Abstract
Endogenous opioids within the central nervous system are postulated to mediate hedonic aspects of feeding behavior. To identify the relevant endogenous opioid receptor ligands, mice lacking one or two of the opioid peptide families β-endorphin, enkephalins or dynorphins were tested for sucrose preference in a two-bottle free-choice drinking paradigm under drug-naïve conditions and following treatment with an opioid antagonist (1 mg/kg naloxone i.p.) or saline. Basal sucrose consumption was unaltered in all of the knockout genotypes compared to their congenic wild-type C57BL/6 littermates during 0.5 and 6 h access to a bottle containing 2, 4, 8, or 16% sucrose and a second bottle containing water. Moreover, all mutant genotypes and wildtype mice exhibited a similar compensatory decrease in overnight food intake following the extra caloric load from 6 h sucrose access. Although these basal responses to sucrose were unaffected by the knockout genotypes, naloxone reduced sucrose consumption by 50% compared to saline treatment during the first 0.5 h in wild-type and β-endorphin knockout mice, but had no effect in enkephalin knockouts, β-endorphin and enkephalin double knockouts, or dynorphin knockouts. These data suggest that naloxone reduces sucrose consumption in wild-type mice by blocking endogenous enkephalin and/or dynorphin signaling, but not β-endorphin. Dynorphin knockouts in the current study had bar-pressing responses for a palatable food reinforcer in an operant procedure under free-feeding conditions similar to wild-type mice while we found in a previous study that β-endorphin and enkephalin knockout mice had reduced motivation to respond (Hayward, et al., 2002). We conclude from these studies directly comparing three strains of opioid peptide knockout mice that enkephalin and dynorphin can modulate sucrose preference but are not necessary to support sucrose consumption. However, dynorphin was not necessary to support wildtype levels of operant responding suggesting that only enkephalin and β-endorphin modulate conditioned food reinforcement.
Keywords: Operant behavior, β-endorphin, enkephalin, dynorphin, knockout mice, ingestive behavior
The plentiful access to calorically dense and highly palatable food choices is a likely contributor to the current obesity epidemic. How ingestion of palatable food is regulated is of great interest to those studying ingestive behavior as well as those studying basic mechanisms of motivation and reward. One of the first neurochemical systems implicated in the hedonics of feeding and obesity was the endogenous opioid system (Belluzzi and Stein, 1977; Mandenoff, et al., 1982; Margules, et al., 1978). In general, opioid antagonists are anorexigenic while opioid agonists are orexigenic [reviewed in (Bodnar, 2004; Glass, et al., 1999)]. The current view is that the endogenous opioid system is predominantly involved in the hedonics of feeding rather than in the regulation of energy homeostasis. This hypothesis is based on a number of studies showing that opioids are more efficacious in animals with ad lib access to food than in animals with restricted access to food and that opioids more potently modulate ingestion of highly palatable foods (Levine, et al., 1995)[reviewed in (Levine, et al., 2003)]. However, it is unlikely that there is a complete separation between energy homeostatic and hedonic processes in feeding since there is significant overlap between systems that control these two processes (Saper, et al., 2002).
Antagonists to the opioid receptors have been instrumental in demonstrating that the endogenous opioid system modulates feeding behavior. Sub-type selective antagonists have demonstrated that each of the three opioid receptors (MOP [mu opioid receptor], DOP [delta opioid receptor], and KOP [kappa opioid receptor]) can modulate intake of solid chow or flavored water depending upon the tastant, route of drug administration and the specific behavioral test (reviewed in (Bodnar, 1996). However, this pharmacological approach has not been able to identify which endogenous opioid peptide is blocked because the endogenous opioids are capable of binding multiple opioid receptor subtypes and multiple opioids bind each receptor subtype. Specifically, β-endorphin binds both the mu and delta receptor with nearly equal affinity, enkephalin binds the delta receptor with the highest affinity though its affinity to the mu receptor is within physiological ranges and dynorphin, which has the highest affinity to the kappa receptor can also bind the mu and delta receptor within physiological ranges (Raynor, et al., 1994). Consequently, it has been difficult to determine which endogenous opioids are involved in the many behaviors that are modulated by the endogenous opioid system even though the biologically relevant receptors can be identifed via antagonists.
To identify the specific endogenous opioid peptides involved in the hedonics of feeding we tested mice lacking any one of the endogenous opioids as a result of targeted mutations in the genes encoding the peptides (i.e., gene “knockout”). We previously showed that mice lacking β-endorphin, enkephalin or both peptides had reduced operant responding for food reinforcers (Hayward, et al., 2002) under a progressive ratio (PR), which was originally developed as a procedure to measure the magnitude of reward (Hodos, 1961). This schedule of reinforcement has been used by us and others as a measure of the hedonics of feeding by measuring the incentive value of food reinforcers (Brennan, et al., 2001; Feifel and Vaccarino, 1990; Hayward and Low, 2001; Hayward, et al., 2002; Rudski, et al., 1994). Here we tested consummatory behavior, independent of appetitive behaviors, by analyzing sucrose consumption in a two-bottle free choice procedure at multiple time points. To broaden our studies with this aspect of ingestive behavior we used a strain of dynorphin knockout mice in addition to the previously described enkephalin and β-endorphin knockout mice.
Experimental Procedures
Animals
The β-endorphin-deficient mice were previously described and shown to specifically lack the β-endorphin peptide without alteration in adrenocorticotropin hormone or α-melanocyte stimulating hormone levels (Rubinstein, et al., 1996) and the enkephalin-deficient mice were previously described and shown to specifically lack all enkephalins (Nitsche, et al., 2002; Ragnauth, et al., 2001). To generate mice for the current studies, the two mutant gene alleles on chromosomes 13 and 4, respectively, were backcrossed simultaneously onto the C57BL/6J genetic background for 11 generations (N11) using double heterozygous mice (Enk+/− End+/−) and alternating sexes for each generation. The four genotypes of mice used in the current study were wildtype (Enk+/+End+/+), β-endorphin-deficient (Enk+/+End−/−), enkephalin-deficient (Enk−/− End+/+) and double knockout (Enk−/− End−/−) mice and were generated by breeding Enk+/− End+/− mice. This breeding strategy was employed so that all subjects used were potential littermates and were raised by parents with identical genotypes. Due to the low frequency of homozygous genotypes generated by this breeding strategy (1/16) there were significant differences in age among the subjects in some of the studies (details are given in Results section) but the genotypes and sexes were evenly balanced across ages. Genotyping was performed by PCR as previously described (Hayward, et al., 2002).
The dynorphin knockout mice (Dyn−/−) were previously described (Sharifi, et al., 2001). The breeding stock used here was acquired from The Jackson Laboratory (Stock #004272F, Strain B6.129S4-Pdyntm1Ute) backcrossed to the C57BL/6J background for 8 generations (N8). We subsequently backcrossed the mutant Dyn allele for one more generation to the C57BL/6J background (N9) and then interbred heterozygous offspring to produce the mice used here. Dynorphin wildtype and knockout mice used in the operant behavior experiments were not used in the two-bottle free-choice experiment although they were of the same generation of backcrossing.
Both male and female mice were used in all studies unless otherwise noted and sex was included as a factor in the overall statistical analyses. All mice were group housed in a 12:12 light: dark cycle until assayed. All procedures were approved by the Institutional Animal Care and Use Committee and followed the Public Health Service guidelines for the humane care and use of experimental animals (NIH Publications No. 80-23).
Drug
Naloxone (Sigma, St. Louis, Mo) was dissolved in sterile 0.9% physiological saline at a concentration of 0.1 mg/ml and mice were injected with a volume of 10ml/kg body weight. All drug injections were intraperitoneal (i.p.).
Food
Daily maintenance food was Rodent Chow Diet #5001 (PMI Feeds Inc, St. Louis MO) [4.5% fat (1.16% linoleic acid, 0.07% linolenic acid, <0.01% arachadonic acid, 0.26% omega-3 fatty acids, 1.5% total saturated fatty acids, 1.58% total monounsaturated fatty acids), 23% protein and 6% fiber; 3.34 g/kcal; Calories provided by 28.0% protein, 12.1% fat, and 59.8% carbohydrates (31.9% starch, 0.23% glucose, 0.3% fructose, 3.68% sucrose, 1.67% lactose)]. The 20 mg reinforcement pellets were custom made (Research Diets, Inc., New Brunswick, NJ), and composed of PicoLab Mouse Diet #5015 (PMI Feeds Inc.) [11% Fat (2.45% linoleic acid, 0.18% linolenic acid, 0.03% arachadonic acid, 0.18% omega-3 fatty acids, 3.7% total saturated fatty acids, 4.28% total monounsaturated fatty acids), 17% Protein, and 3% Fiber; 3.83 kcal/gm; Calories provided by 18.3% protein, 25.8% fat and 55.9% carbohydrates (39.4% starch, 0.16% glucose, 0.15% fructose, 0.7% sucrose and 2.7% lactose)].
Two bottle free choice sucrose drinking
Individually housed mice were presented with two graduated cylinders (10 ml pipettes) equipped with lixit tubes in their home cages. Tap water was first presented in both bottles for two days to acclimate the subjects to the bottles.
The two-bottle free choice paradigm consisted of four-day cycles with mice successively having access to tap water versus sucrose (2%, 4%, 8%, and 16%). All subjects experienced the same concentration of sucrose at the same time in an increasing order so that no subjects developed a preference for a higher concentration of sucrose before being presented with a lower concentration. Drinking tube positions were reversed every two days to control for side preferences and the second day’s reading on each side was used. Two measurements of fluid consumption were averaged for each tastant concentration. Thus a four-day cycle was used to get measurements for each sucrose concentration. Sucrose consumption was measured after 0.5 h and 6 h of access beginning 2 h after light onset. Measurements of consumption were made to the nearest 0.1 ml. Food consumption was also measured daily by weighing the amount of food remaining in each cage and subtracting from the previous day’s food weight. The same subjects that were tested for basal sucrose consumption were subsequently tested for sucrose consumption following naloxone treatment. Naloxone was injected 15 minutes before sucrose tubes were introduced to the cage in the same order of increasing sucrose concentrations as before. Initially a four-day cycle of saline injections was performed with water presentation to acclimate subjects to the injection procedure. The same four-day cycle was used for each sucrose concentration with one test day being treated with saline and the other test day being treated with naloxone with the drug order and drinking tube position balanced among the groups. Thus mice were injected twice with saline and twice with naloxone for each sucrose concentration but only the second day for each drinking tube position was recorded for analysis.
Operant Behavior
I. Apparatus
Four 16 x 14 x 13 cm and four 22 x 18 x 13 cm modular operant chambers (Med Associates Inc., Georgia, VT) designed specifically for use by mice were used in these experiments. Each chamber consisted of a metal grid floor with Plexiglas walls and ceiling contained in a sound attenuated and ventilated cabinet. At the beginning of a session a house light was illuminated and the subjects were presented with two retractable ultrasensitive levers, one on either side of a receptacle into which pellets were dispensed from an elevated hopper. Completion of the instrumental contingency on the active lever dispensed a single food pellet, turned off the house light and turned on a dim white LED stimulus light above the pellet receptacle for 5 sec. following which the house light turned back on and the stimulus LED turned off. The levers remained accessible throughout the session until it ended, at which time they retracted at the same time that the house light was turned off. The positions of the active and inactive levers were counterbalanced between mice but remained consistent between sessions for each mouse.
II. Training & Experimental Procedure
All mice experienced 5 days of restricted food access to maintain each animal between 75–85% of their original body weight (approximately 2 h of access). To avoid food neophobia, 10% of the daily food access included the reinforcement pellets. This restricted daily access to food was enforced during the following steps 2–3.
Seven sessions under a fixed ratio (FR) 1 schedule with the active and inactive lever available and primed with a single food reinforcer prior to each 0.5 h session. Three animals did not meet a criterion of at least 5 lever presses on the active lever after seven shaping sessions. These three mice had one additional 6 hr session by which time all of them met the performance criterion so they were included in subsequent trials. All three of these mice were female, one was Dyn+/+ and the other two were Dyn−/−.
Three sessions under an FR5 (0.5 h sessions)
Food was returned to the home cage and ad lib feeding was reestablished for three days with no operant sessions.
Five sessions under an FR5 while feeding ad lib (0.5 h sessions).
Ten sessions under a PR3 while feeding ad lib. The PR3 schedule increased the number of lever presses by three for each subsequent reinforcer, i.e., the first reinforcer required 3 lever presses, the second reinforcer required 6, the third reinforcer required 9, etc. (3n+3 where n=number of reinforcers received). The breakpoint was defined as the last ratio completed before 15 minutes elapsed without the mouse receiving a reinforcer; i.e., subjects had 15 minutes to complete each ratio level. Thus, the duration of PR sessions varied substantially and were governed by the frequency of responding but averaged approximately 1 h. Experimental sessions were conducted once each day for each animal and took place between 10:00 and 18:00 during the light phase. Subjects were always tested in the same chamber for all operant studies.
Experimental Design and Statistical Analysis
The studies employed mixed factor analysis of variance (ANOVAs with both within-subjects [repeated measures] and between groups factors). The factors for the experiments were the following. Sucrose access for 0.5 h and 6 h in two-bottle free-choice tests without drug treatment: (Genotype X Sex X Sucrose concentration) with genotype and sex treated as between groups factors and sucrose concentration treated as a within subjects factor. Sucrose access for 0.5 h in two-bottle free-choice tests with naloxone treatment: (Genotype X Sex X Sucrose concentration X Drug) with genotype and sex treated as between group factors and sucrose concentration and drug treatment as within subjects factors. Body weight in two-bottle free-choice test: (Genotype X Sex) with both treated as between group factors. Total caloric intake (food plus sucrose) during two-bottle free-choice test: (Genotype X Sex X Sucrose concentration X drug) with genotype and sex treated as between group factors and sucrose concentration and drug treatment treated as within subjects factors. The two-bottle free-choice sucrose drinking data were only reported as the volume of sucrose consumed and preference ratios were not calculated since none of the animals consumed measurable amounts of water at any of the time points measured. Dynorphin wildtype and mutant mice operant behavior training: (Genotype X Sex X training day) with genotype and sex treated as between groups factors and training day treated as a within subjects factor. Dynorphin wildtype and mutant mice operant responding for PR3 breakpoints: (Genotype X Sex X test day) with genotype and sex treated as between groups factors and test day treated as a within subjects factor for a total of 10 test days. Post-hoc simple main effect analyses were conducted where allowed and pairwise comparisons were performed with Fisher’s PLSD when the initial P value was significant. All data were analyzed with StatView 5 for Macintosh (SAS Institute Inc., Cary, NC). Significance was set at P<0.05.
Results
Sucrose consumption in a two-bottle free-choice procedure by opioid knockout mice
Male and female Enk+/+End+/+, Enk+/+End−/−, Enk−/− End+/+ and Enk−/− End−/− mice 12–32 weeks of age (n=8 for each genotype and sex) were tested for sucrose consumption in a two-bottle free-choice procedure. After 0.5 h of access there were no main effects of genotype (F3,56=0.1.97, P=n.s.) on the amount of sucrose consumed (Figure 1A). A main effect of sex was detected (F1,56=28.80, P<0.0001) where female intake was greater but no interaction between sex and genotype was detected. There was a main effect of sucrose concentration (F3,168=133.37, P<0.0001) but there were no significant interactions between this factor and either genotype or sex. Similarly, after 6 h of access there were no main effects of genotype (F3,56=1.22, P=n.s.) where female intake was greater but there was a main effect of sex (F1,56=21.49, P<0.0001) on sucrose consumption (Figure 1B). There was a main effect of sucrose concentration (F3,169=221.76, P<0.0001) but there were no significant interactions between this factor and either genotype or sex.
Figure 1.
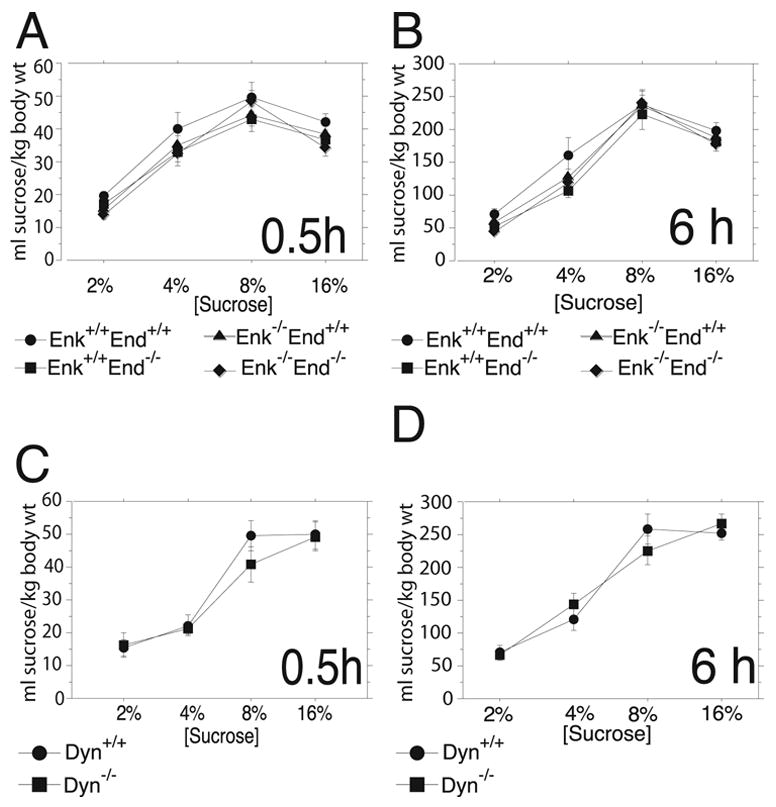
Two-bottle free-choice drinking for four sucrose concentrations by opioid peptide knockout mice, normalized for body weight. (A.) The volume of sucrose consumed/kg body weight (means ± SEM) after 0.5 h of access by Enk+/+End+/+, Enk+/+End−/−, Enk−/− End+/+, and Enk−/− End−/− mice did not differ among genotypes across four concentrations of sucrose. (B.) The volume of sucrose consumed/kg body weight (means ± SEM) after 6 h of access by Enk+/+End+/+, Enk+/+End−/−, Enk−/− End+/+, and Enk−/− End−/− mice did not differ among genotypes across four concentrations of sucrose. (C.) The volume of sucrose consumed/kg body weight (means ± SEM) after 0.5 h of access by Dyn+/+ and Dyn−/− mice did not differ between genotypes across four concentrations of sucrose. (D.) The volume of sucrose consumed/kg body weight (means ± SEM) after 6 h of access by Dyn+/+ and Dyn−/− mice did not differ between genotypes across four concentrations of sucrose.
Male and female Dyn+/+ and Dyn−/− mice 5–7 weeks of age (n=6 for female Dyn+/+ and Dyn−/−, n=11 for male Dyn+/+ and Dyn−/−) were tested for sucrose consumption after 0.5 h of access (Figure 1C). There were no main effects of genotype (F1,30=0.17, P=n.s.) or sex (F1,30=1.14, P=n.s.). There was a main effect of sucrose concentration (F3,90=61.09, P<0.0001) but no significant interactions between this factor and either genotype or sex. Similarly, after 6 h of access there were no main effects of genotype (F1,30=0.20, P=n.s.) but there was a main effect of sex (F1,30=12.54, P=0.001) on sucrose consumption (Figure 1D) where female intake was greater. There was a main effect of sucrose concentration (F3,90=71.15, P<0.0001) but there were no significant interactions between this factor and either genotype or sex. The ages of the two groups of mice (β-endorphin/enkephalin knockouts and dynorphin knockouts) were significantly different (see Methods) so the studies were analyzed separately. Nonetheless, these experiments suggested that all of the mutant genotypes consumed similar amounts of sucrose as their corresponding wildtypes following two different periods of access.
The same male and female Enk+/+End+/+, Enk+/+End−/−, Enk−/− End+/+ and Enk−/− End−/− mice used previously without a drug treatment were then tested with an i.p. injection of saline or 1 mg/kg naloxone 15 min before access to the sucrose and water containing bottles. After 0.5 h of access there was a main effect of genotype (F3,56=3.19, P=0.031) and a main effect of sex (F1,56=12.22, P=0.0009.) on sucrose consumption (Figure 2A–D) where females consumed more sucrose but there were no significant interactions between sex and the other factors. A main effect of the drug treatment was also detected (F1,56=42.30, P<0.0001). Importantly, a significant interaction between genotype and the drug treatment (F3,56=6.82, P=0.0005) was detected, indicating that the drug did not alter sucrose consumption identically among the four genotypes. These results are illustrated in Figure 2 where naloxone significantly decreased sucrose consumption by Enk+/+End+/+ (Figure 2A) and Enk+/+End−/− mice (Figure 2B) at higher sucrose concentrations but not by Enk−/− End+/+ (Figure 2C) or Enk−/− End−/− mice (Figure 2D). A post-hoc analysis supported this finding (Figure 2A–B). A main effect of sucrose concentration was detected (F3,168=79.59, P<0.0001) as well as an interaction between sucrose concentration and the drug treatment (F3,168=3.33, P=0.02) indicating that the drug treatment was not equally effective at all sucrose concentrations. No other interactions were significant. These results demonstrated that enkephalin was necessary for a naloxone-mediated decrease in sucrose consumption. After 6 h of access to sucrose and water bottles there were no detectable effects of naloxone in any of the genotypes (data not shown).
Figure 2.
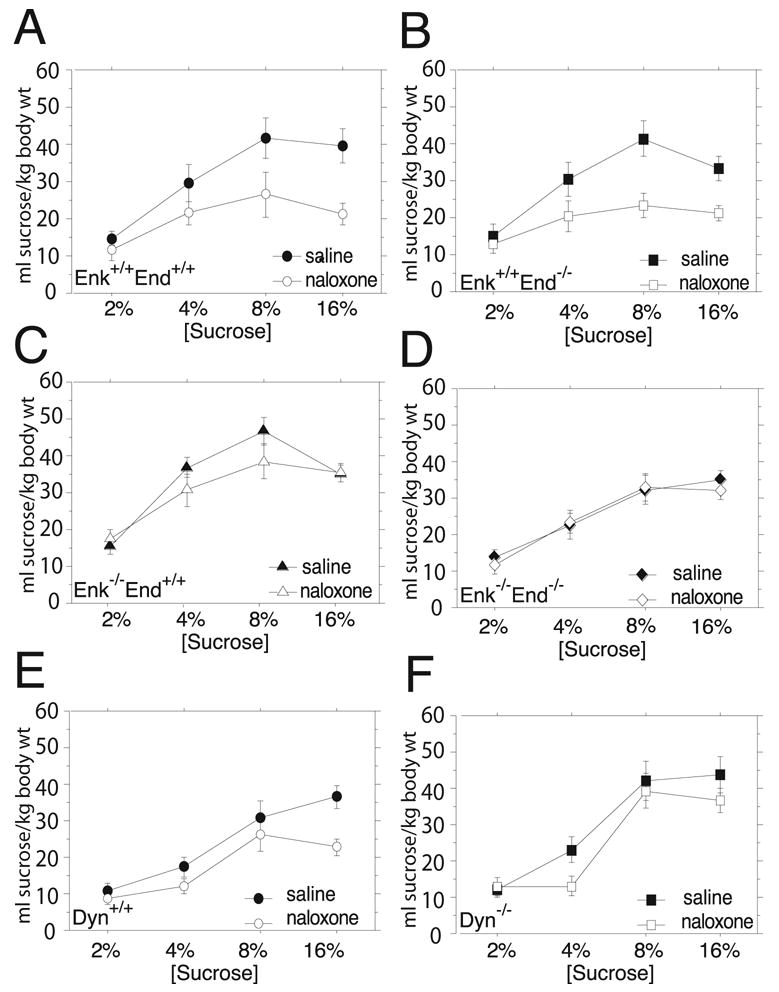
The influence of naloxone on two-bottle free-choice drinking for four sucrose concentrations by opioid peptide knockout mice normalized for body weight. Mice were treated with an i.p. injection of saline or 1 mg/kg of naloxone and the volume of sucrose consumed after 0.5 h was measured. All data are expressed as mean volume/kg body weight (± SEM). (A.) Naloxone significantly reduced consumption of 8% and 16% sucrose by Enk+/+End+/+ mice. (B.) Naloxone significantly reduced 4%, 8%, and 16% sucrose consumption by Enk+/+End−/− mice. (C.) Naloxone did not reduce sucrose consumption at any concentration in Enk−/− End+/+mice. (D.) Naloxone did not reduce sucrose consumption at any concentration in Enk−/− End−/− mice. (E.) Naloxone significantly reduced 16% sucrose consumption by Dyn+/+ mice. (F.) Naloxone did not significantly reduce sucrose consumption at any concentration in Dyn−/− mice. * P<0.05 by Fisher’s PLSD post hoc test on drug treatment effect at one sucrose concentration.
The same male and female Dyn+/+ and Dyn−/− mice used previously without a drug treatment were also tested with an i.p. injection of saline or 1 mg/kg naloxone 15 min before access to the sucrose and water containing bottles. After 0.5 h of access there was a main effect of genotype (F1,30=8.87, P=0.006) and sex (F1,30=8.22, P=0.008.) on sucrose consumption (Figure 2E–F). A main effect of the drug treatment was detected (F1,30=10.09, P=0.003). Although a significant interaction between drug and genotype was not detected (F1,30=0.23, P=n.s) a planned post-hoc analysis determined that naloxone significantly reduced sucrose consumption in Dyn+/+ mice at the highest concentration of sucrose (Figure 2E). The lack of a significant interaction was likely a result of naloxone’s effect on sucrose consumption being significant at only one concentration in Dyn+/+ mice (Figure 2E) whereas in the β-endorphin/enkephalin mutant mouse group naloxone significantly reduced sucrose consumption at two concentrations in Enk+/+End+/+ mice (Figure 2A) and three concentrations in Enk+/+End−/− (Figure 2B). A main effect of sucrose concentration was also detected in the study with Dyn+/+ and Dyn−/− mice (F3,90=54.47, P<0.0001) but no other interactions were detected. These results demonstrated that dynorphin was also necessary for a naloxone-mediated decrease in sucrose consumption. After 6 h of access to sucrose and water bottles the effects of naloxone were not detectable in any of the genotypes (data not shown).
Body weights
The male and female Enk+/+End+/+, Enk+/+End−/−, Enk−/− End+/+ and Enk−/− End−/− mice used in the two-bottle free-choice tests were weighed at the beginning of the experiment. Consistent with previous observations (Appleyard, et al., 2003) the Enk+/+End−/− males and females were significantly heavier than Enk+/+End+/+male and females, respectively (Figure 3A). Interestingly, Enk−/− End−/− male and females were also heavier than Enk+/+End+/+ males and females, respectively. This phenotype was likely the result of the loss of β-endorphin since the loss of enkephalin had no effect on body weight (Figure 3A). These results are supported by the ANOVA analysis, which found a main effect of genotype (F3,56=19.82, P<0.0001) and sex (F1,56=346.58, P<0.0001). We observed that the obese phenotype in the male Enk+/+End−/− and Enk−/− End−/− was greater than in the females (Male Enk+/+End−/− were 15% heavier than Enk+/+End+/+; Male Enk−/− End−/− were 18% heavier than Enk+/+End+/+; Female Enk+/+End−/− were 6% heavier than Enk+/+End+/+; Female Enk−/− End−/− were 8% heavier than Enk+/+End+/+). The finding that the obese phenotype was greater in the Enk+/+End−/− and Enk−/− End−/− males than in the females was supported by a significant interaction between genotype and sex (F3,56=3.59, P=0.019). The male and female Dyn+/+ and Dyn−/− mice used in the two-bottle free-choice tests were weighed at the beginning of the experiment. Dyn−/− male and female body weights did not differ from their respective Dyn+/+ counterparts (Figure 3B). A main effect of sex was detected (F1,30=58.29, P<0.0001) but not genotype (F1,30=1.37, P=n.s) or an interaction between the two factors (F1,30=0.04, P=n.s.). Overall this group weighed much less than the subjects in the β-endorphin/enkephalin mutant mouse group most likely because they were significantly younger.
Figure 3.
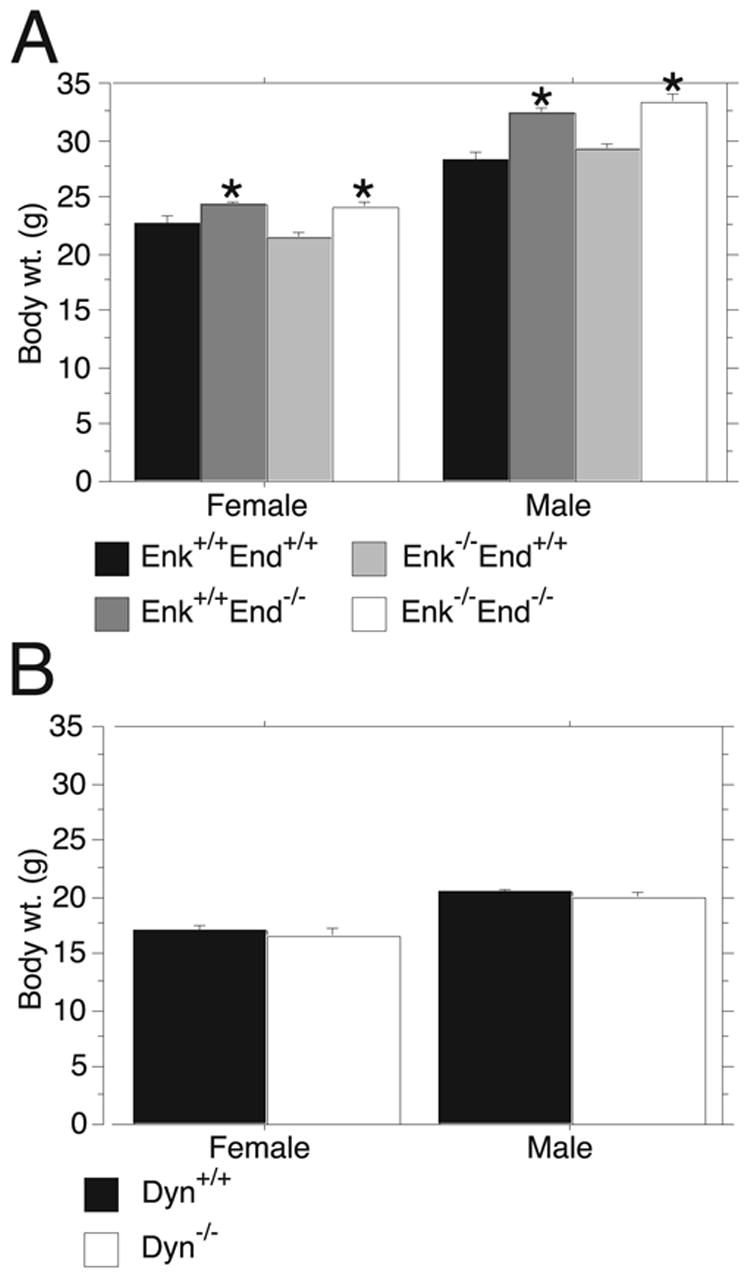
Body weight of the opioid peptide knockout mice in the two-bottle free-choice tests. Mice were weighed at the beginning of the study and mean body weights are given (± SEM). (A.) Female and Male Enk+/+End−/− and Enk−/− End−/− were heavier than their respective wildtypes. *P<0.05 by Fisher’s PLSD post hoc compared to the Enk+/+End+/+. (B.) Female and Male Dyn+/+ and Dyn−/− body weights did not differ between genotypes but differed between sex.
Food eaten during the two-bottle free-choice test
The amount of food eaten overnight (24h) by the male and female Enk+/+End+/+, Enk+/+End−/−, Enk−/− End+/+ and Enk−/− End−/− mice used in the two-bottle free-choice tests was measured during the first phase when no drug was administered. All genotypes decreased their overnight food intake as well as their overall caloric intake as they consumed more sucrose during the day in the two-bottle free-choice tests (Figure 4A–B) This observation was supported by the ANOVA analysis, which found a main effect of sucrose concentration (F3,168=19.85, P<0.0001) on the total caloric intake. No other main effects or significant interactions between any factors were detected. The amount of food eaten overnight (24 h) by the male and female Dyn+/+ and Dyn−/− mice used in the two-bottle free-choice tests not involving drug treatments were also measured throughout the experiment. Similar to the β-endorphin/enkephalin mutant mouse groups, both Dyn+/+ and Dyn−/− mice decreased their overnight food intake as well as their overall caloric intake as they consumed more sucrose during the day in the two-bottle free-choice tests (Figure 4C–D) This observation was supported by the ANOVA analysis which found a main effect of sucrose concentration (F3,90=13.10, P<0.0001) on the amount of food eaten. A main effect of sex was detected (F1,30=10.23, P=0.003), although no interaction between sucrose concentration and sex and any other factor was detected. These data indicated that the daily caloric intake of the mutant genotypes was similar to their wildtype counterparts.
Figure 4.
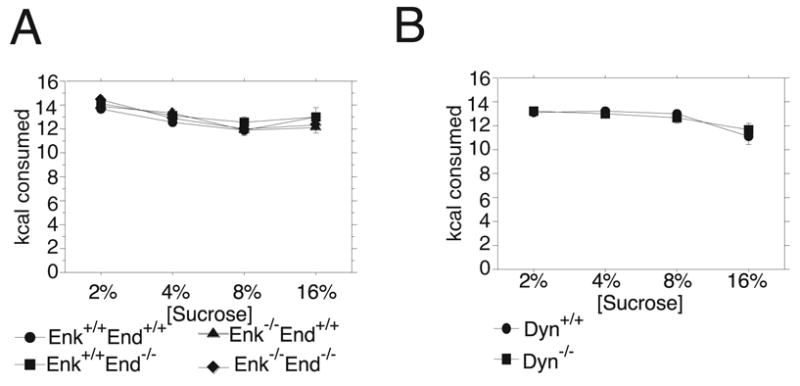
Total daily caloric intake during the two-bottle free-choice tests. Total calories from food and sucrose were calculated and expressed as mean kilocalories ± SEM for each 24 h period. All genotypes had similar daily caloric intake during the testing (A.) Enk+/+End+/+, Enk+/+End−/−, Enk−/− End+/+, and Enk−/− End−/− mice. (B.) Dyn+/+ and Dyn−/− mice.
Operant behavior of endogenous opioid knockout mice
Male and female Dyn+/+ and Dyn−/− mice 8–12 weeks of age (n=10 for each sex and genotype) were trained to bar press in order to receive palatable high-fat food pellets by initially restricting their access to food. After seven days of training all of the subjects had learned to press the correct lever in order to receive a reinforcer (Figure 5A). Analysis by ANOVA found no main effect of sex or genotype but a significant effect of training day (F6,216=16.35, P<0.0001). No significant interactions between any of these factors were detected. These results indicated that Dyn−/− mice had no deficit in acquisition of bar-pressing behavior in an operant chamber. Following restoration of ad lib feeding conditions mice were tested under a FR-5 for 5 days but no main effect of genotype was detected. Similarly, under a PR3 schedule of reinforcement, breakpoints were not significantly different between Dyn+/+ and Dyn−/− mice (Figure 5B) as was evidenced by a lack of a main effect of genotype or sex. There was also no significant difference across the repeated measure of the 10 test days under the PR3 and no significant interactions among any of these measures. These results indicated that Dyn−/− mice had the same motivation to bar press for reinforcers as Dyn+/+ mice.
Figure 5.
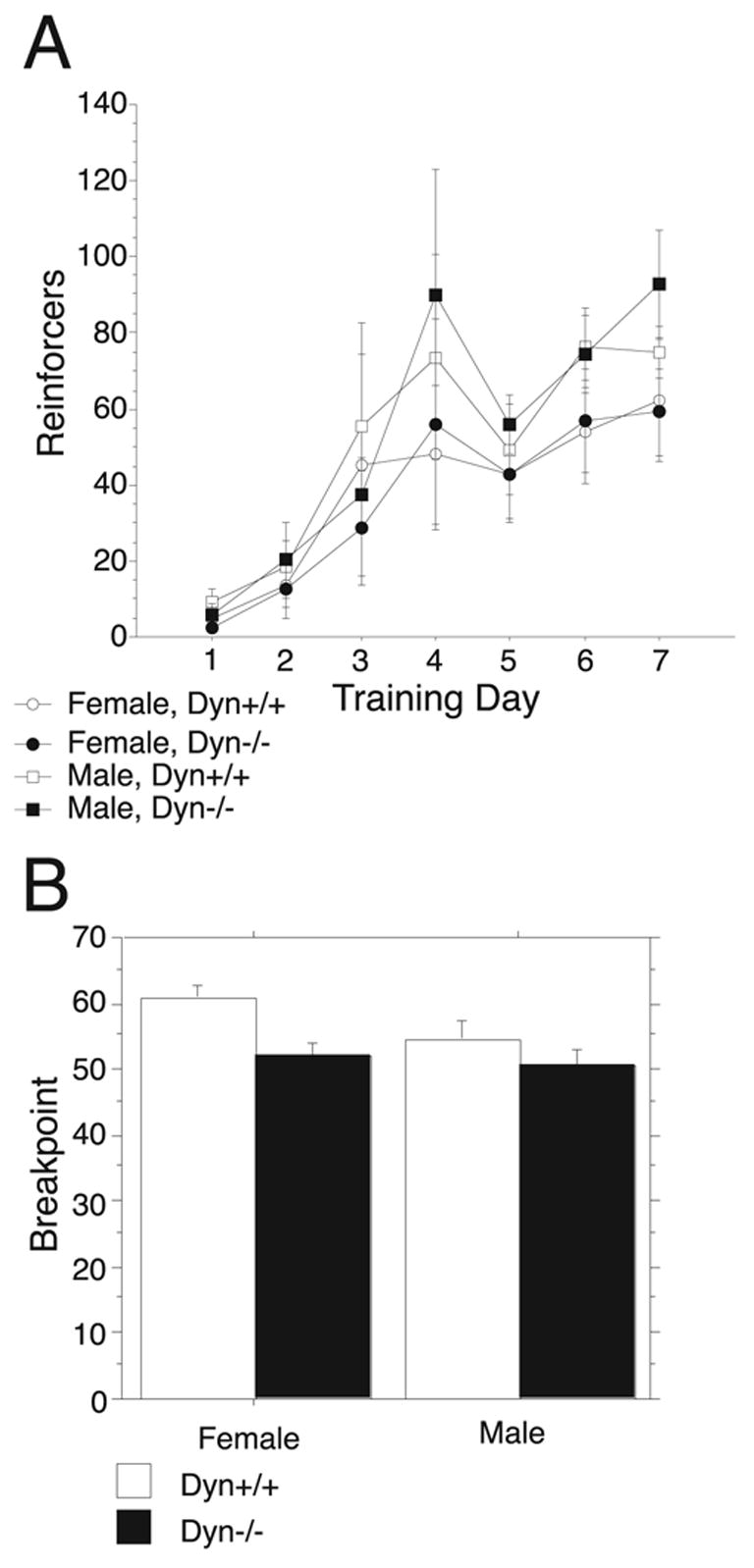
Operant responding for food reinforcers by opioid knockout mice. (A.) Dyn+/+ and Dyn−/− mice were trained to respond under an FR-1 for food pellets for seven days while maintained under restricted access to food in their home cage. Data are expressed as mean number of reinforcers earned (±SEM). Both sexes and genotypes learned to bar press to receive a food pellet at the same rate. (B.) After the completion of training Dyn+/+ and Dyn−/− mice were maintained under ad lib access to food and water in their home cage and tested daily for 10 d under a PR3 schedule of reinforcement. Data are expressed as mean breakpoint across all 10 d (±SEM). Breakpoints under this schedule were not significantly different between genotypes or sexes.
Discussion
We used mutant mice lacking each of the three endogenous opioid peptides and a behavioral paradigm of sucrose preference and consumption by two-bottle free choice drinking to identify which endogenous opioids modulate the hedonics of ingestive behavior (see summary in Table 1). All of the opioid peptide knockout mice strains drank similar amounts of sucrose and preferred higher concentrations of sucrose as their wildtype counterparts after 0.5 h and 6 h of access. However, naloxone decreased sucrose consumption after 0.5 h of access by wildtype and β-endorphin knockout mice but was completely ineffective in mice lacking enkephalin or dynorphin. We also confirmed previous findings that the β-endorphin knockout mice are obese (Appleyard, et al., 2003) but found that neither the enkephalin or dynorphin knockout mice had an obese phenotype, despite the fact that all genotypes appropriately reduced their chow intake in response to the increased caloric load provided by the sucrose solutions (Table 1). Previously, we found that Enk+/+End−/−, Enk−/− End+/+, and Enk−/− End−/− mice all had reduced breakpoints under a PR3, suggesting that the incentive value of food was reduced in these mice compared to wild type Enk+/+End+/+ (Hayward, et al., 2002). In the current study we demonstrated that dynorphin knockout mice had no alteration in their PR3 breakpoint under similar conditions (Table 1).
Table 1.
Summary of Phenotypes for Opioid Peptide Knockout Mice
| Phenotype | β –Endorphin KO | Enkephalin KO | Dynorphin KO |
|---|---|---|---|
| Body weight: ad libitum access to low fat chow | Increasedb | Unchanged | Unchanged |
| Basal sucrose consumption: Two- Bottle Free-Choice, 2%–16% sucrose | Unchangeda,c | Unchangeda,c | Unchanged |
| Compensatory reduction in caloric intake from chow during sucrose access | Unchanged | Unchanged | Unchanged |
| Reduction in sucrose consumption following acute naloxone treatment | Unchanged | Absent | Absent |
| Breakpoint: Operant responding under PR-3 for palatable food reinforcers | Decreasedc | Decreasedc | Unchanged |
The phenotypes for each mutant strain are listed in comparison to wild-type littermates on a uniform congenic C57BL/6 genetic background and are from the current study unless otherwise noted.
Appleyard, et al., 2003 and the current study.
Our current findings are in complete agreement with our previous study where we only tested male incipient congenics with 6 h of access to sucrose. Most studies that have demonstrated an opioid antagonist-mediated reduction in sucrose consumption have only used a brief period of intake (0.5 h – 2 h) and shown that this effect is short-lived. For that reason, we examined sucrose consumption during the first 0.5 h of access as well as after 6 h of access. Regardless of the time of access we found that sucrose consumption was unchanged in all of the mutant genotypes. Additionally, we also examined intake at 1 and 1.5 hours with the same results (data not shown). Previous studies have shown that preference for sugar appears to be highly conserved throughout evolution and largely controlled by the brainstem. For example, decerebrate rats will show preference for sugar water [reviewed in (Berridge, 1996)]. Nonetheless, a large number of studies have shown that blocking endogenous opioids with antagonists reduces sucrose consumption so we tested all of the mutant opioid mouse lines with naloxone to see if this antagonist still reduced sucrose consumption. We found that a dose of naloxone that reduced sucrose consumption in wildtype mice also reduced sucrose consumption in mice lacking β-endorphin. However, naloxone was completely without effect in mice lacking enkephalin or dynorphin. Although basal sucrose consumption was unchanged in all of the mutant genotypes, naloxone was no longer capable of decreasing sucrose consumption in enkephalin and dynorphin knockout mice. It would appear that the endogenous opioids were not necessary for maintaining sucrose consumption but when present, enkephalin and dynorphin can both potentially modulate the intake of sucrose.
Because previous studies have shown that opioid antagonists decrease sucrose intake we were surprised to find that all of the knockout mice, including the double β-endorphin/enkephalin knockouts, had no alteration in basal sucrose consumption. Rather than endogenous opioids being necessary for supporting normal levels of sucrose consumption, these results would suggest that when opioids are present changes in basal opioid activity can change sucrose consumption. Consistent with this hypothesis, a recent study suggested that enkephalin may mediate a tonic hedonic tone (Skoubis, et al., 2005). This also opens the possibility that consumption may not be specifically altered in the enkephalin and dynorphin knockout mice but rather a shift in a tonic hedonic tone, as suggested by experiments using conditioned place aversion to naloxone in enkephalin knockout mice as well as MOP receptor knockout mice (Skoubis, et al., 2005; Skoubis, et al., 2001). In fact, sucrose preference has been used as one method of measuring hedonics so the reduction in sucrose intake in response to naloxone treatment could be because the hedonic value of sucrose was reduced. However, it does not appear that this procedure describes an alteration in a basal hedonic tone because basal sucrose consumption was not altered in any of the knockouts. This could be interpreted as meaning that endogenous opioids do not set a basal hedonic tone, but rather modulate that hedonic tone which may be driven by a major neurotransmitter (e.g., dopamine).
The use of multiple sucrose concentrations allowed for a control in potential differences in threshold detection of sucrose and it seemed clear that in the drug naïve portion of the study there was no alteration in the threshold of sucrose detection. Additionally, this procedure allowed for a control in the naloxone treated subjects since confounding physiological responses to naloxone exist, including decreases in spontaneous locomotor activity (Hayward and Low, 2005) and potential dysphoric responses (reviewed in (Miotto, et al., 2002). Since naloxone did not alter intake of lower concentrations of sucrose it appears that the effects of naloxone on sucrose intake at higher concentrations was not due to a nonspecific decrease in locomotor activity or a general behavioral suppressive effect. The dose of naloxone used here was based on previous studies that conducted a dose response for naloxone (Cleary, et al., 1996). Higher doses of naloxone than we used here could have had more of an effect on general locomotor activity, as we have previously described (Hayward and Low, 2005). Additionally, high concentrations of naloxone could produce effects not specific to opioid receptor blockade. We chose to use a relatively nonspecific opioid antagonist here because the endogenous ligands are not highly specific to any one receptor. This design ensured that we would detect the activity of an opioid even if its effect on sucrose consumption was exerted through binding to multiple opioid receptors.
Similar to our previous studies, we found that both male and female β-endorphin knockout mice were heavier than wild-types (Appleyard, et al., 2003). In addition, we found that subjects lacking both β-endorphin and enkephalin have the same obese phenotype as mice lacking β-endorphin alone, despite the additional loss of enkephalin. Enkephalin knockouts alone as well as dynorphin knockouts weighed the same as their wildtype counterparts. Additionally, all of the genotypes reduced the amount of food eaten overnight while they were ingesting larger amounts of sucrose in the experiments during the day. This finding suggests that all of the opioid genotypes compensated for calories from the sucrose by decreasing their food intake.
It would appear to be a paradox that the loss of β-endorphin, which should stimulate feeding, actually results in hyperphagia and obesity. However, we previously demonstrated that the β-endorphin knockout males had less motivation to bar press for food reinforcers under a PR3 (Hayward, et al., 2002). We argue that these seemingly contradictory phenotypes are likely via independent pathways. For example the weight phenotype may be due to the loss of β-endorphin from the arcuate nucleus or nucleus of the solitary tract projecting to the paraventricular nucleus of the hypothalamus, while the operant behavior phenotype may be due to the loss of endorphinergic neurons modulating the mesoaccumbens dopaminergic pathway at the ventral tegmentum or the nucleus accumbens or from areas whose projections are known to modulate this circuit, such as the amygdala. Different brain regions are likely involved in appetitive and consummatory behaviors and the activity of β-endorphin in these different brain regions may result in the differing phenotypes observed. In support of this hypothesis, sucrose consumption was still modulated by endogenous opioids in the β-endorphin knockouts while it was not in mice lacking enkephalin or dynorphin, genotypes that lacked a weight phenotype. Thus, we did not find a direct correlation between the effects of individual endogenous opioids to modulate sucrose drinking or weight gain even though both measures involve consummatory behavior.
In our previous study on mice lacking β-endorphin and enkephalin we found that the motivation to work for reinforcers was reduced in the mutant subjects only when they had ad lib access to food in their home cage (Hayward, et al., 2002). These data suggested that the hedonics of food were altered rather than an alteration in energy homeostasis. For that reason we only tested the dynorphin knockouts under a PR3 while they were under that same ad lib food availability in the current study. A significant amount of work in the past has concluded that motivational states play an important role in opioid modulation of reward behaviors (reviewed in (Carr, 1996; Nader, et al., 1997)). Nonetheless, the possibility that dynorphin selectively modulates feeding under caloric restriction exists and could be addressed in future studies.
Previous studies have suggested that antagonists selective for the kappa receptor are effective at reducing intake of both sucrose and high-fat foods (Arjune and Bodnar, 1990; Beczkowska, et al., 1992). In the current study we used a highly palatable high-fat reinforcer which, in our experience, all mice prefer more than other types of reward pellets. Thus, it was likely that if there was even a small difference between Dyn−/− and Dyn+/+ then the high-fat pellet would be most likely to detect that difference. We did not directly examine operant behavior for sucrose reinforcement in the dynorphin knockouts so the possibility remains that the loss of this opioid could affect operant responding specifically for a sucrose reinforcer. Although the absence of an operant responding phenotype in the dynorphin knockout mice in this study was surprising, we do not think it was due to the relative palatability of the reinforcer since a highly preferred reinforcer was used and kappa receptor antagonists have been shown to reduce intake of high-fat foods (Arjune and Bodnar, 1990). No previous study to the best of our knowledge has examined kappa receptor specific antagonists in operant behavior. We can conclude from the data presented here that dynorphin is likely not important in appetitive behaviors such as bar pressing for food reinforcers but likely plays a more important role in the consummatory phase such as measured in the two-bottle free choice phase of the study.
Some of the most convincing data suggesting enkephalin, β-endorphin and dynorphin bind to more than one subtype of opioid receptor comes from studies demonstrating changes in opioid receptor binding in the opioid knockout mice. For example, in a separate line of enkephalin knockout mice both MOP and DOP receptors were up-regulated in discrete brain regions including limbic forebrain regions and DOP was up-regulated in striatum (Brady, et al., 1999). Similarly, MOP, DOP and KOP receptors were all found to be up-regulated in discrete regions of a separate line of dynorphin knockout mice (Clarke, et al., 2003). However, we have not found changes in MOP, DOP or KOP receptors in the β-endorphin knockout mouse in selective brain regions including hypothalamus (Slugg, et al., 2000), nucleus accumbens and ventral tegmental area (M.D. Hayward, unpublished data). It is unlikely that changes in receptor levels could explain the observed phenotypes in the current study since presumably the receptors are up-regulated because of the loss of the endogenous ligand. If the up-regulation is the result of a loss of a ligand then it is unlikely that there are other ligands available for binding to the receptor in these discrete brain regions to produce a physiologically significant effect. Nonetheless, these studies do suggest that compensatory changes in neuronal systems occur in the absence of these ligands and so interpretation of our data conducted in knockout mice must be made with this in mind.
A major goal of this study was to identify unique contributions of the endogenous opioids to feeding hedonics by using the two-bottle free-choice test, which tests for consumption in the absence of any measured appetitive behavior. In fact, our procedure was conducted in such a way as to minimize any associative conditioning by alternating the position of the sucrose bottles. We previously found that β-endorphin and enkephalin appear to be important in appetitive behaviors (Hayward, et al., 2002) but in the current study we found that enkephalin and dynorphin were necessary for opioid modulation of sucrose consumption as revealed by receptor blockade with naloxone but β-endorphin did not appear to be necessary. There is considerable data describing the involvement of separate pathways involved in consumption and appetitive behavior. Most recently, two distinct classes of nucleus accumbens neurons have been described that encode palatability and appetitive behaviors respectively (Taha and Fields, 2005). The studies here complement those findings, suggesting that enkephalin and dynorphin may modulate pathways involved in modulating palatability while β-endorphin and enkephalin can modulate pathways involved in appetitive behaviors. In support of this hypothesis it appears that the MOP-R knockout mice show diminished food-anticipatory activity (Kas, et al., 2004) and both β-endorphin and enkephalin are endogenous ligands for the mu receptor.
Neuronal circuits within the brain that have been shown to be important for positive reinforcement by drugs of abuse are likely present to shape behavior around the procurement of natural rewards such as food (reviewed in (Kelley and Berridge, 2002). Other circuits in the brain control feeding through energy homeostasis and there appear to be several possible regions that may serve as an underlying pathway for cross-talk (reviewed in (Saper, et al., 2002). It is well known that food reward can be modulated by feeding state (i.e., deprived or sated). In fact, food restriction can increase the rewarding value of drugs of abuse (reviewed in (Carr, 2002). Additionally, the lateral hypothalamic area (LHA) is well known as a region containing peptides important in feeding (e.g., melanin concentrating hormone, orexin) as well as a region that potently supports electrical self-stimulation. In fact, leptin, the anorectic hormone released from adipocytes, attenuates the ability of fasting to increase self-stimulation of the LHA (Fulton, et al., 2000). Interactions between the LHA and regions important for reward behaviors such as the nucleus accumbens have yet to be described but it is likely that dopaminergic and serotonergic pathways are critical for food reward. The endogenous opioid system is an ideal candidate for potential cross-talk between homeostatic and hedonic aspects of feeding since endogenous opioids modulate both appetitive and consummatory feeding behaviors. Our previous results suggested that β-endorphin and enkephalin are important modulators of behaviors leading to or enabling feeding (i.e., appetitive behaviors) (Hayward, et al., 2002) while the current study indicates that dynorphin and enkephalin can modulate food consumption when they are present but are not necessary for consumption.
Acknowledgments
Supported by NIH grant DA14203.
Abbreviations
- MOP
mu opioid receptor
- DOP
delta opioid receptor
- KOP
kappa opioid receptor
- Enk+/− End+/−
double heterozygous mice
- Enk+/+End+/+
wildtype
- Enk+/+ End−/−
β-endorphin-deficient
- Enk−/− End+/+
enkephalin-deficient
- Enk−/− End−/−
double knockout
- Dyn−/−
dynorphin knockout mice
- FR
fixed ratio
- PR
progressive ratio
- ANOVA
analysis of variance
- i.p.
intraperitoneal
- LHA
lateral hypothalamic area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- Arjune D, Bodnar RJ. Suppression of nocturnal, palatable and glucoprivic intake in rats by the kappa opioid antagonist, norbinaltorphamine. Brain Res. 1990;534:313–316. doi: 10.1016/0006-8993(90)90147-4. [DOI] [PubMed] [Google Scholar]
- Beczkowska IW, Bowen WD, Bodnar RJ. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain Res. 1992;589:291–301. doi: 10.1016/0006-8993(92)91289-q. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Stein L. Enkephaline may mediate euphoria and drive-reduction reward. Nature. 1977;266:556–558. doi: 10.1038/266556a0. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Opioid receptor subtype antagonists and ingestion. In: Cooper SJ, Clifton PG, editors. Drug receptor subtypes and ingestive behaviour. Academic Press Inc; San Diego, CA: 1996. pp. 127–146. [Google Scholar]
- Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Brady LS, Herkenham M, Rothman RB, Partilla JS, Konig M, Zimmer AM, Zimmer A. Region-specific up-regulation of opioid receptor binding in enkephalin knockout mice. Brain Res Mol Brain Res. 1999;68:193–197. doi: 10.1016/s0169-328x(99)00090-x. [DOI] [PubMed] [Google Scholar]
- Brennan K, Roberts DC, Anisman H, Merali Z. Individual differences in sucrose consumption in the rat: motivational and neurochemical correlates of hedonia. Psychopharmacology (Berl) 2001;157:269–276. doi: 10.1007/s002130100805. [DOI] [PubMed] [Google Scholar]
- Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Clarke S, Zimmer A, Zimmer AM, Hill RG, Kitchen I. Region selective up-regulation of micro-, delta- and kappa-opioid receptors but not opioid receptor-like 1 receptors in the brains of enkephalin and dynorphin knockout mice. Neuroscience. 2003;122:479–489. doi: 10.1016/j.neuroscience.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Cleary J, Weldon DT, O'Hare E, Billington C, Levine AS. Naloxone effects on sucrose-motivated behavior. Psychopharmacology (Berl) 1996;126:110–114. doi: 10.1007/BF02246345. [DOI] [PubMed] [Google Scholar]
- Feifel D, Vaccarino FJ. Central injections of growth hormone-releasing factor increase operant responding for food reward. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:813–820. doi: 10.1016/0278-5846(90)90053-j. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Glass MC, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Low MJ. The effect of naloxone on operant behavior for food reinforcers in DBA/2 mice. Brain Res Bull. 2001;56:537–543. doi: 10.1016/s0361-9230(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Low MJ. Naloxone's suppression of spontaneous and food-conditioned locomotor activity is diminished in mice lacking either the dopamine D(2) receptor or enkephalin. Brain Res Mol Brain Res. 2005;140:91–98. doi: 10.1016/j.molbrainres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking beta-endorphin and enkephalin. J Neurosci. 2002;22:8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Kas MJ, van den Bos R, Baars AM, Lubbers M, Lesscher HM, Hillebrand JJ, Schuller AG, Pintar JE, Spruijt BM. Muopioid receptor knockout mice show diminished food-anticipatory activity. Eur J Neurosci. 2004;20:1624–1632. doi: 10.1111/j.1460-9568.2004.03581.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars and fats: the neurobiology of preference. J Nutr. 2003;133:831S–834S. doi: 10.1093/jn/133.3.831S. [DOI] [PubMed] [Google Scholar]
- Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol. 1995;268:R248–252. doi: 10.1152/ajpregu.1995.268.1.R248. [DOI] [PubMed] [Google Scholar]
- Mandenoff A, Fumeron F, Apfelbaum M, Margules DL. Endogenous opiates and energy balance. Science. 1982;215:1536–1538. doi: 10.1126/science.7063865. [DOI] [PubMed] [Google Scholar]
- Margules DL, Moisset B, Lewis MJ, Shibuya H, Pert CB. beta-Endorphin is associated with overeating in genetically obese mice (ob/ob) and rats (fa/fa) Science. 1978;202:988–991. doi: 10.1126/science.715455. [DOI] [PubMed] [Google Scholar]
- Miotto K, McCann M, Basch J, Rawson R, Ling W. Naltrexone and dysphoria: fact or myth? Am J Addict. 2002;11:151–160. doi: 10.1080/10550490290087929. [DOI] [PubMed] [Google Scholar]
- Nader K, Bechara A, van der Kooy D. Neurobiological constraints on behavioral models of motivation. Annu Rev Psychol. 1997;48:85–114. doi: 10.1146/annurev.psych.48.1.85. [DOI] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ, Pfaff DW. Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci U S A. 2001;98:1958–1963. doi: 10.1073/pnas.041598498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yauda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned k-, d-, and m- opioid receptors. Mol Pharm. 1994;45:330–334. [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Jápon M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking β-endorphin by site-directed mutagenesis. Proc Natl Acad Sci USA. 1996;93:2577–2582. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudski JM, Billington CJ, Levine AS. Naloxone's effects on operant responding depend upon level of deprivation. Pharmacol Biochem Behav. 1994;49:377–383. doi: 10.1016/0091-3057(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U. Generation of dynorphin knockout mice. Brain Res Mol Brain Res. 2001;86:70–75. doi: 10.1016/s0169-328x(00)00264-3. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Matthes HW, Walwyn WM, Kieffer BL, Maidment NT. Naloxone fails to produce conditioned place aversion in muopioid receptor knock-out mice. Neuroscience. 2001;106:757–763. doi: 10.1016/s0306-4522(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Slugg RM, Hayward MD, Ronnekleiv OK, Low MJ, Kelly MJ. Effect of the mu-opioid agonist DAMGO on medial basal hypothalamic neurons in beta-endorphin knockout mice. Neuroendocrinology. 2000;72:208–217. doi: 10.1159/000054589. [DOI] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci. 2005;25:1193–1202. doi: 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


