Abstract
Promotion or inhibition of tubulin assembly into microtubules is the standard in vitro assay for evaluating potential antimicrotubule agents. Many agents to be tested are poorly soluble in aqueous solution and require a cosolvent such as DMSO4. However, DMSO itself can promote tubulin assembly, and its inclusion in assays for compounds that induce tubulin assembly complicates interpretation of the results. Substituting GDP for GTP in the exchangeable nucleotide binding site of tubulin produces a less active form of the protein, tubulin-GDP. Here it is shown that tubulin-GDP can be assembled into normal microtubules in DMSO concentrations up to 15% (v/v), and polymerization assays performed under these conditions can be compared with assays run under more standard conditions. Assays for measuring the effective concentration of a ligand for promotion of tubulin assembly (EC50), measuring the concentration for inhibition of tubulin assembly (IC50) by a colchicine site ligand, and for measuring tubulin critical concentrations in the presence of poorly soluble taxol derivatives are illustrated.
Keywords: Taxol, Microtubules, Assembly promotion, Tubulin-GDP, DMSO, Solubility, Critical concentration
Introduction
Microtubules are an important target for antineoplastic chemotherapy [1–3]. Antimicrotubule drug candidates to be tested are frequently introduced into the assembly assay in DMSO [4–7]. This is particularly true for derivatives and analogs of taxol, which is highly insoluble in aqueous solution. Taxol induces the assembly of purified tubulin by lowering the critical concentration of the protein [8,9]. Unfortunately, DMSO also lowers the critical concentration of protein [10–12].
Antimicrotubule agents that are in clinical use or clinical trials are natural products or substances very similar to the natural product [13,14]. The complexities of structures and the physical properties of some of these molecules make the development of simpler, synthetic alternatives an important goal [2,15,16]. Recent progress in understanding the molecular interactions between assembly-promoting ligands such as taxol, epothilone and discodermolide and the receptor site on tubulin has led to the synthesis and testing of some early candidates [17–19]. Initial synthetic ligands are anticipated to be only weakly active, and functional groups that will enhance the solubility may not be built into these molecules. Thus, higher concentrations of the test compounds are required for the biological assays, which may also necessitate higher cosolvent concentrations.
Presented here is a method by which the activity of highly insoluble promoters of tubulin polymerization can be quantitatively evaluated in vitro. DMSO concentrations up to 15% (v/v) can be used. Assays to determine the EC50 of a microtubule promoter and to determine the critical concentration of tubulin in the presence of a promoter are presented. Inhibitors of tubulin assembly can also be assessed in the presence of high concentrations of DMSO.
Materials and Methods
All the chemicals used were analytical grade and purchased from Sigma Chemicals. Stock solutions of 2 mM taxol and 1 mM TG-185-219 (Fig. 1) were made in DMSO. All references to percent DMSO concentration are v/v. The concentration of taxol or podophyllotoxin in DMSO was determined by absorption spectroscopy using ε273 nm = 1700 M−1cm−1 for taxol [4] and ε294 nm = 4538 M−1 cm−1 for podophylltoxin as determined in our lab.
Fig. 1.
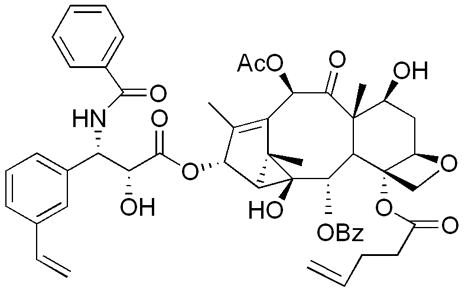
Structure of TG-185-219.
Tubulin Purification and Protein Determination
Tubulin was prepared by two cycles of temperature dependent assembly-disassembly as described by Williams and Lee [20]. The protein solution from the end of the second cycle was subjected to ion exchange chromatography on phosphocellulose resin to yield purified tubulin, which was then drop frozen in liquid nitrogen. The tubulin preparations did not contain MAPs as confirmed by SDS-PAGE [21]. Prior to use, the frozen pellets were gently thawed and then desalted into PME buffer (100 mM PIPES, 1 mM MgSO4, and 2 mM EGTA, pH 6.90 at 25 ºC) by the method of Penefsky [22]. Briefly, tubulin was subjected to gel filtration in 1 mL columns packed with Sephadex G-50 pre-equilibrated in PME. The concentration of tubulin was determined spectrophotometrically on a Hewlett Packard Model 8453 diode array spectrophotometer. The extinction coefficient used to determine tubulin concentration was 1.23 (mg/mL)−1 cm−1 at 278 nm in PME buffer [23].
Solubility of Taxol
The solubility of taxol as a function of DMSO concentration in water was evaluated using HPLC. A calibration curve for taxol was constructed by injecting 20 μL of varying concentrations of taxol in 100% DMSO onto a Phenomenex C-18 column (50% acetonitrile:water, 1 mL/min, detected at 230 nm). Sample solutions were made as follows: a series of a stock solutions of taxol in 100% DMSO were made and diluted with distilled water to yield a final taxol concentration of 800 μM and a final DMSO concentration of 4 – 30% (v/v). Each solution was mixed thoroughly and passed through a 0.45 μm filter. The filtrate was injected into the HPLC and the concentration of taxol in the filtrate was determined from the calibration curve.
Preparation of Tubulin-GDP
Tubulin-GDP in which the exchangeable site (E site) GTP was completely replaced by GDP was prepared by the method of Seckler et al. [24]. Tubulin in PME buffer was incubated with excess GDP (5 mM) for 30 min at 4 ºC. Unbound GTP and excess of GDP were removed by rapid gel filtration on Sephadex G-50 columns pre-equilibrated with PM buffer (100 mM PIPES, 1 mM MgSO4, pH 6.90 at 25 ºC). Tubulin-GDP was evaluated for nucleotide content using HPLC. Nucleotides were extracted from tubulin-GDP and the HPLC analysis of extracts was carried out according to the method of Seckler et al [24]. A final concentration of 0.5 M perchloric acid was used to denature the protein. The supernatant collected after centrifugation was neutralized with 3M KOH and buffered with 1 M K2HPO4, and 0.5 M CH3COOH. The mobile phase used for HPLC analysis was 0.2 M K2HPO4, 0.1 M CH3COOH and 4 mM TBA at a flow rate of 1mL/min. The HPLC analysis of the extracts showed a 1:1 ratio of GTP:GDP.
Polymerization Assay of Tubulin-GTP and Tubulin-GDP
Tubulin polymerization was monitored by apparent light scattering using a Hewlett-Packard 8453 absorption spectrophotometer with a thermostated multicell holder that was maintained at 37 °C with a circulating water bath. Tubulin-GTP or tubulin-GDP in PME buffer was equilibrated in the sample cell and a baseline was recorded. The samples were then incubated with pre-warmed DMSO (concentration according to the requirements of the experiment), taxol or TG-185-219 in DMSO. The reaction was followed by measuring the increase in apparent absorption at 350 nm as a function of time. The extent of assembly was determined from the difference in the initial and plateau absorption values. For the polymerization of tubulin-GTP, 1 mM GTP was included in the cuvette.
Critical Concentration Determination
Critical concentrations were determined by assembly experiments performed at 37 °C. Samples containing different concentrations of tubulin were polymerized and the extent of assembly was defined as the apparent absorption at 350 nm at steady state. Critical concentrations were obtained as the x-intercepts of plots of apparent absorption at 350 nm (ΔA350nm) vs. tubulin concentration.
EC50 Determination of Taxol
The EC50 is the concentration of ligand at which the extent of microtubule assembly is 50% of the maximum value. Tubulin-GDP (5 μM) in PME buffer was polymerized with varying concentrations of taxol at a final concentration of 4% or 15% DMSO. The percentage of polymerization was measured in terms of apparent light scattering at 350 nm, which was plotted against the concentration of taxol. The EC50 values were calculated from nonlinear regression fits of the rectangular hyperbola using the nonlinear regression software in Sigma Plot 2001 (Jandel Scientific).
IC50 Determination of Podophyllotoxin
The IC50 is the total concentration of the inhibitor ligand required to reduce the extent of tubulin polymerization by 50% of its value in the absence of added inhibitor. The IC50 of the assembly inhibitor podophyllotoxin was evaluated as follows: varying concentrations of podophyllotoxin in DMSO were added to 8 μM tubulin-GDP in PME buffer. Polymerization was initiated at 37 °C by addition of taxol in DMSO to a final taxol concentration of 8 μM and final DMSO concentration of 15%. Assembly was monitored by apparent light scattering at 350 nm until a plateau was reached. The percentage of taxol-induced assembly versus the log of podophyllotoxin concentration was plotted and the IC50 value was calculated from nonlinear fit of the sigmoidal plot using regression software in Sigma Plot 2001 (Jandel Scientific). The IC50 of podophyllotoxin for tubulin-GTP was determined in similar fashion except that the final DMSO concentration was maintained at 4% and the concentrations of tubulin-GTP and taxol used were 6 μM each.
Affinity of Taxol for GMPCPP- Microtubules in 4% and 15% DMSO
The affinity of taxol for GMPCPP-stabilized microtubule in the presence of 4% and 15% DMSO was assessed by competition between taxol and a fluorescent derivative of taxol, N-AB-PT, according to the method of Li et al. [25]. Tubulin-GDP was incubated with 100 times the concentration of GMPCPP, a weakly hydrolysable analog of GTP, at 4 ºC for 30 min. The resulting tubulin-GMPCPP was polymerized to microtubules by incubating at 37 ºC for 30 min. To 5 μM of polymerized GMPCPP-tubulin in PME buffer, 5 μM N-AB-PT was added. The above solution was then incubated with 0 – 40 μM of taxol at 37 ºC for 30 min. The final concentration of DMSO was maintained at 4% and 15%, respectively. The emission spectrum of each sample was collected on Jobin Yvon spectrofluorimeter using a thermostated cell holder maintained at 37 ºC. (The excitation and emission maxima for N-AB-PT bound to microtubules were 320 nm and 413 nm, respectively.) The emission of free N-AB-PT was negligible under the experimental conditions. The EC50 of taxol for GMPCPP-microtubules was determined by the plot of (F/F0) versus the log of taxol concentration, where F0 is fluorescence emission intensity of N-AB-PT bound to GMPCPP-microtubules in the absence of taxol and F is the fluorescence emission intensity of N-AB-PT in the presence of varying concentrations of taxol. The apparent dissociation constant for taxol was determined using a one-site competition relation: Ki = EC50/(1+[N-AB-PT]/Kd), where Ki is the dissociation constant for taxol, Kd is the dissociation constant for N-AB-PT with GMPCPP-polymerized tubulin (15 nM, [25]), and [N-AB-PT] is 5 μM. The inverse of Ki is the apparent equilibrium binding constant Ka.
Electron Microscopy
Single drops of the solutions of assembled tubulin were put on a carbon coated 200 mesh copper grid. Care was taken to avoid shearing the microtubules. The grid was negatively stained with 1% (w/v) aqueous uranyl acetate. Electron micrographs were obtained using a Hitachi 7000 TEM operated at 100 kV.
Results and Discussion
The goal of this investigation was to develop conditions under which a high concentration of DMSO could be included in the assembly assay. Optimal conditions are those in which true microtubules are formed and the critical concentration of the protein in the absence of the ligand of interest is moderately high. Such conditions would be conducive to quantitatively assessing assembly promoting efficacies of substances with a wide range of activities.
Effect of DMSO on Tubulin-GTP Polymerization
The polymerization of tubulin proceeds in a cooperative manner through a nucleated condensation mechanism [26], in which no polymerization occurs under a threshold concentration of protein known as the critical concentration. The critical concentration of tubulin is highly dependent on solution conditions and is affected by factors such as the nature of the buffer [27], ionic strength [28], magnesium ion concentration [29], temperature [11], tubulin nucleotide content [4], DMSO [10–12], and antimicrotubule drugs [30]. Typical assay conditions employ a buffer near neutral pH, 1–10 mM Mg2+, 0.1 to 1.0 mM GTP (which makes “tubulin-GTP”) and temperatures between 25 and 37 °C [31].
Taxol is soluble in pure water up to ~2 μM [32], which is significantly lower than the tubulin concentrations used in standard assembly assays (5–10 μM), so a cosolvent must be included. Standard assays of taxol analogs in our lab are performed using a low, fixed concentration of DMSO in all samples (4% or less) [33,34]. Taxol is soluble up to about 40 μM in this cosolvent mixture (Fig. 2). It is known that 8–10% DMSO lowers the critical concentration of tubulin 8–10 fold [12]; it is seen in Table 1 that even the lower concentration of DMSO (4%) can significantly decrease the critical concentration of tubulin-GTP. Under these experimental conditions, taxol decreased the critical concentration of tubulin by a factor of 14.
Fig. 2.
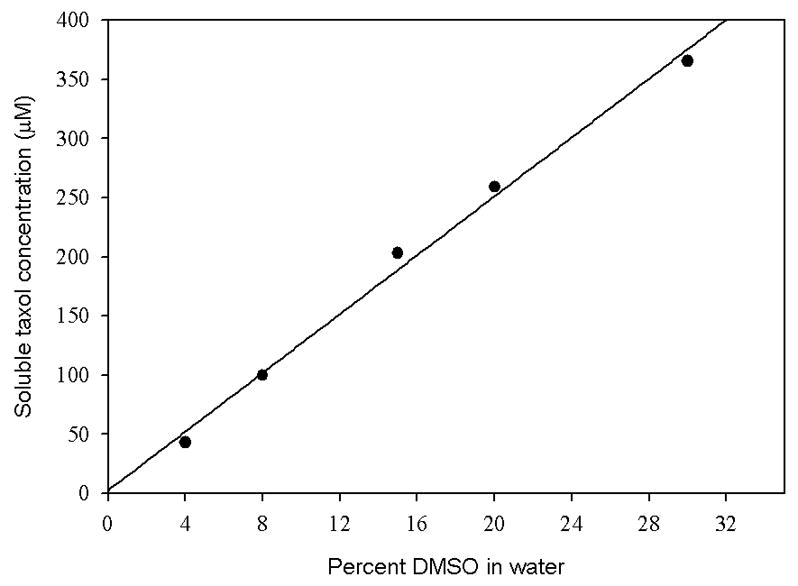
Solubility of taxol as a function of DMSO concentration. The concentration of taxol soluble at different concentrations of DMSO was determined as described under Materials and Methods.
Table 1.
Critical concentrations of tubulin with and without taxol in varying concentrations of DMSO
| Tubulin sample | Critical concentration, μM | ||
|---|---|---|---|
| 0% DMSO | 4% DMSO | 15% DMSO | |
| Tubulin-GTP | 20.4 ± 0.34 | 5.4 ± 0.49 | 0.93 ± 0.02 |
| Tubulin-GDP | >30 μMa | >30 μM | 6.9 ± 0.39 |
| Tubulin sample and taxolb | |||
| Tubulin-GTP | NDc | 0.37 ± 0.07a | NDd |
| Tubulin-GDP | NDc | 2.0 ± 0.15 | 0.58 ± 0.12 |
Values taken from reference 32
Concentration of taxol is equal to that of tubulin
Taxol is not sufficiently soluble in pure buffer to perform this measurement.
Critical concentration too close to zero to be accurately determined.
Higher amounts of DMSO cosolvent increased the solubility of taxol; however, higher concentrations of DMSO further lower the critical concentration of the protein. Increasing the DMSO concentration to 15% decreased the critical concentration of tubulin-GTP to 0.93 μM. If the efficacy factor for taxol is the same in 15% DMSO as in 4% DMSO, the critical concentration of tubulin under these conditions would be 0.07 μM. This tubulin concentration was deemed to be too low to be reliably detected.
Effect of DMSO on Tubulin-GDP Polymerization
Tubulin-GTP has two nucleotide binding sites, E and N, bound to GTP, which renders the protein into a conformation favorable for assembly into microtubules. Replacing the E site GTP in tubulin by GDP produces tubulin-GDP, which possesses a conformation that is unfavorable for microtubule formation [35,36]. In fact, no assembly of tubulin-GDP is observed under normal assay conditions at protein concentrations up to 30 μM and DMSO concentrations up to 4% [4,33,37]. Tubulin-GDP is assembly-competent in the presence of taxol (4,33 and Table 1). Therefore the effect of 15% DMSO on tubulin-GDP assembly was evaluated.
It was observed that DMSO at 15% induced a tubulin-concentration-dependent enhancement in the rate and extent of tubulin-GDP polymerization. The polymerization profile appeared to be normal with the characteristic lag, elongation and steady state phases of tubulin assembly (Fig. 3). Electron microscopy confirmed that the assembled polymer contained broad ribbons and microtubules, both of which are normally produced in the presence of DMSO (data not shown) [10]. The critical concentration of tubulin-GDP for 15% DMSO was found to be 6.9 μM, which is near that of tubulin-GTP in 4% DMSO. Importantly, the critical concentration of tubulin-GDP in the presence of taxol in 15% DMSO was 0.58 μM, which is about 12-fold smaller than that in the absence of taxol. The relative activity of taxol is similar in both assays (tubulin-GTP with 4% DMSO, and tubulin-GDP with 15% DMSO); therefore, it is reasonable to use either assay to evaluate the relative potency of assembly-promoting substances.
Fig. 3.
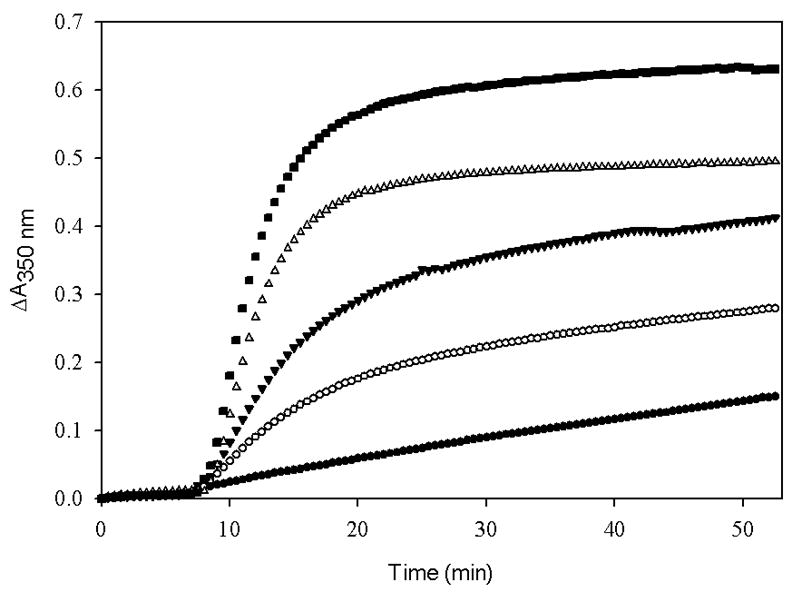
Polymerization of tubulin-GDP, 15 μM (■), 13.5 μM (▽), 12 μM (▼), 10.5 μM (○) and 9 μM (●) induced by 15% DMSO. Tubulin-GDP samples in PME buffer were incubated with pre-warmed 15% DMSO at 37 °C for 53 min and the extent of assembly was monitored in terms of apparent absorption at 350 nm.
Fig. 4 is an illustration of the effect of ligand insolubility on the outcome of polymerization experiments. At a concentration of 40 μM, taxol is completely soluble in 4% and 15% DMSO. The same amount of TG-185-219, a taxol analog, is completely soluble in 15% DMSO but is only minimally soluble in 4% DMSO. It is clear that the activity of the taxol analog would be underestimated in the standard assay.
Fig. 4.
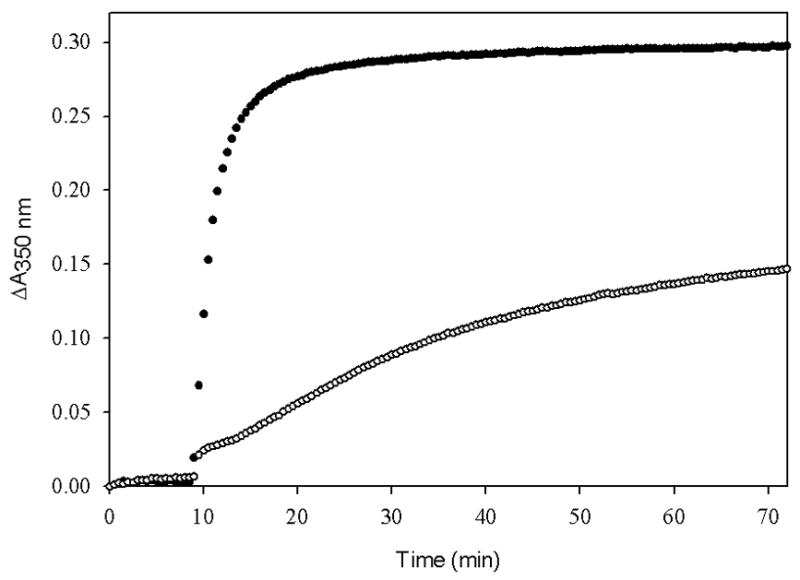
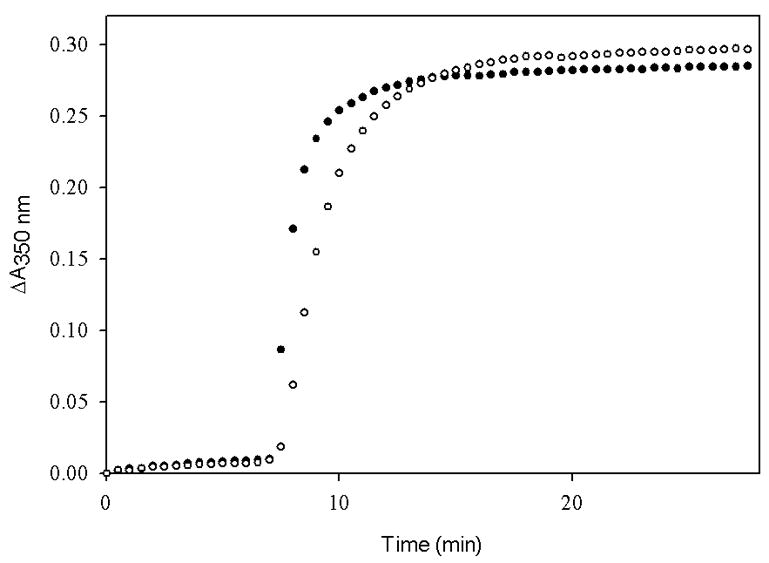
The extent of polymerization of tubulin-GDP induced by taxol (●) and TG-185-219 (○). Tubulin-GDP (5 μM) in PME buffer was incubated with 40 μM taxol or TG-185-219 in the presence of (A) 4% DMSO or (B) 15% DMSO at 37 °C and the polymerization was monitored as apparent absorption at 350 nm.
EC50 of Taxol
Many investigators use a tubulin assembly assay for antimicrotubule agents in which the protein concentration is constant and the ligand concentration is varied. The concentration of ligand that induces tubulin to assemble to 50% of a maximum level (EC50) is established and used to determine relative activities (6,7,33,34,38). These assays do not provide direct assessment of the affinity of the ligand for the protein, because the activity is a function of both the affinity of the ligand for tubulin and the efficacy of the ligand on protein polymerization (4,39), but since they are simple to perform they remain in wide use. Fig. 5 shows that the effect of DMSO on these EC50 experiments is to shift the curve to the left; i.e., decreasing EC50 from 4.2 ± 0.54 μM in 4% DMSO to 0.54 ± 0.16 in 15% DMSO. The difference in EC50 values is most likely due to the effect of DMSO on the critical concentration of the protein, since the affinity of taxol for GMPCPP-microtubules is unaffected by changing the DMSO concentration from 4% to 15% (Fig. 6).
Fig. 5.
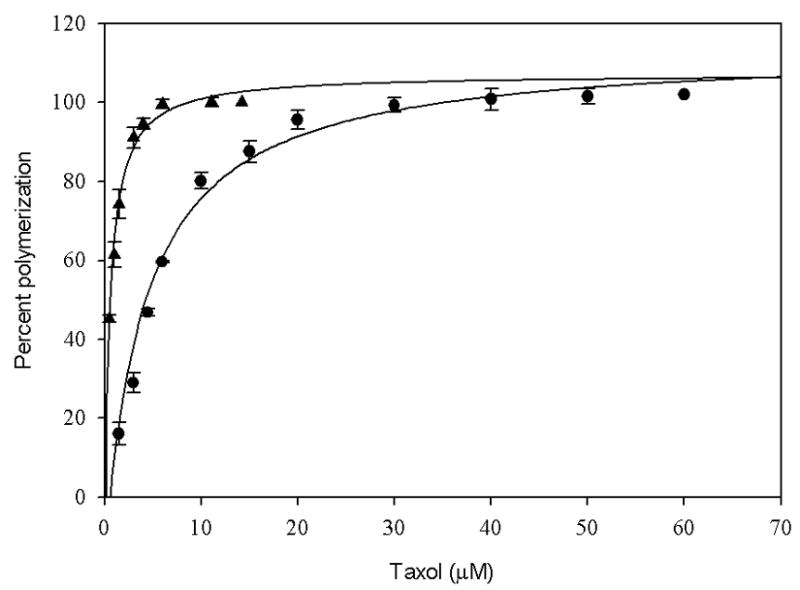
EC50 of taxol for tubulin-GDP in the presence of 15% DMSO (▲) and 4% DMSO (●). Tubulin-GDP samples (5μM) in PME buffer were incubated with different concentrations of taxol in 15% DMSO and 4% DMSO at 37 °C for 54 min. The percentage polymerization was measured in terms of the apparent absorption at 350 nm. The EC50 of taxol in 4% and 15% DMSO was 4.2 ±0.54 μM and 0.54 ± 0.16 μM respectively. The values are an average of three independent determinations.
Fig. 6.
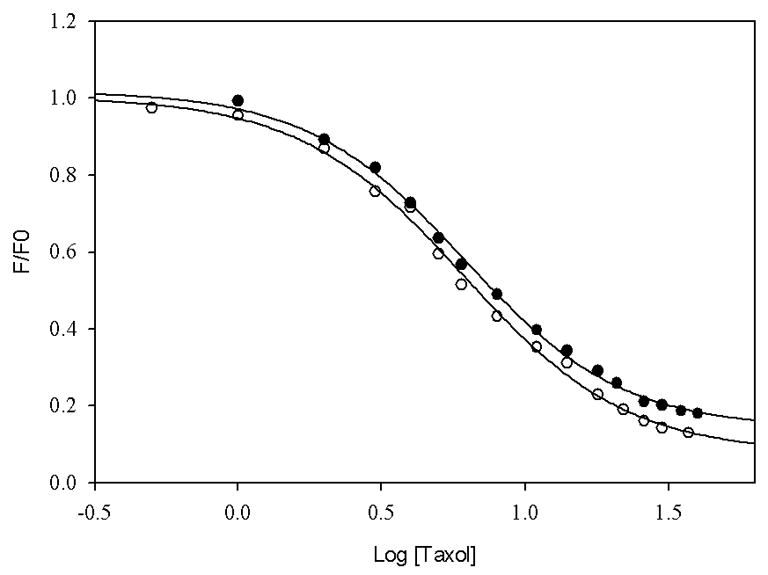
Affinity of taxol for GMPCPP-microtubules in the presence of 4% DMSO (○) and 15% DMSO (●). Affinity constants were determined by competition of taxol with N-AB-PT, a fluorescent analog of taxol towards GMPCPP-microtubules, in 4% and 15% DMSO, respectively. The affinity constants of taxol were identical within experimental error (6.1 × 107 M−1).
IC50 of Podophyllotoxin
High concentrations of DMSO may also be used to evaluate the activities of microtubule inhibiting agents. Inhibition of taxol-induced tubulin assembly by podophyllotoxin was evaluated to assess the method. Podophyllotoxin was chosen because it is a well-characterized inhibitor of tubulin assembly that binds at the colchicine site [40] but it does not suffer from the very slow tubulin-binding kinetics characteristic of colchicine binding to tubulin [41]. The IC50 values were determined using either tubulin-GTP in 4% DMSO or tubulin-GDP in 15% DMSO (Fig. 7) as described under ‘Materials and Methods’. The IC50 value of podophyllotoxin for tubulin-GTP in 4% DMSO and that for tubulin-GDP in 15% DMSO was 4.19 μM or 5.25 μM, respectively. Note that the ratio of IC50 values (0.80) of podophyllotoxin for tubulin-GTP in 4% DMSO to that for tubulin-GDP in 15% DMSO is about the same as the ratio of critical concentration (0.78) of tubulin-GTP in 4% DMSO to that of tubulin-GDP in 15% DMSO. These data indicate that the results from the tubulin-GDP polymerization assay using high concentrations of DMSO can be compared to results of a more standard tubulin-GTP polymerization assay, provided that the critical concentration of tubulin under each solution condition is measured.
Fig. 7.
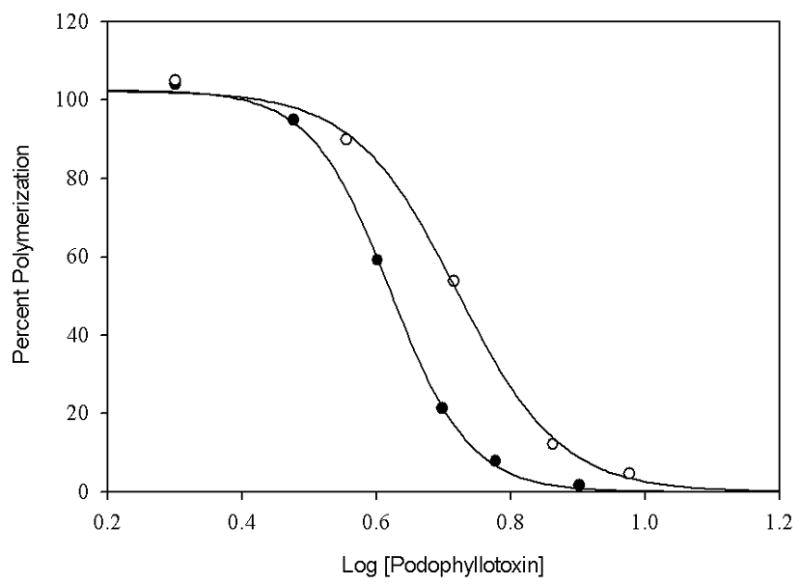
IC50 of podophyllotoxin for tubulin-GDP in 15% DMSO (○) and tubulin-GTP in 4% DMSO (●). Tubulin-GDP samples (8μM) in PME buffer with 15% DMSO were treated with increasing concentration of podophyllotoxin and polymerized with 8 μM taxol at 37oC. Tubulin-GTP samples (6μM) in PME buffer with 4% DMSO were treated in the same way and polymerized with 6 μM taxol under identical conditions. The percent polymerization was measured in terms of the apparent absorption at 350 nm.
Summary
Poor solubility of substances that may affect microtubule assembly can hamper quantitative evaluation of their potencies. Assembly assays can be performed in solutions containing up to 15% DMSO as a cosolvent, provided that the less active tubulin-GDP is used.
Acknowledgments
We thank Mr. Henry Eichelberger for assistance in obtaining the electron micrographs. This work was financially supported by the National Cancer Institute (Grant No. CA-69571 to D.G.I.K).
Abbreviations used
- DMSO
dimethyl sulfoxide
- EC50
Effective Concentration – 50% of maximal activity
- MAPs
microtubule associated proteins
- PIPES
piperazine-1,4-bis (2-ethanesulfonic acid)
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TBA
tetrabutylammonium dihydrogen phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhalla KN. Microtubule – targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 2.Wilson L, Jordan MA. New microtubule/tubulin-targeted anticancer drugs and novel chemotherapeutic strategies. J Chemother. 2004;4(16 Suppl):83–85. doi: 10.1179/joc.2004.16.Supplement-1.83. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Giannakakou P. Targeting microtubules for cancer chemotherapy. Curr Med Chem: Anti-Cancer Agents. 2005;5:65–71. doi: 10.2174/1568011053352569. [DOI] [PubMed] [Google Scholar]
- 4.Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry. 1993;32:2747–2755. doi: 10.1021/bi00062a003. [DOI] [PubMed] [Google Scholar]
- 5.Verdier-Pinard P, Wang Z, Mohanakrishnan AK, Cushman M, Hamel E. A steroid derivative with paclitaxel-like effects on tubulin polymerization. Mol Pharm. 2000;57:568–575. doi: 10.1124/mol.57.3.568. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Schilling JK, Ravindra R, Bane S, Kingston DGI. Syntheses and bioactivities of macrocyclic paclitaxel bis-lactones. Bioorg Med Chem. 2004;23:6147–6161. doi: 10.1016/j.bmc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Sampath D, Greenberger LM, Beyer C, Hari M, Liu H, Baxter M, Yang S, Rios C, Discafani C. Preclinical pharmacologic evaluation of MST-997, an orally active taxane with superior in vitro and in vivo efficacy in paclitaxel- and docetaxel-resistant tumor models. Clin Cancer Res. 2006;12:3459–3469. doi: 10.1158/1078-0432.CCR-05-2349. [DOI] [PubMed] [Google Scholar]
- 8.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 9.Parness J, Horwitz SB. Taxol binds to polymerized tubulin in vitro. J Cell Biol. 1981;91:479–487. doi: 10.1083/jcb.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himes RH, Burton PR, Gaito JM. Dimethyl sulfoxide-induced self-assembly of tubulin lacking associated proteins. J Biol Chem. 1977;252:6222–6228. [PubMed] [Google Scholar]
- 11.Robinson J, Engelborghs Y. Tubulin polymerization in dimethyl sulfoxide. J Biol Chem. 1982;257:5367–5371. [PubMed] [Google Scholar]
- 12.Algaier J, Himes RH. The effects of dimethyl sulfoxide on the kinetics of tubulin assembly. Biochim Biophys Acta. 1988;954:235–243. doi: 10.1016/0167-4838(88)90078-7. [DOI] [PubMed] [Google Scholar]
- 13.Mooberry SL, Tien G, Hernandez AH, Plubrukarn A, Davidson BS. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999;59:653–660. [PubMed] [Google Scholar]
- 14.Nagle A, Hur W, Gray NS. Antimitotic agents of natural origin. Curr Drug Targets. 2006;7:305–326. doi: 10.2174/138945006776054933. [DOI] [PubMed] [Google Scholar]
- 15.Miller ML, Roller EE, Zhao RY, Leece BA, Ab O, Baloglu E, Goldmacher VS, Chari RV. Synthesis of taxoids with improved cytotoxicity and solubility for use in tumor-specific delivery. J Med Chem. 2004;47:4802–4805. doi: 10.1021/jm049705s. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Strobl JS, Bane S, Schilling JK, McCracken M, Chatterjee SK, Rahim-Bata R, Kingston DG. Design, synthesis, and bioactivities of steroid-linked taxol analogues as potential targeted drugs for prostate and breast cancer. J Nat Prod. 2004;67:152–159. doi: 10.1021/np030296x. [DOI] [PubMed] [Google Scholar]
- 17.Isbrucker RA, Gunasekera SP, Longley RE. Structure-activity relationship studies of discodermolide and its semisynthetic acetylated analogs on microtubule function and cytotoxicity. Cancer Chemother Pharmacol. 2001;48:29–36. doi: 10.1007/s002800100287. [DOI] [PubMed] [Google Scholar]
- 18.Dabydeen DA, Florence GJ, Paterson I, Hamel E. A quantitative evaluation of the effects of inhibitors of tubulin assembly on polymerization induced by discodermolide, epothilone B, and paclitaxel. Cancer Chemother Pharmacol. 2004;53:397–403. doi: 10.1007/s00280-003-0755-0. [DOI] [PubMed] [Google Scholar]
- 19.Cachoux F, Isarno T, Wartmann M, Altmann KH. Total synthesis and biological assessment of benzimidazole-based analogues of epothilone A: ambivalent effects on cancer cell growth inhibition. Chembiochem. 2006;1:54–57. doi: 10.1002/cbic.200500351. [DOI] [PubMed] [Google Scholar]
- 20.Williams RC, Jr, Lee JC. Preparation of tubulin from brain. Methods Enzymol. 1982;85:376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Penefsky HS. Preparation of nucleotide-depleted F1 and binding of adenine nucleotides and analogs to the depleted enzyme. Methods Enzymol. 1979;55:377–380. doi: 10.1016/0076-6879(79)55048-4. [DOI] [PubMed] [Google Scholar]
- 23.Detrich HW, III, Williams RC., Jr Reversible dissociation of the alpha beta dimer of tubulin from bovine brain. Biochemistry. 1978;17:3900–3907. doi: 10.1021/bi00612a002. [DOI] [PubMed] [Google Scholar]
- 24.Seckler R, Wu GM, Timasheff SN. Interactions of tubulin with guanylyl- (beta-gamma- methylene) diphosphonate: formation and assembly of a stoichiometric complex. J Biol Chem. 1990;265:7655–7661. [PubMed] [Google Scholar]
- 25.Li Y, Edsall R, Jr, Jagtap PG, Kingston DGI, Bane S. Equilibrium Studies of a Fluorescent Paclitaxel Derivative Binding to Microtubules. Biochemistry. 2000;39:616–623. doi: 10.1021/bi992044u. [DOI] [PubMed] [Google Scholar]
- 26.Johnson KA, Borisy GG. Kinetic analysis of microtubule self-assembly in vitro. J Mol Biol. 1977;117:1–31. doi: 10.1016/0022-2836(77)90020-1. [DOI] [PubMed] [Google Scholar]
- 27.Lee JC, Timasheff SN. In Vitro reconstitution of calf brain microtubules: effects of solution variables. Biochemistry. 1977;16:1754–1764. doi: 10.1021/bi00627a037. [DOI] [PubMed] [Google Scholar]
- 28.Arakawa T, Timasheff SN. The mechanism of action of Na glutamate, lysine HCl, and piperazine- N,N'-bis(2-ethanesulfonic acid) in the stabilization of tubulin and microtubule formation. J Biol Chem. 1984;259:4979–4986. [PubMed] [Google Scholar]
- 29.Herzog W, Weber K. In vitro assembly of pure tubulin into microtubules in the absence of microtubule-associated proteins and glycerol. Proc Natl Acad Sci USA. 1977;74:1860–1864. doi: 10.1073/pnas.74.5.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamel E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem Biophys. 2003;38:1–21. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 31.Barron DM, Chatterjee SK, Ravindra R, Roof R, Baloglu E, Kingston DGI, Bane S. A fluorescence-based high-throughput assay for antimicrotubule drugs. Anal Biochem. 2003;315:49–56. doi: 10.1016/s0003-2697(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 32.Dordunoo SK, Burt HM. Solubility and stability of taxol: effects of buffers and cyclodextrins. Int J Pharm. 1996;133:191–201. [Google Scholar]
- 33.Chatterjee SK, Barron DM, Vos S, Bane S. Baccatin III induces assembly of purified tubulin into long microtubules. Biochemistry. 2001;40:6964–6970. doi: 10.1021/bi002816i. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee SK, Laffray J, Patel P, Ravindra R, Qin Y, Kuehne ME, Bane SL. Interaction of tubulin with a new fluorescent analogue of vinblastine. Biochemistry. 2002;41:14010–14018. doi: 10.1021/bi026182m. [DOI] [PubMed] [Google Scholar]
- 35.Howard WD, Timasheff SN. GDP state of tubulin: stabilization of double rings. Biochemistry. 1986;25:8292–8300. doi: 10.1021/bi00373a025. [DOI] [PubMed] [Google Scholar]
- 36.Melki R, Carlier MF, Pantaloni D, Timasheff SN. Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry. 1989;28:9143–9152. doi: 10.1021/bi00449a028. [DOI] [PubMed] [Google Scholar]
- 37.Diaz JF, Menendez M, Andreu JM. Thermodynamics of ligand-induced assembly of tubulin. Biochemistry. 1993;32:10067–10077. doi: 10.1021/bi00089a023. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta S, Boge TC, Liu Y, Hepperle M, Georg GI, Himes RH. Probing the environment of tubulin-bound paclitaxel using fluorescent paclitaxel analogues. Biochemistry. 1997;36:5179–5184. doi: 10.1021/bi962891m. [DOI] [PubMed] [Google Scholar]
- 39.Edler MC, Buey RM, Gussio R, Marcus AI, Vanderwal CD, Sorensen EJ, Díaz JF, Giannakakou P, Hamel E. Cyclostreptin ( FR182877), an antitumor tubulin-polymerizing agent deficient in enhancing tubulin assembly despite its high affinity for the taxoid site. Biochemistry. 2005;44:11525–11538. doi: 10.1021/bi050660m. [DOI] [PubMed] [Google Scholar]
- 40.Cortese F, Bhattacharyya B, Wolff J. Podophyllotoxin as a probe for the colchicine binding site of tubulin. J Biol Chem. 1977;252:1134–1140. [PubMed] [Google Scholar]
- 41.Hastie SB. Interactions of colchicine with tubulin. Pharmacol Ther. 1991;51:377–401. doi: 10.1016/0163-7258(91)90067-v. Review. [DOI] [PubMed] [Google Scholar]


