Abstract
Febrile non-hemolytic and allergic reactions are the most common transfusion reactions, but usually do not cause significant morbidity. In an attempt to prevent these reactions, US physicians prescribe acetaminophen or diphenhydramine premedication before more than 50% of blood component transfusions. Acetaminophen and diphenhydramine are effective therapies for fever and allergy respectively, so their use in transfusion has some biologic rationale. However, these medications also have potential toxicity, particularly in ill patients, and in the studies performed to date, they have failed to prevent transfusion reactions. Whether the benefits of routine prophylaxis with acetaminophen and diphenhydramine outweigh their risks and cost requires re-examination, particularly in light of the low reaction rates reported at many institutions even when pre-medication is not prescribed.
The most common acute adverse reactions to blood-component transfusions, febrile non-hemolytic transfusion reactions (FNHTRs) and allergic reactions, are fortunately among the least harmful. Blood recipients with FNHTRs experience fever (often defined as a temperature rise ≥ 1°C above baseline) and/or rigors within 3 hours of transfusion. Allergic reactions are most often associated with the development of urticaria or other rash, pruritus, wheezing, or angioedema within several hours of transfusion.1–8 These reactions are temporally limited, self-resolving, and generally pose little risk of causing lasting harm. Mild reactions often consist of a limited increase of temperature without other symptoms or a localized urticarial exanthem. In moderate and severe reactions, rigors and fever may be severe with rapid onset and associated with other symptoms, and urticaria may be extensive and painful or include respiratory or other systemic symptoms.
How an incipient urticarial or FNHTR will progress cannot be predicted on the basis of its initial presentation. Further, it is often not possible to distinguish the symptoms of mild reactions from early symptoms of other more consequential problems such as sepsis, hemolytic reactions, or anaphylaxis. Therefore, the earliest indication of a reaction mandates discontinuation of a transfusion and, except for the mildest urticarial reactions, its termination. Increased clinical and laboratory monitoring is necessary, and infusion of replacement blood may be required. Blood replacement is associated with additional transfusion-associated risks and may deplete low blood inventories. The development of repeated reactions, even when mild, in patients receiving chronic transfusions may warrant further prophylaxis through the subsequent modification of blood products by leukoreduction, volume-reduction, saline-resuspension, or washing of cellular products. These steps require increased effort and cost, and may reduce product potency. Because allergic and febrile non-hemolytic reactions affect patient welfare, laboratory management, and the cost of patient care, prevention is an important goal.
The most common bedside approach for the prevention of febrile non-hemolytic and urticarial transfusion reactions is premedication with an antipyretic and an antihistamine, most commonly acetaminophen and diphenhydramine. The use of premedication before transfusion is widespread. For example, Ezidiegwu et al.1 reported a premedication rate of 80% in a large US hospital. At a Canadian institution, Patterson et al.2 reported a rate of 73%, which decreased to 50% after implementation of institutional guidelines. At our institution, where most transfusions are administered to pediatric oncology patients, we have observed a rate of 68%.3
Based on these results, it can be concluded that millions of transfusion recipients are premedicated each year to prevent transfusion reactions. Yet the efficacy of such premedication has not been rigorously tested. We review the causes of and rationale for pre-medication in febrile non-hemolytic and allergic transfusion reactions, the use and toxicities of acetaminophen and diphenhydramine premedication, as well as existing data on the utility of premedication in the management of transfusion reactions.
INCIDENCE AND RISK FACTORS
Frequency of Allergic and Febrile Non-hemolytic Reactions
A recipient’s risk of developing a FNHTR or allergic reaction after transfusion is unknown. A review of the literature showed substantial variability in reaction rates after platelet and RBC transfusions (Table 1). The reported rate of FNHTRs to platelets ranged from 0.09% to >27%, a greater than 300-fold difference! The risk of an allergic reaction to platelets ranged from 0.09% to 21%, a similarly remarkable difference of greater than 200-fold. Differences in inter-institutional FNHTR rates cannot be explained by the implementation of pre-storage leukoreduction, a practice reported to have reduced FNHTR rates in several4–6 but not all7 studies. Further, FNHTR reaction rates at some institutions where leukoreduction is performed are greater than those at other institutions where it is not. For instance, Patterson et al.2 reported a greater than 10% FNHTR rate for platelet transfusions after the initiation of universal leukoreduction, whereas Yazer et al.4 reported a rate of 0.45% without universal leukoreduction. It therefore seems likely that the discrepancy in reported rates results from the combined effect of multiple variables, potentially including differences in premedication use, patient characteristics, product manufacture, storage time, reporting rates, reaction definitions, and monitoring standards.
Table 1.
Allergic and Febrile Non-hemolytic Transfusion Reaction Rates
| RBCs (%) | Platelets (%) | ||||
|---|---|---|---|---|---|
| Study | Febrile | Allergic | Febrile | Allergic | Blood product preparation |
| DaPonte 200551 | 0.42 | 0.12 | |||
| 0.09 | 0.17 | Pre-storage LR | |||
| Sanders 20053 | 0.36 | 0.61 | 0.21 | 0.86 | Pre-storage LR |
| King 20046 | 0.37 | 0.15 | |||
| 0.19 | 0.17 | Pre-storage LR | |||
| Paglino 20045 | 0.34 | 0.09 | 2.18 | 0.49 | |
| 0.18 | 0.09 | 0.15 | 0.35 | Pre-storage LR | |
| Ezidiegwu 20041 | 0.13 | 0.09 | |||
| Yazer 20044 | 0.33 | 0.45 | |||
| 0.19 | 0.11 | Pre-storage LR | |||
| Domen 200321 | 0.03 | 0.09 | |||
| Couban 200252 | 12 | 5 | |||
| 7 | 6 | Plasma depleted | |||
| 5 | 6 | Pre-storage LR | |||
| Heddle 200253 | 21.3 | 0 | Plasma depleted | ||
| 6.4 | 4.1 | PC, Pre-storage LR | |||
| 7.7 | 4.8 | SDP, Pre-storage LR | |||
| Ibojie 20027 | 0.35 | ||||
| 0.23 | Pre-storage LR | ||||
| Uhlmann 200154 | 0.12 | 0.04 | |||
| 0.08 | 0.06 | Pre-storage LR | |||
| Patterson 20002 | 23.80 | 2.4 | FNHTR estimated as total – urticarial reactions | ||
| Sarkodee-Adoo 199855 | 2.6 | 1.5 | |||
| Riccardi 199756 | 0.95 | 0.26 | |||
| Anderson 199757 | 17.1 | PRP-PC | |||
| 3.8 | Buffy coat PC | ||||
| 3.1 | SDP | ||||
| Muylle 199658 | 9.3 | PRP-PC | |||
| 2.7 | Buffy coat PC | ||||
| Federowicz 199659 | 2.15 | 0.51 | 1.58 | 3.7 | |
| 1.10 | 0.41 | 1.73 | 3.7 | Pre-storage LR | |
| Dzieczkowski 199560 | 2.15 | 0.51 | 1.58 | 3.7 | Pre-storage process LR for platelets only |
| Oskanen 199461 | 4.6 | 21 | |||
| Mangano 199162 | 14 | Single donor | |||
| 27.2 | PC | ||||
| 13.5 | Bedside leukoreduction | ||||
| Chambers 199063 | 21.4 | PC | |||
| 8.4 | SDP |
When more than one rate is listed for a single study, the major difference between study groups is also listed. Studies otherwise vary in product type and manufacturing. RBC, red blood cells; LR, leukocyte reduced; FNHTR, febrile non-hemolytic transfusion reaction; PC, platelet concentrate; SDP, single donor platelet; PRP, platelet rich plasma
The data compiled in Table 1 may be used to crudely estimate reaction incidence. The median reported rates for FNHTR are 4.6% for platelets (n=29 data points) and 0.33% for RBCs (n=17 data points). For allergic reactions, median reported rates are 3.7% for platelets (n=17 data points) and 0.15% for RBCs (n=13 data points). Although these numbers are crude estimates and the actual incidence will vary between institutions, they nevertheless provide a rough guideline for the overall frequency of FNHTR and allergic reactions. Patients in the United States received approximately 14,000,000 units of RBCs in 2001.8 Assuming a 0.48% rate for both FNHTR and allergic reactions, approximately 67,000 reactions to RBCs occurred in the U.S. that year. In 1999, an estimated 1,500,000 platelet transfusions were performed.9 Assuming an 8.3% reaction rate, an estimated 124,500 FNHTR and allergic reactions resulted. The medical and financial toll implied by these numbers, even considering their imprecision, is impressive.
PATHOPHYSIOLOGY OF FEBRILE NON-HEMOLYTIC TRANSFUSION REACTIONS
FNHTRs present in clinically diverse ways.10 They vary in severity, extent of fever, timing of fever development, presence of rigors, and in their association with additional symptoms such as hypo- or hypertension, flushing, GI irritability, headache, and occasional respiratory distress. Indeed, fever itself is sometimes dissociated from symptoms otherwise consistent with FNHTRs, and fever can also occur in association with other types of transfusion reactions. The variability in clinical presentation of FNHTRs suggests that they are not a single entity. The current commonly applied reaction classification scheme may therefore combine pathophysiologically distinct entities into a single category based on a common spectrum of symptoms. Reaction classifications that alternatively segregate reaction types have been proposed, but have not been widely adopted.10
Although the pathophysiology of FNHTR has not been fully elucidated, release of fever-promoting cytokines appears to be the common responsible downstream event.10 Cytokine production may, but need not, occur after blood infusion. Leukocytes secrete an array of cytokines during blood storage. Platelet units, which are stored at room temperature, and which may contain large numbers of leukocytes, are particularly susceptible to the accumulation of biologically significant quantities of cytokines. Cytokine content increases in proportion to the leukocyte content and time of storage.11 In a classic study establishing a role for cytokine production during blood storage in FNHTRs, Heddle et al.12 separated stored platelets into their cellular and plasma components before transfusion. Plasma and cells were transfused separately using a randomized crossover technique. The transfusion of platelet unit-derived plasma was associated with the development of a FNHTR significantly more often than was the transfusion of the cellular component. Further studies demonstrated that various pro-inflammatory cytokines and biological response modifiers accumulate in stored platelets, including histamine, interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor α.10,13 Cytokine accumulation was seen to a lesser degree in RBCs, which unlike platelets are refrigerated during storage.
The hypothesis that passive infusion of leukocyte-generated cytokines can induce FNHTR suggests that removal of leukocytes from blood components before storage should reduce the risk of FNHTRs. Indeed, pre-storage leukoreduced platelets accumulate few leukocyte-derived cytokines.11,14 Use of these products has reduced the incidence of FNHTRs in many institutions (Table 1). Interestingly, it has not eliminated these reactions, and some centers report no change in reaction incidence when comparing rates before and after the implementation of leukoreduction.
Cytokines from Plasma, Platelets, and Red Blood Cells in FNHTR
One possible complementary cause of FNHTRs is the release of cytokines or other biologic response modifiers from platelets, RBCs, or plasma. Indeed, pre-storage leukoreduced platelet units accumulate several platelet-derived cytokines and biological response modifiers during storage.15 Anaphylatoxins C3a and C5a, derived from the proteolysis of plasma complement C3 and C5, accumulate in plasma-containing components during storage.16,17 At the time of infusion, contact-dependent mechanisms may promote the activation of kininogens and release of bradykinin18. Thus progressive activation of the non-leukocytic moieties in stored blood components may lead to the generation and accumulation of bio-active compounds. However, it remains to be established whether, how, and which of these biological response modifiers participate in the pathogenesis of FNHTRs.
Leukoagglutinins in FNHTRs
Several observations indicate that passively infused cytokines are not required for FNHTRs. Limited quantities of cytokines accumulate in RBCs or leukoreduced components, yet these products may still be associated with severe reactions. Infused cytokines and biological response modifiers would be anticipated to induce rapid reactions after infusion. However, FNHTR may occur as late as several hours after transfusion. Finally, if passive cytokine infusion was primarily responsible for FNHTRs, it would be anticipated that the risk of FNHTRs would be largely recipient-independent. However, some recipients show increased susceptibility to FNHTRs.
Recipient or donor leukoagglutinins are believed to bear primary responsibility for many of these residual reactions. In one study, 5 patients who had experienced FNHTRs and who had leukoagglutinins received either a buffy-coat–rich fraction of blood, or buffy-coat–depleted blood.19 The buffy coats induced severe febrile reactions, whereas the leukopoor components were not associated with such reactions. Interestingly, many of the symptoms of the reactions were delayed and fever was not observed until 1 to 2 hours after the transfusion was started. How leukoagglutinins mediate FNHTRs is uncertain. Leukocytes themselves are responsible for the production of many of the cytokines and bioactive compounds that mediate the symptoms of FNHTRs. Antibodies may stimulate leukocytes, promoting their production of these substances. Alternatively, leukoagglutinins, after binding to target leukocytes, may fix complement or activate cellular immune mechanisms that promote destruction of the coated leukocytes. This destruction may be associated with an inflammatory response and the release of effector molecules responsible for FNHTRs. This latter scenario would imply that leukocyte-specific agglutinins should not exclusively be responsible for FNHTRs. Antibodies specific to other cell types should be able to activate similar pro-inflammatory pathways. Indeed, immune-mediated hemolytic reactions caused by anti-RBC antibodies share many of the features of severe FNHTRs. Some evidence suggests that anti-platelet antibodies may similarly provoke FNHTRs20.
In summary, several mechanisms appear to be capable of causing reactions currently classified as FNHTRs. Downstream, all involve the generation of pro-inflammatory cytokines and response modifiers that induce fever and associated symptoms. How the interplay of the different pathophysiologic mechanisms and recipient-specific characteristics leads to the ultimate phenotype of the reactions, however, remains unknown.
PATHOPHYSIOLOGY OF ALLERGIC TRANSFUSION REACTIONS
Clinical Manifestations of Allergic Transfusion Reactions
Like FNHTRs, allergic reactions have highly variable clinical manifestations. Allergic reactions may present as isolated pruritis, limited numbers of anatomically localized hives, or disseminated urticaria. In some instances they include systemic allergic symptoms such as bronchoconstriction, hypotension, anxiety, or gastrointestinal distress. Domen and Hoeltge21 reviewed 273 allergic reactions and identified skin manifestations that included urticaria, maculopapular rash, periorbital edema, erythema, flushing, and pruritis or combinations of these manifestations. Five percent of reactions were associated with fever, and some with hypotension. Almost one-tenth of reactions (9.5%) lacked skin manifestations; these primarily involved pulmonary symptoms.
IgE in Allergic Transfusion Reactions
The term “allergy” is typically used to indicate Type I hypersensitivity responses, which are mediated by IgE antibodies. IgE is produced when antigen-specific Th2 T-cells, secreting IL-4 and IL-13, stimulate activated B-lymphocytes to change the antibody isotype they produce from IgM to IgE.22 Evidence exists that at least some allergic transfusion reactions are mediated by this pathway; however, much remains speculative regarding the actual mechanisms underlying allergic reactions. Antigens presented transmucosally, at low dose, and in the absence of some bacterial or virally derived immune stimuli, are more likely to induce a Th2 response and thus promote the development of IgE. Genetic factors can also lead to a proclivity toward atopy and IgE production.23 Polymorphisms in IL-4 and its receptor and other genes may also play a role in the induction of Th2 immunity and susceptibility to allergy.
IgE binds tightly to a high-affinity antibody receptor present on mast cells and basophils. When a multivalent allergen binds the cell-associated IgE, it crosslinks the receptor and activates the mast cell. The mast cell releases a variety of mediators (Table 2); the net effect of which is to increase the flow of cells and fluid into tissues. This results in the classic wheal and flare reaction in the skin, airway constriction, edema, mucus production in the respiratory tract, and gastrointestinal irritability that causes diarrhea and vomiting.
Table 2.
Role of Mast- Cell–Derived Mediators in Allergic Transfusion Reactions
| Mediator | Some Effects |
|---|---|
| Histamine | Increased vascular permeability and smooth muscle contraction |
| Heparin | |
| Leukotrienes | Increased vascular permeability, smooth muscle contraction, leukocyte infiltration, leukocyte activation |
| Platelet activating factor | |
| IL-4 | Th2 T-cell differentiation |
| IL-13 | |
| IL-5 | Eosinophil activation |
| Chemokines | Attract neutrophils, macrophages, and other inflammatory cells |
IgG in Allergic Transfusion Reactions
Although IgE is considered dominantly responsible for allergic reactions, it is not exclusively so. IgG antibodies may induce allergic or anaphylactic symptoms. Complement fixation to IgG mediates the release of C3a and C5a anaphylatoxins, which act directly on target cells or by promoting the release of vasoactive compounds from mast cells. Indeed, anaphylaxis that develops in response to IgA, haptoglobin, or C4 can result from reactions of the iso- or alloantigen with IgG rather than IgE.24,25
Allergens Responsible for Allergic Transfusion Reactions
The allergens that are responsible for most allergic reactions have not been described. In cases such as those involving anaphylactic responses to IgA, haptoglobulin, and C4, the recipient may lack the infused antigen or may express allelically distinct versions of it. In other cases, the antigen may be an extraneous substance present in blood. For instance, infusion of blood from a donor taking penicillin may induce an allergic reaction in a patient with penicillin sensitivity.26 It is also possible for infused antibodies to cause allergic reactions in recipients with, or exposed to, a cognate allergen. Such cases have been observed, for example, when cephalothin-specific antibodies have been infused into recipients using this drug.27 Indeed, it has been similarly demonstrated that sensitivity to grass antigen may be passively transferred through IgE present in blood products.28
Because the intravenous infusion of an allergen or antibody through the transfusion of blood components is necessarily a systemic event, the localized manifestations of many allergic reactions are an unexpected and interesting feature. They suggest that the presence of atopy is insufficient for a global reaction. It is possible that mild allergic reactions are limited by the localized presence of high concentrations of specific IgE to transfused allergens. Alternatively, transfused antibody may react with localized deposits of antigen. It is also possible that tissue-specific susceptibility features can explain the asymmetrical skin manifestations observed with allergic transfusion reactions.
Antibody Independent Activation of Mast Cells
Mast cells can be activated through immunoglobulin-independent mechanisms, although the role of these mechanisms in allergic transfusion reactions is not established. Various organic bases can stimulate mast cells. For example, vancomycin can induce histamine release, resulting in “red man syndrome.” Basic polypeptides, such as those present in venoms or released after tissue injury, can also activate mast cells.29 Several basic compounds able to activate mast cells can accumulate during blood storage, including the anaphylatoxins C3a and C5a, and cytokines such as IL-1, IL-8, and platelet factor 4. It is possible that these or other substances in blood can provoke or modulate allergic reactions.
ACETAMINOPHEN
Mechanism of Action
Body temperature is regulated through the activity of hypothalamic neurons. In the classic model of fever, pyrogens such as IL-1, IL-6, and TNF are produced at a site of inflammation and released into the circulation, from which they stimulate the thermoregulatory center of the anterior hypothalamus to produce prostaglandin E2 (PGE2). PGE2 adjusts the body’s thermal rheostat, inducing fever. Indeed, the infusion of recombinant pyrogenic cytokines is able to induce fever. Newer data suggest that pro-inflammatory compounds can also induce fever through the activation of Toll-like receptors on the vascular endothelium supplying the thermoregulatory center 30,31 or through the stimulation of signaling through noradrenergic nerves.32,33 Regardless of the mechanism, production of PGE2 is important for fever development. Cyclooxygenase, an enzyme that participates in the transformation of lipids into prostaglandins, is crucial for PGE2 production and fever.
Acetaminophen, or N-acetyl-p-aminophenol, is effective against fever because of its direct inhibitory effect against cyclooxygenase.34 Cyclooxygenase also plays a role in pain responses, platelet activation, inflammation, and many other crucial activities. Acetaminophen has both antipyretic and analgesic effects, but only weak anti-inflammatory effects. High concentrations of peroxides within inflammatory lesions suppress acetaminophen activity, limiting its ability to suppress cyclooxygenase.
The poor anti-inflammatory properties of acetaminophen distinguish it from aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) that similarly act by inhibiting cyclooxygenase. It is also distinguished from aspirin because it does not adversely affect platelet function or bleeding time, a significant advantage when used as premedication for blood transfusions. Additionally, it does not irritate or erode the stomach lining, or noticeably influence the cardiovascular or respiratory systems. Acetaminophen’s limited spectrum of activity, combined with its rapid absorption from the gastrointestinal tract (it peaks within 30 to 60 minutes of oral administration) and its short half life (approximately 2 hours) are important reasons that it is preferred as an antipyretic in patients receiving transfusion.
Risks Associated with the Use of Acetaminophen
Acetaminophen is among the most commonly used medications worldwide; in the U.S., it was the most commonly prescribed and over-the-counter drug between 1998 and 1999 35. Acetaminophen may be used alone or combined with other active ingredients. Its wide use implies safety, and although acetaminophen has a good safety record considering the number of doses consumed, it does have substantial potential for toxicity that may be increased in some ill patients. Acetaminophen’s primary toxicity stems from one of its metabolites, N-acetyl-p-quinoneimine (NAPQI).36 Approximately 90% to 95% of ingested acetaminophen is metabolized by conjugation with glucuronides and sulfates, which boosts urinary excretion. In the liver, the remaining 5% is normally converted into NAPQI through the action of the cytochrome P450 system, primarily CYP2E1. NAPQI is electrophilic and highly reactive. It is detoxified within cells through conjugation with the sulfhydryl moiety of glutathione. If an overdose occurs, glutathione is depleted, limiting detoxification. Sulfate stores are similarly consumed, limiting alternative metabolic pathways. The accumulating NAPQI reacts with other sulfhydryls within the hepatocyte, such as those within proteins. Hepatic toxicity may result directly from this protein damage, or by the accumulation of reactive oxygen species and pro-inflammatory cytokines after the initial insult. Acetaminophen overdose currently accounts for nearly half of all cases of acute hepatic failure in the United States.37 Liver failure may also lead to renal failure through the hepatorenal syndrome or through the direct effects of NAPQI generated in the kidneys by renal cytochrome oxidase.
The typical pre-transfusion dose of acetaminophen is 650 mg for adults and 10 mg/kg for children. Yet, the smallest documented lethal dose in an adult is only roughly 15 times this, or 10 g. The risk of hepatotoxicity becomes substantial at even smaller doses, 7.5 g in the adult or 150 mg/kg in children. Further, chronic acetaminophen ingestion may induce hepatotoxicity when doses as low as 4 g for an adult or 90 mg/kg for children daily are exceeded. This represents only 6 to 9 doses of acetaminophen per day. In our experience in a pediatric oncology center, it is not unusual for some allogeneic stem cell transplant recipients to receive this number of transfusions in a single day. If each transfusion were preceded by premedication, toxicity could result. This concern is exacerbated by the fact that many multiply transfused patients are ill and may have hepatic and metabolic abnormalities that enhance the generation or toxicity of NAPQI. For this reason acetaminophen premedication is best avoided in the patient with liver disease, and the use of acetaminophen in the hospitalized patient should be monitored.
Outpatients receiving blood products may also be self-administering acetaminophen or acetaminophen-containing products for other reasons. Interestingly, in one recent study most cases of acute liver failure secondary to acetaminophen toxicity were non-intentional.37 Patients may not recognize the harmful effects of acetaminophen, or even be aware that they are receiving this medication before transfusion. They may also not be aware that acetaminophen is a common component of many over-the-counter medications and that cumulative toxicity may result from its use in different medications. Further, not all types of toxicity associated with this frequently used medication may be known. For instance, some data have associated frequent acetaminophen use with the development of allergies and asthma.38 The common misconception that over-the-counter medications are inherently safe may lead to the conclusion that more medicine is better, increasing the risk of an overdose and toxic effects. Direct toxicity due to acetaminophen administered as a pre-transfusion medication has not been reported. Nevertheless, this medication should only be used with due consideration of its potential for adverse effects.
DIPHENHYDRAMINE
Mechanism of Action
Histamine, or β-aminoethylimidazole, is an important effector molecule in the allergic response.34 After activation of mast cells by the stimulation of FcεRI or other receptors, the contents of secretory granules containing histamine are released into the extracellular space. Histamine plays multiple roles, only some of which are significant for allergic transfusion reactions. Dilation of small blood vessels and increased vascular permeability are responsible for the flushing and tissue edema that occur with allergic reactions. These effects may also decrease vascular resistance, thereby diminishing blood pressure; however, that may be tempered by histamine’s activity in constricting larger blood vessels. Histamine also constricts the bronchial smooth muscle, which may induce the wheezing and respiratory distress apparent in some allergic reactions. It is important to note that histamine is only one of several bioactive mediators secreted by activated mast cells (Table 2). Also released by these cells are leukotrienes, kinins, and other biological response mediators. As a result, inhibition of the histamine response would be expected to have a substantial, although incomplete, effect in the prevention or treatment of allergic reactions.
There are three types of histamine receptors: H1, H2, and H3. Diphenhydramine acts selectively on the H1 receptor. An ethylamine moiety within the drug associates with the receptor and stabilizes it in an inactive conformation. H1 receptors are responsible for the smooth muscle and vascular effects of histamine; by blocking these, diphenhydramine is able to counteract related symptoms. GI absorption of diphenhydramine is more gradual than that of acetaminophen, and peak concentrations are not achieved until 2 to 3 hours after oral administration. For this reason, intravenous administration of diphenhydramine is often the preferred route pre-transfusion. The antihistaminic effects last approximately 4 to 6 hours in healthy adults, although they may be longer in the presence of liver disease and shorter in children or after upregulation of hepatic microsomal enzymes.
Risks of Diphenhydramine
Diphenhydramine, like acetaminophen, is widely used; between 1998 to 1999, it was the sixth most commonly used medication in the United States.35 Unlike acetaminophen, which has no discernible adverse effect in most recipients, diphenhydramine commonly causes substantial impairment. Diphenhydramine has anticholinergic as well as antihistaminic effects and can penetrate into the CNS, where it often causes drowsiness, decreased alertness, and impaired cognitive performance.39 In some recipients, paradoxically, restlessness and nervousness develops. Diphenhydramine is in a class of antihistamines most likely to induce impairment. Patients receiving diphenhydramine perform more poorly on driving tests than do those with blood alcohol levels exceeding the legal limit40, and have an increased risk of serious injury.41 Therefore the routine use of diphenhydramine may have substantial adverse effects on quality of life, and it may increase the risk of inadvertent injury and diminish work or school performance. In a small percentage of patients, diphenhydramine and other first-generation antihistamines have also been associated with cardiotoxicity and arrhythmias.42 As for acetaminophen, the over-the-counter classification of diphenhydramine may lull medical practitioners and patients into underestimating this medication’s adverse effects. It is important to consider toxicities when deciding whether to use this premedication before a transfusion.
PREMEDICATION WITH ACETAMINOPHEN AND DIPHENHYDRAMINE
Prevalence of routine premedication
Premedication with acetaminophen and diphenhydramine is the most commonly used approach to reduce the incidence of FNHTR and allergic reactions to blood products; it is used in 50% to 80% of transfusions in the US and Canada.1,3,43,44 In a study of 7,900 transfusions administered in a pediatric cancer center, no premedication was administered before 2,521 (32%) transfusions; acetaminophen alone was used as premedication in 1,064 (13%), diphenhydramine alone in 1,271 (16%), and both drugs in 3,044(38%). The strongest predictor of who would receive premedication was whether the patient had been premedicated for a previous transfusion. An informal survey of colleagues at our institution confirmed that when ordering a transfusion, the standard practice was to use the same premedications that had been used previously. Indeed, practitioners appear unlikely to review the medical record to determine why a patient has been receiving premedications. This suggests that establishing centralized systems that facilitate communication of premedication needs may improve ordering practice.
Clinical Studies of the Utility of Premedication in the Prevention of Transfusion Reactions
Despite the fact that millions of transfusion recipients per year are premedicated to prevent transfusion reactions, the efficacy of premedication has not been rigorously tested. The only randomized, double-blind, placebo-controlled clinical trial of premedication use showed no difference in the rate of FNHTR with or without premedication.44 The trial included 98 transfusions in 51 adult oncology patients. The rate of reactions was 15.2% in patients premedicated with placebo versus 15.4% in those premedicated with 650 mg acetaminophen orally and 25 mg diphenhydramine intravenously (P =0.94).44 A retrospective study of 7,900 transfusions administered to 385 pediatric patients with cancer or who required hematopoietic stem cell transplantation assessed the effect of premedication on the rates of allergic and FNHTR.3 In this study, patients received exclusively pre-storage leukoreduced, irradiated blood products, including only single-donor apheresis platelets. The incidence of allergic reactions was 0.75%; of FNHTR, 0.28%. Allergic reactions were associated with 0.9% of transfusions in which diphenhydramine was administered, compared to 0.56% of those in which it was not; febrile reactions occurred in 0.37% of cases with acetaminophen premedication and 0.18% of those without it. Neither difference was statistically significant in multivariable analysis.
These data suggest that acetaminophen and diphenhydramine are not effective in diminishing the incidence of febrile or allergic reactions. In contrast, in one study of almost 120,000 transfusions with 80% use of acetaminophen premedication, the overall rate of FNHTR was only 0.09%, low by all standards. However, no comparison group was studied to isolate the effect of premedication.1
Prevention of Febrile and Allergic Reactions in Patients with a History of Previous Reactions
In an attempt to decrease subsequent reactions, premedications are frequently prescribed in patients who have had a previous transfusion reaction. This practice was not supported by the data of Sanders et al., which showed no difference in reaction rates with premedication use, even when patients had a history of 2 or more reactions (Figures 1 and 2).3 Furthermore, allergic and febrile reactions were no more common in patients with a previous reaction than in those who had no reaction. One limitation of this retrospective study and the current literature is that the effect of pre-medication on reaction severity has not been analyzed. Therefore, it is unknown whether the premedications may have decreased the severity of reactions, even if they did not affect incidence.
Figure 1.
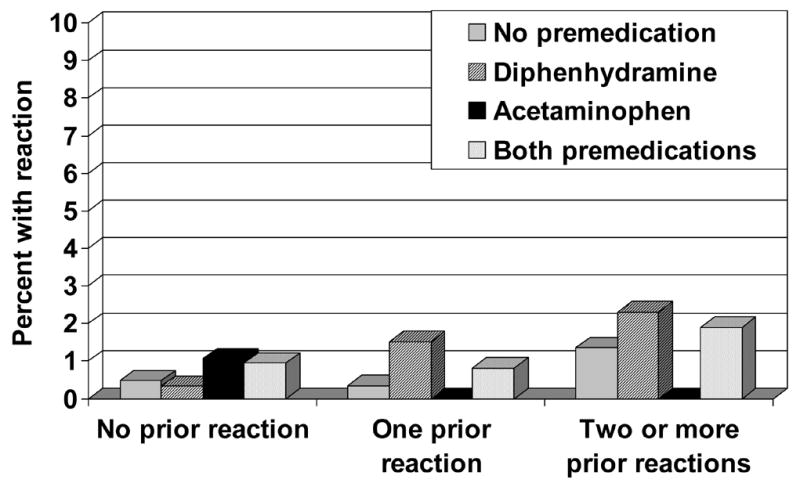
Allergic transfusion reactions according to the number of previous transfusion reactions and premedications used. Allergic transfusion reactions are uncommon, even when a patient has had 2 or more previous allergic transfusion reactions. The rate of reaction is not affected by premedication with diphenhydramine or acetaminophen (p-value not significant for any subgroup versus any other).3
Figure 2.
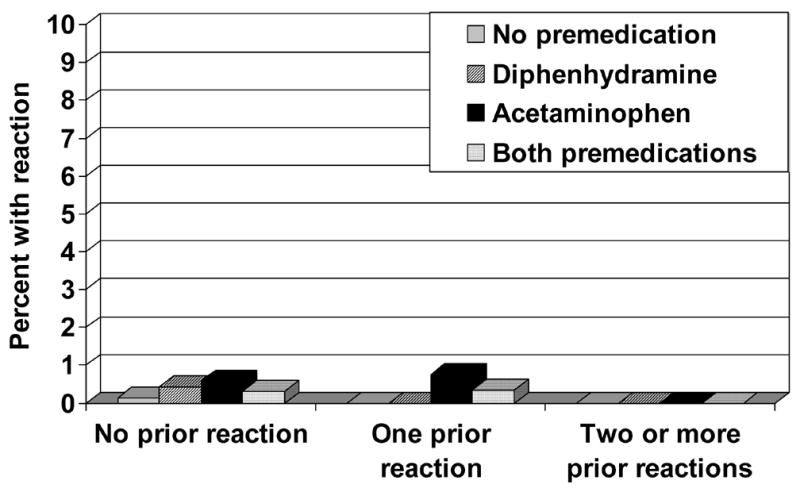
Febrile transfusion reactions according to the number of previous transfusion reactions and premedications used. Febrile nonhemolytic transfusion reactions are uncommon, even when a patient has had 2 or more previous febrile reactions. The rate of reaction is not affected by premedication with diphenhydramine or acetaminophen (p-value not significant for any subgroup versus any other).3
There are alternatives to pre-medication with acetaminophen and diphenhydramine to prevent reactions. For instance, in patients with recurrent or severe allergic reactions, the use of washed or plasma-reduced blood products may be helpful.45,46 Corticosteroid premedication is also sometimes used in patients with a history of severe allergic reactions, although its efficacy for this purpose has not been demonstrated in clinical trials.
Areas for Further Research
Much remains to be learned about the pathophysiology and prevention of allergic and FNHTR (Table 3). To definitively demonstrate the efficacy of premedication with acetaminophen and diphenhydramine in patients receiving transfusions, a large, prospective, randomized trial would be necessary. However, given the low incidence of reactions at most centers such a trial would require a very large number of patients, would be costly to perform, and is probably unnecessary. In fact, even if premedication reduced reactions by 50%, it would only decrease the absolute risk of reaction from approximately 1% to 0.5% at our institution. Routine premedication would have to be used 200 times to prevent a single reaction (Figure 3)! Because most reactions are mild and easily treated and the medications administered have potential toxicity, the rationale for routine premedication must be re-considered.
Table 3.
Future Research Directions
| Clinical question | Study needed | Comments |
|---|---|---|
| 1. Does premedication with acetaminophen and diphenhydramine prevent or diminish the severity of reactions when leukocyte-filtered blood products are transfused? | A large, multi-center, randomized, placebo-controlled study | A very large sample size is required to answer this question, because the rate of reaction is low when single-donor leukocyte-filtered blood products are used. A grading system for the severity of transfusion reactions is needed. |
| 2. What is the utility and optimal dose and schedule of glucocorticoid premedication for patients with severe or recurrent reactions? | Randomized trial in high-risk patients | |
| 3. What blood product preparation factors affect the rate of transfusion reactions? | The effects of volume reduction, washing, HLA-matching, and product age on reaction rates must be elucidated by carefully conducted trials. | Many studies have examined these factors for blood products without pre-storage leukofiltration, but further evaluation is needed to determine their effect in transfusion of pre-storage leukoreduced products. |
| 4. What determines whether a health care provider will prescribe premedications? | Qualitative study of factors that affect the decisions of providers | One small randomized trial and several observational studies have shown no benefit to premedication, yet the practice remains widespread. What evidence and intervention would be required to bring about a change in practice? |
| 5. How can the severity of transfusion reactions be graded? | Consensus of experts | A grading system similar to the Common Terminology Criteria for Adverse Events published by the US National Cancer Institute would be helpful. |
Figure 3.
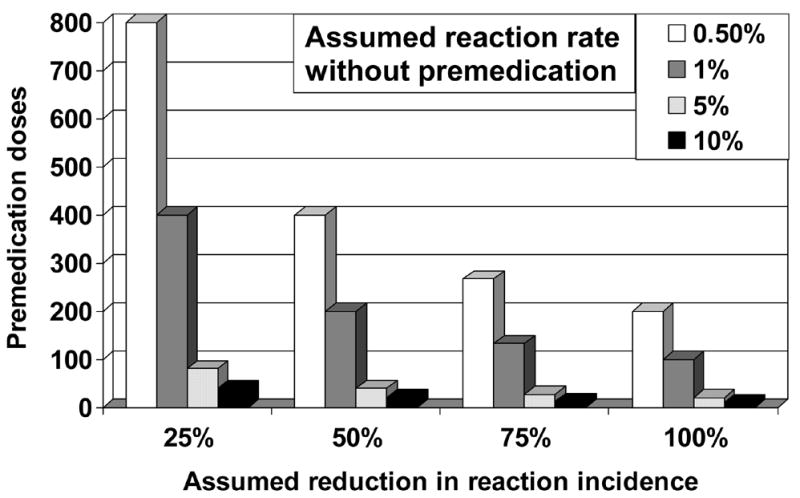
Pre-medication doses needed to prevent one transfusion reaction. The number of premedication doses needed to prevent a single transfusion reaction is displayed as a function of the baseline risk of transfusion reaction and the percentage reduction in the incidence of reaction when premedication is used. No study has shown that premedication reduces the rate of febrile non-hemolytic or allergic transfusion reactions, so the true reduction in reaction incidence may be zero.
Summary of Risks and Costs of Premedication
The routine practice of premedication is not entirely benign. Acetaminophen is well known to cause hepatotoxicity with acute overdose,47 and has also been reported to cause hepatic injury after repeated doses in the mildly supratherapeutic range.48 Diphenhydramine has substantial effects on memory, psychomotor performance, and mood.49,50 While both medications are relatively inexpensive, routine premedication may result in substantial cumulative costs, both in drug purchases and in expenditure of pharmacy and nursing time. At St. Jude Children’s Research Hospital, where most diphenhydramine premedication is given intravenously, we estimate that premedication with acetaminophen and diphenhydramine annually requires more than 800 hours of pharmacist time and 700 hours of nursing time, and that it costs more than $15,000 for drug acquisition per year. The low incidence of febrile and allergic reactions, evidence suggesting that premedication is not effective, and the costs and risks of premedication lead us to conclude that routine premedication before transfusion is not good prophylaxis, and bad practice. A role for the directed use of premedication in subsets of patients requires additional study, though limited data suggest that even in patients with a history of allergic or FNHTR, diphenhydramine and acetaminophen may have limited efficacy.
Footnotes
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC) and by National Institutes of Health Cancer Center Support Grant CA-21765.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ezidiegwu CN, Lauenstein KJ, Rosales LG. Febrile nonhemolytic transfusion reactions. Management by premedication and cost implications in adult patients Arch Pathol Lab Med. 2004;128:991–995. doi: 10.5858/2004-128-991-FNTR. [DOI] [PubMed] [Google Scholar]
- 2.Patterson BJ, Freedman J, Blanchette V. Effect of premedication guidelines and leukoreduction on the rate of febrile nonhaemolytic platelet transfusion reactions. Transfus Med. 2000;10:199–206. doi: 10.1046/j.1365-3148.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanders RP, Maddirala SD, Geiger TL. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. Br J Haematol. 2005;130:781–787. doi: 10.1111/j.1365-2141.2005.05670.x. [DOI] [PubMed] [Google Scholar]
- 4.Yazer MH, Podlosky L, Clarke G. The effect of prestorage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion. 2004;44:10–15. doi: 10.1046/j.0041-1132.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 5.Paglino JC, Pomper GJ, Fisch GS. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 6.King KE, Shirey RS, Thoman SK. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44:25–29. doi: 10.1046/j.0041-1132.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 7.Ibojie J, Greiss M, Urbaniak SJ. Limited efficacy of universal leucodepletion in reducing the incidence of febrile non-haemolytic reactions in red cell transfusion. Transfus Med. 2002;12:387–389. doi: 10.1046/j.1365-3148.2002.00401_2.x. [DOI] [PubMed] [Google Scholar]
- 8.Pitocco C, Sexton TR. Alleviating blood shortages in a resource-constrained environment. Transfusion. 2005;45:1118–1126. doi: 10.1111/j.1537-2995.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MT, Wallace EL. Blood collection and transfusion in the United States in 1999. Transfusion. 2005;45:141–148. doi: 10.1111/j.1537-2995.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- 10.Heddle NM. Pathophysiology of febrile nonhemolytic transfusion reactions. Curr Opin Hematol. 1999;6:420–426. doi: 10.1097/00062752-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Stack G, Snyder EL. Cytokine generation in stored platelet concentrates. Transfusion. 1994;34:20–25. doi: 10.1046/j.1537-2995.1994.34194098597.x. [DOI] [PubMed] [Google Scholar]
- 12.Heddle NM, Klama L, Singer J. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331:625–628. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 13.Muylle L, Laekeman G, Herman AG. Histamine levels in stored platelet concentrates: Relationship to white cell content. Transfusion. 1988;28:226–228. doi: 10.1046/j.1537-2995.1988.28388219148.x. [DOI] [PubMed] [Google Scholar]
- 14.Stack G, Baril L, Napychank P. Cytokine generation in stored, white cell-reduced, and bacterially contaminated units of red cells. Transfusion. 1995;35:199–203. doi: 10.1046/j.1537-2995.1995.35395184274.x. [DOI] [PubMed] [Google Scholar]
- 15.Wadhwa M, Seghatchian MJ, Dilger P. Cytokines in WBC-reduced apheresis PCs during storage: a comparison of two WBC-reduction methods. Transfusion. 2000;40:1118–1126. doi: 10.1046/j.1537-2995.2000.40091118.x. [DOI] [PubMed] [Google Scholar]
- 16.Schleuning M, Bock M, Mempel W. Complement activation during storage of single-donor platelet concentrates. Vox Sang. 1994;67:144–148. doi: 10.1111/j.1423-0410.1994.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 17.Gyongyossy-Issa MI, McLeod E, Devine DV. Complement activation in platelet concentrates is surface-dependent and modulated by the platelets. J Lab Clin Med. 1994;123:859–868. [PubMed] [Google Scholar]
- 18.Takahashi TA, Abe H, Hosoda M. Bradykinin generation during filtration of platelet concentrates with a white cell-reduction filter. Transfusion. 1995;35:967. doi: 10.1046/j.1537-2995.1995.351196110903.x. [DOI] [PubMed] [Google Scholar]
- 19.Brittingham TE, Chaplin H., Jr Febrile transfusion reactions caused by sensitivity to donor leukocytes and platelets. J Am Med Assoc. 1957;165:819–825. doi: 10.1001/jama.1957.02980250053013. [DOI] [PubMed] [Google Scholar]
- 20.de Rie MA, van der Plas-van Dalen CM, Engelfriet CP. The serology of febrile transfusion reactions. Vox Sang. 1985;49:126–134. doi: 10.1111/j.1423-0410.1985.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 21.Domen RE, Hoeltge GA. Allergic transfusion reactions: an evaluation of 273 consecutive reactions. Arch Pathol Lab Med. 2003;127:316–320. doi: 10.5858/2003-127-0316-ATR. [DOI] [PubMed] [Google Scholar]
- 22.Janeway CA, Jr, Travers P, Walport M, Schlomchik MJ. Immunobiology. Garland Science; New York: 2005. 6 ed. [Google Scholar]
- 23.Hoffjan S, Nicolae D, Ober C. Association studies for asthma and atopic diseases: A comprehensive review of the literature. Respir Res. 2003;4:14. doi: 10.1186/1465-9921-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilstad CW. Anaphylactic transfusion reactions. Curr Opin Hematol. 2003;10:419–423. doi: 10.1097/00062752-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Lambin P, Le Pennec PY, Hauptmann G. Adverse transfusion reactions associated with a precipitating anti-C4 antibody of anti-Rodgers specificity. Vox Sang. 1984;47:242–249. doi: 10.1111/j.1423-0410.1984.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 26.Michel J, Sharon R. Non-haemolytic adverse reaction after transfusion of a blood unit containing penicillin. Br Med J. 1980;280:152–153. doi: 10.1136/bmj.280.6208.152-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Branch DR, Gifford H. Allergic reaction to transfused cephalothin antibody. JAMA. 1979;241:495–496. [PubMed] [Google Scholar]
- 28.Johansson SG, Nopp A, van HM. Passive IgE-sensitization by blood transfusion. Allergy. 2005;60:1192–1199. doi: 10.1111/j.1398-9995.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- 29.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 31.Yamagata K, Matsumura K, Inoue W. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills EM, Rusyniak DE, Sprague JE. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. J Mol Med. 2004;82:787–799. doi: 10.1007/s00109-004-0591-7. [DOI] [PubMed] [Google Scholar]
- 33.Blatteis CM, Li S, Li Z. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostaglandins Other Lipid Mediat. 2005;76:1–18. doi: 10.1016/j.prostaglandins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Brunton L, Lazo J, Parker K. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw Hill; 2005. 11 ed. [Google Scholar]
- 35.Kaufman DW, Kelly JP, Rosenberg L. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 36.Kaplowitz N. Acetaminophen hepatoxicity: What do we know, what don’t we know, and what do we do next? Hepatology. 2004;40:23–26. doi: 10.1002/hep.20312. [DOI] [PubMed] [Google Scholar]
- 37.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 38.Allmers H. Frequent acetaminophen use and allergic diseases: is the association clear? J Allergy Clin Immunol. 2005;116:859–862. doi: 10.1016/j.jaci.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Casale TB, Blaiss MS, Gelfand E. First do no harm: Managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003;111:S835–S842. doi: 10.1067/mai.2003.1550. [DOI] [PubMed] [Google Scholar]
- 40.Weiler JM, Bloomfield JR, Woodworth GG. Effects of fexofenadine, diphenhydramine, and alcohol on driving performance: A randomized, placebo-controlled trial in the Iowa driving simulator. Ann Intern Med. 2000;132:354–363. doi: 10.7326/0003-4819-132-5-200003070-00004. [DOI] [PubMed] [Google Scholar]
- 41.Finkle WD, Adams JL, Greenland S. Increased risk of serious injury following an initial prescription for diphenhydramine. Ann Allergy Asthma Immunol. 2002;89:244–250. doi: 10.1016/S1081-1206(10)61950-3. [DOI] [PubMed] [Google Scholar]
- 42.Sharma AN, Hexdall AH, Chang EK. Diphenhydramine-induced wide complex dysrhythmia responds to treatment with sodium bicarbonate. Am J Emerg Med. 2003;21:212–215. doi: 10.1016/s0735-6757(02)42248-6. [DOI] [PubMed] [Google Scholar]
- 43.Heddle NM, Klama LN, Griffith L. A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion. 1993;33:794–797. doi: 10.1046/j.1537-2995.1993.331094054613.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang SE, Lara PN, Jr, Lee-Ow A. Acetaminophen and diphenhydramine as premedication for platelet transfusions: A prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70:191–194. doi: 10.1002/ajh.10119. [DOI] [PubMed] [Google Scholar]
- 45.Buck SA, Kickler TS, McGuire M. The utility of platelet washing using an automated procedure for severe platelet allergic reactions. Transfusion. 1987;27:391–393. doi: 10.1046/j.1537-2995.1987.27587320530.x. [DOI] [PubMed] [Google Scholar]
- 46.Perrotta PL, Snyder EL. Non-infectious complications of transfusion therapy. Blood Rev. 2001;15:69–83. doi: 10.1054/blre.2001.0151. [DOI] [PubMed] [Google Scholar]
- 47.Hinson JA, Reid AB, McCullough SS. Acetaminophen-induced hepatotoxicity: Role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev. 2004;36:805–822. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- 48.Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: hepatoxicity after multiple doses in children. J Pediatr. 1998;132:22–27. doi: 10.1016/s0022-3476(98)70479-2. [DOI] [PubMed] [Google Scholar]
- 49.Verster JC, Volkerts ER, van Oosterwijck AW. Acute and subchronic effects of levocetirizine and diphenhydramine on memory functioning, psychomotor performance, and mood. J Allergy Clin Immunol. 2003;111:623–627. doi: 10.1067/mai.2003.63. [DOI] [PubMed] [Google Scholar]
- 50.O’Hanlon JF, Ramaekers JG. Antihistamine effects on actual driving performance in a standard test: a summary of Dutch experience, 1989–94. Allergy. 1995;50:234–242. doi: 10.1111/j.1398-9995.1995.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 51.Da PA, Bidoli E, Talamini R. Pre-storage leucocyte depletion and transfusion reaction rates in cancer patients. Transfus Med. 2005;15:37–43. doi: 10.1111/j.1365-3148.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 52.Couban S, Carruthers J, Andreou P. Platelet transfusions in children: Results of a randomized, prospective, crossover trial of plasma removal and a prospective audit of WBC reduction. Transfusion. 2002;42:753–758. doi: 10.1046/j.1537-2995.2002.00070.x. [DOI] [PubMed] [Google Scholar]
- 53.Heddle NM, Blajchman MA, Meyer RM. A randomized controlled trial comparing the frequency of acute reactions to plasma-removed platelets and prestorage WBC-reduced platelets. Transfusion. 2002;42:556–566. doi: 10.1046/j.1537-2995.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 54.Uhlmann EJ, Isgriggs E, Wallhermfechtel M. Prestorage universal WBC reduction of RBC units does not affect the incidence of transfusion reactions. Transfusion. 2001;41:997–1000. doi: 10.1046/j.1537-2995.2001.41080997.x. [DOI] [PubMed] [Google Scholar]
- 55.Sarkodee-Adoo CB, Kendall JM, Sridhara R. The relationship between the duration of platelet storage and the development of transfusion reactions. Transfusion. 1998;38:229–235. doi: 10.1046/j.1537-2995.1998.38398222865.x. [DOI] [PubMed] [Google Scholar]
- 56.Riccardi D, Raspollini E, Rebulla P. Relationship of the time of storage and transfusion reactions to platelet concentrates from buffy coats. Transfusion. 1997;37:528–530. doi: 10.1046/j.1537-2995.1997.37597293886.x. [DOI] [PubMed] [Google Scholar]
- 57.Anderson NA, Gray S, Copplestone JA. A prospective randomized study of three types of platelet concentrates in patients with haematological malignancy: corrected platelet count increments and frequency of nonhaemolytic febrile transfusion reactions. Transfus Med. 1997;7:33–39. doi: 10.1046/j.1365-3148.1997.d01-73.x. [DOI] [PubMed] [Google Scholar]
- 58.Muylle L, Wouters E, Peetermans ME. Febrile reactions to platelet transfusion: The effect of increased interleukin 6 levels in concentrates prepared by the platelet-rich plasma method. Transfusion. 1996;36:886–890. doi: 10.1046/j.1537-2995.1996.361097017174.x. [DOI] [PubMed] [Google Scholar]
- 59.Federowicz I, Barrett BB, Andersen JW. Characterization of reactions after transfusion of cellular blood components that are white cell reduced before storage. Transfusion. 1996;36:21–28. doi: 10.1046/j.1537-2995.1996.36196190511.x. [DOI] [PubMed] [Google Scholar]
- 60.Dzieczkowski JS, Barrett BB, Nester D. Characterization of reactions after exclusive transfusion of white cell-reduced cellular blood components. Transfusion. 1995;35:20–25. doi: 10.1046/j.1537-2995.1995.35195090654.x. [DOI] [PubMed] [Google Scholar]
- 61.Oksanen K, Ebeling F, Kekomaki R. Adverse reactions to platelet transfusions are reduced by use of platelet concentrates derived from buffy coat. Vox Sang. 1994;67:356–361. doi: 10.1111/j.1423-0410.1994.tb01273.x. [DOI] [PubMed] [Google Scholar]
- 62.Mangano MM, Chambers LA, Kruskall MS. Limited efficacy of leukopoor platelets for prevention of febrile transfusion reactions. Am J Clin Pathol. 1991;95:733–738. doi: 10.1093/ajcp/95.5.733. [DOI] [PubMed] [Google Scholar]
- 63.Chambers LA, Kruskall MS, Pacini DG. Febrile reactions after platelet transfusion: The effect of single versus multiple donors. Transfusion. 1990;30:219–221. doi: 10.1046/j.1537-2995.1990.30390194340.x. [DOI] [PubMed] [Google Scholar]


