Abstract
Purpose
To study the possibility of generating photoreceptors through programming RPE transdifferentiation by examining cell differentiation after transplantation into the developing chick eye.
Methods
RPE was isolated, and the cells were dissociated, cultured, and guided to transdifferentiate by infection with retrovirus expressing neuroD (RCAS-neuroD), using RCAS–green fluorescence protein (GFP) as a control. The cells were then harvested and microinjected into the developing eyes of day 5 to day 7 chick embryos, and their development and integration were analyzed.
Results
Cells from the control culture integrated into the host RPE. When grafted cells were present in large number, multilayered RPE-like tissues were formed, and the extra tissues consisted of grafted cells and host cells. None of the cells from the control culture expressed photoreceptorspecific genes. In contrast, most cells from RCAS-neuroD–infected culture remained depigmented. A large number of them expressed photoreceptor-specific genes, such as visinin and opsins. Antibodies against red opsin decorated the apical tips and the cell bodies of the grafted, transdifferentiating cells. In the subretinal space, visinin+ cells aligned along the RPE or an RPE-like structure. When integrated into the host outer nuclear layer, grafted cells emanated elaborate, axonal arborization into the outer plexiform layer of the host retina.
Conclusions
Cultured RPE cells retained their remarkable regenerative capabilities. Cells guided to transdifferentiate along the photoreceptor pathway by neuroD developed a highly ordered cellular structure and could integrate into the outer nuclear layer. These data suggest that, through genetic programming, RPE cells could be a potential source of photoreceptor cells.
Photoreceptors are the primary sensory neurons residing in the outer nuclear layer (ONL) of the vertebrate retina. Photoreceptor degeneration is a common cause of human visual impairments resulting from light damage, genetic changes, and aging. The low quality of life of persons with severe vision loss and the unfortunate nonrenewable nature of photoreceptors have inspired investigations ranging from photoreceptor rescue1,2 to photoreceptor replacement either with prosthetic devices3 or through biologic approaches such as retinal regeneration4-6 and cell transplantation.7,8 In the teleost retina, injuries induce progenitor/stem cells at the ciliary marginal zone to produce new retinal cells, including photoreceptors.9-11 However, such an inborn stem cell–based regeneration mechanism seems to be lacking in the retina of higher vertebrates. This has spurred a number of studies aiming to replace ailing photoreceptors with exogenous cells, including stem cells of nonretinal origin. A recent study in mnd mice, which have a condition of photoreceptor degeneration, showed that neuralized mouse embryonic stem cells grafted into the vitreous migrate to the neural retina and reach the outer plexiform layer (OPL), where they express photoreceptor-specific genes.12
The retinal pigment epithelium (RPE) is developmentally and anatomically close to the neural retina. Unlike retinal neurons, RPE cells are nonneural and can reenter the cell cycle on stimulation. Furthermore, their progenies may differentiate into cell types other than RPE.13-18 Classical experiments show that embryonic chick RPE at early developmental stages can be triggered to transdifferentiate into a neural retina.19 This RPE-to-retina transdifferentiation occurs in vivo and in vitro under the induction of fibroblast growth factors.20-24 Making use of these properties of RPE, we have recently begun to explore the possibility of using RPE as a potential source of retinal neurons. Chick RPE cells are dissociated, cultured, and infected by retrovirus RCAS expressing a proneural gene. Later, the culture is analyzed for de novo expression of neural properties. We found that cultured, dissociated RPE cells undergo transdifferentiation toward various types of retinal neurons under the induction of different proneural bHLH genes,25-28 including neuroD, which encodes a transcriptional factor in the basic helix-loop-helix (bHLH) family and is homologous to Drosophila proneural gene atonal. In the retina, neuroD is mostly expressed in photoreceptor cells and rod progenitors of teleost fish, with some expression in amacrine cells.25,29,30 Gain- and loss-of-function studies in the mouse and the chick show that neuroD plays an instrumental role in photoreceptor development25,31 and is required specifically for photoreceptor survival.29 When ectopically expressed, neuroD induces RPE cells to transdifferentiate selectively toward a photoreceptor phenotype. In culture, the transdifferentiation is substantial, judging from the presence of inner segment–like processes and the expression of an array of photoreceptor genes.25,32 Nevertheless, it is unknown how these cells would behave and to what extent they would differentiate along the photoreceptor pathway once they encounter the three-dimensional milieu (or niche) offered by the local environment of the eye. As an initial step to address this question, we grafted transdifferentiating cells into the developing chick eye. We found that transdifferentiating cells continued their transdifferentiation along the photoreceptor pathway in the subretinal space, the choroid, and the vitreous. Some of the grafted cells integrated into the host ONL and emanated axons with elaborate arborization in the OPL. We also found that RPE cells exhibited remarkable self-organization activities; not only were grafted cells incorporated into the host tissue, multilayered RPE-like tissues were formed from host cells and grafted cells. The self-organization (or regeneration) and the transdifferentiation properties of RPE cells render them unique for studies of photoreceptor rescue and replacement.
Materials and Methods
Chick Embryos
Fertilized, pathogen-free chicken eggs were purchased (Spafas, North Franklin, CT). Fertilized line 72 eggs were produced at the Avian Disease and Oncology Laboratory (United States Department of Agriculture Agricultural Research Service [USDA-ARS]). All use of animals adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and to the procedures and policies set by the institutional review board at the University of Alabama at Birmingham.
RPE Cell Culture
RPE was isolated from embryonic day (E)6 chick embryos and was cultured as dissociated cells. To induce transdifferentiation toward photoreceptor cells, replication-competent retrovirus RCAS expressing neuroD (RCAS-neuroD) was added to the RPE cell culture at approximately 50% confluence, as previously described.25 RCAS-GFP was used as a negative control. The RCAS is a derivative of the Schmidt-Ruppin strain of Rous sarcoma virus with A envelope [RCAS(A)]33 and can infect chick lines that are resistant only to the E-subgroup of avian retroviruses.34 After an additional 4 days in vitro, cells were harvested with trypsin/EDTA treatment followed by centrifugation. Approximately 1 × 106 cells from one 35-mm dish were resuspended in 50 μL culture medium. Fast green was added to visualize the flow of cell suspension into the eye during grafting.
Grafting Cells into the Developing Eyes
Approximately 2 × 104 cells (1 μL) were microinjected into the subretinal space or the vitreous at E5.5 and E7.5. Briefly, 2 mL egg white was withdrawn from the pointy end of the egg to allow the embryo to detach from the shell. Then a 1-cm2 incision was made on the shell. Through the incision, the embryo was accessed by a micro-manipulated micropipette attached to a pneumatic pump with a positive pressure delivery system. Flow and the delivery of cell suspension into the developing eye were monitored under a dissecting microscope.
Immunohistochemistry
Embryonic chick eyes were fixed between E11 and E18 with ice-cold 4% paraformaldehyde. Cryosections of 8 to 10 μm were collected. Monoclonal antibody RA4 (1:1000 dilution) was a gift from Steven McLoon (University of Minnesota). Monoclonal antibodies against visinin (clone 7G4, 1:500; developed by Constance Cepko), against LIM (clone 4F2; 1:50; developed by Thomas Jessell), and against AP2α (3B5; 1:50; developed by Trevor Williams) were obtained from the Developmental Studies Hybridoma Bank (University of Iowa). Antibody against viral protein p27 (1:1000 dilution) was purchased (Spafas), as were polyclonal antibodies against red opsin (1:200; Chemicon, Temecula, CA), monoclonal antibodies against rhodopsin (Chemicon), and monoclonal antibodies against PSD-95 (7E3-1B8; 1:200; Sigma). Standard immunocytochemistry was performed with fluorophore-conjugated secondary antibodies (Molecular Probes, Eugene, OR).
In Situ Hybridization
The expression of RxRγ was analyzed with in situ hybridization. A fragment of 813 base pairs of RxRγ was RT-PCR amplified from retina RNA and cloned into pGEM-T, and its sequence was verified in agreement with the published information.35 This fragment covered 618 nucleotides of the 3′ coding sequence and 195 nucleotides of the 3′ untranslated region in messenger RNA (UTR). Digoxigenin-labeled antisense RNA probes were synthesized with the use of a commercial kit (Roche, Basel, Switzerland) according to the manufacturer's instructions. In situ hybridization was carried out with cryosections 8 to 10 μm thick, as previously described.36
Results
Integration of Cultured RPE Cells into the Host Tissue
To achieve ectopic expression of exogenous genes in cultured RPE cells, replication-competent retrovirus RCAS(A)33 was used in combination with pathogen-free chick embryos (Spafas) from which primary RPE cell cultures were derived. During the initial phase of culture, when cells were reentering the cell cycle, RPE cells lost their pigment and exhibited a fibroblastlike morphology (Figs. 1A-C). As the culture progressed and became confluent, cells became repigmented and appeared hexagonal, a morphology typical of RPE cells (Fig. 1D). Nonetheless, repigmentation was partial compared with initial cells (compare Figs. 1D and 1A).
Figure 1.
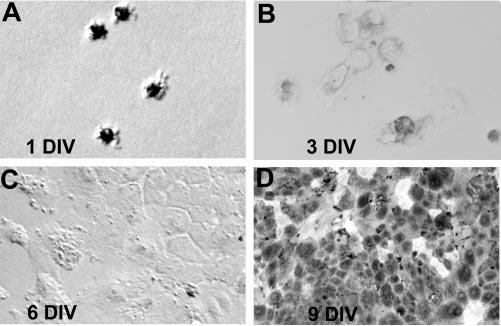
Morphologies of RPE cells at different time points of culture as indicated. Note the heavily pigmented cells at the initial stage of culture (A), the depigmented cells during subsequent culture phases (B–C), and the repigmented cells in a confluent culture (D). DIV, days in vitro. Original magnifications, ×200.
Cells from virally infected RPE cultures were harvested and microinjected into the developing chick eye (Fig. 2) at E5.5 and E7.5. In most experiments, embryos of line 72 were used as recipients. Cells in line 72 embryos lacked receptors for RCAS(A) virus; thus, viral spreading from grafted cells into host cells did not occur. This made it possible to unambiguously identify the injected cells in the host tissues by immunostaining for viral protein p27. When the recipient eyes were analyzed at E11 to E18, cells from the control culture infected with RCAS-GFP were found to integrate into the host RPE tissue (Fig. 3, arrows). Those integrated cells were indistinguishable from the host cells in terms of morphology and pigmentation. They extended digitlike processes into the subretinal space co-occupied by the outer segments of photoreceptors (Figs. 3C, 3D; arrowheads). Integration into the single-layered host RPE was observed when grafted cells were present in small numbers. No incorporation into the neural retinal was found with the control cells.
Figure 2.
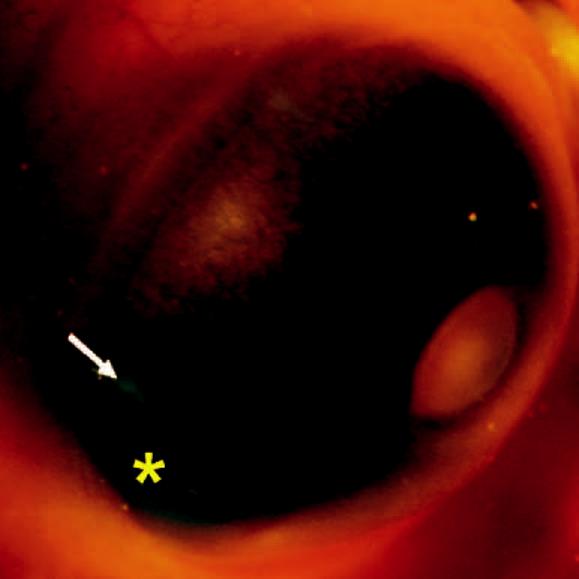
Cell suspension (star) microinjected into the subretinal space of E5.5 chick eye. Arrow: retina displaced by the cell suspension.
Figure 3.
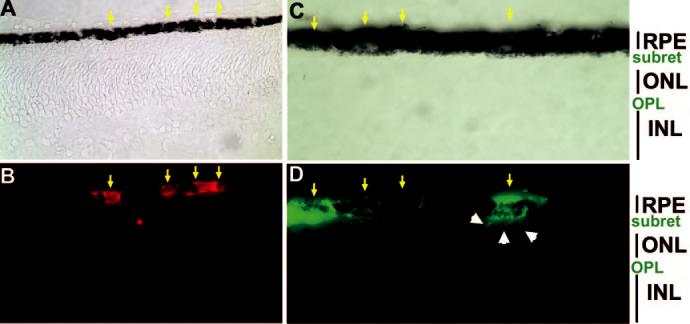
Integration of cultured RPE cells into the host RPE tissue. Shown are bright-field views (A, C) or immunodetection of grafted cells (B, D) in line 72 retina at E11 (A, B) and E17 (C, D). Cells were harvested from RPE cell cultures infected with RCAS-GFP and microinjected into the subretinal space at E7.5 (A, B) or E5.5 (C, D). Arrows: grafted (p27+) cells localized within the host RPE tissue. Arrowheads: digitlike processes extending from a grafted cell into the subretinal space (subret). Original magnifications: (A–B) ×400; (C–D) ×1000.
Formation of Extra Layers of RPE-like Tissues
At the injection site, where a large number of grafted cells were present, multilayered RPE-like structures were formed (Figs. 4A, 4C, stars), especially with cells from the control culture. This was observed in all the eyes (n = 16) examined that had more than 20 grafted cells at the injection site in one retinal section. Often, grafted cells were “enveloped” by RPE tissues (Figs. 4A-D). Immunostaining showed these multiple layers of RPE-like tissues composed of p27+ (Fig. 4, yellow arrows) and p27− (Fig. 4, white arrows) cells, indicating that grafted cells and host cells participated in their formation. None of the cells from the control culture expressed photoreceptor markers, such as visinin (Fig. 4E), RXRγ, red opsin, or rhodopsin (data not shown).
Figure 4.
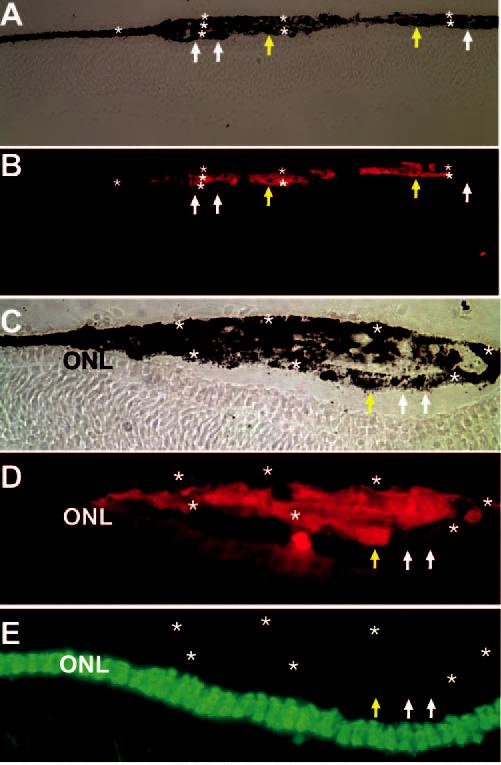
Presence of multiple layers of RPE-like tissues (stars) at the injection sites of control cells infected with RCAS-GFP and lack of visinin expression in these cells. Shown are bright-field views (A, C)or immunostaining for p27 (B, D) and visinin (E) of cross-sections of E11.5 line 72 recipient eyes. Extra tissues were composed of host cells (p27−, white arrows) and grafted cells (p27+, yellow arrows). Original magnifications: (A–B) ×200; (C–E) ×400.
Distribution of Transdifferentiating Cells Grafted into the Subretinal Space
On transduction by RCAS-neuroD, cultured RPE cells undergo transdifferentiation toward the photoreceptor pathway. This transdifferentiation is demonstrated by the de novo appearance of cells with photoreceptor-like morphologies and expressing an array of photoreceptor genes.25,32,37 According to the timeline of the viral spread and transdifferentiation process,25 we harvested donor cells 4 days after the administration of the virus. This was to ensure thorough viral infection of the culture and to keep the progression of transdifferentiation under in vitro conditions to a minimum.
As observed with the control RPE culture, we found that some cells from RPE cultures infected with RCAS-neuroD integrated into the host RPE and participated in the formation of multiple layers of RPE-like tissues. This was expected because not all cells underwent transdifferentiation. We estimated that less than 30% of the cells in the culture ultimately underwent detectable transdifferentiation toward photoreceptor cells under the experimental conditions. Notably, compared with the control cultures (Figs. 4C, 4D), fewer cells from the transdifferentiating cultures partook of the formation of layered, RPE-like structures (Figs. 5A, 5B). In fact, most cells from RCAS-neuroD–infected cultures remained depigmented (Figs. 5, 6).
Figure 5.
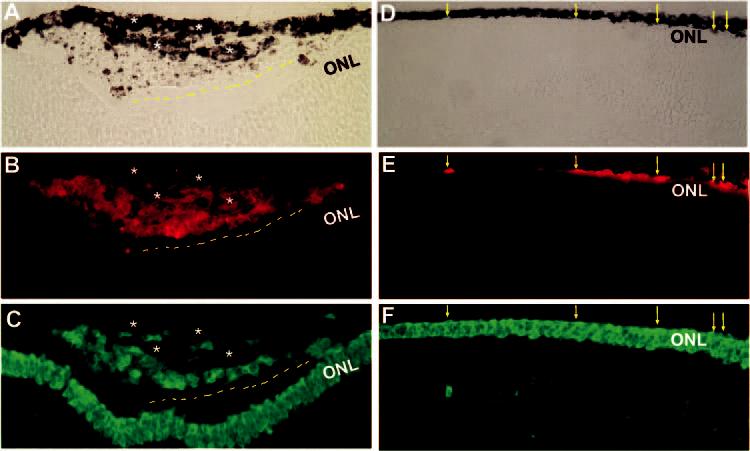
Distribution and expression of photoreceptor marker visinin in grafted, transdifferentiating cells in E11.5 line 72 retinas (5 days after grafting on E7.5). Shown are bright-field views (A, D) or double-immunostaining for p27 (B, E) and visinin (C, F). When grafted cells were present in large number, a primitive organization of visinin+ cells (dashed line) appeared along the newly formed RPE-like tissue (A–C). When present in small number, grafted cells remained in the subretinal space (D–F), and some were visinin+ (arrows). Stars: multilayered RPE-like tissues. Original magnifications: (A–C) ×400; (D–F) ×200.
Figure 6.
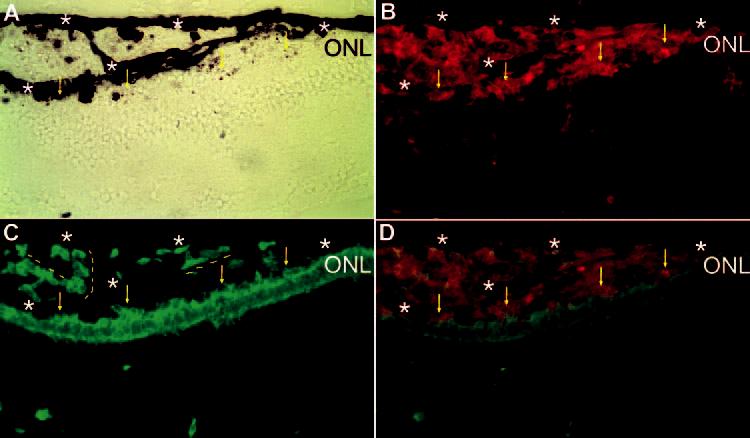
Attachment of grafted (p27+, red) and transdifferentiating (visinin+, green) cells to the ONL and rudimentary alignment of the cells along the extra RPE-like tissues. Shown is double labeling of a cross-section of E17 eye (Spafas pathogen-free embryos) for p27 (B) and visinin (C). Bright-field view (A) and simultaneous view of the double labeling (D). Dashed line: rudimentary alignment of the cells along the extra RPE-like tissues. Arrows: grafted, transdifferentiating (double-labeled) cells attached to the ONL of the host retina. Stars: RPE or RPE-like tissues. Original magnifications, ×400.
A large number of cells from RCAS-neuroD–infected cultures were positive for visinin, a marker for cones (Figs. 5C, 5F). The distribution of visinin+ cells was not totally random. Instead, it showed a discernible arrangement, however primitive, along the RPE-like structures (Figs. 5A-C, 6 [dashed lines]), reminiscent of the RPE-ONL organization in developing chick retina. Unexpectedly, with cells microinjected into E7.5 eyes, no obvious incorporation into the ONL of the host retina was found, despite their abundance in the subretinal space (Figs. 5A-C, 6). Lack of incorporation into the host retina was also observed at regions with only a few grafted cells present (Figs. 5D-F). In this case, grafted cells, including visinin + cells, remained in the subretinal space. The morphologic orientation of the visinin+ cells (Figs. 5D-F, arrows) indicated that their cell bodies laid parallel, rather than perpendicular, to the host RPE.
The visinin+ grafted cells were aligned along RPE-like structures in various orientations, from parallel to perpendicular to the existing RPE (Figs. 6A, 6C [dashed lines]). When positioned next to the retina, visinin+ cells aligned along the ONL of the host retina (Figs. 6C, 6D, arrows).
Distribution of Cells Placed in the Vitreous
Cells placed in the vitreous showed little dispersion. No discernible RPE-like structures were observed with cells from the control culture infected with RCAS-GFP (Figs. 7A, 7B) or cultures infected with RCAS-neuroD (Figs. 7D, 7E). As in the subretinal space, a large number of cells from a RCAS-neuroD– infected culture were visinin+ (Figs. 7D, 7E), whereas no such cells were found with the control culture (Fig. 7C). Unlike in the subretinal space, most of the visinin+ cells in the vitreous appeared as aggregates (Figs. 7F, 7G).
Figure 7.
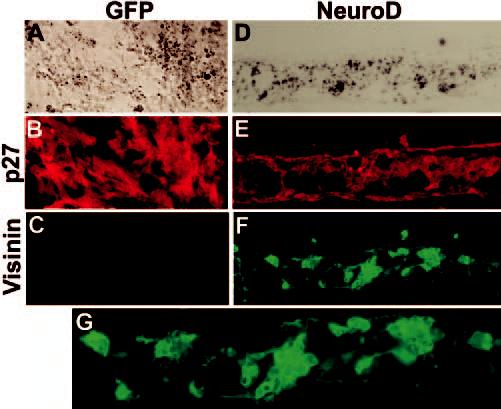
Distribution and expression of visinin in cells injected into the vitreous. Shown are double-staining for p27 (B, E) and visinin (C, F, G) of E17 eyes receiving cells from the control culture (A–C) or transdifferentiating cells (D–G). (G) is a view of (F) at higher magnification. A larger number of cells from RPE cultures infected with RCAS-neuroD were visinin+ (F, G), whereas no visinin+ cells were observed in the control (C). Note the lack of formation of RPE-like tissues among the grafted cells in the vitreous. Original magnifications: (A–F) ×200; (G) ×400.
Expression of Markers for Advanced Photoreceptor Differentiation
As the first step in assessing the developmental potential of grafted, transdifferentiating cells, we harvested recipient eyes at E17 and E18 (the latest reliable time point possible; experimental embryos died before hatching, possibly because of insufficient nutrients given that 2 mL egg white was taken out before microinjection). Immunohistochemistry and in situ hybridization showed that grafted cells expressed red opsin (Fig. 8), rhodopsin, RXRγ, or synaptic protein PSD-95 (data not shown). Red opsin+ cells were present in the subretinal space (Figs. 8C, 8D, 8F, s), choroid (Figs. 8C-E, c), and vitreous (Figs. 8G, 8H, v). In normal E18 retina, anti–red opsin immunostaining decorated the very apical ends—the outer segments—of red cones, with no detectable staining of their cell bodies (Figs. 8A, 8B). Decorated outer segments were aligned in an orderly arrangement between the ONL and the RPE. In grafted cells, however, strong immunostaining was found in cell bodies (Figs. 8D-H). In some cells, immunostaining marked the apical tips (Figs. 8E-H, yellow arrows). This decoration of the apical, outer segment–like structure was observed in cells placed in the subretinal space, the choroid (Figs. 8D, 8E), and the vitreous (Figs. 8G, 8H).
Figure 8.
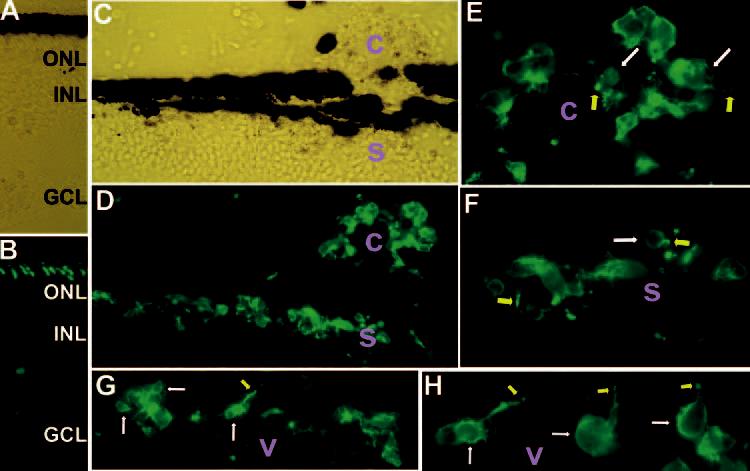
Expression of red opsin in transdifferentiating cells placed in the subretinal space (s), the choroid (c), and the vitreous (v). Shown are immunostaining of E18 recipient eyes that received grafted cells at E7.5. In E18 retina (A; bright-field view), the anti–red opsin antibody decorated the outer segments (B). At the injection site (C; bright-field view), however, such a well-formed order of the red opsin+ outer segments of host photoreceptors was not apparent (D). A large number of grafted cells were red opsin+ in the subretinal space (s in D, F), the choroid (c in D, E), and the vitreous (v in G, H). Arrows: cell bodies (white)or outer segment–like structures (yellow). Original magnifications: (A–D, G) ×400; (E, F, H) ×1000.
The mislocalization of red opsin in grafted cells could have been caused, at least in part, by a lack of appropriate, intimate physical contact with the RPE. If this were the case, then the host photoreceptor cells at the injection site would also have displayed impaired development because their intimate interaction with the RPE was disrupted. Indeed, the well-formed order of the red opsin+ outer segments observed in normal retina (Fig. 8B) was lacking at the injection site (Fig. 8D). Furthermore, host photoreceptor cells at the injection site often lacked oil droplets and outer segments, which were well formed in adjacent cells (Fig. 9). The expression of photoreceptor genes was also affected: the number of cells expressing advanced markers, such as red opsin, rhodopsin, and PSD-95, was reduced at the injection sites compared with the adjacent regions (data not shown).
Figure 9.
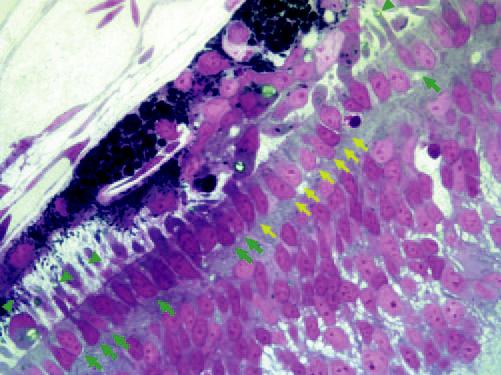
Semithin section of E17 retina showing a lack of outer segment in host photoreceptor cells (yellow arrows) at the injected site. Adjacent photoreceptors with well-formed outer segments (green arrows). Oil droplet observed in well-developed photoreceptors (arrowheads). Original magnification, ×1000.
Under in vitro conditions, photoreceptor-like cells are the major, if not the only, product of neuroD-induced RPE trans-differentiation.25,37 To examine whether this outcome was altered by the local environment of the developing eye, we performed immunostaining for markers that identified nonphotoreceptor neurons. We found a background level25,37 of expression of RA4 (a ganglion cell marker38) and no expression of LIM (a marker for horizontal cells39) or AP2α (a marker for amacrine cells40) among the grafted cells.
Incorporation into the ONL
The lack of obvious retinal incorporation of the transdifferentiating cells grafted at E7.5 into the subretinal space prompted additional experiments with younger embryos as recipients. Transdifferentiating cells, or cells from the control RPE cell culture, were microinjected into the subretinal space at E5.5, when peak photoreceptor genesis commenced,41 and the experimental eyes were harvested at E14 and E17 for analyses. With the younger embryos as recipients, some of the transdifferentiating cells localized in the ONL of the host retina at both E14 (Fig. 10A) and E17 (Figs. 10B, 10C, 10D). Incorporated cells displayed elaborate axonal arborization in the OPL (Figs. 10A, 10D). No grafted cells were found in the inner nuclear layer or the ganglion cell layer.
Figure 10.
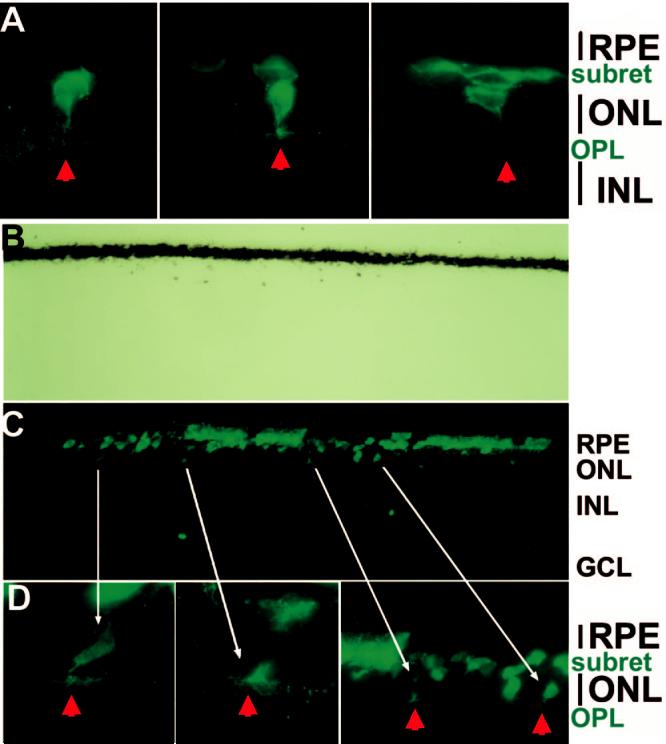
Integration into the ONL of integrated, transdifferentiating cells and their emanation of elaborate axonal arborization in the OPL. Shown are p27+ cells in E14 (A) and E17 (B,C,D) eyes of line 72 embryos that had, at E5.5, received cells from RPE cell culture infected with RCAS-neuroD. (B) Bright-field view of (C).Arrowheads: axonal arborization in the OPL of grafted cells. Long arrows: corresponding cells in the images of different magnifications. Original magnifications: (A, D) ×1000; (B, C) ×400.
Discussion
We found that cultured RPE cells integrated into the host RPE tissue when grafted in the vicinity. Cells that integrated into the host appeared indistinguishable from the endogenous RPE cells, judging from their morphology under light microscopy and the extension of digitlike processes into the subretinal space occupied by the photoreceptor outer segments. These results indicate the similarity of cultured RPE cells to the endogenous ones. When present in a large number, grafted cells were “enveloped” by multiple layers of RPE-like tissues that appeared to be composed of host cells and grafted cells. The formation of layers of RPE-like tissues consisting of host and grafted cells showed the remarkable regeneration capability of the RPE, an attribute supplementary to its rescuing photoreceptor from degeneration reported in Royal College of Surgeons rats and RPE65−/− mice.42-48
To examine the possibility of generating photoreceptor cells from RPE through guided transdifferentiation, we grafted transdifferentiating cells into developing chick eyes and then examined their distribution and differentiation. At the time of cell grafting, no overt transdifferentiation was apparent, judged by morphologic criteria under an inverted microscope and by immunostaining or in situ hybridization for the expression of an early photoreceptor marker, visinin.25 After grafting into the eye, a large number of cells expressed photoreceptor markers and developed photoreceptor-like morphologies with no expression of non–photoreceptor markers. This suggested that those cells continued their transdifferentiation along the photoreceptor pathway in the in vivo environment. Continued transdifferentiation was also evidenced by the lack of repigmentation most cells placed in the subretinal space, where control RPE cells were heavily repigmented.
Continued transdifferentiation of RPE cells along the photoreceptor pathway was observed when the cells were placed at three locations: the subretinal space, the choroid, and the vitreous. These three locations have been explored in other cell transplantation studies. Although it is difficult to quantify the similarities and the differences of milieu at the three locations, we observed less organization of the control cells into RPE-like structure in the vitreous than in the subretinal space. This difference could be attributed to the physical and spatial constraints of the subretinal space only permitting longitudinal organization of the grafted cells into sheetlike structures. Differences in biochemical composition might have affected the differentiation and behavior of grafted cells. Importantly, the development of photoreceptor-like morphologies and the expression of photoreceptor markers, including visinin, RXRγ, and red opsin, were observed with transdifferentiating cells at all three locations. This suggests the transdifferentiation potential was set by neuroD and was not significantly affected by the microenvironments at all three locations, however different they might have been. These findings are consistent with neuroD playing an important role in photoreceptor development and survival,25,29,31 but do not mean location is not important. On the contrary, the expression of photoreceptor genes, or the survival of the transdifferentiating cells, at all locations might have benefited from their proximity to the retina. We did not observe that cells farther away from the eye expressed photoreceptor genes, even though during grafting many cells ended in those “far away” locations. Thus, location could be an important aspect in the survival and development of grafted cells. The subretinal space may be superior to others for maturation into functional photoreceptors. In fact, our data indicated the importance of intimate interaction with the RPE for proper photoreceptor differentiation because impaired differentiation of the host photoreceptors was observed at the injection site. In the subretinal space, cells from transdifferentiating cultures showed rudimentary alignment along the RPE-like tissues, reminiscent of the RPE-ONL organization in the retina. Clearly, rigorous studies are needed to investigate whether cells from the transdifferentiating culture can form the RPE-photoreceptor organization of the retina.
Cells from the RCAS-neuroD–infected culture, but not the RCAS-GFP–infected culture, were found in the ONL. Further, the incorporated cells emanated elaborate processes into the OPL that resembled axonal arborization. These findings indicate that some cells from the transdifferentiating culture oriented themselves correctly and were incorporated into the host retina. Of note, incorporation was observed with grafting at E5.5 but not at E7.5, even though retinal neurogenesis was active at both stages. One underlying cause for the difference could be the number of photoreceptors already present at the time of grafting. Peak photoreceptor genesis begins on E5.5.41 At this stage, the retina might be more receptive to cells transdifferentiating along the photoreceptor pathway. By E7.5, most photoreceptor cells have already been produced, and the retina might become less accommodative to exogenous cells. This is consistent with published studies reporting integration into the host retina, when cells are transplanted into the developing, diseased, or injured retina.49-52 Given that photo-receptor-like cells are the major product of neuroD-induced RPE transdifferentiation,25,37 the demonstration of incorporation into the ONL and continued differentiation of transdifferentiating cells along the photoreceptor pathway raises the possibility of exploring this system as an alternative in cell replacement studies.
Acknowledgments
The authors thank Steven McLoon for monoclonal antibody RA4; Stephen Hughes for retroviral vector, RCAS, and shuttle vector Cla12Nco; and James Kimble for helpful discussion.
Footnotes
Supported by National Institutes of Health/National Eye Institute (NEI) Grant EY11640 and by Research to Prevent Blindness (RPB). LL is a Kelman Postdoctoral Scholar of the International Retinal Research Foundation. WM is a Postdoctoral Trainee of NEI Training Grant T32. S-ZW is a recipient of the RPB Dolly Green Scholar Award.
Disclosure: L. Liang, None; R.-T. Yan, None; W. Ma, None; H. Zhang, None; S.-Z. Wang, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. $1734 solely to indicate this fact.
References
- 1.Milam AH. Strategies for rescue of retinal photoreceptor cells. Curr Opin Neurobiol. 1993;3:797–804. doi: 10.1016/0959-4388(93)90156-s. [DOI] [PubMed] [Google Scholar]
- 2.Luthert PJ, Chong NH. Photoreceptor rescue. Eye. 1998;12:591–596. doi: 10.1038/eye.1998.149. [DOI] [PubMed] [Google Scholar]
- 3.Zrenner E, Miliczek KD, Gabel VP, et al. The development of subretinal microphotodiodes for replacement of degenerated photoreceptors. Ophthalmic Res. 1997;29:269–280. doi: 10.1159/000268025. [DOI] [PubMed] [Google Scholar]
- 4.Raymond PA. Retinal regeneration in teleost fish. Ciba FoundSymp. 1991;160:171–186. doi: 10.1002/9780470514122.ch9. [DOI] [PubMed] [Google Scholar]
- 5.Stenkamp DL, Cameron DA. Cellular pattern formation in the retina: retinal regeneration as a model system. Mol Vis. 2002;8:280–293. [PubMed] [Google Scholar]
- 6.Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–936. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 7.Aramant RB, Seiler MJ. Retinal transplantation—advantages of intact fetal sheets. Prog Retin Eye Res. 2002;21:57–73. doi: 10.1016/s1350-9462(01)00020-9. [DOI] [PubMed] [Google Scholar]
- 8.Young MJ. Stem cells in the mammalian eye: a tool for retinal repair. APMIS. 2005;113:845–857. doi: 10.1111/j.1600-0463.2005.apm_334.x. [DOI] [PubMed] [Google Scholar]
- 9.Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Cameron DA. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis Neurosci. 2000;17:789–797. doi: 10.1017/s0952523800175121. [DOI] [PubMed] [Google Scholar]
- 11.Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- 12.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24:274–283. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eguchi G. Instability in cell commitment of vertebrate pigmented epithelial cells and their transdifferentiation into lens cells. In: Moscona AA, Monroy A, editors. Current Topics in Developmental Biology. Academic Press, Inc.; New York: 1986. pp. 21–37. [DOI] [PubMed] [Google Scholar]
- 14.Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330:68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- 15.Dutt K, Scott M, Sternberg PP, Linser PJ, Srinivasan A. Transdifferentiation of adult human pigment epithelium into retinal cells by transfection with an activated H-ras proto-oncogene. DNA Cell Biol. 1993;12:667–673. doi: 10.1089/dna.1993.12.667. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat pigment epithelium to neural retina. Brain Res. 1995;677:300–310. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]
- 17.Zhao S, Rizzolo LJ, Barnstable CJ. Differentiation and transdifferentiation of the retina pigment epithelium. Int Rev Cytol. 1997;171:225–265. doi: 10.1016/s0074-7696(08)62589-9. [DOI] [PubMed] [Google Scholar]
- 18.Araki M, Yamao M, Tsudzuki M. Early embryonic interaction of retinal pigment epithelium and mesenchymal tissue induces conversion of pigment epithelium to neural retinal fate in the silver mutation of the Japanese quail. Dev Growth Differ. 1998;40:167–176. doi: 10.1046/j.1440-169x.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- 19.Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- 20.Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- 21.Pittack C, Jones M, Reh TA. Basic fibroblast growth factor induces retinal pigment epithelium to generate neural retina in vitro. Development. 1991;113:577–588. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- 22.Guillemot F, Cepko CL. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1992;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- 23.Opas M, Dziak E. bFGF-induced transdifferentiation of RPE to neuronal progenitors is regulated by the mechanical properties of the substratum. Dev Biol. 1994;161:440–454. doi: 10.1006/dbio.1994.1043. [DOI] [PubMed] [Google Scholar]
- 24.Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124:805–816. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- 25.Yan RT, Wang SZ. neuroD induces photoreceptor cell overproduction in vivo and de novo generation in vitro. J Neurobiol. 1998;36:485–496. [PMC free article] [PubMed] [Google Scholar]
- 26.Yan RT, Ma WX, Wang SZ. neurogenin2 elicits the genesis of retinal neurons from cultures of non-neural cells. Proc Natl Acad Sci USA. 2001;98:15014–15019. doi: 10.1073/pnas.261455698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma W, Yan RT, Xie W, Wang SZ. bHLH genes cath5 and cNSCL1 promote bFGF-stimulated RPE cells to transdifferentiate towards retinal ganglion cells. Dev Biol. 2004;265:320–328. doi: 10.1016/j.ydbio.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 28.Xie W, Yan RT, Ma W, Wang SZ. Enhanced retinal ganglion cell differentiation by ath5 and NSCL1 coexpression. Invest Ophthalmol Vis Sci. 2004;45:2922–2928. doi: 10.1167/iovs.04-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennesi ME, Cho JH, Yang Z, et al. BETA2/NeuroD1 null mice: a new model for transcription factor-dependent photoreceptor degeneration. J Neurosci. 2003;23:453–461. doi: 10.1523/JNEUROSCI.23-02-00453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hitchcock P, Kakuk-Atkins L. The basic helix-loop-helix transcription factor neuroD is expressed in the rod lineage of the teleost retina. J Comp Neurol. 2004;477:108–117. doi: 10.1002/cne.20244. [DOI] [PubMed] [Google Scholar]
- 31.Yan RT, Wang SZ. Requirement of neuroD for photoreceptor formation in the chick retina. Invest Ophthalmol Vis Sci. 2004;45:48–58. doi: 10.1167/iovs.03-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan RT, Wang SZ. Expression of an array of photoreceptor genes in chick embryonic RPE cell cultures under the induction of neuroD. Neurosci Lett. 2000;280:83–86. doi: 10.1016/s0304-3940(99)01003-4. [DOI] [PubMed] [Google Scholar]
- 33.Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fekete DM, Cepko CL. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowe A, Eage NS, Brickell PM. A member of the RXR nuclear receptor family is expressed in neural-crest-derived cells of the developing chick peripheral nervous system. Development. 1991;111:771–778. doi: 10.1242/dev.111.3.771. [DOI] [PubMed] [Google Scholar]
- 36.Li CM, Yan RT, Wang S-Z. Misexpression of cNSCL1 disrupts retinal development. Mol Cell Neurosic. 1999;14:17–27. doi: 10.1006/mcne.1999.0765. [DOI] [PubMed] [Google Scholar]
- 37.Yan RT, Wang SZ. Differential induction of gene expression by basic fibroblast growth factor and neuroD in cultured retinal pigment epithelial cells. Vis Neurosci. 2000;17:157–164. doi: 10.1017/s0952523800171172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waid DK, McLoon SC. Immediate differentiation of ganglion cells following mitosis in the developing retina. Neuron. 1995;14:117–124. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Wang JH, Xiang M. Specific expression of the LIM/home-odomain protein Lim-1 in horizontal cells during retinogenesis. Dev Dyn. 2000;217:320–325. doi: 10.1002/(SICI)1097-0177(200003)217:3<320::AID-DVDY10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Bisgrove DA, Godbout R. Differential expression of AP-2alpha and AP-2beta in the developing chick retina: repression of R-FABP promoter activity by AP-2. Dev Dyn. 1999;214:195–206. doi: 10.1002/(SICI)1097-0177(199903)214:3<195::AID-AJA3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Belecky-Adams T, Cook B, Adler R. Correlations between terminal mitosis and differentiated fate of retinal precursor cells in vivo and in vitro: analysis with the “window-labeling” technique. Dev Biol. 1996;178:304–315. doi: 10.1006/dbio.1996.0220. [DOI] [PubMed] [Google Scholar]
- 42.Li LX, Turner JE. Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988;47:911–917. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 43.Lopez R, Gouras P, Kjeldbye H, et al. Transplanted retinal pigment epithelium modifies the retinal degeneration in the RCS rat. Invest Ophthalmol Vis Sci. 1989;30:586–588. [PubMed] [Google Scholar]
- 44.Lavail MM, Li L, Turner JE, Yasumura D. Retinal pigment epithelial cell transplantation in RCS rats: normal metabolism in rescued photoreceptors. Exp Eye Res. 1992;55:555–562. doi: 10.1016/s0014-4835(05)80168-x. [DOI] [PubMed] [Google Scholar]
- 45.Little CW, Castillo B, DiLoreto DA, et al. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Invest Ophthalmol Vis Sci. 1996;37:204–211. [PubMed] [Google Scholar]
- 46.Coffey PJ, Girman S, Wang SM, et al. Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat Neurosci. 2002;5:53–56. doi: 10.1038/nn782. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Lu B, Wood P, Lund RD. Grafting of ARPE-19 and Schwann cells to the subretinal space in RCS rats. Invest Ophthalmol Vis Sci. 2005;46:2552–2560. doi: 10.1167/iovs.05-0279. [DOI] [PubMed] [Google Scholar]
- 48.Gouras P, Kong J, Tsang SH. Retinal degeneration and RPE transplantation in Rpe65(−/−) mice. Invest Ophthalmol Vis Sci. 2002;43:3307–3311. [PubMed] [Google Scholar]
- 49.Takahashi M, Palmer TD, Takahashi J, Gage FH. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol Cell Neurosci. 1998;12:340–348. doi: 10.1006/mcne.1998.0721. [DOI] [PubMed] [Google Scholar]
- 50.Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH. Neuronal differentiation and morphological integration of hippo-campal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16:197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- 51.Van Hoffelen SJ, Young MJ, Shatos MA, Shatos MA, Sakaguchi DS. Incorporation of murine brain progenitor cells into the developing mammalian retina. Invest Ophthalmol Vis Sci. 2003;44:426–434. doi: 10.1167/iovs.02-0269. [DOI] [PubMed] [Google Scholar]
- 52.Seiler MJ, Aramant RB. Transplantation of neuroblastic progenitor cells as a sheet preserves and restores retinal function. Semin Ophthalmol. 2005;20:31–42. doi: 10.1080/08820530590921873. [DOI] [PubMed] [Google Scholar]


