Abstract
In general, scorpion β-toxins have been well examined. However, few in-depth studies have been devoted to species selectivity and affinity comparisons on the different voltage-activated Na+ channels since they have become available as cloned channels that can be studied in heterologous expression systems. As a result, their classification is largely historical and dates from early in vivo experiments on mice and cockroach and fly larvae.
In this study, we aimed to provide an updated overview of selectivity and affinity of scorpion β-toxins towards voltage-activated Na+ channels of vertebrates or invertebrates. As pharmacological tools, we used the classic β-toxins AaHIT, Css II, Css IV, Css VI and Ts VII and tested them on the neuronal vertebrate voltage-activated Na+ channel, rNav1.2a. For comparison, its invertebrate counterpart, DmNav1, was also tested. Both these channels were expressed in Xenopus laevis oocytes and the currents measured with the two-electrode voltage-clamp technique. We supplemented this data with several binding displacement studies on rat brain synaptosomes. The results lead us to propose a general classification and a novel nomenclature of scorpion β-toxins based on pharmacological activity.
Keywords: scorpion β-toxins, voltage-activated Na+ channels, species-selectivity, Centruroides suffusus suffusus, Androctonus australis Hector
Introduction
Voltage-activated Na+ channels (VASCs) are membrane-spanning proteins responsible for the rising phase of action potentials in excitable cells. The central, pore-forming α-subunit of VASCs consists of four homologous domains (DI–DIV, each containing 6 segments: S1–S6) and can be associated with up to four known different β-subunits (Catterall, 2000; Yu et al., 2003). To date, ten mammalian and six invertebrate VASCs have been cloned (Catterall et al., 2005; Zuo et al., 2006) and most of them have been functionally expressed. Since VASCs play a pivotal physiological role, they are targeted by toxins of various organisms. Until now, toxins have been used to describe eight different receptor sites on the α-subunit (French and Terlau, 2004).
Scorpion long-chain neurotoxins targeting VASCs are single chain polypeptides composed of 60–76 amino acids cross-linked by four disulphide bridges. They have been divided into two classes, α- and β-toxins (Couraud et al., 1982). The scorpion α-toxins inhibit the inactivation process by binding to site 3 of the VASC which is located in the extracellular linkers of DIV thereby preventing the normal gating movements of the S4 segment of DIV. Scorpion α-like toxins belong to the same group but distinguish themselves by acting on both mammals and insects. However, they do not bind to rat brain synaptosomes (Gordon et al., 1996; Gordon, 1998; Possani et al., 1999). The nomenclature of these α-like toxins is primarily based on the results of binding displacement studies, while the properties of α- and α-like toxins from an electrophysiological point of view are in fact alike (Couraud et al., 1982).
β-toxins bind to receptor site 4 of the VASC located in the extracellular linkers of DII, thereby modifying the activation process of the channel. It was demonstrated that the hyperpolarizing shift in the voltage dependence of activation caused by scorpion β-toxins is largely promoted after the channel is primed with a preceding depolarizing prepulse in the presence of the toxin (Cahalan, 1975; Cestele et al., 1998; Cestele and Catterall, 2000; Mejri et al., 2003). Recent research also indicated that distinct β-toxins can produce resurgent currents in Nav1.6 by trapping the voltage sensor in DII (Schiavon et al., 2006).
The group of scorpion β-toxins consists of mammalian-selective and insect-selective peptides. Historically, the insect-selective toxins are subdivided into two categories according to their effect on blow fly larvae and amino acid sequences. The first category of insect-selective β-toxins induces an immediate reversible fast contraction paralysis upon injection and is therefore called excitatory toxins. Typical examples include AaHIT from the Androctonus australis Hector and Bj-xtrIT from the Buthotus judaicus. The second category comprises the depressant insect-selective toxins which induce a slow progressive onset of paralysis preceded by a short transient phase of contraction. Typical depressant toxins include BjIT2 from Buthotus judaicus and LqqIT2 from Leiurus quinquestriatus quinquestriatus (Pelhate and Zlotkin, 1982; Zlotkin et al., 1985; Zlotkin et al., 1991; Moskowitz et al., 1998; Froy et al., 1999; Gurevitz et al., 2001; Gordon et al., 2003; Cohen et al., 2004; Karbat et al., 2004). Previous studies on VASCs have already highlighted the importance of DII as a whole and more specific residues like Pro782 and Glu779 in DII S1–S2; Glu837, Leu840 and Gly845 in DII S3–S4, Arg850 and Arg853 in DII for binding of the β-toxin to the channels (Cestele et al., 1998; Cestele and Catterall, 2000; Cestele et al., 2001; Mantegazza and Cestele, 2005). Mutagenesis studies on β-toxins themselves have revealed a number of residues critical for the pharmacological activity (see Figure 1) (Froy et al., 1999; Hassani et al., 1999). A putative pharmacophore involved in the interaction of scorpion β-toxins with receptor site 4 on the VASC was reported by Cohen et al. 2004. In this study, the surface of the scorpion β-toxin Bj-xtrIT from the scorpion Buthotus judaicus was analyzed by point mutagenesis. This analysis highlighted a functional discontinuous surface composed of non-polar and charged amino acids clustered around the main α-helical motif and the C-tail. Among the charged residues, Glu30 is a putative hot-spot and is shielded from bulk solvent by a hydrophobic gasket (Tyr26 and Val34), a phenomenon also observed with scorpion α-like toxins (Ye et al., 2005). Furthermore, another study confirmed the mandatory role for toxin action of Glu30 together with Glu15 (Karbat et al., 2004). In 2005, Cohen et al. 2005 reported the successful recombinant expression for Css IV fused to a His-tag. They stated that this His-Css IV had a similar binding affinity for, and effect on Na+ currents of rNav1.2a as that of the recombinant wild-type toxin devoid of His-tag. However, it should be stressed that the binding affinity of the native Css IV is still much higher (between 0.05 and 0.1 nM, Martin-Eauclaire, unpublished data). Nevertheless, by dissecting His-Css IV, a functionally important surface was reported to consist of: 1) a cluster of residues associated with the α-helix, which includes a putative hot-spot (Glu28); 2) a hydrophobic cluster associated mainly with the β2 and β3 strands; 3) a single residue (Trp58) in the C-tail and 4) a negatively charged residue (Glu15) involved in voltage sensor trapping. All aforementioned residues are indicated in Figure 1.
Figure 1.

Sequence alignment based on cystine residues (ClustalX 1.8). Relative amino acid location is shown at the bottom (absolute location is used in text). Conserved residues are marked with *. Sequences are divided into three groups: toxins with reported activity on mammals, mammals and insects or insects. For each group, a representative toxin was chosen of which previously studied residues are indicated in red (Froy et al., 1999; Hassani et al., 1999; Cohen et al., 2004; Karbat et al., 2004; Cohen et al., 2005). Amidated C-tails are indicated. Accession numbers: P01497 for AaHIT, P08900 for Css II, P60266 for Css IV, P60267 for Css VI and AAB29120 for Ts VII.
Since the classification of scorpion β-toxins is largely historically-based and dates from early in vivo experiments on mice, cockroach and fly larvae, in this study we provide an updated: 1) overview of these toxins with regard to their VASC selectivity and 2) classification based on pharmacological function of scorpion β-toxins towards vertebrates or invertebrates in this study.
Methods
Toxins
Toxins II (Css II), IV (Css IV) and VI (Css VI) from the Mexican scorpion Centruroides suffusus suffusus, Toxin VII or γ (Ts VII or Ts γ ) of the Brazilian scorpion Tityus serrulatus and the anti-insect toxin (AaHIT) from the North African scorpion Androctonus australis were purified as previously described (Bechis et al., 1984; De Lima et al., 1986; Martin et al., 1987; De Lima et al., 1989; Ceard et al., 1992).
Competition studies
Css IV was 125I-labelled using the lactoperoxidase-catalyzed method and purified by batch-wise resuspension with a Dowex 1-X8 ion exchanger. The synaptosomal fraction (P2) from adult rat brains was prepared as previously described in the presence of protease inhibitors (Legros et al., 2005). Synaptosomes were kept frozen in liquid nitrogen until use. Incubation buffer was (in mM): Hepes 25, glucose 10, choline chloride 140, KCl 5.4, MgSO4 0.8, 0.1 % bovine serum albumine (BSA), adjusted to pH 7.2 with Tris base. The washing buffer consisted of 5 mM HEPES adjusted to pH 7.2 with Tris base. Competition experiments (3.5 μg/assay) were carried out in 600 μl buffer, incubated for 2 hrs at 37°C. The free ligand was separated by filtration over through GF/B glass fiber-filters pre-treated with BSA (20 %, w/v). The filters were washed twice with 5 ml ice cold washing buffer. The radioactivity retained on the filters was counted on a gamma counter (Crystal II, Packard). The non-specific binding was determined in the presence of 0.1 μM (final concentration) of native toxin. The data were analysed using PRISM (GraphPad).
Voltage-activated sodium channel expression
For the expression in Xenopus laevis oocytes, the DmNav1/pGH19-13-5 plasmid, tipE/pGH19 plasmid and rBrainIIa (rNav1.2a)/pLCT1 plasmid were linearised with NotI and transcribed with the T7 mMESSAGE-mMACHINE kit (Ambion, U.S.A.) (Noda et al., 1986; Auld et al., 1988; Isom et al., 1992; Feng et al., 1995; Warmke et al., 1997). β1/pSP64T was first linearised with EcoRI. Next, capped cRNA’s were synthetized from the linearised plasmid using the large-scale SP6 mMESSAGE-mMACHINE transcription kit (Ambion, U.S.A.). The harvesting of oocytes from anaesthetized female Xenopus laevis frogs was as previously described (Ye et al., 2005). Oocytes were injected with 50 nl of cRNA at a concentration of ±1 ng nl−1 (1:1 mixture for both rNav1.2a/β1 and DmNav1/tipE) using a Drummond micro-injector (U.S.A.). The solution used for incubating the oocytes contained (in mM): NaCl 96, KCl 2, CaCl2 1.8, MgCl2 2 and HEPES 5 (pH 7.4), supplemented with 50 mg l−1 gentamycin sulphate and 180 mg l−1 theophyllin.
Electrophysiological studies
Two-electrode voltage-clamp recordings were performed at room temperature (18° – 22° C) using a GeneClamp 500 amplifier (Molecular Devices, USA) controlled by a pClamp data acquisition system (Molecular Devices, USA). Whole-cell currents from oocytes were recorded 2 to 4 days after injection. Voltage and currents electrodes were filled with 3 M KCl. Resistances of both electrodes were kept as low as possible (< 0.5 MΩ). Bath solution composition was (in mM): NaCl 96, KCl 2, CaCl2 1.8, MgCl2 2 and HEPES 5 (pH 7.4). Using a four-pole low-pass Bessel filter, currents were filtered at 1 kHz and sampled at 5 kHz. Leak subtraction was performed using a -P/4 protocol. From a holding potential of −90 mV, cells were depolarised with a three-step protocol based on a previous report by Borges et al. (Borges et al., 2004). The first and last depolarisation of 25 ms duration ranged from −70 mV to +40 mV in steps of 10 mV. The second depolarisation to a voltage ranging between −10 mV and 0 mV (depending on the VASC) was used to prime the channels ensuring maximal binding of the β-toxin to the channel. The third segment of 25 ms at −120 mV ensured recovery from inactivation. Repetition interval was 3 seconds. The peak currents elicited in the third depolarisation were plotted as a function of voltage, resulting in a current-voltage relationship (I–V curve). This dataset was used for assessing toxin effect on channel activation. Curves were fit using pClamp8 (Molecular Devices, U.S.A.) and Origin software (OriginLab Corp., U.S.A.).
Results
Inhibition of 125I-Css IV binding by other scorpion toxins at equilibrium conditions
Results of competition experiments on rat brain synaptosomes show that the equilibrium binding of 125I-Css IV was completely prevented by the studied β-toxins. The following values were obtained: Ts VII (K0.5=0.003 nM), Css VI (K0.5=0.01 nM), Css IV (K0.5=0.04 nM) and Css II (K0.5=10 nM) (see Figure 2).
Figure 2.

Inhibition of 125I-Css IV binding by other scorpion toxins at equilibrium conditions (n ≥ 3). The equilibrium binding of 125I-Css IV was completely prevented by the studied β-toxins. The following values were obtained: Ts VII (K0.5=0.003 nM), Css VI (K0.5=0.01 nM), Css IV (K0.5=0.04 nM) and Css II (K0.5=10 nM). The scorpion α-toxin AaH II, which binds to site 3 on the VASC, and the insect-selective toxin AaHIT, which only binds to site 4 on insect VASCs, were unable to displace 125I-Css IV at a concentration of 1 μM. Bars represent SEM.
It has been reported before that 125I-Css II binds specifically to site 4 on rat brain synaptosomes and that 125I-AaHIT only binds to insect synaptosomes (see Table 1). In our hands, there was indeed no observed competition between these two toxins. Ts VII is a link between the two types of toxins since it binds with high affinity in both systems. As a consequence, we propose that they all recognize the same site (site 4) on rat brain synaptosomes. In contrast, the well-studied scorpion α-toxin AaH II, which binds to site 3 on the VASC, and the insect-selective toxin AaHIT, which only binds to site 4 on insect VASCs, were unable to displace 125I-Css IV, even at a concentration of 1 μM.
Table 1.
Comparison of the binding constants in the literature of five β-scorpion toxins on synaptosome preparations from rat brain, fly head, and cockroach nerve cord. (De Lima et al., 1986; Martin et al., 1987; De Lima et al., 1989; Ceard et al., 1992). Nd = not done.
| Toxin | 125I-Css II bound to rat brain synaptosomes KD (nM) | 125I-AaHIT bound to fly head synaptosomes KD (nM) | 125I-AaHIT bound to cockroach nerve cord synaptosomes KD (nM) |
|---|---|---|---|
| Css II | 3.30a | >10 000c | >10 000d |
| Css IV | 0.13 a | nd | nd |
| Css VI | 0.08a | 50c | nd |
| Ts VII | 0.08b | 0.08c | 0.002d |
| AaHIT | >10 000c | 0.3c | 0.3d |
Effect of the studied toxins on cloned VASCs expressed in Xenopus laevis oocytes
Using the two-electrode voltage clamp (TEVC) technique on Xenopus laevis oocytes a pharmacological comparison was made to study the effects of the five classic toxins on currents recorded from two different cloned VASCs, DmNav1/tipE and rNav1.2a/β1 (see Figure 3). We used the Drosophila VASC, DmNav1, co-expressed with its auxiliary subunit tipE in Xenopus oocytes as model for invertebrates. As neuronal counterpart in vertebrates, rNav1.2a was studied in combination with just the β1 subunit since this was the only one available at that time. rNav1.2a was chosen in order to obtain a close correlation between the electrophysiological results on cloned channels and the binding data obtained on rat brain synaptosomes since they contain ±80 % of rNav1.2. It should be stressed that there are some caveats inherent to using oocytes and recombinant channels: assembly may not be similar to native channels (e.g. subunit composition) and post-translational processing might be different.
Figure 3.

Bar diagram indicating the toxin-induced Na+ current increase (if present) at a voltage of −40 mV for DmNav1/tipE (grey bars) and rNav1.2a/β1 (black bars). Factor of current increase at −40 mV is deduced from the normalized current-voltage (I–V) relationship before (□) and after (Δ) addition of the studied toxin (shown above the bars). These curves are constructed using the dataset after priming of the channel (third depolarisation, see inset left upper corner). In addition, traces at the voltage of maximal Na+ influx (depending on the I–V relationship of the VASC; from −20 to 0 mV) are displayed in order to show another typical effect of scorpion β-toxins, namely a small reduction in current. Data of toxins that did not affect a particular channel are not shown. Toxin concentration in all cases was 5 μM to obtain maximum effect. Error bars represent S.E.M. (n ≥ 3). Genbank accession number for Nav1.2: X03639 (Noda et al., 1986; Auld et al., 1988); for DmNav1: M32078 (Warmke et al., 1997).
To evaluate and compare toxin effect (hyperpolarizing shift in activation) between different channels and toxins, we chose to show a bar diagram indicating the toxin-induced Na+ current increase (if present) at a fixed voltage of −40 mV. Furthermore, Figure 3 shows the normalized current-voltage relationships before (□) and after (Δ) addition of the studied toxin from which the bardiagram is deduced. These relationships are based on the dataset after priming the channel (third depolarisation). Steady-state activation curves were also derived from these data and a fit with a Boltzmann equation yielded V1/2 (voltage of half maximal activation) values which are reported in the text. In addition, Figure 3 displays traces at or near the voltage of maximal Na+ influx in order to show another typical effect of scorpion β-toxins, namely a small reduction in current. Data of toxins that did not affect a particular channel are not shown. Toxin concentration in all cases was 5 μM to obtain maximum effect.
At a concentration of 5 μM, AaHIT only affected DmNav1/tipE indicating its insect-selectivty. The current was slightly reduced (≤ 10%) and the channel activated at a voltage that was about 10 mV more negative than the unaffected channel. At −40 mV, the inward current increased 5.3 ± 0.6 times. The shift in V1/2 (=ΔV1/2) was −2.2 ± 0.8 mV. Both Css II and Css VI clearly affected the vertebrate rNav1.2a/β1 VASC. A clear shift in activation is present at 5 μM and a small block of the current is seen (≤10%). At −40 mV, inward currents increased by a factor of 3.6 ± 1.6 (ΔV1/2 = −3.8 ± 0.9) and 9.5 ± 1.3 (ΔV1/2 = −6.0 ± 1.4) for Css II and Css IV, respectively. However, a small hump in the I–V curve of DmNav1/tipE was seen between −50 mV and −30 mV when 5 μM Css IV was present (current increase at −40 mV was 3.8 ± 0.5; no shift in V1/2) which could represent the toxin binding to only a fraction of the VASC population at this concentration. The rest of the I–V curve was identical to the standard conditions as if no toxin was added. Interestingly, this feature was not present when only 1 μM of toxin was added while this concentration still caused the same effect on rNav1.2a/β1. Therefore, it seems that Css II (see also Table 1) and Css IV reveal a preference for mammalian VASCs. Ts VII had much more drastic effects on both rNav1.2a/β1 and DmNav1/tipE. Besides the small block of Na+ current, a large hyperpolarizing shift in activation was seen on both VASCs when 5 μM of the toxin was applied. At −40 mV, inward currents increased by a factor of 6.4 ± 1.7 (ΔV1/2 = −22.0 ± 3.1) and 16.8 ± 2.1 (ΔV1/2 = −17.3 ± 4.4) for DmNav1/tipE and rNav1.2a/β1, respectively. On DmNav1/tipE, a puzzling small but significant shift (p<0.05) in reversal potential indicating a change in ion selectivity was seen. Ts VII acts on both vertebrate and invertebrate channels which is in concordance with previous studies (see Table 1).
Discussion
In this paper, we present the first overview of the target-specificity of five classical scorpion β-toxins correlating displacement studies and electrophysiological tests on two different neuronal VASCs: one from vertebrates, rNav1.2a and one from insects, DmNav1. These two channels are expressed in Xenopus laevis oocytes together with their corresponding subunit (β1 and tipE, respectively). When compared to the literature, our results are in concordance with other (mostly binding) studies. AaHIT is only active on DmNav1/tipE while Css II, Css IV and Css VI only seem to affect rNav1.2a/β1 at concentrations up to 1 μM. Ts VII shifts the activation voltage of both channels to more negative potentials. Previous binding studies concur with this result (see Table 1).
Based on the available physiological data and our new experiments on oocytes, we propose that the scorpion β-toxin group can be subdivided according to biological activity into three groups (see Figure 4): 1) βm-toxins active on mammals (e.g. Css II, IV and VI); 2) βi-toxins active on insects (e.g. AaHIT, LqqIT2) and 3) βlike-toxins active on both mammals and insects (e.g. Ts VII, Lqhβ1). The βi-toxins can be further subdivided into two categories according to their effect on blow fly larvae: a) the depressant insect-selective toxins and b) the excitatory toxins. The term βlike was chosen because of the analogy with the scorpion α-toxin group where α-like toxins are active on both mammals and insects. β-like has also been used before in a different context (Froy and Gurevitz, 2003). In this previous study it was suggested that, in the ancient world, the ancestral long-chain scorpion toxins affecting VASCs developed into β-like toxins, which most likely developed into α- and β-toxins before the separation of South America from Africa. Because of the different standpoint, no confusion or contradiction is to be expected with our classification and nomenclature.
Figure 4.
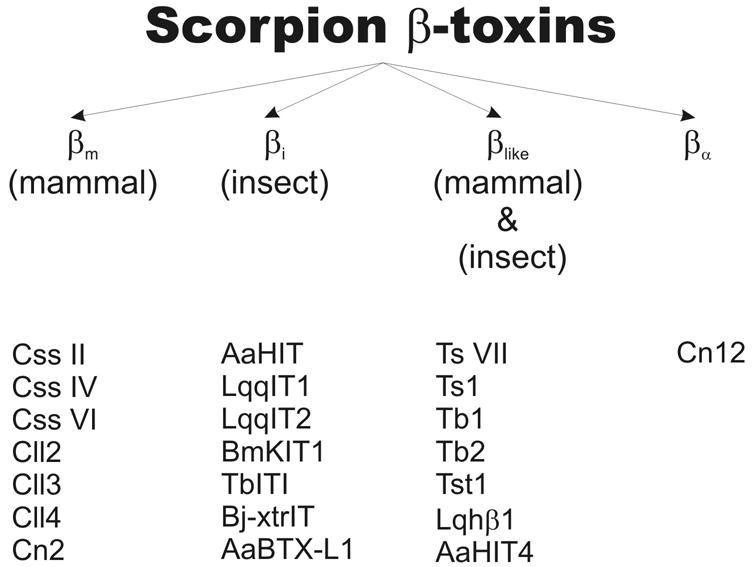
Overview of the proposed nomenclature of scorpion β-toxins with several toxins as examples.
Cn12, a peptide from Centruroides noxius was purified in 2004 (del Rio-Portilla et al., 2004). This toxin appeared to possess a typical β-toxin sequence but an α-like physiological activity. With respect to our proposed nomenclature, Cn12 can therefore be classified in another group, namely βα .
Recenty, several Birtoxin analogues were identified in the venom of Androctonus australis (Martin-Eauclaire et al., 2005). They are similar in size to long-chain scorpion toxins but are only reticulated by three disulfide bridges. With the results of this study, this relatively new structural group of Birtoxin-related toxins is increasing but the peptides described so far exhibit highly divergent biological activities. AaBTX-L1, which was studied here, seems to only affect the insect VASC and can therefore be classified as a scorpion βi-toxin.
Acknowledgments
We would like to thank the following persons: M.S. Williamson, IACR-Rothamsted, UK for sharing the DmNav1 and tipE clone; A.L. Goldin, Univ. of California, Irvine, USA for sharing rNav1.2a and S.H. Heinemann, Friedrich-Schiller-Universität Jena, Germany for sharing the β1 subunit. F.Bosmans is an honorary Fellow of the Belgian American Educational Foundation.
Abbreviations
- VASCs
voltage-activated Na+ channels
- Css
Centruroides suffusus suffusus
- AaH
Androctonus australis Hector
- Ts
Tityus serrulatus
- TEVC
two-electrode voltage clamp
Footnotes
Current address: Molecular Physiology and Biophysics Section, Porter Neuroscience Research Center, Bldg. 35-3B 211, NINDS, NIH, Bethesda, MD 20892, USA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel alpha subunit with novel gating properties. Neuron. 1988;1:449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Bechis G, Sampieri F, Yuan PM, Brando T, Martin MF, Diniz CR, Rochat H. Amino acid sequence of toxin VII, a beta-toxin from the venom of the scorpion Tityus serrulatus. Biochem Biophys Res Commun. 1984;122:1146–1153. doi: 10.1016/0006-291x(84)91211-7. [DOI] [PubMed] [Google Scholar]
- Borges A, Alfonzo MJ, Garcia CC, Winand NJ, Leipold E, Heinemann SH. Isolation, molecular cloning and functional characterization of a novel beta-toxin from the Venezuelan scorpion, Tityus zulianus. Toxicon. 2004;43:671–684. doi: 10.1016/j.toxicon.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Cahalan MD. Modification of sodium channel gating in frog myelinated nerve fibres by Centruroides sculpturatus scorpion venom. J Physiol. 1975;244:511–534. doi: 10.1113/jphysiol.1975.sp010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Ceard B, De Lima ME, Bougis PE, Martin-Eauclaire MF. Purification of the main beta-toxin from Tityus serrulatus scorpion venom using high-performance liquid chromatography. Toxicon. 1992;30:105–110. doi: 10.1016/0041-0101(92)90506-z. [DOI] [PubMed] [Google Scholar]
- Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- Cestele S, Qu Y, Rogers JC, Rochat H, Scheuer T, Catterall WA. Voltage sensor-trapping: enhanced activation of sodium channels by beta-scorpion toxin bound to the S3–S4 loop in domain II. Neuron. 1998;21:919–931. doi: 10.1016/s0896-6273(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Cestele S, Scheuer T, Mantegazza M, Rochat H, Catterall WA. Neutralization of gating charges in domain II of the sodium channel alpha subunit enhances voltage-sensor trapping by a beta-scorpion toxin. J Gen Physiol. 2001;118:291–302. doi: 10.1085/jgp.118.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Karbat I, Gilles N, Froy O, Corzo G, Angelovici R, Gordon D, Gurevitz M. Dissection of the functional surface of an anti-insect excitatory toxin illuminates a putative "hot spot" common to all scorpion beta-toxins affecting Na+ channels. J Biol Chem. 2004;279:8206–8211. doi: 10.1074/jbc.M307531200. [DOI] [PubMed] [Google Scholar]
- Cohen L, Karbat I, Gilles N, Ilan N, Benveniste M, Gordon D, Gurevitz M. Common features in the functional surface of scorpion beta-toxins and elements that confer specificity for insect and mammalian voltage-gated sodium channels. J Biol Chem. 2005;280:5045–5053. doi: 10.1074/jbc.M408427200. [DOI] [PubMed] [Google Scholar]
- Couraud F, Jover E, Dubois JM, Rochat H. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon. 1982;20:9–16. doi: 10.1016/0041-0101(82)90138-6. [DOI] [PubMed] [Google Scholar]
- De Lima ME, Martin-Eauclaire MF, Hue B, Loret E, Diniz CR, Rochat H. On the binding of two scorpion toxins to the central nervous system of the cockroach Periplaneta americana. Insect Biochem. 1989;19:413–422. [Google Scholar]
- De Lima ME, Martin MF, Diniz CR, Rochat H. Tityus serrulatus toxin VII bears pharmacological properties of both beta-toxin and insect toxin from scorpion venoms. Biochem Biophys Res Commun. 1986;139:296–302. doi: 10.1016/s0006-291x(86)80112-7. [DOI] [PubMed] [Google Scholar]
- del Rio-Portilla F, Hernandez-Marin E, Pimienta G, Coronas FV, Zamudio FZ, Rodriguez de la Vega RC, Wanke E, Possani LD. NMR solution structure of Cn12, a novel peptide from the Mexican scorpion Centruroides noxius with a typical beta-toxin sequence but with alpha-like physiological activity. Eur J Biochem. 2004;271:2504–2516. doi: 10.1111/j.1432-1033.2004.04181.x. [DOI] [PubMed] [Google Scholar]
- Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- French RJ, Terlau H. Sodium channel toxins--receptor targeting and therapeutic potential. Curr Med Chem. 2004;11:3053–3064. doi: 10.2174/0929867043363866. [DOI] [PubMed] [Google Scholar]
- Froy O, Gurevitz M. New insight on scorpion divergence inferred from comparative analysis of toxin structure, pharmacology and distribution. Toxicon. 2003;42:549–555. doi: 10.1016/s0041-0101(03)00236-8. [DOI] [PubMed] [Google Scholar]
- Froy O, Zilberberg N, Gordon D, Turkov M, Gilles N, Stankiewicz M, Pelhate M, Loret E, Oren DA, Shaanan B, Gurevitz M. The putative bioactive surface of insect-selective scorpion excitatory neurotoxins. J Biol Chem. 1999;274:5769–5776. doi: 10.1074/jbc.274.9.5769. [DOI] [PubMed] [Google Scholar]
- Gordon D, Ilan N, Zilberberg N, Gilles N, Urbach D, Cohen L, Karbat I, Froy O, Gaathon A, Kallen RG, Benveniste M, Gurevitz M. An 'Old World' scorpion beta-toxin that recognizes both insect and mammalian sodium channels. Eur J Biochem. 2003;270:2663–2670. doi: 10.1046/j.1432-1033.2003.03643.x. [DOI] [PubMed] [Google Scholar]
- Gordon D, Martin-Eauclaire MF, Cestele S, Kopeyan C, Carlier E, Khalifa RB, Pelhate M, Rochat H. Scorpion toxins affecting sodium current inactivation bind to distinct homologous receptor sites on rat brain and insect sodium channels. J Biol Chem. 1996;271:8034–8045. doi: 10.1074/jbc.271.14.8034. [DOI] [PubMed] [Google Scholar]
- Gordon D, Savarin P, Gurevitz M, Zinn-Justin S. Functional anatomy of scorpion toxins affecting sodium channels. J Toxicol Toxin Rev. 1998;17:131–159. [Google Scholar]
- Gurevitz M, Gordon D, Ben-Natan S, Turkov M, Froy O. Diversification of neurotoxins by C-tail 'wiggling': a scorpion recipe for survival. Faseb J. 2001;15:1201–1205. doi: 10.1096/fj.00-0571hyp. [DOI] [PubMed] [Google Scholar]
- Hassani O, Mansuelle P, Cestele S, Bourdeaux M, Rochat H, Sampieri F. Role of lysine and tryptophan residues in the biological activity of toxin VII (Ts gamma) from the scorpion Tityus serrulatus. Eur J Biochem. 1999;260:76–86. doi: 10.1046/j.1432-1327.1999.00152.x. [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- Karbat I, Cohen L, Gilles N, Gordon D, Gurevitz M. Conversion of a scorpion toxin agonist into an antagonist highlights an acidic residue involved in voltage sensor trapping during activation of neuronal Na+ channels. Faseb J. 2004;18:683–689. doi: 10.1096/fj.03-0733com. [DOI] [PubMed] [Google Scholar]
- Legros C, Ceard B, Vacher H, Marchot P, Bougis PE, Martin-Eauclaire MF. Expression of the standard scorpion alpha-toxin AaH II and AaH II mutants leading to the identification of some key bioactive elements. Biochim Biophys Acta. 2005;1723:91–99. doi: 10.1016/j.bbagen.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Cestele S. Beta-scorpion toxin effects suggest electrostatic interactions in domain II of voltage-dependent sodium channels. J Physiol. 2005;568:13–30. doi: 10.1113/jphysiol.2005.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Eauclaire MF, Ceard B, Bosmans F, Rosso JP, Tytgat J, Bougis PE. New "Birtoxin analogs" from Androctonus australis venom. Biochem Biophys Res Commun. 2005;333:524–530. doi: 10.1016/j.bbrc.2005.05.148. [DOI] [PubMed] [Google Scholar]
- Martin MF, Garcia y Perez LG, el Ayeb M, Kopeyan C, Bechis G, Jover E, Rochat H. Purification and chemical and biological characterizations of seven toxins from the Mexican scorpion, Centruroides suffusus suffusus. J Biol Chem. 1987;262:4452–4459. [PubMed] [Google Scholar]
- Mejri T, Borchani L, Srairi-Abid N, Benkhalifa R, Cestele S, Regaya I, Karoui H, Pelhate M, Rochat H, El Ayeb M. BotIT6: a potent depressant insect toxin from Buthus occitanus tunetanus venom. Toxicon. 2003;41:163–171. doi: 10.1016/s0041-0101(02)00246-5. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Herrmann R, Jones AD, Hammock BD. A depressant insect-selective toxin analog from the venom of the scorpion Leiurus quinquestriatus hebraeus--purification and structure/function characterization. Eur J Biochem. 1998;254:44–49. doi: 10.1046/j.1432-1327.1998.2540044.x. [DOI] [PubMed] [Google Scholar]
- Noda M, Ikeda T, Kayano T, Suzuki H, Takeshima H, Kurasaki M, Takahashi H, Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986;320:188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Pelhate M, Zlotkin E. Actions of insect toxin and other toxins derived from the venom of the scorpion Androctonus australis on isolated giant axons of the cockroach (Periplaneta americana) J Exp Biol. 1982;97:67–77. doi: 10.1242/jeb.97.1.67. [DOI] [PubMed] [Google Scholar]
- Possani LD, Becerril B, Delepierre M, Tytgat J. Scorpion toxins specific for Na+-channels. Eur J Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Schiavon E, Sacco T, Cassulini RR, Currola G, Tempia F, Possani LD, Wanke E. Resurgent current and voltage sensor trapping enhanced activation by a beta-scorpion toxin solely in Nav1.6 channel. Significance in mice Purkinje neurons. J Biol Chem. 2006;281(29):20326–20337. doi: 10.1074/jbc.M600565200. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Reenan RA, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Van der Ploeg LH, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology. J Gen Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Bosmans F, Li C, Zhang Y, Wang DC, Tytgat J. Structural basis for the voltage-gated Na+ channel selectivity of the scorpion alpha-like toxin BmK M1. J Mol Biol. 2005;353:788–803. doi: 10.1016/j.jmb.2005.08.068. [DOI] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotkin E, Eitan M, Bindokas VP, Adams ME, Moyer M, Burkhart W, Fowler E. Functional duality and structural uniqueness of depressant insect-selective neurotoxins. Biochemistry. 1991;30:4814–4821. doi: 10.1021/bi00233a025. [DOI] [PubMed] [Google Scholar]
- Zlotkin E, Kadouri D, Gordon D, Pelhate M, Martin MF, Rochat H. An excitatory and a depressant insect toxin from scorpion venom both affect sodium conductance and possess a common binding site. Arch Biochem Biophys. 1985;240:877–887. doi: 10.1016/0003-9861(85)90098-0. [DOI] [PubMed] [Google Scholar]
- Zuo XP, He HQ, He M, Liu ZR, Xu Q, Ye JG, Ji YH. Comparative pharmacology and cloning of two novel arachnid sodium channels: Exploring the adaptive insensitivity of scorpion to its toxins. FEBS Lett. 2006;580:4508–4514. doi: 10.1016/j.febslet.2006.07.024. [DOI] [PubMed] [Google Scholar]


