Abstract
Quiescent satellite cells represent pluripotent stem cells capable of differentiating into other lineages. To define the potential changes in adhesion and motility in these differentiating cells, we utilized an established model system of murine-derived satellite cells induced with BMP2 to undergo osteoblastic differentiation. When mouse myogenic satellite cells were treated with BMP2, myogenesis was inhibited, and interaction with extracellular matrix ligands was altered. α7 integrin expression was rapidly downregulated with attenuation of adhesion and migration on laminin substrates. BMP2 also induced α2 integrin expression with increased adhesion and motility on collagen substrates as the pluripotent myoblasts develop into the osteogenic lineage. We examined the effect of BMP2 on α7 promoter activity in myoblasts using a CAT reporter gene. BMP2 was found to suppress integrin expression through a transcriptional mechanism. The results identify a novel role for BMP2 in modulating satellite cell integrin expression and altering their interactions with the microenvironment during osteoblastic differentiation.
Keywords: satellite cell, BMP2, differentiation, α7 integrin, gene promoter
INTRODUCTION
Adult stem cells interact with their surrounding microenvironment, including the extracellular matrix (ECM). The stem cell ECM is unique to each type of tissue and provides not only scaffolding for support and organization but also generates signals needed for survival, proliferation, and differentiation [1, 2]. It is now accepted that the ECM is encrypted with vital information deciphered by multiple adhesion receptors that include the integrins. These transmembrane receptors bind specific ligands in the ECM that then triggers a host of downstream signaling pathways regulating several important events. For example, in vitro studies have shown that the substratum can influence whether myogenic cells develop into functional myotubes [3, 4]. During muscle development, it is known that myogenic differentiation depends on the interaction between progenitors and the surrounding ECM [5, 6].
Precursor myoblasts utilize adhesive interactions with specific laminins (Ln), heterotrimeric proteins composed of α, βand γ chains [7, 8]. Laminins play an important role in the growth, locomotion, and differentiation of myoblasts [9–12], and are mediated by integrin and other surface adhesion receptors [13]. The Ln-binding α7 integrin, as a dimer with β1 integrin, is expressed in mouse skeletal muscle as early as E10.5 d of development, and mediates myoblast motility on Ln substrates, is associated with costameres and forms the myotendinous and neuromuscular junctions in mature muscle [14–18].
In this current study, we investigated how cell-matrix interactions are altered following the conversion of pluripotent muscle satellite stem cells to an osteoblastic lineage. BMP2 is known to not only inhibit the terminal differentiation of myoblasts, but also induces an osteogenic phenotype. We show here that Ln-binding integrin receptors are modulated as cells develop into osteoblasts. Our studies provide new insights into the biological responses of satellite cells to BMP2 and demonstrate that adhesive and migratory functions are dramatically altered as myogenic cells shift to the osteogenic lineage. Such changes in cell behavior would be important during the recruitment of different cell types during tissue repair and regeneration.
Materials and Methods
Cell culture and differentiation protocol
C2C12 and MM14 mouse myoblast cell lines were maintained as previously described [15, 16]. For the current studies, cells were plated onto 6-well tissue culture plates at a density of 1.5×105 cells/cm2. Cells were cultured for 7 d with or without BMP2 (300 ng/ml, Peprotech Inc.). To examine alkaline phosphatase activity, cultures were processed as described previously [19].
Flow cytometry and immunofluorescence staining
Standard procedures for flow cytometry were followed[16, 19]. Cells (10 6/ml) were incubated with predetermined optimal concentrations of primary antibodies, washed, and incubated with secondary FITC-conjugated fluorescein-labeled antibodies (affinity-purified goat anti-hamster or anti-rat antibodies [Jackson Immunoresearch Labs]). Monoclonal antibodies, including anti-mouse β1 (Ha2/11), anti-mouse α1 (Ha31/8), anti-mouse α2 (Ha1/29), anti-mouse α3 (clone 42), rat anti-mouse α5 (CD49E), rat anti-α6 (GoH3), and anti-mouse α7 (CY8), were used against mouse integrin subunits [15, 20].
For immunofluorescence staining, cells were seeded (1.5×105 cells/cm2) on coverslips for 7 d with or without BMP2 (300 ng/ml), and fixed with 2% paraformaldehyde in PBS. Cells were permeabilized with 0.4% Triton X-100 and blocked with 10% normal goat serum. Cells were incubated with primary antibodies (CD49b: anti-α2, CY8: anti-α7) followed by staining with FITC or Rhodamine labeled anti-rat or anti-mouse IgG, and viewed with a Zeiss Axio Vert 200M microscope.
Reverse Transcription-polymerase Chain Reaction (RT-PCR)
The protocol for RT-PCR has been described previously [19]. After BMP2 treatment as described above, total RNA was isolated using RNeasy Mini Kit). The amount of RNA was equalized with a human-β actin competitive PCR kit (Takara Shuzo Co.). Samples of RNA were subjected to PCR using gene-specific primers which were derived from previously published sequences. The sequences of the PCR primers used for amplification were: Osteocalcin, fwd 5’-CTGAGTCTG ACA AAGCCT TC-3’, rev 5’-GCTGTGACATCCATACTTGC-3’ (314 bp fragment); Runx2, fwd 5’-GGCAAGATGAGCGACGTGAG-3’, rev5’-ATCTGACTCTG TCCTTGTGG-3’ (767 bp fragment); Desmin, fwd 5’-AAGGCCAAACTACAGGAGGA-3’, rev 5’-CATGTTGTTGCTGCGTAGCC-3’ (832 bp fragment); MyoD, fwd 5’-AGGCTCTGCTGCGCGACCA-3’, rev 5’-GGTGCTCTGA GAGGTTGGCTGCA-3’ (315 bp fragment); α7 integrin, fwd 5’-CCAACCCCAG AGCTGGCTGCT-3’, rev 5’-ATCCAGCTCA TCACGGATGGC-3’ (350 bp fragment); α2 integrin, fwd 5’-GCGTGTGGACAT CAG-3’, rev 5’-AGAAGCCGAGCT TCC-3’ (1225 bp fragment); GAPDH, fwd 5’-CCTGCACCAC CAACTGCTTA-3’, rev 5’-CTTACTCCTTGGAGGCCATGTAG-3’ (558 bp fragment). PCR reactions were performed for 25 cycles in a thermal cycler.
Cell adhesion and migration assays
The previously described assay was used [16] for the analysis of cell adhesion. Single-cell suspensions were prepared in culture medium, added in triplicate to 96-well plates, and then incubated for 40 min laminin-1 (Ln-1) or 30 min collagen type I (Col-I) at 37°C. The number of cells bound to the substrates was estimated with the absorbance read at 562 nm after staining with crystal violet. Cells bound to Ln-1 or Col-I for 2 h were used to indicate 100% attachment. Background cell adhesion to 0.5% BSA-coated wells was subtracted. The effect of specific antibody and incubation time was tested by pre-incubating the cells with the antibody on ice for 60 min prior to assay.
Cell migration was assayed as described previously [21]. The undersides of the transwell (8μm pore) were precoated with Ln-1 (5μg/ml) or Col-I (1μg/ml). Next, cells were loaded onto the upper chamber of the transwell, and the lower chamber was filled with serum-free medium. Cells were incubated for 3 h at 37°C, fixed with paraformaldehyde, and stained with crystal violet. The effect of specific antibody was tested by pre-incubating the cells with the optimal dilutions of purified antibody on ice for 30 min prior to the assay. Cells that had migrated through the filter were counted and averaged from ten randomly chosen microscopic fields using a 20x objective.
Transient transfections and promoter assays
The full length construct p2.8 kb of the α7 integrin promoter has been described [22]. C2C12 cells were plated at 1.5×105 cells in 6-well plates and transfected 16 h later using Lipofectmine Plus (Invitrogen). Equal amounts of DNA (either p2.8 kb α7 construct or the empty vector pCAT) were transfected in duplicate wells; total DNA transfected per well ranged from 2 to 4μg. Transfection efficiency was normalized by co-transfection of 80 ng of pRLβ-galactosidase as internal control. After 48 h, the cells processed for CAT ELISA (Promega). The day after transfection the medium was replaced with or without BMP2 (300ng/ml). Lysates were prepared after 7 days and CAT promoter assays were performed; β-galactosidase activity was determined using Tropix (Applied Biosystems) on a luminometer.
Results and Discussion
Induction of osteogenic differentiation
Both the MM14 and C2C12 myoblasts are well characterized for their potential for myogenic differentiation and capability for forming mature myotubes in culture. These two cell lines were originally derived from adult muscle satellite cells. They both express muscle-specific markers and are competent for differentiating into myotubes and incorporate into functional muscle fibers after transplantation in vivo. In addition, the MM14 cells have been reported to exhibit different migratory phenotypes on Ln verses fibronectin substrates while C2C12 cells have been widely used for studies of myoblast differentiation and transplantation fate [12, 23].
Here we have analyzed the capacity of C2C12 and MM14 cells for their multipotent potential along osteoblastic lineages. Myoblasts were grown in the presence or absence of BMP2, an inducer of the osteogenic pathway. Both cells were able to form myotubes (Fig. 1A,a,c). When either MM14 or C2C12 cells were treated with BMP2, they failed to differentiate into myotubes and instead differentiated along the osteogenic pathway as revealed by induction of alkaline phosphatase (Fig. 1A,b,d). The cells remained as nonfused mononuclear polygonal cells. A majority of BMP2 treated cells strongly expressed the alkaline phosphatase, whereas control cells did not. These results confirm that both MM14 and C2C12 myoblasts when treated with BMP2 underwent osteogenic differentiation.
Figure 1. BMP2 induces osteogenic differentiation in C2C12 and MM14 myoblasts.
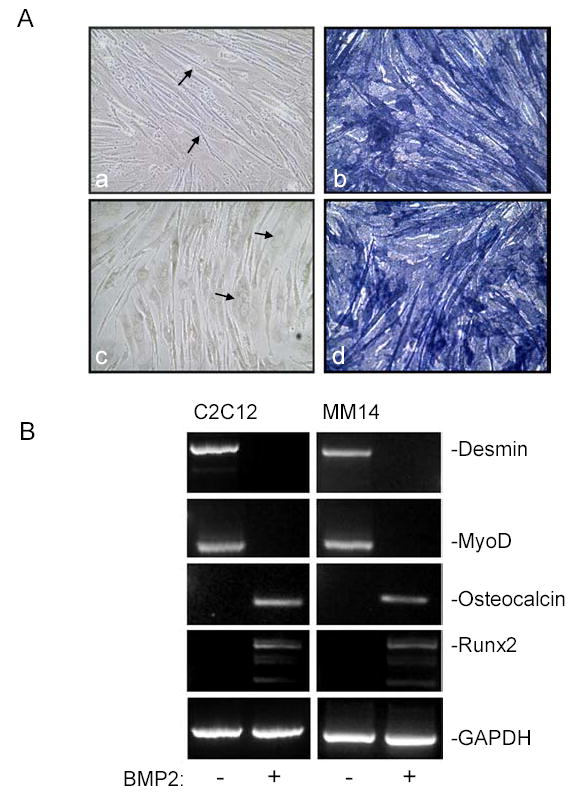
(A) The C2C12 (a,b) and MM14 (c,d) cells were cultured (1.5×105 cells/cm2) under myogenic conditions (a, c) or treated with BMP2 (300 ng/ml) (b,d) for 7 d. Cultures were processed for alkaline phosphatase activity to identify osteogenic lineage (Materials and Methods). In the absence of BMP2, numerous multinucleated myotubes were visible (a,c, arrows) but these cells were negative for enzyme activity. In the presence of BMP2 strong alkaline phosphatase activity alkaline phosphatase (b,d). (B) Cells were cultured for 7 days as above with or without BMP2 and processed for RT-PCR using primers specific for GAPDH, osteocalcin, Runx2, desmin, and MyoD. BMP2 produced a decrease in the myogenic desmin and MyoD expression but induced osteogenic markers osteocalcin and Runx2.
To further define the potential of mouse MM14 and C2C12 myoblasts to differentiate along the osteogenic pathway, BMP2 treated myoblasts were analyzed by RT-PCR for expression of osteocalcin and Runx2, both markers for osteoblasts. Compared to the GAPDH control, osteocalcin and Runx2 were strongly detected in BMP2-treated myoblasts while expression was absent in untreated cells (Fig. 1B). The expression of common myogenic markers such as desmin and MyoD following osteogenic differentiation was also examined. Desmin and MyoD were detected in control cells while loss of expression was observed in BMP2 treated myoblasts (Fig. 1B). These results further support that BMP2 can induce mouse C2C12 and MM14 myoblasts along osteogenic differentiation pathway while inhibiting myogenesis.
BMP2-induced changes in adhesion and motility
ECM proteins are important for tissue-specific functions [24, 25]. These structural proteins contribute to the unique properties that define the niche for each tissue type, and help maintain stem cell function and specification [1]. For the most part, the integrin expression profile reflects the type of differentiated cell type found in tissue subtypes. Consequently, we tested myoblasts for their ability to adhere to different ECM molecules and examined if this adhesion was altered during osteogenic differentiation. Adhesion to Ln-1 or Col-I substrates was assessed after BMP2 treatment and in the presence of function-perturbing mAbs to integrin receptors,. For C2C12 cells, anti-α7 mAb substantially inhibited cell adhesion, while anti-β1 mAb and the combination of anti-α6 and anti-α7 mAb completely blocked Ln adhesion (Fig. 2A). Following induction of differentiation with BMP2 treatment, C2C12 adhesion to Ln substrates dramatically declined (Fig. 2A). Adherence was sensitive to anti-α2 mAb but also to anti-β1. This is consistent with α2 integrin mediating the relatively poor adhesion to Ln in BMP2 treated cells. In contrast, anti-α7 had no effect on adhesion to Ln.
Figure 2. Adhesion and migration of mouse myoblasts is altered by BMP2.
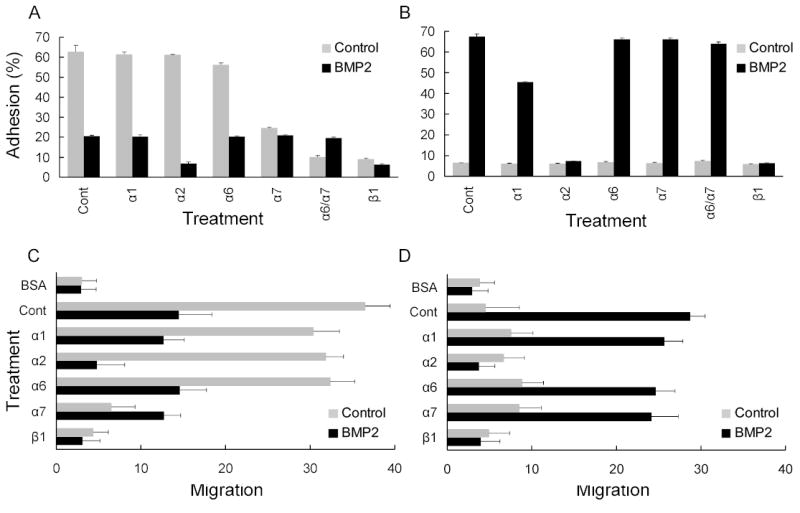
Adhesion of C2C12 (gray bars) and BMP2-treated cells (black bars) on (A) Ln-1 (5μg/ml) or (B) Col-I (1μg/ml) were assayed in the absence (control) or presence of the indicated anti-integrin antibodies as described in Materials and Methods. Data are presented as a percentage of the total input cell number. Bars show S.D. Migration of C2C12 (gray bars) and BMP2-treated cells (black bars) on (C) Ln-1 (5μg/ml) and (D) Col-I (1μg/ml) was assayed in the absence (control) or presence of the indicated anti-integrin antibodies. C2C12 cells were added to upper chamber and incubated for 3 h. Motility was estimated by counting the number of cells that migrated to the undersides of the membranes. The results are averages of the at least ten random microscopic fields. Bars show S.D.
On Col-I substrates, C2C12 cells displayed poor adhesion and only a minor fraction of the cells adhered to this substrate (Fig. 2B). However, following BMP2 induction, there was a strong increase in adhesion to Col-I (Fig. 2B) that was blocked by anti-α2 and by anti-α1 mAb to a lesser extent; other anti-α chain mAbs had no effect. Complete inhibition of adhesion was achieved with anti-β1 mAb. Similar results were also obtained for the MM14 myoblasts (not shown).
BMP2 affects on cell motility with Ln-1 or Col-I substrates was also examined. For Ln, the C2C12 myoblasts showed a strong locomotive response (Fig. 2C,D). Consistent with the high expression of the α7 integrin, migration on Ln-1 was blocked with the anti-α7 mAb, and as expected, anti-β1 mAb was effective in inhibiting motility. Following treatment with BMP2, however, cell migration on Ln-1 was reduced as compared to controls (Fig. 2C). The migration response was mediated by the α2 receptor, which is well known to function as a Ln receptor, a collagen receptor, or both [26]. On Col-I substrates, C2C12 cells typically showed poor migration, but with BMP2, migration on Col-I dramatically increased (Fig. 2D). This increase was dependent on the α2 integrin receptor, since antibodies to either α2 or β1 subunits completely abolished motility. For the MM14 cells, nearly identical results were observed (data not shown).
Modulation of integrin expression following BMP2 treatment revealed the reason for the inversed behavior in adhesion and migration on the two substrates. As such, changes in integrin expression following induction were assessed by FACS. For C2C12 cells, we found a diverse set of integrin α chains with low levels of α1, α2 and α5, significant levels of α3 and α6, and abundant α7 integrin expression (Fig. 3A). A similar repertoire of integrins was expressed on MM14 cells (not shown). Following BMP2 treatment, there was a dramatic loss of α7 integrin (Fig. 3A,B). Also, there was a reciprocal increase in the α2 integrin, the dominant Col-I receptor used by osteoblasts. Analysis of the dot plot data for α2 and α7 integrins shows that the majority of the cell population had inverted their relative integrin expression levels following BMP2 treatment. A minor subset of cells did not show complete induction of the α2 and loss of α7 receptor expression, suggesting that this small fraction of cells remained committed to muscle lineage and did not switch their specification. Similar results were obtained by immunofluoresent staining showing that in most cells, positive staining of the α7 integrin was lost following BMP2 treatment whereas α2 showed a strong increase after the treatment (Fig. 3C).
Figure 3. BMP2 modulates adhesion receptor expression.
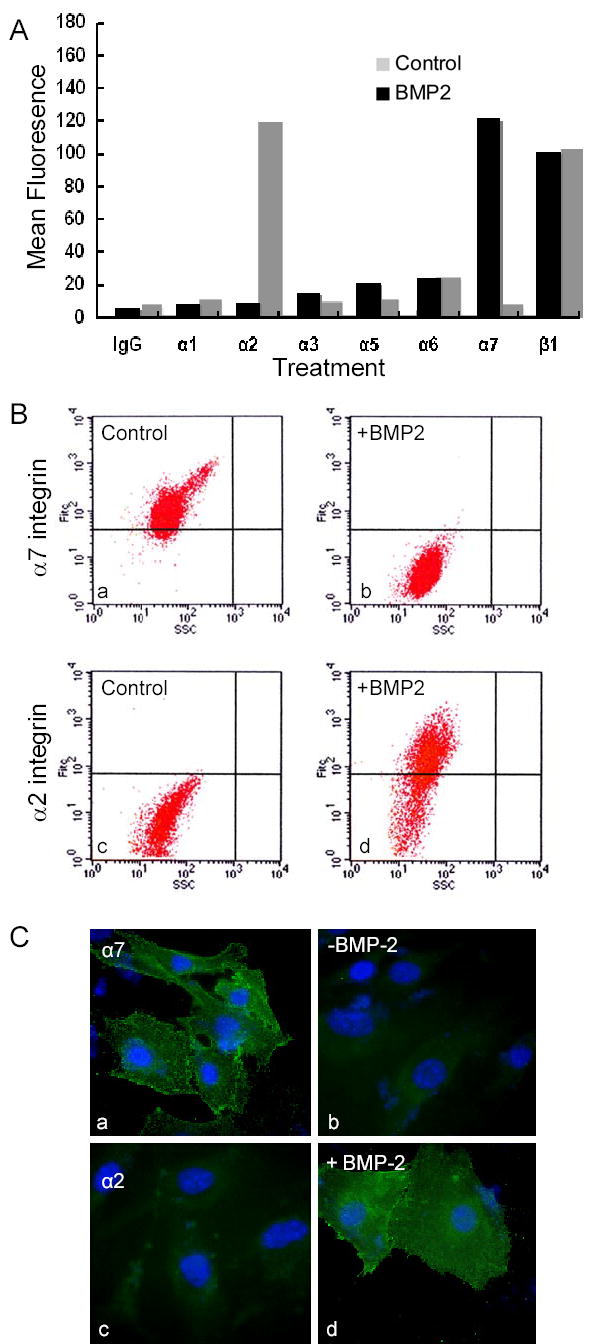
(A) Flow cytometry analysis of integrin expression for C2C12 cells (Materials and Methods) cultured in the absence (black bars) and presence (grey bars) of BMP2 for 7 d. Values from controls of secondary antibody alone were subtracted to give the mean fluorescence intensity. Note that high expression of α7 integrin in C2C12 cells is lost following BMP2 treatment whereas expression of α2 integrin is strongly induced. (B) Analysis of integrin expression by 2D dot plot for shows the (a) control cells are positive for α7 and (c) negative for α2, but following BMP2 treatment (b) α7 level is near background and (d) α2 integrin is strongly induced in most cells. (C) Immunofluorescence staining of C2C12 cells shows that strong staining of α7 integrin in control myoblasts (a) is lost following BMP2 treatment (b) whereas poorly expressed α2 (c) is strongly induced by BMP2 treatment (d).
Thus, following BMP2 induced conversion to the osteogenic lineage, cells expressed the α2 integrin, which is needed to bind to interstitial collagen matrix of bone, and thereby maintaining a fully differentiated phenotype. We confirmed that BMP2-induced alterations in the integrin profile seem to correlate with the changes in the adhesive and motile. Not only does BMP2 dramatically downregulate α7 integrin levels in C2C12 (and MM14) myoblasts, but induced a reciprocal increase in the α2 integrin, the dominant Col-I receptor for osteogenic cells.
BMP2 downregulated α7 integrin expression at the level of the promoter
Next, we tested gene expression by RT-PCR of Ln-I-binding α7 and Col-I-binding α2 integrins following BMP2-mediated differentiation into the osteogenic lineage (Fig. 4A). The results indicate that compared to GAPDH controls, BMP2 downregulated the α7 integrin gene in myoblasts, and induced a reciprocal increase in the α2 integrin expression (Fig.4A).
Figure 4. α7 gene expression and promoter activity following osteogenic differentiation.
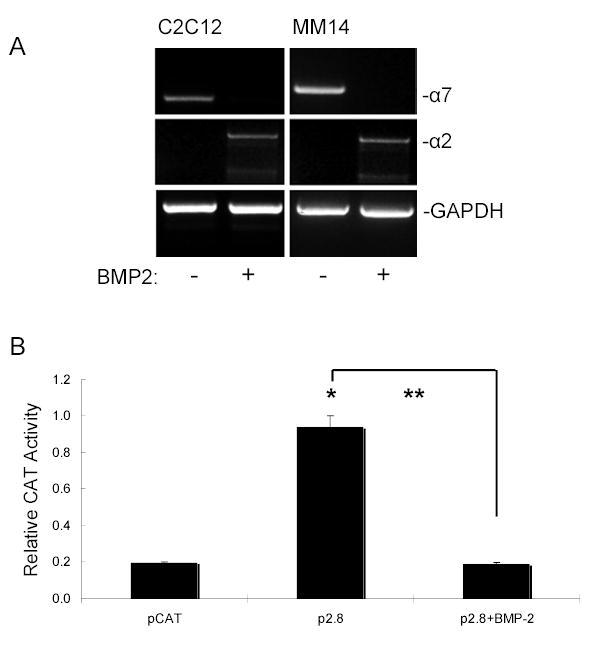
(A) BMP2-induced changes in integrin mRNA. Cells were cultured for 7 days as in Fig. 1 above with or without BMP2 and processed for RT-PCR using primers specific for GAPDH, α7, and α2. Compared to control cultures, BMP2 produced a decrease in α7 but induced α2 integrin under osteogenic conditions. (B) The α7 promoter activity was measured using the CAT receptor gene driven by wild-type α7 promoter (pCAT2.8). C2C12 myoblast cells were transiently transfected with the full length construct of mouse α7 integrin promoter and processed after treatment with BMP2. CAT promoter activity was measured and expressed as activity relative to that of empty vector pCAT. Transfection efficiency was normalized with co-transfection of the β-gal vector as an internal control. Data represents the mean of at least three separate experiments with error bar indicating S.D. * p < 0.01 vs. control; ** p < 0.005 as indicated by the bracket.
Therefore, both FACS and RT-PCR analysis showed that BMP2 induced myoblasts to develop into the osteogenic pathway with a nearly complete loss of expression of the α7 integrin. To confirm decreased transcription was responsible for this change in mRNA levels, we checked promoter activity in myoblasts using the CAT reporter gene driven by the full-length 2.8-kb mouse α7 promoter. C2C12 cells were transiently transfected with deletion constructs of mouse α7 integrin promoter and cell lysates were tested for CAT promoter activity expressed as activity relative to that of empty vector pCAT (Fig. 4B). In cells treated with BMP2, α7 promoter activity was nearly completely suppressed indicating that BMP2 regulates α7 integrin expression through a transcriptional mechanism.
Conclusion
The current study has identified that α7 integrin may be a specific marker that defines myoblasts with pluripotent stem cell potential. Also, modulation of integrin receptor expression occurs in parallel with the conversion of myoblasts cells to osteogenic lineage. These alterations in adhesion are potentially important for the optimization of cell functions such as adhesion, motility, remodeling, and repair of interstitial matrix molecules. Similar changes in adhesion receptors must occur in embryonic and other adult stem cells as they develop into highly differentiated cell types. More work is needed to define these changes. α7 integrin is rapidly lost following BMP2-induction. We have established that the mechanism for this suppression is at the transcriptional level. Since α7 levels appear to be an important marker of myoblasts with ‘stem cell’ characteristics and since α7 levels seem to be modulated by BMP2 treatment, we are interested in defining the mechanism of this regulation. In addition, it will be interesting to artificially downregulate α7 and determine if receptor loss facilitates osteogenic differentiation. Myoblasts bind to Ln-1 through the α7 receptor, but lack the α2 receptor and bind poorly to interstitial Col-I. In contrast, osteoblasts tend to favor binding to Col-I scaffolding through the α2 receptor and fail to express the α7 receptor. Regulation of α7 levels by BMP2 at the level of the promoter may be through direct or indirect mechanisms. BMP2 could affect the myogenic MRF family of factors including MyoD, myf5, myogenin, MRF4 or have an effect on the MEF2 family of proteins. It is also possible that Smads bind directly or through a protein complex to modulate expression of the α7 promoter. Future studies will investigate the mechanism by which BMP2 downregulates α7 integrin.
Acknowledgments
The skilled technical assistance by Baomei Liu and Eric Gschweng are gratefully acknowledged. These studies were supported by a grant from the National Institutes of Health (R01 DE15404).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 2.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 3.Hauschka SD, Konigsberg IR. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966;55:119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff R, Holtzer H. Effect of Mitotic Inhibitors on Myogenesis in Vitro. Journal of Cell Biology. 1968;36:111. &. [PubMed] [Google Scholar]
- 5.Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 6.Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–14590. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- 7.Timpl R, Brown JC. The laminins. Matrix Biol. 1994;14:275–281. doi: 10.1016/0945-053x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 8.Wewer UM, Engvall E. Laminins. Methods Enzymol. 1994;245:85–104. doi: 10.1016/0076-6879(94)45007-2. [DOI] [PubMed] [Google Scholar]
- 9.Kuhl U, Timpl R, von der Mark K. Synthesis of type IV collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Dev Biol. 1982;93:344–354. doi: 10.1016/0012-1606(82)90122-1. [DOI] [PubMed] [Google Scholar]
- 10.Foster RF, Thompson JM, Kaufman SJ. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev Biol. 1987;122:11–20. doi: 10.1016/0012-1606(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 11.Goodman SL, Risse G, von der Mark K. The E8 subfragment of laminin promotes locomotion of myoblasts over extracellular matrix. J Cell Biol. 1989;109:799–809. doi: 10.1083/jcb.109.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von der Mark K, Ocalan M. Antagonistic effects of laminin and fibronectin on the expression of the myogenic phenotype. Differentiation. 1989;40:150–157. doi: 10.1111/j.1432-0436.1989.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 13.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman SJ, George-Weinstein M, Foster RF. In vitro development of precursor cells in the myogenic lineage. Dev Biol. 1991;146:228–238. doi: 10.1016/0012-1606(91)90462-c. [DOI] [PubMed] [Google Scholar]
- 15.Yao CC, Ziober BL, Squillace RM, Kramer RH. Alpha7 integrin mediates cell adhesion and migration on specific laminin isoforms. J Biol Chem. 1996;271:25598–25603. doi: 10.1074/jbc.271.41.25598. [DOI] [PubMed] [Google Scholar]
- 16.Yao CC, Ziober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the alpha 7 integrin receptor. J Cell Sci. 1996;109:3139–3150. doi: 10.1242/jcs.109.13.3139. Pt 13. [DOI] [PubMed] [Google Scholar]
- 17.von der Mark H, Durr J, Sonnenberg A, von der Mark K, Deutzmann R, Goodman SL. Skeletal myoblasts utilize a novel beta 1-series integrin and not alpha 6 beta 1 for binding to the E8 and T8 fragments of laminin. J Biol Chem. 1991;266:23593–23601. [PubMed] [Google Scholar]
- 18.Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K. Beta 1D integrin displaces the beta 1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J Cell Biol. 1996;132:211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozeki N, Lim M, Yao CC, Tolar M, Kramer RH. alpha7 integrin expressing human fetal myogenic progenitors have stem cell-like properties and are capable of osteogenic differentiation. Exp Cell Res. 2006 doi: 10.1016/j.yexcr.2006.09.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziober BL, Chen YQ, Ramos DM, Waleh N, Kramer RH. Expression of the alpha7beta1 laminin receptor suppresses melanoma growth and metastatic potential. Cell Growth Differ. 1999;10:479–490. [PubMed] [Google Scholar]
- 21.Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269:31807–31813. [PubMed] [Google Scholar]
- 22.Ziober BL, Kramer RH. Identification and characterization of the cell type-specific and developmentally regulated alpha7 integrin gene promoter. J Biol Chem. 1996;271:22915–22922. doi: 10.1074/jbc.271.37.22915. [DOI] [PubMed] [Google Scholar]
- 23.Blau HM, Hughes SM. Retroviral lineage markers for assessing myoblast fate in vivo. Adv Exp Med Biol. 1990;280:201–203. doi: 10.1007/978-1-4684-5865-7_22. [DOI] [PubMed] [Google Scholar]
- 24.Rocha V, Ringo DL, Read DB. Casein production during differentiation of mammary epithelial cells in collagen gel culture. Exp Cell Res. 1985;159:201–210. doi: 10.1016/s0014-4827(85)80049-5. [DOI] [PubMed] [Google Scholar]
- 25.Reznikoff CA, Loretz LJ, Pesciotta DM, Oberley TD, Ignjatovic MM. Growth kinetics and differentiation in vitro of normal human uroepithelial cells on collagen gel substrates in defined medium. J Cell Physiol. 1987;131:285–301. doi: 10.1002/jcp.1041310302. [DOI] [PubMed] [Google Scholar]
- 26.Hemler ME, Elices MJ, Chan BM, Zetter B, Matsuura N, Takada Y. Multiple ligand binding functions for VLA-2 (alpha 2 beta 1) and VLA-3 (alpha 3 beta 1) in the integrin family. Cell Differ Dev. 1990;32:229–238. doi: 10.1016/0922-3371(90)90035-u. [DOI] [PubMed] [Google Scholar]


