Abstract
Heat shock proteins exert their beneficial effects via basically two modes of action depending on their relative location within the host. Intracellular heat shock proteins found within cells serve a cytoprotective role by chaperoning naïve, misfolded and/or denatured proteins in response to stressful stimuli by a process known as the stress response. However, stressful stimuli also induce the release of intracellular heat shock proteins into the extracellular milieu and circulation. The extracellular heat shock protein proteins serve a cytostimulatory role by initiating immune responses designed to fend off microbial infection and destroy neoplastic transformed cells. This review will briefly cover recent advances into elucidating the mechanism(s) by which stress induces the release of heat shock proteins into the circulation, how it initiates immune responses and suggest the possible biological significance of circulating Hsp to the host.
Keywords: Cancer, chaperokine, heat shock proteins, inflammation, receptors, signal transduction
INTRODUCTION
Heat shock proteins (Hsp) were originally described for their role as chaperones induced by temperature shock as well as various other kinds of stress including environmental (U.V. radiation, heat shock, heavy metals and amino acids), pathological (bacterial, parasitic infections or fever, inflammation, malignancy or autoimmunity) or physiological stresses (growth factors, cell differentiation, hormonal stimulation or tissue development), that induced a marked increase in intracellular Hsp synthesis known as the stress response [1]. This is achieved by activating the trimerization and nuclear translocation of cytoplasmic heat shock factor-1 (HSF-1) to the heat shock element (HSE) within the nucleus and consequent transcription of Hsp. By binding unfolded, misfolded or mutated peptides or proteins and transporting them to the endoplasmic reticulum (ER), Hsp prevent potential aggregation and/or death. Within the ER the peptides are released in an ATP-dependent fashion and refolded [2]. Recently an additional role has been ascribed to Hsp as danger signals produced and released when cells are under stress and as activators of the immune system (Fig. 1). This review will focus mainly on the Hsp70 family, which constitutes the most conserved and best studied class of all heat shock proteins. The human Hsp70 family consists of at least 12 members (for review see [3]). The best known Hsp70 family members are the constitutively expressed Hsp70 (Hsc70 or Hsp73; molecular weight of 73 kDa), the stress inducible Hsp70 (Hsp70 or Hsp72; molecular weight of 72 kDa), the mitochondrial Hsp70 (Hsp75; molecular weight of 75 kDa), and the endoplasmic reticulum Hsp70 (Grp78; molecular weight of 78 kDa). This review will briefly delve recent advances in our understanding of how stress induces the release of Hsp72 into the circulation. We will also discuss the steps required for the initiation of an immune response and its biological significance in the context of host defense.
Fig. (1).
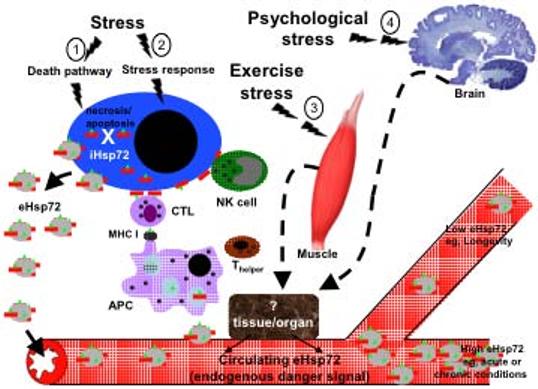
Model system for stress-induced release of Hsp72. Depending on the intensity and kind of stressful stimuli the following down stream effects can occur. 1) Non physiological stress (e.g., trauma or infection with lytic virus) will activate the cellular death pathway and result in intracellular (iHsp72) release into the circulation, refereed to as the passive release hypothesis. 2) Physiological, environmental, pathological (except infection with lytic virus) stress activates the stress response by triggering the trimerization and nuclear translocation of cytoplasmic HSF-1 and subsequent transcription of intracellular Hsp72 (iHsp72; red rod). The increased iHsp72 protects the cell from cell death under certain conditions. iHsp72 chaperones peptides (green triangle) and is expressed on the cell surface and released into the extracellular milieu within exosomes and free Hsp72. 3) Exercise stress or 4) Psychological stress stimulates the release of Hsp72 into the circulation via a still unknown tissue and/or organ. The released extracellular Hsp72 (eHsp72) make their way into the circulation where it acts as an endogenous danger signal. Antigen presenting cells (monocytes, macrophages, B cells, dendritic cells, langerhans cells) bind eHsp72 to specific receptors and stimulates a signal transduction cascade that results in the initiation of an immune response characterized by the upregulation of pro-inflammatory cytokines, chemokines, nitric oxide and costimulatory molecules. The eHsp72 is internalized by APC and the chaperoned peptide is processed and presented in the context of MHC class I to cytotoxic T lymphocytes (CTL), which lyse peptide bearing cells. Circulating eHsp72 induces NK cells migration and the expression of Hsp72 on the surface of stressed cells activates NK lytic functions. As an endogenous danger signal, circulating eHsp72 found in high concentrations is a good indicator of acute or chronic conditions, low eHsp72 concentrations have been linked to exceptional longevity.
MECHANISMS OF Hsp RELEASE: PASSIVE RELEASE HYPOTHESIS
Gallucci and colleagues initially demonstrated that dendritic cells (DC) are stimulated by endogenous signals received from stressed, viral-infected or necrosis-induced cells, but not by healthy cells or cells undergoing apoptosis [4]. In a series of elegantly performed experiments, Basu and co-workers later reported that heat shock proteins including gp96, calreticulin, Hsp90 and Hsp72 are released from cells by necrotic but not apoptotic cells [5]. These authors demonstrated that necrosis induced by freeze thaw, but not apoptosis induced by irradiation resulted in the release of Hsp into the culture supernatant, respectively [5-8]. During apoptotic cell death, the contents of the cell are not released into the external milieu but are packaged neatly into apoptotic bodies which are efficiently scavenged by neighboring professional phagocytes. However, necrotic cell death results in the discharge of intracellular contents into the extracellular milieu thereby liberating heat shock proteins (for review see [6, 9]). These results make the necrosis hypothesis an attractive explanation for the mechanism by which heat shock proteins are released into the circulation (Fig. 1).
A condition in which necrotic cell death clearly contributes to the release of Hsp72 from cells is after severe trauma. In a study by Pittet and colleagues a significant upregulation in circulating serum Hsp72 can be measured in severely traumatized patients as early as 30 minutes after injury [10]. Increased circulating serum Hsp72 has also been measured in patients after coronary artery bypass grafting [11, 12]. Importantly, circulating serum Hsp72 has been suggested as a marker of myocardial damage, and reported to have a role in the inflammatory response after acute myocardial infarction (AMI) [13]. Other conditions in which elevated levels of circulating serum Hsp72 has been demonstrated is in renal disease [14], hypertension [15], atherosclerosis [16], aging [17], and sickle cell disease [18]. However, in these conditions although necrosis is proposed as the mechanism of release, conclusive experimental data is still lacking.
A more conclusive study was designed in which in situ killing of tumor cells using suicide gene transfer to generate death by a non-apoptotic pathway was shown to be associated with high immunogenicity and induction of Hsp [19]. The most conclusive reports to demonstrate that necrosis accounts for Hsp release is found following infection with lytic viruses. In a study by Moehler and coworkers, it was demonstrated that parvovirus-mediated cell killing enhances tumor immunogenicity by Hsp72 release and contributes to the anti-tumor effect of parvoviruses [20]. Although these authors did not directly demonstrate that H1-induced cell killing and its associated Hsp72 release, promotes the loading and maturation of antigen presenting cells and by extension triggers tumor specific immune responses. One can speculate that the release of Hsp72 that could facilitate priming of T cells specific for viral antigens.
Taken together, the passive release hypothesis clearly is an important mechanism by which Hsp72 is released into the circulation. However, is it the only mechanism? An additional mechanism for Hsp release is now proposed as being of equal importance in the release of Hsp72 into the circulation.
MECHANISM OF Hsp RELEASE: ACTIVE RELEASE HYPOTHESIS
The active release hypothesis has been proposed as an additional mechanism to the passive release hypothesis. Three lines of evidence strongly support this hypothesis. First, as early as 1998 Pockley and coworkers demonstrated the presence of soluble Hsp72 and antibodies against Hsp72 in the peripheral circulation of normal individuals [21]. Second, Guzhova and colleagues demonstrated that Hsp72 is released by glia cells in the absence of necrotic cell death [22]. Third, and extremely compelling, psychological stress induced by exposure of a Sprague Dawley rat to a cat results in the release of Hsp72 into the circulation [23]. In this study, a rat was put in glass cage and a cat placed on top of the cage. This form of psychological stress induced a marked increase in circulating Hsp72, as judged by the classical Hsp72 sandwich ELISA. The terrified rat did not run around the cage thereby negating the possibility that damage to the muscles played a part in increased Hsp72 release. Subsequent studies have demonstrated that Hsp72 is released by B cells [24] and peripheral blood mononuclear cells [25] under non necrotic conditions.
Using conditions that will not induce significant cell death, our group showed that IFN-γ and IL-10 induce the active release of constitutively expressed Hsp70 also designed, Hsc70 or Hsp73 from tumors [26]. However, these initial studies did not address the mechanism underlying Hsp72 release. Recently, our group [27, 28] and others [29] have begun to elucidate the mechanism of active release of iHsp72 from viable cells (Fig. 1). In our study, we demonstrated that certain pro-inflammatory cytokines normally found in high concentrations within inflammatory foci including IFN-γ, IL-10 but not the anti-inflammatory cytokine TGF-β1, mediate the active release of Hsp72. We further showed that whereas some eHsp72 could be found as free Hsp72, a proportion of eHsp72 was released within exosomes [27]. Exosomes are internal vesicles of multivesicular bodies (MVB) released into the extracellular milieu upon fusion of multivesicular bodies MVB with the cell surface [30-32]. In addition to containing Hsp72 [27, 28], exosomes are highly packed with immunostimulatory mediators including MHC class I and II [30-32] and costimulatory molecules [33]. Additionally, we demonstrated that Hsp72 is released by a non classical protein transport pathway and that intact surface membrane lipid rafts are required for efficient stress-induced Hsp72 release [27, 28]. These studies were recently confirmed in B cells [24]. Studies by Lancaster & Febbraio recently demonstrate that exosomes provide the major pathway for secretory vesicular release of Hsp72 [29]. However, using methyl-β-cyclodextrin (the cholesterol depleting agent) to disrupt lipid raft function, these authors were unable to confirm a role for lipid rafts in stress-induced Hsp72 release from human peripheral blood mononuclear cells PBMC [29]. In order to address the cellular location of Hsp72 after stress, a recent study demonstrated that newly synthesized Hsp72 protein localizes within the Golgi region of HeLa cells and also concentrates on the surface of the plasma membrane and in the ruffled zone of migrating cells [34].
Taken together, these studies suggest that the active release hypothesis is an important mechanism by which Hsp72 is released into the circulation. However, studies remain to be performed that conclusively demonstrate that T cell responses are primed in response to active release of Hsp72 especially in the case of psychological stress or exercise.
Hsp70 RECEPTOR
The prerequisite for the initiation of an immune response is that eHsp72 must bind to specific receptors on the surface of specialized cells and stimulate a cascade of signals that eventually induces the production and release of immune modifiers which in turn changes the immune status of the cell and by extension the host. However, not all cells bind heat shock proteins. eHsp72 has been shown to bind selectively and with high specificity and affinity to a number of cells including natural killer (NK) cells [35-37], dendritic cells (DC) [38, 39], macrophages, peripheral blood monocytes [40-42], and B cells [43]. In contrast, T lymphocytes do not bind Hsp72 [43].
The number of receptors known to bind eHsp72 is increasing. Our group was the first to demonstrate that the Toll-like receptors 2 and 4 with their cofactor CD14 is a receptor for Hsp72 [39]. These results were independently corroborated by others [44]. Presently, the list of putative Hsp72 receptors now includes the scavenger receptor, CD36 [45, 46], the co-stimulatory molecule, CD40 [47], the low-density lipoprotein receptor-related protein CD91 [48-51], Lox-1 [45, 52] and SR-A, another member of the scavenger superfamily [53, 54].
Hsp70 SIGNALING AND ANTIGEN PRESENTATION
Once eHsp72 is bound to its specific surface receptor, two interrelated events occur. First, signal transduction cascades are activated. Second, the eHsp72 is endocytosed and the chaperoned peptides are presented to the antigen presentation pathway.
Our group was first to demonstrate that 10 seconds after eHsp72 binds to human monocytes there is a rapid intracellular Ca2+ ([Ca2+]i) flux [41]. This is an important signaling step that distinguishes Hsp72- from LPS-induced signaling, since treatment of APC with LPS does not result in [Ca2+]i flux [55]. Thirty-minutes after [Ca2+]i flux there is evidence of I-κBα phosphorylation [41]. Activation of NF-κB is regulated by its cytoplasmic inhibitor, I-κBα via phosphorylation at Serine 32 (Ser-32) and 36 (Ser-36) which targets it for degradation by the proteosome and releases NF-κB to migrate to the nucleus and activate the promoter of target genes [56]. As early as 30 minutes post exposure to eHsp72, I-κBα is phosphorylated at Serine 32 (Ser-32) and 36 (Ser-36) resulting in the release and nuclear translocation of NF-κB [41]. We performed in vitro studies using the HEK293 model system and revealed that Hsp72-induced NF-κB promoter activity is MyD88-dependant, CD14-dependant and is transduced via both TLR2 and TLR4 [39]. Further, the presence of both TLR2 and TLR4 synergistically stimulates Hsp72-induced cytokine production [39]. In addition, we demonstrated a synergistic activation of the NF-κB promoter by co-expression of both TLR2 and TLR4 is MyD88-independent, suggesting an alterative pathway by which eHsp72 stimulates cells of the immune system. Independent studies by Vabulas and colleagues, corroborated these findings [44].
Following receptor-mediated endocytosis heat shock proteins carrying their peptide load is transported via clathrin-coated pits to endosomes where it colocalizes with both MHCI and MHCII [57]. The exact mechanism by which cross-presentation occurs has not been completely elucidated. However, eHsp72 may traffic through various intracellular compartments, which results in peptide release into the cytoplasm and re-presentation on the cell surface associated with MHC proteins (for review see [58]). eHsp72 has been shown to deliver peptides to MHC class I molecules through the cross-presentation pathway [59]. Following internalization of eHsp72, chaperoned peptides maybe released into the cytoplasm and processed by the classical antigen representation pathways. However, cross-presentation may proceed through a recently described alternative route which involves a specialized MHC class I structure known as the ER/phagosome fusion compartment, which is highly sufficient in inducing antigen cross-presentation [60].
CHAPEROKINE ACTIVITY OF eHsp72
Chaperokine, is a term recently coined to better describe the unique function of eHsp72 as both chaperone and cytokine (for review, see Refs. [61, 62]). The consequence of binding and signaling is the stimulation of a potent and long lasting immune response. eHsp72 induces a plethora of immune responses and the list continues to grow. Table 1 lists some immune effector functions that are attributed to various sources of Hsp70 including recombinant bacterial, viral and parasitic sources, Hsp70 from human, mouse and non-human primate tissue and their targets (Table 1). Briefly, as early as 2-4 hours post exposure of APC to eHsp72, there is significant release of cytokines including TNF-α, IL-1β, IL-6 and IL-12 [39, 41] and GM-CSF [63]; nitric oxide, a potent apotogenic mediator [64]; chemokines including MIP-1, MCP-1 and RANTES [64, 65]. This part of the immune response does not require peptide, since both peptide-bearing and non peptide-bearing eHsp72 is capable of inducing pro-inflammatory cytokine production by APCs [66]. However, peptide is required for specific CD8+ CTL responses [67-69]. eHsp72 induces the DC maturation by augmenting the surface expression of CD40, CD83, CD86 and MHC class II molecules on DC [5, 39, 70, 71] and migration of DC [72] and NK cells [28] (Fig. 1).
Table 1.
Immune Effect of Hsp70
| Source of Hsp70 | Target | Immune Function | Ref. |
|---|---|---|---|
| Rec human and mouse | human monocyte human monocytes and monocyte-derived DC human DC human DC mouse DC |
cytokine release CC chemokines co-stimulatory molecules maturation signal antigen presentation, cytokine release |
[39, 41] [76] [39] [39] [66, 77] |
| Rec mycobacterium tuberculosis Hsp70-p24 fusion protein |
mouse immunization | humoral and cellular immune response | [78-80] |
| Rec DNA vaccine | murine encephalitis | protective immunity | [81] |
| Hyperthermia | mouse measles virus | protective immunity | [82] |
| Adenovirus | murine tumors | protective immunity | [83] |
| Plasmodium falciparum | mouse immunization | serum Ab, cytokine release | [84] |
| Rec MSP1 fused to Hsc70 | plasmodium yoelii sporozoite-infected mice | protective immunity | [85] |
| Mouse liver & kidney | mouse DC mouse PEC CD8+ CTL |
nitric oxide release, migration cross presentation protective immunity |
[64, 72] [86] [87-89] |
| Mouse and recombinant Non-human primates |
melanoma CD8+CTL |
cross-presentation beta chemokines |
[90-92] [65] |
| Non-human primates rec Hsp70- CCR5 fusion |
HIV | protective immunity | [93, 94] |
| Human tumors | human DC | cross presentation | [69, 70] |
| Human exosomes | human DC human NK cells |
cytokine release, co-stimulatory molecules, maturation signal migration |
[27, 28] [28] |
| Human tumors | cancer | tumor regression | [67, 68, 95, 96] |
CCR, chemokine receptor; CTL, Cytotoxic T Lymphocytes; DC; Dendritic Cells; HIV, Human Immunodeficiency Virus; MSP, Merozoite Surface Protein; NK, Natural Killer; PEC, Peritoneal Exudates Cells; Rec, Recombinant.
CIRCULATING eHsp72: BIOLOGICAL SIGNIFICANCE
Whether Hsp72 enters the circulation via an active or passive release mechanism – what is the role of eHsp72 in circulation? The danger theory postulates that immune activation involves danger/non-danger molecular recognition schemas and suggests that innate immune cells are activated by danger signals that are derived from stressed or damaged self-proteins [73, 74]. It is now widely accepted that eHsp72 fit this criteria. The hypothesis is further reinforced by studies showing that circulating eHsp72 is increased and upregulated in diseased conditions including renal disease [14], hypertension [15], atherosclerosis [16] and sickle cell disease [18]. However, intriguing questions still remain as to the role of increased circulating eHsp72 during psychological stress like that demonstrated when a Sprague Dawley rat is exposed to a cat [23]. Or studies demonstrating that the human brain is able to release eHsp72 into the circulation in response to exercise [75] (Fig. 1).
In these situations, is it possible that circulating eHsp72 is priming the immune system to real or perceived danger? These are important questions, the answers to which will provide a keen insight into numerous psychological and pathophysiological conditions. However, extensive studies and a concerted effort that brings together Hsp researchers from such disparate fields as immunology, molecular biology, cell biology, neurophysiology and psychology utilizing new breakthrough technologies including the recently deciphered human genome and proteomics is required before conclusive answers can be given.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institute of Health grant RO1CA91889, Scott & White Clinic, the Texas A&M University System Health Science Center College of Medicine, the Central Texas Veterans Health Administration and an Endowment from the Cain Foundation.
ABBREVIATIONS
- APC
Antigen presenting cells
- CTL
Cytotoxic T lymphocytes
- eHsp72
Extracellular Hsp72
- Hsp72
Inducible form of the seventy-kilo Dalton heat shock protein
- IFN-γ
Interferon-gamma
- iHsp72
Intracellular Hsp72
- IL
Interleukin
- TLR
Toll-like receptors
REFERENCES
- 1.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 2.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–49. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 3.Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–8. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 5.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava PK. Hypothesis: controlled necrosis as a tool for immunotherapy of human cancer. Cancer Immun. 2003;3:4. [PubMed] [Google Scholar]
- 7.Basu S, Srivastava PK. Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones. 2000;5:443–51. doi: 10.1379/1466-1268(2000)005<0443:hsptfo>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava PK, Amato RJ. Heat shock proteins: the ‘Swiss Army Knife’ vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–7. doi: 10.1016/s0264-410x(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 9.Calderwood SK. Chaperones and slow death - a recipe for tumor immunotherapy. Trends Biotechnol. 2005;23:57–9. doi: 10.1016/j.tibtech.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52:611–7. doi: 10.1097/00005373-200204000-00001. discussion 17. [DOI] [PubMed] [Google Scholar]
- 11.Dybdahl B, Wahba A, Haaverstad R, et al. On-pump versus off-pump coronary artery bypass grafting: more heat-shock protein 70 is released after on-pump surgery. Eur J Cardiothorac Surg. 2004;25:985–92. doi: 10.1016/j.ejcts.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Dybdahl B, Wahba A, Lien E, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–90. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- 13.Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- 15.Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20:1815–20. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Pockley AG, Georgiades A, Thulin T, de Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42:235–8. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- 17.Terry DF, McCormick M, Andersen S, et al. Cardiovascular disease delay in centenarian offspring: role of heat shock proteins. Ann N Y Acad Sci. 2004;1019:502–5. doi: 10.1196/annals.l297.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adewoye AH, Klings ES, Farber HW, et al. Sickle cell vaso-occlusive crisis induces the release of circulating serum heat shock protein-70. Am J Hematol. 2005;78:240–2. doi: 10.1002/ajh.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998;4:581–7. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 20.Moehler MH, Zeidler M, Wilsberg V, et al. Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum Gene Ther. 2005;16:996–1005. doi: 10.1089/hum.2005.16.996. [DOI] [PubMed] [Google Scholar]
- 21.Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–77. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 22.Guzhova I, Kislyakova K, Moskaliova O, et al. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 23.Fleshner M, Campisi J, Amiri L, Diamond DM. Cat exposure induces both intra- and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology. 2004;29:1142–52. doi: 10.1016/j.psyneuen.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–8. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 25.Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–7. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 26.Barreto A, Gonzalez JM, Kabingu E, Asea A, Fiorentino S. Stress-induced release of HSC70 from human tumors. Cell. Immunol. 2003;222:97–104. doi: 10.1016/s0008-8749(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 27.Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative Mechanism by which IFN-{gamma} Enhances Tumor Recognition: Active Release of Heat Shock Protein 72. J Immunol. 2005;175:2900–12. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gastpar R, Gehrmann M, Bausero MA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–47. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–55. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 30.Zitvogel L, Fernandez N, Lozier A, et al. Dendritic cells or their exosomes are effective biotherapies of cancer. Eur J Cancer. 1999;35(Suppl 3):S36–8. doi: 10.1016/s0959-8049(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 31.Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 32.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–7. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 34.Schneider EM, Niess AM, Lorenz I, Northoff H, Fehrenbach E. Inducible hsp70 expression analysis after heat and physical exercise: transcriptional, protein expression, and subcellular localization. Ann N Y Acad Sci. 2002;973:8–12. doi: 10.1111/j.1749-6632.2002.tb04598.x. [DOI] [PubMed] [Google Scholar]
- 35.Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R. CD3- large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood. 1995;86:1374–82. [PubMed] [Google Scholar]
- 36.Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J. Immunol. 1997;158:4341–50. [PubMed] [Google Scholar]
- 37.Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–79. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- 38.Reed RC, Nicchitta CV. Chaperone-mediated cross-priming: a hitchhiker's guide to vesicle transport (review) Int J Mol Med. 2000;6:259–64. doi: 10.3892/ijmm.6.3.259. [DOI] [PubMed] [Google Scholar]
- 39.Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 40.Sondermann H, Becker T, Mayhew M, Wieland F, Hartl FU. Characterization of a receptor for heat shock protein 70 on macrophages and monocytes. Biol Chem. 2000;381:1165–74. doi: 10.1515/BC.2000.144. [DOI] [PubMed] [Google Scholar]
- 41.Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000;6:435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 42.Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptide-bearing and peptide-negative preparations function as chaperokines. Cell Stress & Chaperones. 2000;5:425–31. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold-Schild D, Hanau D, Spehner D, et al. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 1999;162:3757–60. [PubMed] [Google Scholar]
- 44.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–12. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 45.Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura T, Hinagata J, Tanaka T, et al. HSP90, HSP70, and GAPDH directly interact with the cytoplasmic domain of macrophage scavenger receptors. Biochem Biophys Res Commun. 2002;290:858–64. doi: 10.1006/bbrc.2001.6271. [DOI] [PubMed] [Google Scholar]
- 47.Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277–85. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat. Immunol. 2000;1:151–55. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 49.Binder RJ, Karimeddini D, Srivastava PK. Adjuvanticity of alpha 2-macroglobulin, an independent ligand for the heat shock protein receptor CD91. J Immunol. 2001;166:4968–72. doi: 10.4049/jimmunol.166.8.4968. [DOI] [PubMed] [Google Scholar]
- 50.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci USA. 2004;101:6128–33. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–51. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 52.Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–60. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 53.Berwin B, Hart JP, Rice S, et al. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003;22:6127–36. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haworth R, Platt N, Keshav S, et al. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–9. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLeish KR, Dean WL, Wellhausen SR, Stelzer GT. Role of intracellular calcium in priming of human peripheral blood monocytes by bacterial lipopolysaccharide. Inflammation. 1989;13:681–92. doi: 10.1007/BF00914312. [DOI] [PubMed] [Google Scholar]
- 56.Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–6. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 57.Singh-Jasuja H, Toes RE, Spee P, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J. Exp. Med. 2000;191:1965–74. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calderwood SK, Theriault JR, Gong J. Message in a bottle: Role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol. 2005;35:2518–27. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- 59.Baker-LePain JC, Reed RC, Nicchitta CV. ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr Opin Immunol. 2003;15:89–94. doi: 10.1016/s0952791502000067. [DOI] [PubMed] [Google Scholar]
- 60.Houde M, Bertholet S, Gagnon E, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–6. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 61.Asea A. Chaperokine-induced signal transduction pathways. Exerc. Immunol. Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- 62.Asea A, Henderson B, Pockley AG. The Extracellular Biology of Molecular Chaperones. Cambridge University Press; London (in press): 2004. Exogenous Hsp70: principles and application of the chaperokine activity of Hsp70. [Google Scholar]
- 63.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 64.Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J. Immunol. 2002;168:2997–3003. doi: 10.4049/jimmunol.168.6.2997. [DOI] [PubMed] [Google Scholar]
- 65.Lehner T, Bergmeier LA, Wang Y, Tao L, Sing M, Spallek R, van der Zee R. Heat shock proteins generate beta-chemokines which function as innate adjuvants enhancing adaptive immunity. Eur J Immunol. 2000;30:594–603. doi: 10.1002/1521-4141(200002)30:2<594::AID-IMMU594>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 66.Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptide-bearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5:425–31. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava PK. Immunotherapy for human cancer using heat shock protein-Peptide complexes. Curr Oncol Rep. 2005;7:104–8. doi: 10.1007/s11912-005-0035-8. [DOI] [PubMed] [Google Scholar]
- 68.Srivastava PK. Heat shock protein-based novel immunotherapies. Drug News Perspect. 2000;13:517–22. doi: 10.1358/dnp.2000.13.9.858479. [DOI] [PubMed] [Google Scholar]
- 69.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–8. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 70.Noessner E, Gastpar R, Milani V, et al. Tumor-derived heat shock protein 70 peptide complexes are cross- presented by human dendritic cells. J. Immunol. 2002;169:5424–32. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- 71.Singh-Jasuja H, Scherer HU, Hilf N, et al. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur. J. Immunol. 2000;30:2211–5. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 72.Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165:6029–35. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- 73.Matzinger P. An innate sense of danger. Semin. Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 74.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 75.Lancaster GI, Moller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–80. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Whittall T, McGowan E, et al. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol. 2005;174:3306–16. doi: 10.4049/jimmunol.174.6.3306. [DOI] [PubMed] [Google Scholar]
- 77.Millar DG, Garza KM, Odermatt B, et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat. Med. 2003;9:1469–76. doi: 10.1038/nm962. [DOI] [PubMed] [Google Scholar]
- 78.Suzue K, Young RA. Heat shock proteins as immunological carriers and vaccines. Exs. 1996;77:451–65. doi: 10.1007/978-3-0348-9088-5_30. [DOI] [PubMed] [Google Scholar]
- 79.Suzue K, Young RA. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J. Immunol. 1996;156:873–9. [PubMed] [Google Scholar]
- 80.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–51. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen W, Lin Y, Liao C, Hsieh S. Modulatory effects of the human heat shock protein 70 on DNA vaccination. J Biomed Sci. 2000;7:412–9. doi: 10.1007/BF02255816. [DOI] [PubMed] [Google Scholar]
- 82.Oglesbee MJ, Pratt M, Carsillo T. Role for heat shock proteins in the immune response to measles virus infection. Viral Immunol. 2002;15:399–416. doi: 10.1089/088282402760312296. [DOI] [PubMed] [Google Scholar]
- 83.Huang XF, Ren W, Rollins L, et al. A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. Cancer Res. 2003;63:7321–9. [PubMed] [Google Scholar]
- 84.Qazi KR, Wikman M, Vasconcelos NM, Berzins K, Stahl S, Fernandez C. Enhancement of DNA vaccine potency by linkage of Plasmodium falciparum malarial antigen gene fused with a fragment of HSP70 gene. Vaccine. 2005;23:1114–25. doi: 10.1016/j.vaccine.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 85.Kawabata Y, Udono H, Honma K, et al. Merozoite surface protein 1-specific immune response is protective against exoerythrocytic forms of Plasmodium yoelii. Infect Immun. 2002;70:6075–82. doi: 10.1128/IAI.70.11.6075-6082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Binder RJ, Harris ML, Menoret A, Srivastava PK. Saturation, competition, and specificity in interaction of heat shock proteins (hsp) gp96, hsp90, and hsp70 with CD11b+ cells. J. Immunol. 2000;165:2582–7. doi: 10.4049/jimmunol.165.5.2582. [DOI] [PubMed] [Google Scholar]
- 87.Li Z, Srivastava PK. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 1993;12:3143–51. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–6. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heike M, Blachere NE, Srivastava PK. Protective cellular immunity against a spontaneous mammary carcinoma from ras transgenic mice. Immunobiology. 1994;190:411–23. doi: 10.1016/S0171-2985(11)80612-1. [DOI] [PubMed] [Google Scholar]
- 90.Casey DG, Lysaght J, James T, Bateman A, Melcher AA, Todryk SM. Heat shock protein derived from a non-autologous tumour can be used as an anti-tumour vaccine. Immunology. 2003;110:105–11. doi: 10.1046/j.1365-2567.2003.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang XY, Chen X, Manjili MH, Repasky E, Henderson R, Subjeck JR. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res. 2003;63:2553–60. [PubMed] [Google Scholar]
- 92.Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer. 2003;105:226–31. doi: 10.1002/ijc.11058. [DOI] [PubMed] [Google Scholar]
- 93.Bogers WM, Bergmeier LA, Ma J, et al. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. Aids. 2004;18:25–36. doi: 10.1097/00002030-200401020-00003. [DOI] [PubMed] [Google Scholar]
- 94.Bogers WM, Bergmeier LA, Oostermeijer H, et al. CCR5 targeted SIV vaccination strategy preventing or inhibiting SIV infection. Vaccine. 2004;22:2974–84. doi: 10.1016/j.vaccine.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 95.Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006 doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 96.Srivastava PK, Udono H. Heat shock protein-peptide complexes in cancer immunotherapy. Curr Opin Immunol. 1994;6:728–32. doi: 10.1016/0952-7915(94)90076-0. [DOI] [PubMed] [Google Scholar]


