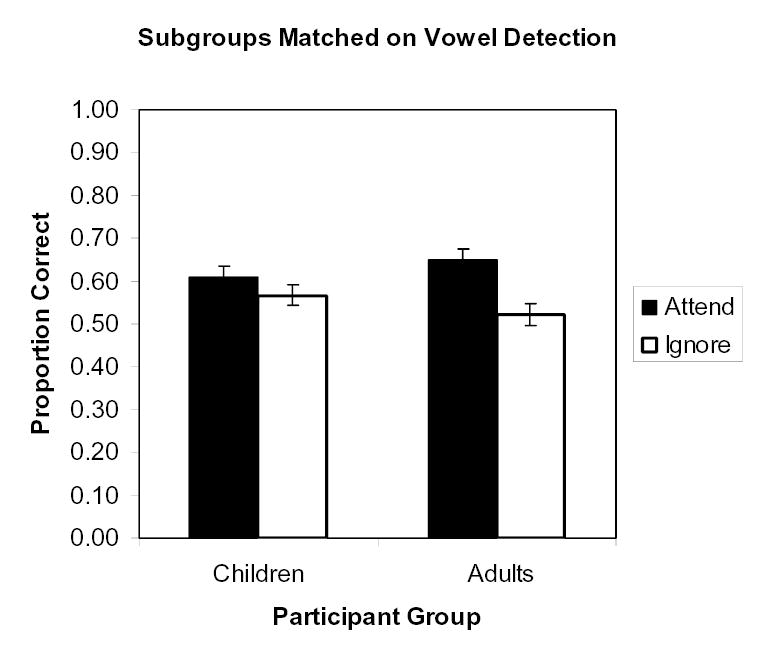
The interaction of age group and attention in the list recall tasks, for 30 children and 30 adults matched for performance (d’) on the vowel-detection task. Error bars are standard errors.
Another indication that children were not as able to modulate attention as adults were comes from a comparison of performance on ignored auditory lists compared to performance on lists that did not include visual stimuli at all. In confirmation of what is shown in Figure 2, an analysis of proportion correct for these two types of lists in the two age groups (with list length as a second within-subject factor) produced a condition × age group interaction, F(1, 102) = 26.87, p < .001, ηp2 = .21. In children, performance on ignored auditory lists (M = .75, SEM = .02) was somewhat poorer than on auditory-only lists (M = .86, SEM = .01), but in adults, performance on ignored auditory lists (M = .64, SEM = .02) was much poorer than on auditory-only lists (M = .87, SEM = .01). Newman Keuls tests showed the difference to be significant at both age groups. Still, the interaction shows that the “ignore auditory” instructions were more successful in the adult group. Moreover, the same interaction was significant in the subsample matched for vowel detection performance, F(1, 58) = 13.96, p < .001, ηp2 = .19. (In children M = .73 & .86 for ignored-auditory and auditory-only lists, respectively, SEM = .03 & .02; for adults, M = .61 & .86, respectively, and SEM = .03 & .02). Newman-Keuls tests again showed the condition effect to be significant at both age groups. Of course, there may be some component of interference from visual stimuli that is not based on attention. Therefore, the comparison of attended versus ignored lists presented previously, which showed no condition difference in children and a large difference in adults, may be a purer indication of group differences in attention control.
We also compared the condition in which attention was directed to the auditory channel and this channel was then tested with the condition in which only an auditory channel was presented. An ANOVA of proportion correct in the two age groups (using the full sample, N = 52 per group) with the condition (attend auditory versus auditory only) and list length (span-4 through span) as within-subject factors produced no main effect of age group or interaction of age group with any other factors, F < 1.05 in each case. Across both groups, performance was better for the auditory-only condition than for the attend-to-auditory condition, F(1, 102) = 69.25, p < .001, ηp2 = .41 (children, M = .86 vs. .79; adults, .87 vs. .79). This suggests that there may be some type of resource that is inevitably allocated to the visual stimuli that are to be ignored. For example, phonological memory for spoken items may suffer interference when people automatically and silently read the ignored letters and produce phonological representations of them. This is the converse of the cross-modal Stroop-like effect (Cowan & Barron, 1987; Elliott, Cowan, & Valle-Inclan, 1998) and the irrelevant-speech or irrelevant-sound effect (Colle & Welsh, 1976; Jones, 1999; Salamé & Baddeley, 1982), in which spoken stimuli interfere with printed words. It suggests that not only spoken stimuli but also printed verbal stimuli can have automatic access to the phonological system, as one might conclude also from the original effect of Stroop (1935).
In principle, it would be possible for a participant to close his or her eyes when the visual modality was to be ignored (counter to the task instructions). However, we found that, on every visual trial in both age groups, every participant correctly recalled at least one printed letter, indicating that participants’ eyes could not have been closed throughout the presentation. Moreover, as Figure 3 shows, for ignored visual lists there was a large effect of the list length with very small error bars, which could occur only if the stimuli were rather consistently seen.
Visual array task
Hits refer to detection of a change between the standard and comparison arrays and false alarms refer to answers indicating that there was a change when in fact there was not. Collapsed across set sizes, the mean hit rate of children in the visual array task was .68 (SD = .17) and the mean false alarm rate was .30 (SD = .18). For adults, the mean hit rate was slightly higher at .73 (SD = .10) and the mean false alarm rate was considerably lower at .17 (SD = .09). The main analyses for this task were, however, capacity estimates as described above. An important characteristic of the estimate is that it should not change as a function of the set size above the capacity limit. Figure 5 plots one type of estimate (Cowan, 2001; Cowan et al., 2005, Appendix A), which was fairly constant across set sizes for the children and fairly constant for adults with set sizes above 4. Some adults may have a capacity higher than 4, resulting in a ceiling effect for the estimate at that set size. Table 1 shows that this estimate increased markedly, and significantly, from children to adults. In contrast, the estimates using the original method of Pashler (1988) were less constant across the four set sizes: for the children, 2.75, 3.05, 3.18, and 3.35, respectively and, for the adults, 3.39, 4.36, 4.86, and 4.46.
Figure 5.
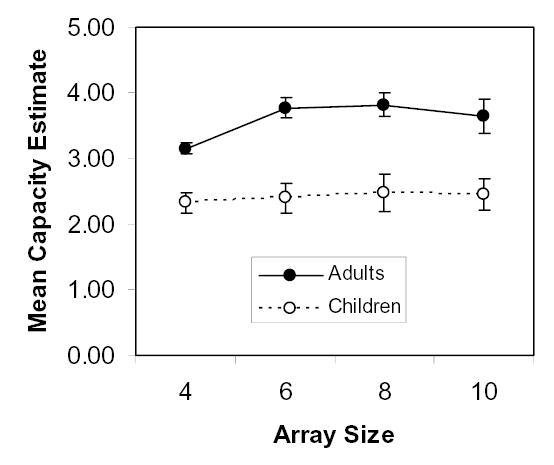
Estimates of adults’ and children’s working memory capacity for items in visual arrays of different set sizes (x axis), based on the capacity estimation formula of Cowan and colleagues (Cowan, 2001; Appendix A of Cowan et al., 2005). Error bars are standard errors.
Aptitude tests
The aptitude test scores, summarized in Table 1, show that there were marked improvements from children to adults in the absolute level of performance, both on the vocabulary test and on the pattern analysis test. Nevertheless, the age-adjusted scores were not significantly different in children versus adults. Therefore, group differences reasonably can be attributed to maturational differences rather than sample selection differences.
Correlations and Regressions
Selected measures were used to characterize the extent to which potential indices were related to intelligence, and to each other. To characterize intelligence, we used a score equal to the sum of z scores for the verbal and performance measures. In order to compare the benefit of attention in the list-recall tasks to other measures, we devised a single score that would reflect that benefit. This score, termed the “attention benefit ratio,” was taken as the average proportion correct on trials in which the modality to be attended was also tested, divided by the average proportion correct on trials in which the modality to be attended turned out not to be the one tested. Given that the shortest (span-3) list conditions generally yielded lower standard deviations than the other three conditions, it was excluded and this ratio was based on the averages across the three longer list conditions. This ratio for the children was 1.08 (SD = 0.29), and for the adults it was 1.34 (SD = 0.36). The difference was highly significant, t(102) = 3.96, p < .001. We also included the d’ measure of vowel detection for visually-presented letters. This is a possible index of vigilance and the adequacy of attention to the visual modality during the bimodal presentation of lists. We included the average capacity estimate on the visual array task (the revised estimate), which presumably indicates the scope of attention (Cowan et al., 2005). Finally, we included the visual and auditory spans (the more sensitive, average number correct measure) and performance in the auditory-only condition following span.
Correlations between these measures are shown separately for the adults and children, in Table 2. This table shows different patterns of correlations for the two age groups, for reasons that are not difficult to understand.
Table 2.
Correlations Between Selected Measures
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. IQ Composite | .95/.90 | .18 | .13 | .44* | .45* | .38* | .08 |
| 2. Attention Benefit (Lists) | .47* | .70/.67 | .17 | .05 | .07 | −.22 | .01 |
| 3. Vowel Detection (Lists) | −.08 | .02 | .88/.92 | .39* | −.02 | .05 | .03 |
| 4. Visual Arrays | .52* | .34* | .16 | .69/.78 | .34* | .28* | .25 |
| 5. Visual span | .25 | .41* | .15 | .38* | .68/.71 | .66* | .20 |
| 6. Auditory span | .23 | .18 | .16 | .23 | .45* | .74/.78 | −.08. |
| 7. Auditory-only | −.08 | .26 | .15 | .16 | −.22 | −.29* | .77/.68 |
Note. N=52 for all correlations. The correlations below the diagonal are for adults and those above the diagonal are for children. “Attention benefit” refers to the attention benefit ratio described in the text. The basis of the attention benefit and auditory-only measures was the mean proportion correct for all list lengths except the shortest (Span-3). Auditory and visual spans refer to the average number correct. On the diagonal in bold: reliability statistics for adults/children. All are Cronbach’s Alpha measures except those for the IQ composite, which are Kuder-Richardson Formula 2 scores from the manual of Thorndike et al. (1986, p. 39) averaged across the Vocabulary and Pattern Analysis tests (vocabulary, .96/.93; pattern analysis, .94/.87).
p < .05, 2-tailed
Adults
In the adults (below the diagonal), both the attention benefit ratio and the visual-array task performance showed substantial, significant correlations with intelligence. These two different attention measures also were correlated with one another. Given that the attention benefit is an attention-control measure and the visual array task is a scope-of-attention measure (Cowan et al., 2005), this appears to indicate that these two attention constructs have something in common.
It is useful to dissect performance into the conditions contributing to the attention benefit ratio. In adults, the average of the ignored-modality conditions contributing to this ratio were negatively correlated with the intelligence composite, r =−.45, p < .001. That is, better performance in this condition was found for individuals with lower IQ composites. However, it was not correlated with array comparisons, r =−.17, n.s., and the average of the attended-modality conditions was not correlated with either intelligence, r = .14, n.s., or array comparisons, r = .23, n.s.
To address the question of how much variance in the prediction of intelligence is shared between the scope of attention and the control of attention in adults, a regression approach was used. When the attention benefit ratio and visual-array performance scores were used together to predict intelligence, 37% of the variance in intelligence was accounted for. On the basis of that, along with regressions in which only one of the predictors was used, it could be calculated that 12% of the variance in intelligence was accounted for by both tasks together; another 10%, uniquely by the attention benefit ratio; and another 15%, uniquely by visual-array performance. The unique contributions of both variables and their joint contributions all were significant, p < .01. On this basis, it can be concluded that the portions of variance in intelligence related to the control of attention and the scope of attention appear to be rather separate mechanisms, at least as measured by these constructs.
Vowel detection did not prove to tap an important source of individual differences related to these other concepts, perhaps because it was so easy for adults (Table 1). Also, as in many other studies with adults, the measures simply involving serial recall of a list (auditory span, visual span, and auditory-only recall) were not good predictors of intelligence (cf. Daneman & Merikle, 1996).
Children
The pattern of correlations was different for children (Table 2, above the diagonal). The attention benefit ratio was not correlated with the other measures, in contrast to the adults. When correlations were examined separately for ignored lists (correlations with IQ composite and array comparison performance) and for attended lists (with the same two variables), none of these correlations was significant (r = .02, .08, .23, & .14, respectively; n.s. in each case). The problem was probably not a lack of reliability for any of the measures; as shown in Table 2, the reliabilities were not very different from those obtained in adults. Inspection of the children’s scores showed that several outliers (three with benefit ratios above 1.7, and one with a benefit ratio of below 0.7) had mid-range IQ composite scores. However, the attention benefit ratios did fall into a narrower range in children, as noted above (SD = .29 in children versus .36 in adults).
In order to understand the meaning of the attention benefit ratio, a median split on this variable was carried out separately in the children and the adults, and an ANOVA for attended and ignored lists across all list lengths was examined in each age group. In neither age group was a higher attention benefit ratio associated with significantly better memory performance overall. In adults, the overall proportion correct with a low ratio was .60; with a high ratio, .63. In children, the proportion with a low ratio was .59; with a high ratio, .58. In contrast, the IQ composite was significantly lower in participants with a low attention benefit ratio than with a high ratio (children, −1.52 vs. −1.13; adults, 0.98 vs. 1.67), though this difference was significant only in adults (t = 3.10) and not in children (t = 1.12). Therefore, it appears that the attention benefit ratio reflects the ability or willingness to follow the attention instructions, not the ability to remember items in the lists. It must be noted that this pattern of results does not imply that young children cannot control attention; indeed, other literature suggests that they can do so to some extent (e.g., Gomes et al., 2000; Lane & Pearson, 1982). The result suggests only that making use of selective attention for mnemonic purposes demanded a level of control that was generally beyond what most of the children, and many of the adults, could manage.
As in the adults, in the children there was a substantial, significant correlation between intelligence and visual-array task performance, again confirming the results of Cowan et al. (2005, Experiment 2). The vowel-detection task performance was related to visual-array performance, but not to intelligence. This common variance might be based on some visual-modality-specific perceptual ability that is used to spot colors in arrays and vowels in visually-presented sequences.
Cowan et al. (2005) found that, in contrast to the conventional wisdom based on the adult literature, simple auditory span correlated well with intelligence measures in children too young to engage in sophisticated rehearsal (see also Hutton & Towse, 2001). The children in the present paper are at the upper end of this range (see, for example, Ornstein & Naus, 1978) and, similar to Cowan et al., memory for lists in children (visual and auditory spans and performance in the auditory-only condition) were significantly related to intelligence, as shown in Table 2. These results suggest that, when rehearsal is not possible, even simple span tasks may index the scope of attention.
It also is possible for non-attentional, domain-specific components of storage, like the phonological and visuospatial buffers of Baddeley (1986), to contribute to intelligence measures. To determine how much of the contribution of visual-array performance versus span for spoken and printed verbal materials arose from a common pool of variance versus domain-specific skills, a regression on the IQ composite measure was carried out. The regressions indicated that 10% of the variance in IQ was shared between spans and the visual array task, another 11% was unique to the span tasks, and another 9% was unique to the visual arrays task. The unique and joint contributions of the span and visual-array variables all were significant at p < .05 or better. A further breakdown showed that, of the 10% of variance in common with the visual-array task, 7% could be attributed to both the auditory digit and the visual letter span tasks together and the other 3%, uniquely to the visual letter span task. Similarly, of the 11% of the variance unique to the span tasks, 6% could be attributed to both span tasks together, 4% to the visual letter span task alone, and only 1% to the spoken digit span task alone. These regression analyses indicate that the amodal, presumably attention-demanding part of memory storage in children exists but is complemented by modality-specific storage.
Discussion
Many recent studies of working memory have used a procedure in which individuals in the high and low quartiles of working-memory span are compared in performance on various tasks (e.g., Bleckley et al., 2003; Conway et al., 2001; Kane et al., 2001; Kane & Engle, 2003; Unsworth et al., 2004). The present study used a developmental comparison between children and adults as an extreme-group comparison, partly to determine if analogues to the results observed in past studies of high- and low- span individuals could be obtained, including better control of attention in high-span individuals (like Kane et al., 2001) and, in these individuals, a greater impact of dividing attention on the recall of incidental materials (like Conway et al., 2001). Indeed, that was the case. Important within-group correlations with an intelligence index also were obtained. A summary of results will be followed by a more in-depth discussion of the theoretical implications.
In a task of selective attention in which either the attended or unattended modality was then tested, there was a pronounced age difference. Whereas adults performed much better on spoken or printed lists that were fully attended than when attention was split, that was not the case in children. This age difference in the use of attention is clearly evident in both of the slightly different task arrangements used to examine the recall of spoken lists (Figure 2), on one hand, and printed lists (Figure 3), on the other. Most strikingly, even when performance on the primary attention task (vowel detection) was matched between age groups, adults were much more able to use full attention for superior encoding and memory, as shown in Figure 4. In children, the differential allocation of attention during encoding during a dual task just did not make much difference for memory.
On the basis of these findings, it seems reasonable to conclude that it is the internal control of attention in the service of memory that was poorer in children. Even when they attended to the visual stimuli enough to carry out the primary visual task (vowel detection) as well as adults, this did not leave them able to use this direction of attention for the benefit of memory for the printed letters.
In a visual array comparison task, the capacity estimates were considerably higher in adults than in children and closely replicated the findings of Cowan et al. (2005). There are still some important details to work out in future research, given that Ross-Sheehy, Oakes, and Luck (2003) found higher capacity estimates in infants than we did in children. However, the procedures were very different in these studies. (The changing stimulus was a series of arrays with a change in the color of a different object in each array in the series, and therefore it would be possible to achieve perfect detection even if only a subset of the items were processed.) It is a considerable challenge to find comparable procedures that could allow conclusions about the full life span development of visual working memory in this sort of task.
Within-age correlations and regressions were quite revealing. Importantly, the present result replicated the finding of Cowan et al. (2005) that performance on the array-comparison task, taken as an indication of the capacity or scope of attention, correlates with intelligence in both adults and children. Also, in the adult group, the amount of benefit from attention to a modality in the list-memory task (in keeping with the instructions) had a high correlation with the intelligence measures. The level of attention demanded by the task may have been too high to distinguish among children as clearly, and thus they did not show the effect.
This is one of the first studies in which the correlation that is to be expected (e.g., by Engle et al., 1999) between performance in an attention-allocation task and intelligence measures actually has been directly observed (see also Schweizer & Moosbrugger, 2004). It is especially important inasmuch as another recent study (Friedman et al., in press) observed correlations between intelligence and one executive function of working memory (updating), but not between intelligence and two others that also are attention-related (shifting and inhibition). The present findings indicate that selective attention to one modality in a bimodal presentation, which is conceptually related to inhibition, also can correlate with intelligence. The explanation for the implied discrepancy with Friedman et al. (in press) must await further research.
It was found that adults who recalled more of the to-be-ignored modality were relatively low in the IQ composite measure (whereas memory for to-be-attended material did not correlate with the IQ measure). This is comparable to the finding of Conway et al. (2001) that individuals who recalled their name in a to-be-ignored auditory channel were relatively low in an operation span measure of working memory. The contribution of the present finding is to establish this point across both modalities, and for intelligence as opposed to working memory as a distinguishing factor.
The amount of variance in intelligence shared between the attention-control (selective attention) and scope-of-attention (array comparison) tasks in adults was found to be substantial, though less than complete. In particular, 11% of the variance of the two tasks was shared between them (i.e., the squared correlation), and 12% of the variance in intelligence was shared with both of these attention-related tasks in common (based on a regression). This was about a third of the 37% of the variance in intelligence picked up by the two attention-related tasks together, including both joint and unique sources of variance. The use of tasks related to different aspects of attention thus seems quite promising as a way to assist our understanding of intelligence.
In the children, who were young enough that rehearsal strategies are not fully mature (Bjorklund & Douglas, 1997; Ornstein & Naus, 1978), there was a correlation between unimodal list recall procedures and intelligence, whereas those correlations were not significant in adults. This replicates the pattern observed by Cowan et al. (2005). Simple list recall may partly reflect the scope of attention in children too young to engage in sophisticated rehearsal. Indeed, two of the list-recall measures, visual span and auditory-only list recall, were significantly related to visual-array performance (the primary measure of the scope of attention) in children, but not in adults (see Table 1). A substantial amount (10%) of the variance in intelligence was accounted for by three simple memory tasks in common (the two span tasks and visual array comparisons), though code-specific, visual and verbal types of memory both made additional, unique contributions to the prediction of intelligence of about the same magnitude as their joint contribution.
Theoretical Implications
The present results have important implications for theories of working memory, attention, intelligence, and their development in childhood. It supports current theories that tie working memory to attention and reconciles those that emphasize the control of attention (e.g., Engle et al., 1999; Kane et al., 2001) with those that emphasize attention as storage (e.g., Cowan et al., 2005; cf. Anderson, 1983; Just & Carpenter, 1992; Lovett, Reder, & Lebière, 1999). In the most-often-cited theory of working memory (Baddeley, 1986; Baddeley & Logie, 1999), passive storage buffers that hold information automatically and effortlessly are controlled by central executive processes, which consume attention and use it to influence memory encoding and retrieval. In a more recent update of that model (Baddeley, 2000, 2001), there is also storage of more abstract information in a mechanism termed the episodic buffer, the attention requirements of which have not been specified. Thus, the updated model of Baddeley does not necessarily contradict the premise of Cowan (1988, 1995, 1999, 2001) and Cowan et al. (2005) stating that attention can be used not only to control information (as in the present selective attention task), but also to retain information (as in the present visual array comparison task).
Storage of information in the focus of attention presumably supplements storage in the passive buffers of Baddeley (1986) or the activated portion of long-term memory of Cowan (1988). Storage in the focus of attention presumably makes the information more resistant to interference. For example, Cowan, Johnson, and Saults (2005) carried out a task in which, on every trial, a probe word was to be judged present or absent from a list that had been presented. Proactive interference from previous lists with semantically similar words occurred for lists that were presumably too long to be held in the focus of attention (those with 6 or 8 words), but not for lists that were presumably short enough to be held in the focus of attention (those with 3 or 4 words). Resistance to interference is important because passive buffers or the activated portion of long-term memory may not have this property. For example, in the array comparison task (Luck & Vogel, 1997), the second array may overwrite the first before a decision can be made, so retention of an interference-resistant form of memory is crucial for successful performance of the task.
According to a slightly different view (Oberauer, 2002, 2005), the focus of attention holds only 1 item and is surrounded by a fringe of up to 3 or 4 other items. This view was based on studies in which only a subset of the presented items is eligible for updating and only one of the eligible items was last selected for updating. However, a way to explain such data without postulating multiple discrete levels of attention (the small focus and its larger fringe) is to postulate that the focus of attention is capable of holding 3 to 5 independent chunks (Cowan, 2001), but with an internal prioritization of the items in the focus of attention. Such a prioritization was already required to account for the finding that probed reaction time often differs as a function of serial position in a list, even for lists of 1 to 4 items that can be held in the focus of attention (e.g., McElree & Dosher, 1989).
Other theoreticians have attempted to explain individual and developmental differences in working memory and intellectual aptitude. What became the standard view was described for individual differences by Daneman and Carpenter (1980) and for developmental differences by Case et al. (1982). In this view, given that working memory includes both storage and processing components, it is necessary to use working memory tests that include both storage and processing components. The view was considered successful inasmuch as the correlations between storage-and-processing tests of working memory and tests of intellectual aptitudes were much higher than the correlations between simple storage tests of working memory and these aptitude tests. However, Cowan et al. (2005) showed that comparable high correlations could be obtained with tests that did not include a separate storage component, provided that it was not possible to carry out rehearsal processes. The presumed reason was that the focus of attention was needed more heavily for storage when rehearsal could not be used, in which case the measure assessed the focus of attention. The present findings reproduce this conclusion by showing, once more, that high correlations with intelligence were obtained for a storage-only task for which rehearsal is not feasible (visual array comparisons).
In recent years, several theorists have suggested that individual and developmental differences in working memory result from differences in the functions of attention. Engle et al. (1999) and Kane et al. (2001) suggested that what is critical is the ability to control attention. Similarly, others (Gernsbacher, 1993; Hasher, Stolzfus, Zacks, & Rypma, 1991; May, Hasher, & Kane, 1999; Lustig et al., 1999) have suggested that what is critical for working memory is the ability to inhibit irrelevant but often salient information, and therefore to make the most efficient use of storage. Inhibition can be viewed as an example of the control of attention. Cowan et al. (1995) suggested instead that what is critical is, more generally, the capacity of the focus of attention, which can zoom in to maintain a goal in the presence of interference (e.g., remembering to name the color and not read the word aloud in the task of Stroop, 1935), zoom out to apprehend and retain concurrently as many items as possible (e.g., remembering colored spots in an array in the task of Luck & Vogel, 1997), or adopt a configuration that is intermediate, attempting to handle both multiple-item retention and interference concurrently (e.g., recalling the part of a list presented in a particular print color if the retrieval cue is presented in a different color; see Bunting & Cowan, 2005).
However, the present work helps to resolve two important issues that remain open. First, it confirms that the control of attention (Kane et al., 2001), and not only attention as used for storage (Cowan et al., 2005; Morey & Cowan, 2004, 2005) or updating (Friedman et al., in press), can be a good predictor of intelligence. Second, it addresses the relation between the control of attention and the use of attention as a storage device. These two concepts, represented by a selective attention task and a visual array comparison task, respectively, were seen to be partly independent and partly overlapping in their relation to each other and to intelligence. This is also a good way to think of these two concepts theoretically. Cowan (1995, 1999) argued that the control of attention is represented by a neural system that heavily includes frontal lobe structures, whereas the focus of attention (where the information is held) is represented by a neural system that more heavily includes parietal lobe structures. The argument was based on both differences in the types of impairment that accompany damage to frontal versus parietal areas (Schacter, 1989), and evidence from early neuroimaging studies (Posner & Peterson, 1990) and has been confirmed by more recent research (e.g., Postle et al., 1999). Yet, these two neural systems work together quite closely, and are often referred to in recent literature as a common frontal-parietal system. It therefore makes sense neurally, as well as behaviorally, to think of the control of attention and the focus of attention as mechanisms that are partly overlapping and partly independent from one another.
The present work may also have implications for the measurement and theory of intelligence, as summarized for example by Wilhelm and Engle (2005). The traditional approach has been to include in tests of intelligence any subtest that correlates well with success in a criterion measure, such as job or school performance. However, different subtests are likely to emphasize different subsets of skills that may contribute to success (attentional control, memory storage, knowledge, strategic thinking, reasoning, and so on). It would be quite helpful to have tests that are known to indicate particular critical abilities, rather than a wide swath of abilities taken together. Given that the combination of attentional control and attentional storage of information appears to correlate well with intelligence measures, it is certainly worth asking in future research how well these aspects of attention and working memory predict success in school or in the work place.
The present data and other literature lead to expectations that the best predictors of intellectual aptitude will change across childhood. There are at least two bases of expected change. First, as children learn to use verbal rehearsal mechanisms in middle and late childhood, simple measures of memory storage of verbal lists (e.g., digit span) should become poorer predictors of intelligence (cf. Cowan et al., 2005). Second, as frontal lobe areas in the brain mature during childhood and adolescence (e.g., Kane & Engle, 2002; Rabinowicz, 1980; Yakovlev & Lecours, 1967) and the accompanying improvement in the control of attention takes place (e.g., Guttentag, 1997; Lane & Pearson, 1982), the variety of attention-control tasks that can be used to predict intelligence should increase. Inter-task correlations in the present data set (Table 2) appear to reflect these trends.
In sum, the present study illustrates that the relation between attention and intelligence is real, and that it extends to children as a group that have much lower spans than adults. It should be a priority to carry out further research to investigate different “flavors” of attention task: those that reflect the control of attention, and those that reflect its scope, or capacity to apprehend multiple items simultaneously.
Acknowledgments
This work was carried out with the support of NIH grant R01 HD-21338 to Cowan. Address correspondence to Nelson Cowan, Department of Psychological Sciences, University of Missouri, 18 McAlester Hall, Columbia, MO 65211. E-mail: CowanN@missouri.edu.
References
- Anderson, J.R. (1983). The architecture of cognition. Cambridge, MA: Harvard University Press.
- Atkinson, R. C., & Shiffrin, R. M. (1968). Human memory: A proposed system and its control processes. In K. W. Spence & J. T. Spence (Eds.), The psychology of learning and motivation: Advances in research and theory (Vol. 2, pp. 89–195). New York: Academic Press.
- Baddeley, A.D. (1986). Working memory. Oxford Psychology Series #11. Oxford: Clarendon Press.
- Baddeley A. The episodic buffer: a new component of working memory? Trends in cognitive sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The magic number and the episodic buffer. Behavioral and Brain Sciences. 2001;24:117–118. [Google Scholar]
- Baddeley, A., & Hitch, G.J. (1974). Working memory. In G. Bower (ed.), Recent advances in learning and motivation, Vol. VIII. New York: Academic Press.
- Baddeley, A.D., & Logie, R.H. (1999). Working memory: The multiple-component model. In A. Miyake & P. Shah (eds.), Models of Working Memory: Mechanisms of active maintenance and executive control. Cambridge, U.K.: Cambridge University Press. (pp. 28–61)
- Bjorklund, D.F., & Douglas, R.N. (1997). The development of memory strategies. In N. Cowan (ed.), The development of memory in childhood. Hove, East Sussex, UK: Psychology Press. (pp. 201–246)
- Bleckley MK, Durso FT, Crutchfield JM, Engle RW, Khanna M. Individual differences in working memory capacity predict visual attention allocation. Psychonomic Bulletin & Review. 2003;10:884–889. doi: 10.3758/bf03196548. [DOI] [PubMed] [Google Scholar]
- Bunting MF, Cowan N. Working memory and flexibility in awareness and attention. Psychological Research. 2005;69:412–419. doi: 10.1007/s00426-004-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R, Kurland DM, Goldberg J. Operational efficiency and the growth of short-term memory span. Journal of Experimental Child Psychology. 1982;33:386–404. [Google Scholar]
- Cherry EC. Some experiments on the recognition of speech, with one and with two ears. The Journal of the Acoustical Society of America. 1953;25:975–979. [Google Scholar]
- Cocchini G, Logie RH, Della Sala S, MacPherson SE, Baddeley AD. Concurrent performance of two memory tasks: Evidence for domain-specific working memory systems. Memory & Cognition. 2002;30:1086–1095. doi: 10.3758/bf03194326. [DOI] [PubMed] [Google Scholar]
- Colle HA, Welsh A. Acoustic masking in primary memory. Journal of Verbal Learning and Verbal Behavior. 1976;15:17–32. [Google Scholar]
- Conlin JA, Gathercole SE, Adams JW. Children’s working memory: Investigating performance limitations in complex span tasks. Journal of Experimental Child Psychology. 2005;90:303–317. doi: 10.1016/j.jecp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Conway RA, Cowan N, Bunting MF. The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin & Review. 2001;8:331–335. doi: 10.3758/bf03196169. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Cowan N, Bunting MF, Therriault DJ, Minkoff SRB. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30:163–183. [Google Scholar]
- Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information processing system. Psychological Bulletin. 1988;104:163–191. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- Cowan, N. (1995). Attention and memory: An integrated framework. Oxford Psychology Series, No. 26. New York: Oxford University Press. (Paperback edition: 1997)
- Cowan, N. (1999). An embedded-processes model of working memory. In A. Miyake & P. Shah (eds.), Models of Working Memory: Mechanisms of active maintenance and executive control. Cambridge, U.K.: Cambridge University Press. (pp. 62–101)
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan N, Barron A. Cross-modal, auditory-visual Stroop interference and possible implications for speech memory. Perception & Psychophysics. 1987;41:393–401. doi: 10.3758/bf03203031. [DOI] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, Conway ARA. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Johnson TD, Saults JS. Capacity limits in list item recognition: Evidence from proactive interference. Memory. 2005;13:293–299. doi: 10.1080/09658210344000206. [DOI] [PubMed] [Google Scholar]
- Cowan N, Nugent LD, Elliott EM, Ponomarev I, Saults JS. The role of attention in the development of short-term memory: Age differences in the verbal span of apprehension. Child Development. 1999;70:1082–1097. doi: 10.1111/1467-8624.00080. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning & Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Daneman M, Merikle PM. Working memory and language comprehension: A Meta-Analysis. Psychonomic Bulletin & Review. 1996;3:422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- Doyle A. Listening to distraction: A developmental study of selective attention. Journal of Experimental Child Psychology. 1973;15:100–115. doi: 10.1016/0022-0965(73)90134-3. [DOI] [PubMed] [Google Scholar]
- Duff SC, Logie RH. Processing and storage in working memory span. Quarterly Journal of Experimental Psychology. 2001;54 A:31–48. doi: 10.1080/02724980042000011. [DOI] [PubMed] [Google Scholar]
- Elliott EM, Cowan N, Valle-Inclan F. The nature of cross-modal, color-word interference effects. Perception & Psychophysics. 1998;60:761–767. doi: 10.3758/bf03206061. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The reading span test and its predictive power for reading comprehension ability. Journal of Memory and Language. 2004;51:136–158. [Google Scholar]
- Friedman, N.P., Miyake, A., Corley, R.P., Young, S.E., DeFries, J.C., & Hewitt, J.K. (in press). Not all executive functions are related to intelligence. Psychological Science. [DOI] [PubMed]
- Gathercole SE, Baddeley AD. Evaluation of the role of phonological STM in the development of vocabulary in children: A longitudinal study. Journal of Memory and Language. 1989;28:200–213. [Google Scholar]
- Gernsbacher MA. Less skilled readers have less efficient suppression mechanisms. Psychological Science. 1993;4:294–298. doi: 10.1111/j.1467-9280.1993.tb00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes H, Molholm, Christodoulou C, Ritter W, Cowan N. The development of auditory attention in children. Frontiers in Bioscience. 2000;5:d108–120. doi: 10.2741/gomes. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Guttentag, R. (1997). Memory development and processing resources. In N. Cowan (ed.), The development of memory in childhood. Hove, East Sussex, UK: Psychology Press. (pp. 247–274)
- Hasher L, Stolzfus ER, Zacks RT, Rypma B. Age and inhibition. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1991;17:163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Hutton UMZ, Towse JN. Short-term memory and working memory as indices of children’s cognitive skills. Memory. 2001;9:383–394. doi: 10.1080/09658210042000058. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Restricted attentional capacity between sensory modalities. Psychonomic Bulletin & Review. 1999;6:87–92. doi: 10.3758/bf03210813. [DOI] [PubMed] [Google Scholar]
- Jones D. The cognitive psychology of auditory distraction: The 1997 BPS Broadbent Lecture. British Journal of Psychology. 1999;90:167–187. [Google Scholar]
- Just M, Carpenter PA. A capacity theory of comprehension: Individual differences in working memory. Psychological Review. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway ARA, Engle RW. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity, proactive interference, and divided attention: Limits on long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:336–358. doi: 10.1037//0278-7393.26.2.336. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Lane DM, Pearson DA. The development of selective attention. Merrill-Palmer Quarterly. 1982;28:317–337. [Google Scholar]
- Lépine R, Barrouillet P, Camos V. What makes working memory spans so predictive of high level cognition ? Psychonomic Bulletin & Review. 2005;12:165–170. doi: 10.3758/bf03196363. [DOI] [PubMed] [Google Scholar]
- Lovett, M.C., Reder, L.M., Lebière, C. (1999). Modeling working memory in a unified architecture: An ACT_R perspective. In, A. Miyake, & P. Shah (Eds.), Models of working memory: Mechanisms of active maintenance and executive control (pp. 135–182). Cambridge: Cambridge University Press.
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. Journal of Experimental Psychology: General. 2001;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Kane MJ. The role of interference in memory span. Memory & Cognition. 1999;27:759–767. doi: 10.3758/bf03198529. [DOI] [PubMed] [Google Scholar]
- McElree B, Dosher BA. Serial position and set size in short-term memory: The time course of recognition. Journal of Experimental Psychology: General. 1989;118:346–373. [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review. 1956;63:81–97. [PubMed] [Google Scholar]
- Miyake, A., & Shah, P. (eds.) (1999). Models of Working Memory: Mechanisms of active maintenance and executive control. Cambridge, U.K.: Cambridge University Press.
- Moray N. Attention in dichotic listening: Affective cues and the influence of instructions. Quarterly Journal of Experimental Psychology. 1959;11:56–60. [Google Scholar]
- Morey CC, Cowan N. When visual and verbal memories compete: Evidence of cross-domain limits in working memory. Psychonomic Bulletin & Review. 2004;11:296–301. doi: 10.3758/bf03196573. [DOI] [PubMed] [Google Scholar]
- Morey CC, Cowan N. When do visual and verbal memories conflict? The importance of working-memory load and retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:703–713. doi: 10.1037/0278-7393.31.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer K. Access to information in working memory: exploring the focus of attention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:411–421. [PubMed] [Google Scholar]
- Oberauer K. Control of the contents of working memory—A comparison of two paradigms and two age groups. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:714–728. doi: 10.1037/0278-7393.31.4.714. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Demmrich A, Mayr U, Kliegl R. Dissociating retention and access in working memory: An age-comparative study of mental arithmetic. Memory & Cognition. 2001;29:18–33. doi: 10.3758/bf03195737. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Lange E, Engle RE. Working memory capacity and resistance to interference. Journal of Memory and Language. 2004;51:80–96. [Google Scholar]
- Ornstein, P.A., & Naus, M.J. (1978). Rehearsal processes in children’s memory. In P.A. Ornstein (ed.), Memory development in children. Hillsdale, NJ: Erlbaum. (pp. 69–99)
- Pashler H. Familiarity and visual change detection. Perception & Psychophysics. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Penney CG. Modality effects and the structure of short-term verbal memory. Memory & Cognition. 1989;17:398–422. doi: 10.3758/bf03202613. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Enns JT, Brodeur D. The development of selective attention: A life_span overview. Acta Psychologica. 1994;86:227–272. doi: 10.1016/0001-6918(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Posner MI, Peterson SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D’Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proceedings of the National Academy of Science. 1999;96:12959–12964. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Druzgal TJ, D’Esposito M. Seeking the neural substrates of visual working memory storage. Cortex. 2003;39:927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- Rabinowicz, T. (1980). The differentiate maturation of the human cerebral cortex. In F. Falkner & J.M. Tanner (Eds.), Human Growth, V. 3: Neurobiology and Nutrition. NY: Plenum Press.
- Rosen VM, Engle RW. The role of working memory capacity in retrieval. Journal of Experimental Psychology: General. 1997;126:211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- Ross-Sheehy S, Oakes SM, Luck SJ. The development of visual short-term memory capacity in infants. Child Development. 2003;74:1807–1822. doi: 10.1046/j.1467-8624.2003.00639.x. [DOI] [PubMed] [Google Scholar]
- Salamé P, Baddeley A. Disruption of short-term memory by unattended speech: Implications for the structure of working memory. Journal of Verbal Learning and Verbal Behavior. 1982;21:150–164. [Google Scholar]
- Schacter, D.L. (1989). On the relation between memory and consciousness: Dissociable interactions and conscious experience. In H.L. Roediger & F.I.M. Craik (eds.), Varieties of memory and consciousness: Essays in Honor of Endel Tulving. Hillsdale, NJ: Erlbaum.
- Schweizer K, Moosbrugger H. Attention and working memory as predictors of intelligence. Intelligence. 2004;32:329–347. [Google Scholar]
- Stevanovski, B., & Jolicoeur, P. (2003, November). Attentional limitations in visual short-term memory. Poster presented at the annual convention of the Psychonomic Society, Vancouver, British Columbia, Canada.
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short_term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Thorndike, R.L., Hagen, E.P., & Sattler, J.M. (1986). The Stanford-Binet Intelligence Scale: Fourth Edition: Guide for administering and scoring the fourth edition. Chicago, IL: The Riverside Publishing Company.
- Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- Turner ML, Engle RW. Is working memory capacity task dependent? Journal of Memory and Language. 1989;28:127–154. [Google Scholar]
- Unsworth N, Schrock JC, Engle RW. Working memory capacity and the antisaccade task: Individual differences in voluntary saccade control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:1302–1321. doi: 10.1037/0278-7393.30.6.1302. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:749–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Wilhelm, O., & Engle, R.W. (eds.) (2005). Handbook of understanding and measuring intelligence. London: Sage.
- Wood N, Cowan N. The cocktail party phenomenon revisited: How frequent are attention shifts to one’s name in an irrelevant auditory channel? Journal of Experimental Psychology: Learning, Memory, & Cognition. 1995;21:255–260. doi: 10.1037//0278-7393.21.1.255. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK, Luck SJ. Attention is not unitary. Behavioral and Brain Sciences. 2001;24:153–154. [Google Scholar]
- Yakovlev, P.I., & Lecours, A.R. (1967). The myelinogenetic cycles of regional maturation of the brain. In A. Minkowski (Ed.), Regional development of the brain in early life. Oxford, England: Blackwell.


