Abstract
Recent studies indicate that chronic inflammation plays a pathogenic role in both the central nervous system (CNS) and periphery in Alzheimer’s disease (AD). We have screened for cytokines differentially produced by peripheral blood mononuclear cells (PBMCs) isolated from subjects with mild cognitive impairment (MCI) and mild AD subjects who had progressed from MCI using a commercially available cytokine array. Following determination of expressed cytokines, we quantified levels of the proinflammatory cytokines TNF-α, IL-6, and IL-8, and the antiinflammatory cytokine IL-10 using flow cytometry. We have found a significant increase in the levels of IL-6, IL-8, and IL-10 produced by PBMCs stimulated for 24 hours with phytohemagglutinin (PHA) in MCI subjects compared to healthy elderly controls. However, in PBMCs stimulated for 48 h with lipopolysaccharide (LPS), lower TNF-α/IL-10, IL-6/IL-10, and IL-8/IL-10 ratios were seen in MCI subjects. There were no differences in plasma levels of IL-8 between aged controls, MCI, and mild AD, and the levels of circulating IL-6 and IL-10 were below detection limits. Our data indicate that changes in cytokine production by PBMCs may be detected early in MCI, and an alteration of the immune response may precede clinical AD.
Keywords: Cytokines, Inflammation, Alzheimer’s disease, Mild cognitive impairment, Flow cytometry
1. Introduction
A chronic inflammatory process has been implicated in the neuropathology of Alzheimer’s disease (AD), the most common form of dementia in the elderly. Both neural and systemic alterations in the immune response have been reported (McGeer and McGeer, 1998; Neuroinflammation Working Group, 2000; Richartz et al., 2005). Interleukin-1 (IL-1) is associated with amyloid-β (Aβ) in senile plaques, hallmarks of AD neuropathology, (Griffin et al., 1989, 1995) and also increases the synthesis and translation of the mRNA for amyloid precursor protein (APP), the processing of which produces Aβ (Goldgaber et al., 1989; Rogers et al., 1999). Blood cells express APP mRNA and protein (Allen et al., 1991; Schlossmacher et al., 1992), and APP mRNA is increased in peripheral blood mononuclear cells (PBMCs) from AD patients compared with cells from healthy elderly controls (Jiang et al., 2003). This could potentially be a source for abnormal amyloid deposited in the brain (Allen et al., 1991; Reale et al., 2005). On the other hand, exposure of Aβ to cultured human monocytes was shown to stimulate the production and release of IL-1β and interleukin-1 receptor antagonist (IL-1Ra) (Meda, 1999). Compared to controls, lymphocytes from AD patients are less responsive to stimulation with amyloid peptides which could suggest T cell anergy (Trieb et al., 1996). However, it is unclear whether circulating amyloid is deposited in the brain and causes neuroinflammation or immune activation to abnormal amyloid peptide in the brain then triggers the peripheral immune response (Reale et al., 2005). Inflammatory signaling pathways exist from the brain to the periphery, but the mechanism of induction is unknown (De Simoni et al., 1990, 1995; Gottschall et al., 1992; Song et al., 1999).
IL-1, IL-6, and IL-10 polymorphisms have been associated with altered cytokine levels and risk for AD (Arosio et al, 2004; Nicoll et al., 2000; Papassotiropoulos et al., 1999). A recent report demonstrated decreased production of the proinflammatory cytokines IL-6, IL-12, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) in whole blood cell cultures of AD patients compared to age-matched controls, suggesting an attenuated immune response (Richartz et al., 2005). Moreover, decreased production of the anti-inflammatory cytokine IL-10 in PBMCs stimulated with Aβ peptides was seen in AD (Arosio et al., 2004). However, other studies have shown elevated levels of TNF-α, IL-1β, IL-6, and IL-10 (Lombardi et al., 1999) and higher proto anti-inflammatory cytokine ratios, both IL-1β/IL-10 and IL-6/IL-10, released from LPS stimulated whole blood cultures from AD patients compared to controls, suggesting a proinflammatory phenotype in AD (Remarque et al., 2001). These seemingly contradictory reports may be partly explained by changes in inflammatory phenotype that are specific to certain disease stages as demonstrated by a study that found decreased production of IL-1β and IL-6 by stimulated blood cells in severe AD but not mild to moderate AD patients (Sala et al., 2003).
Increasing evidence suggests that peripheral modification of inflammatory factors may occur early during the development of AD (Galimberti et al., 2005; Huberman et al., 1994; Tarkowski et al., 2003). This may explain the results of several epidemiological studies that suggest that non-steroidal anti-inflammatory drugs (NSAIDs) protect against AD but do not have therapeutic effects (in t’ Veld et al., 2001; McGeer et al., 1996; Pasinetti and Pompl, 2002). Many therapeutic studies target those with advanced stages of AD at which point inflammatory processes may have already done the damage. Mild cognitive impairment (MCI) describes a pre-clinical stage of AD, applied to a transitional period between normal aging and early AD (Petersen, 2004). An altered immune profile has also been found in MCI patients. Serum monocyte chemotactic protein-1 (MCP-1), a beta chemokine, was shown to be upregulated in MCI and mild AD but not in severe AD patients as compared with controls (Galimberti et al., 2005). Furthermore, both MCI and AD patients who had progressed from MCI had higher TNF-α (proinflammatory cytokine) to TGF-β (anti-inflammatory cytokine) ratios in CSF when compared to healthy controls, indicating a higher proinflammatory state in patients at risk for AD (Tarkowski et al., 2003). Alterations in the immune response in MCI would suggest that inflammatory events may precede the clinical development of AD.
Characterizing similar immune alterations in subjects with MCI, which represents a preclinical stage of AD, will help clarify whether cytokine dysregulation is a cause or consequence of AD as well as identifying potential biomarkers for MCI in easily accessible peripheral blood cells. Our hypothesis is that alterations in peripheral immune function, specifically cytokine production, are early events in the development of AD. The purpose of this study was to detect cytokines that may be involved early in the pathogenesis of AD by first screening for cytokines produced by PBMCs isolated from healthy aged controls, subjects with MCI, and subjects who had progressed to mild AD from MCI, using a commercially available array of 20 cytokines involved in inflammation. Following determination of expressed cytokines, we quantified levels of the proinflammatory cytokines TNF-α, IL-6, and IL-8 and the anti-inflammatory cytokine IL-10 produced by unstimulated and stimulated (with PHA or LPS) PBMCs from controls, MCI, and mild AD subjects using flow cytometry. In general, PHA stimulates lymphocyte proliferation, while LPS stimulates monocytes to release IL-1 and TNF-α (Hsu and Wen, 2002; Tyrsted and Munch-Petersen, 1977; Zhu et al., 2000). Reports that demonstrate a difference in PBMC cytokine production between AD patients and controls have used different stimulants including PHA and LPS and various time points, including 24 and 48 hour incubations (Richartz et al., 2005; Remarque et al., 2001; Huberman et al., 1994). Because we designed this study as a comprehensive assessment of alterations in MCI subjects that have been previously reported in AD patients, both stimulants and time points were used. Examining PBMC response to different stimuli will allow us to determine the relative effect of both cell types in an environment that allows for their interaction and more closely reflects the in vivo state. To our knowledge, this is the first study to examine the PBMC cytokine profile from MCI subjects.
2. Materials and methods
2.1. Subjects
Blood samples were obtained from 31 subjects with MCI (mean age 75.06 ± 1.34, male/female 15/16), seven mild AD subjects who had their blood drawn after they had progressed to mild AD from MCI (79.14 ± 0.99, 3/4), and 21 healthy aged controls (73.10 ± 1.57, 8/13). There was no significant difference in age between MCI subjects and controls. However, the mild AD group was statistically older than the control group (p < 0.05). All subjects included in the study have undergone extensive physical and psychometric evaluations including blood tests, MRI, mini-mental state examination (MMSE), Logical Memory I and II tests, and clinical dementia rating (CDR). MCI was diagnosed following the guidelines by Peterson (2004). Classification of MCI is based on either abnormal memory determined by the Logical Memory II subscale or a CDR memory score of 0.5-1.0 which is based both on performance and informant data. All MCI cases were subtyped as amnestic multiple domain MCI.
Subjects with dementia, neurological disease, history of major head trauma, DSM-IV major depression within the past year, any unstable medical condition, history of schizophrenia, and history of alcohol or substance abuse were excluded. Subjects taking neuroleptics, chronic anxiolytics, or sedative hypnotics were also excluded from the study.
Protocols for collecting blood samples were approved by the Institutional Review Board of Loma Linda University School of Medicine and informed consent was obtained from all subjects.
2.2. Cell isolation and culture conditions
Blood was drawn from each subject into 10 ml heparin Vacutainer tubes from BD Biosciences (San Diego, CA). PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum, nonessential amino acids, 1 mM sodium pyruvate, 50 U/ml penicillin and 50 mg/ml streptomycin in 12 well plates at 106 cells/ml. Cells were cultured for 24 or 48 hours at 37 °C in 5% CO2 in complete culture medium alone or stimulated with 2 μg/ml phytohemagglutinin (PHA) or 10 μg/ml lipopolysaccharide (LPS), concentrations and time points commonly used to optimally induce cytokine production (Arosio et al., 2004; Huberman et al., 1994; Lombardi et al., 1999). After incubation, samples were centrifuged and supernatants collected and stored at -70 °C until assayed.
2.3 Cytokine array
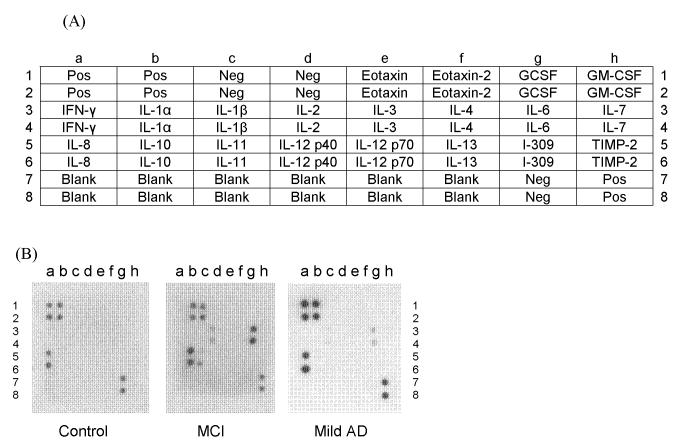
Human cytokine array membranes coated with 20 specific cytokine antibodies (Fig. 1A; ChemiArray™ Human Inflammation Antibody Array I, Chemicon, Temecula, CA) were probed with cell culture supernatants from PBMCs stimulated for 48 hours with LPS from controls, MCI, and mild AD according to the manufacturer’s instructions. This array uses a solid-phase sandwich technique to screen for multiple cytokines at once and is based on capture and detection antibodies directed against different cytokines in a combination ELISA/Immunoblotting method. Samples are incubated on a membrane coated with immobilized antibodies against different cytokines to which a cocktail of biotinylated cytokine antibodies are added to form the antibody “sandwich”. Then, HRP-conjugated streptavidin is added and membranes visualized with chemiluminescence, each duplicate spot on the membrane corresponding to a different cytokine. Briefly, membranes were blocked by incubating with the blocking buffer for 30 min and then incubated with sample for 1.5 h, both at room temperature. Membranes were washed with Wash Buffer I (3x, 5min each) then with Wash Buffer II (2x, 5min each) and incubated with biotin-conjugated antibodies overnight at 4°C. Finally, membranes were washed, incubated with HRP-conjugated streptavidin for 2 h at room temperature, and incubated with detection buffer for 2 min. Membranes were exposed to X-ray film (Biomax XAR film, Kodak, New Haven, CT) and developed. Average signal density for each cytokine was determined by densitometric analysis using ChemiImager 440 (Alpha Innotech, San Leandro, CA), and signals were normalized to the positive controls on the same membrane.
Fig 1.
(A) Alignment of 20 cytokines on the human cytokine array membrane. Abbreviations : GCSF: granulocyte colony stimulating factor; GM-CSF: granulocyte macrophage-colony stimulating factor; IFN: interferon; IL: interleukin; TIMP: tissue inhibitors of metalloproteinase.
(B)Representative examples of cytokine array membranes probed with the cell culture supernatants of 48 h LPS stimulated PBMCs from mild AD, MCI, and control. Each dot represents immunoreactive staining for the respective cytokines.
2.4. Cytokine measurement in cell culture supernatant by flow cytometry
Levels of IL-12p70, TNF-α, IL-10, IL-6, IL-1β, and IL-8 in cell culture supernatants were determined using the Cytometric Bead Array Assay (Becton Dickinson Biosciences, San Diego, CA), according to the manufacturer’s instructions by flow cytometry (FACSort flow cytometer and CellQuest software, Becton Dickinson, Sunnyvale, CA). The instrument was calibrated with CaliBrite beads before each analysis (BD Biosciences, San Diego, CA). Since IL-1β/IL-10 and IL-6/IL-10 ratios have previously been used as indicators of the inflammatory phenotype (Remarque et al., 2001), ratios of each of the proinflammatory cytokines TNF-α, IL-6, IL-1β, and IL-8 to the anti-inflammatory cytokine IL-10 were also calculated to assess propensity to inflammation.
2.5. Cytokine measurement in plasma by ELISA
Plasma samples were collected and analyzed for IL-6, IL-8, and IL-10 using commercially available ELISA kits (Endogen, Woburn, MA) according to the manufacturer’s instructions. The assay sensitivity was <1 pg/ml for IL-6, <2 pg/ml for IL-8, and <3 pg/ml for IL-10.
2.6. Statistical analysis
Data are presented as means ± S.E.M. Statistical analysis was performed using the SPSS 12.0.1 software. Since cytokines are known to have a non-Gaussian distribution (De Luigi et al., 2002; Lombardi et al., 1999), differences among cytokine production were evaluated using the non-parametric Mann-Whitney U test. A P value of <0.05 was chosen to indicate significance.
3. Results
3.1. Cytokine array
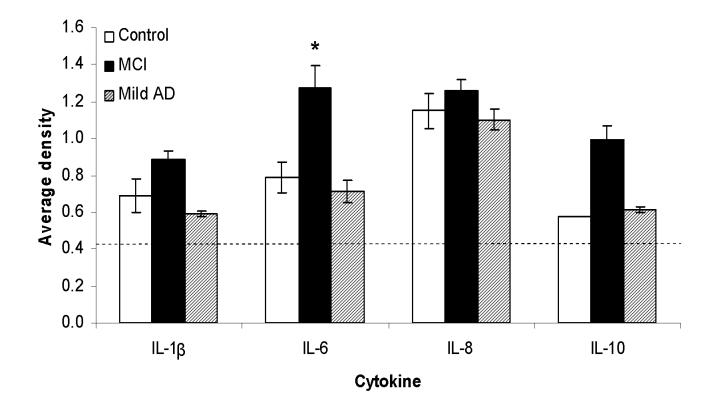
Cell culture supernatants from PBMCs, stimulated for 48 hours with LPS, from elderly controls (n = 6), MCI (n = 4), and mild AD subjects (n = 3) were screened for cytokines involved in inflammation. Out of the 20 cytokines assayed for, one or more subjects expressed IL-1β, IL-2, IL-6, IL-8, and IL-10 (Fig. 1B). However, only one mild AD case out of three, two control subjects out of six, and no MCI subjects expressed IL-2. Therefore, IL-2 was excluded from further study. Only one out of six control cases showed detectable levels of IL-10 expression, but all four MCI patients and two out of three mild AD cases expressed IL-10. IL-6 production by LPS stimulated PBMCs from MCI subjects was significantly higher compared to elderly controls (p < 0.03) (Fig. 2). No significant differences were seen in the other cytokines, only a trend indicating lower IL-1β and IL-6 levels in mild AD versus MCI.
Figure 2.
Densitometric quantification of cytokine levels from the cytokine arrays in elderly controls (n=6), MCI (n=4), and mild AD subjects (n=3). To compare results between different membranes, the signals (average density) for each cytokine were normalized to the positive controls on each membrane. Two out of three mild AD cases had detectable levels of IL-10 expression, but only one out of seven control cases showed IL-10 expression. All MCI subjects showed detectable of IL-1β, IL-6, IL-8, and IL-10. Dashed lines indicate the detection limit (0.53), *p < 0.03, significantly different from controls.
3.2. IL-12p70, TNF-α, IL-10, IL-6, IL-1β, and IL-8 production by PBMCs
The levels of IL-6, IL-8, and IL-10 produced by PBMCs stimulated for 24 h with PHA were significantly higher in MCI patients compared to elderly controls (p < 0.03 for all three cytokines) (Table 1). This difference disappears after cells are cultured for 48 h. In contrast, there were no significant differences between groups in absolute cytokine concentrations when PBMCs were stimulated with LPS. However, pro- to anti-inflammatory cytokine ratios, TNF-α/IL-10, IL-6/IL-10, and IL-8/IL-10, after stimulation with LPS for 48 h were significantly lower in MCI compared to controls (p < 0.03, p < 0.01, and p < 0.05, respectively) (Table 2). No differences in cytokine ratios were seen in PHA stimulated PBMCs between groups as expected since both the proinflammatory cytokines IL-6 and IL-8 and anti-inflammatory cytokine IL-10 were increased in MCI compared to controls. Cytokine levels were below the limit of detection in unstimulated PBMCs cultured for 24 or 48 h except for IL-8, and IL-8 levels did not differ between elderly controls, MCI, and mild AD subjects (data not shown).
Table 1.
Cytokine production by PBMCs from elderly controls, MCI, and mild AD subjects stimulated for 24 h or 48 h with PHA or LPS. All data are presented as mean (SEM) and are expressed in pg/ml for IL-12p70, TNF-α, IL-10, IL-6, IL-1β, and IL-8; *p < 0.03, significantly different from controls; **p < 0.03, significantly different from MCI; ND, non-detectable.
| IL-12p70 | TNF-α | IL-10 | IL-6 | IL-1β | IL-8 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24h PHA | n | mean (SEM) | n | mean (SEM) | n | mean (SEM) | n | mean (SEM) | n | mean (SEM) | n | mean (SEM) |
| Control | 15 | 2.31(0.35) | 15 | 12.06(2.63) | 15 | 3.47(0.17) | 14 | 18.27(10.10) | 15 | 10.37(2.21) | 14 | 1410(270.3) |
| MCI | 22 | 2.54(0.41) | 22 | 46.88(13.79) | 22 | 17.88(6.63)* | 22 | 584.8(271.5)* | 22 | 24.64(7.02) | 19 | 10532(3170)* |
| Mild AD | 7 | 2.56(0.52) | 7 | 12.04(3.55) | 7 | 4.19(0.82) | 7 | 20.41(10.13) | 7 | 12.44(3.86) | 6 | 921.1(398.6)** |
| 48h PHA | ||||||||||||
| Control | 16 | 2.72(0.51) | 16 | 63.13(28.59) | 16 | 35.66(14.60) | 16 | 1203(569.8) | 12 | 35.18(14.84) | 16 | 18836(7804) |
| MCI | 19 | 3.26(0.44) | 19 | 127.8(47.88) | 19 | 51.96(16.06) | 19 | 1478(667.5) | 17 | 65.71(31.86) | 17 | 27351(8431) |
| Mild AD | 5 | ND | 5 | 43.54(22.43) | 5 | 30.58(24.67) | 5 | 144.4(96.11) | 2 | ND | 5 | 22548(152363) |
| 24h LPS | ||||||||||||
| Control | 17 | 5.14(1.07) | 17 | 722.5(169.8) | 17 | 307.0(108.0) | 16 | 10862(3482) | 16 | 1559(474.0) | 16 | 41088(13477) |
| MCI | 20 | 2.73(0.30) | 20 | 558.6(167.3) | 20 | 281.2(122.2) | 16 | 8298(3341) | 19 | 1196(349.9) | 15 | 39075(16399) |
| Mild AD | 7 | 2.49(0.59) | 7 | 300.5(150.8) | 7 | 164.5(108.4) | 6 | 2390(1525) | 7 | 895.5(538.8) | 6 | 11014(6742) |
| 48h LPS | ||||||||||||
| Control | 18 | 3.87(0.73) | 18 | 253.6(153.8) | 18 | 246.6(116.3) | 17 | 9479(4481) | 18 | 1208(509.1) | 14 | 52822(23277) |
| MCI | 22 | 4.47(0.66) | 22 | 160.6(44.56) | 22 | 381.6(115.7) | 17 | 9565(2946) | 21 | 1686(381.5) | 16 | 61903(19590) |
| Mild AD | 6 | 3.33(0.93) | 6 | 98.07(46.22) | 6 | 263.2(171.8) | 5 | 5199(3944) | 6 | 1184(731.4) | 5 | 35876(27158) |
Table 2.
Cytokine parameters of PBMCs from healthy aged controls, MCI, and mild AD subjects stimulated for 24 h or 48 h with LPS or PHA. All data are presented as mean (SEM), *p < 0.03, significantly different from controls.
| Ratio | TNF-α/IL-10 | IL-6/IL-10 | IL-1β/IL-10 | IL-8/IL-10 | ||||
|---|---|---|---|---|---|---|---|---|
| 24h PHA | n | mean (SEM) | n | mean (SEM) | n | mean (SEM) | n | mean (SEM) |
| Control | 15 | 3.35(0.65) | 14 | 5.46(3.07) | 15 | 3.08(0.68) | 14 | 393.8(67.31) |
| MCI | 22 | 3.74(0.88) | 22 | 18.71(5.92) | 22 | 2.38(0.40) | 19 | 766.5(161.3) |
| Mild AD | 7 | 2.97(0.93) | 7 | 5.34(3.06) | 7 | 3.42(1.09) | 6 | 273.3(120.4) |
| 48h PHA | ||||||||
| Control | 16 | 2.59(0.63) | 16 | 10.66(4.86) | 12 | 1.66(0.21) | 16 | 529.0(90.47) |
| MCI | 19 | 2.97(0.62) | 19 | 19.34(6.22) | 17 | 1.69(0.32) | 17 | 548.1(103.7) |
| Mild AD | 5 | 3.31(0.83) | 5 | 7.90(3.13) | 2 | 0.69(0.62) | 5 | 985.7(272.1) |
| 24h LPS | ||||||||
| Control | 17 | 6.83(1.40) | 16 | 78.10(10.52) | 16 | 15.77(3.03) | 16 | 348.8(52.26) |
| MCI | 20 | 5.21(0.74) | 16 | 75.78(17.34) | 19 | 12.93(2.08) | 15 | 272.5(36.54) |
| Mild AD | 7 | 5.76(1.37) | 6 | 76.15(16.22) | 7 | 13.65(3.70) | 6 | 469.6(95.78) |
| 48h LPS | ||||||||
| Control | 18 | 2.71(0.39) | 17 | 95.69(9.98) | 18 | 13.15(2.23) | 14 | 650.7(92.41) |
| MCI | 22 | 1.78(0.51)* | 17 | 62.66(14.50)* | 21 | 10.30(1.55) | 16 | 365.9(65.38)* |
| Mild AD | 6 | 1.50(0.46) | 5 | 69.43(13.28) | 6 | 6.57(1.80) | 5 | 573.3(122.6) |
3.3. IL-6, IL-8, and IL-10 levels in plasma measured by ELISA
There were no significant differences in the levels of IL-8 between elderly controls (n = 13, average ± SEM = 10.2 ± 4.5 pg/ml), MCI subjects (n = 15, average ± SEM = 6.2 ± 2.5 pg/ml), and mild AD subjects (n = 7, average ± SEM = 9.9 ± 4.1 pg/ml). No detectable levels of IL-6 and IL-10 were found in plasma in any of the three groups.
4. Discussion
In our investigation of cytokine production by cultured PBMCs from healthy elderly controls, MCI and mild AD subjects, we found that levels of IL-6, IL-8, and IL-10 were significantly higher in MCI subjects compared to controls when cells were stimulated with PHA for 24 h but not 48 h. Since chronic inflammation is implicated in AD, PBMC response to stimulants was examined at two time points, 24 and 48 h. The inability to detect differences at 48 h after PHA stimulation may be due to cytokine release reaching a plateau in both groups and could suggest that cells from MCI subjects are more easily stimulated to produce cytokines but that production in controls reach similar levels to MCI over time. The concomitant increase in the anti-inflammatory cytokine IL-10 may be in response to increased proinflammatory cytokines IL-6 and IL-8 as IL-10 inhibits most cytokines involved in inflammation, including IL-1, IL-6, TNF-α, and IL-8 (Grutz, 2005; Moore et al., 2001). No differences in absolute cytokine concentrations were seen when cells were stimulated with LPS at either time point. However, stimulation with LPS for 48 h resulted in TNF-α/IL-10, IL-6/IL-10, and IL-8/IL-10 ratios significantly lower in MCI compared to controls which may indicate a faster attenuation in the production of inflammatory cytokines with respect to IL-10. This is in contrast to cells stimulated with PHA. PHA stimulates the proliferation of lymphocytes, increasing DNA polymerase activity after incubation for 15 h (Tyrsted and Munch-Petersen, 1977), while LPS stimulates monocytes to release IL-1 and TNF-α (Hsu and Wen, 2002; Zhu et al., 2000). Even though the effect of interactions between cell types cannot be excluded since both lymphocytes and monocytes were cultured together, the differential effect of PHA and LPS suggests alterations in cytokine production in MCI specific to immune cell type.
It has been shown that PHA stimulated PBMCs of moderately severe AD patients secrete significantly higher levels of IL-2 compared to mild AD and age-matched controls while levels of IL-1β do not differ between the groups (Huberman et al., 1994). Also, Lindberg et al. (2005) have found no differences in CSF or serum levels of the soluble form of IL-1 receptor type II (sIL-1RII) in both the early and late stages of AD when compared to controls and have suggested that the IL-1 system appears intact in both the early and late stages of AD. Similarly, we did not find any significant difference in IL-1β production between elderly controls, MCI and mild AD subjects. TNF-α and IL-6 release from blood cells stimulated with LPS was shown to be downregulated in AD (De Luigi et al., 2002). This is in agreement with our findings that TNF-α/IL-10 and IL-6/IL-10 ratios were lower in MCI compared to controls, showing that depressed production of these proinflammatory cytokines with respect to IL-10 in LPS but not PHA stimulated PBMCs can be detected early in the preclinical stage of AD. The lack of differences we found between controls and mild AD patients must be interpreted with caution since the sample size was small. However, our findings of inflammatory markers changing in MCI and returning near normal levels in mild AD are in agreement with reports that show increased chemokine interferon-γ-inducible protein 10 in CSF from MCI but not AD (Galimberti et al. 2006), decreased production of cytokines in whole blood cultures from AD (Richartz et al., 2005), and decreased IL-1β, IL-6, and TNF-α release from LPS stimulated peripheral blood from severe AD patients but no difference between mild and moderate AD patients and controls (Sala et al., 2003). This also correlates with the findings of Griffin et al. (1995) in which IL-1α+ microglia associated with plaques were found to increase in earlier plaque stages but then decrease in later plaque stages of AD, suggesting that microglia and inflammation play early roles in plaque evolution. However, inflammatory processes in the brain may not have a simple direct relationship with that in the periphery and evidence points to a more complex relationship between brain and peripheral inflammation.
In sum, the results indicate that alterations in cytokine production by PBMCs can be detected in MCI and could precede the development of clinical AD. However, this may not be entirely explained by a shift toward the proinflammatory phenotype as the anti-inflammatory cytokine IL-10 was increased along with IL-6 and IL-8 when cells were stimulated with PHA. Interestingly, the trend in PHA stimulated cytokine production seems to peak in MCI subjects and return to control or below control levels once they progress to early AD. This is in agreement with findings that intrathecal inflammation is seen before the development of AD (Tarkowski et al., 2003). Paganelli et al. (2002) have found that the level of serum TNF-α is significantly lower in mild-moderate AD compared to severe AD and vascular dementia suggesting a difference in the cytokine profile at different stages of AD as well as between other types of dementia. Our data support the hypothesis that inflammation is an early event rather than a late consequence of the disease (Galimberti et al., 2005; Tarkowski et al., 2003).
IL-6 is released in response to TNF-α and IL-1 but persists longer than these cytokines in plasma and so is a useful indicator of proinflammatory cytokine activation (Song and Kellum, 2005). At the same time, the involvement of IL-6 in several diseases with an inflammatory component, and the heterogeneity of MCI subjects resulting in a high SEM of IL-6 production seen in our study would preclude the use of IL-6 by itself as a biomarker for MCI. Most likely a panel of markers, including IL-6, IL-10, IL-8, and other potential markers of MCI such as serum MCP-1 (Galimberti et al., 2005) will be needed to selectively diagnostic MCI. Production of IL-6, IL-1, TNF-α, and IL-8 but not IL-10 in microglia is stimulated by Aβ (Franciosi et al., 2005; Nagai et al., 2001). In peripheral blood monocytes, Aβ stimulates the release of IL-1β, interleukin-1 receptor antagonist (IL-1Ra) but not of IL-6, IL-10, and TGF-β (Meda, 1999). Also, B cells from AD patients have been demonstrated to produce Aβ reactive autoantibodies (Gaskin et al., 1993). Song et al. (2001) have shown that intracerebroventricular injection of Aβ into mice cause increased levels of IL-6 in both brain and plasma which implicates that deposition of Aβ in the CNS may be linked to altered peripheral immune responses. Furthermore, transgenic mice chronically expressing human APP have decreased lymphocyte proliferation and production of cytokines in response to Aβ (Monsonego et al., 2001). Together with the finding that lymphocytes from AD patients are less responsive to stimulation with amyloid peptides compared to controls (Trieb et al., 1996), this could suggest that chronic Aβ exposure leading to cellular immune hyporesponsiveness to Aβ may exacerbate its accumulation in AD (Sala et al., 2003). To our knowledge, there have been no studies that have examined modulation of cytokine release from PBMCs by Aβ in MCI subjects. We will focus our future work on the effects of Aβ on PBMC cytokine production in MCI.
In conclusion, our results show an increased production of the proinflammatory cytokines, IL-6 and IL-8, and the anti-inflammatory cytokine IL-10 in MCI patients compared to elderly controls. This indicates an alteration in the cytokine profile in the preclinical stages of AD which may help to elucidate the role of inflammation and also aid in the design of therapeutics for this devastating disease.
Acknowledgements
We would like to thank Wayne Kelln and Judy Holbeck for technical support and Dr. Steven Yellon for help with the manuscript. This work was supported by the National Institute on Aging, National Institutes of Health (NIH) grant AG20948.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JS, Murphy GM, Jr., Eng LF, Stultz KE, Davies HD, Pickford LB, Tinklenberg JR. Alzheimer’s disease: beta-amyloid precursor protein mRNA expression in mononuclear blood cells. Neurosci. Lett. 1991;132:109–112. doi: 10.1016/0304-3940(91)90445-y. [DOI] [PubMed] [Google Scholar]
- Arosio B, Trabattoni D, Galimberti L, Bucciarelli P, Fasano F, Calabresi C, Cazzullo CL, Vergani C, Annoni G, Clerici M. Interleukin-10 and interleukin-6 gene polymorphisms as risk factors for Alzheimer’s disease. Neurobiol. Aging. 2004;25:1009–1015. doi: 10.1016/j.neurobiolaging.2003.10.009. [DOI] [PubMed] [Google Scholar]
- De Luigi A, Pizzimenti S, Quadri P, Lucca U, Tettamanti M, Fragiacomo C, De Simoni MG. Peripheral inflammatory response in Alzheimer’s disease and multiinfarct dementia. Neurobiol. Dis. 2002;11:308–314. doi: 10.1006/nbdi.2002.0556. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Sironi M, De Luigi A, Manfridi A, Mantovani A, Ghezzi P. Intracerebroventricular injection of interleukin 1 induces high circulating levels of interleukin 6. J. Exp. Med. 1990;171:1773–1778. doi: 10.1084/jem.171.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni MG, Del Bo R, De Luigi A, Simard S, Forloni G. Central endotoxin induces different patterns of interleukin (IL)-1 beta and IL-6 messenger ribonucleic acid expression and IL-6 secretion in the brain and periphery. Endocrinology. 1995;136:897–902. doi: 10.1210/endo.136.3.7867598. [DOI] [PubMed] [Google Scholar]
- Franciosi S, Choi HB, Kim SU, McLarnon JG. IL-8 enhancement of amyloid-beta (Abeta 1-42)-induced expression and production of pro-inflammatory cytokines and COX-2 in cultured human microglia. J. Neuroimmunol. 2005;159:66–74. doi: 10.1016/j.jneuroim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol. Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.10.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, Guidi I, Blankenstein MA, Bresolin N, Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- Gaskin F, Finley J, Fang Q, Xu S, Fu SM. Human antibodies reactive with beta-amyloid protein in Alzheimer’s disease. J. Exp. Med. 1993;177:1181–1186. doi: 10.1084/jem.177.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc. Natl. Acad. Sci. USA. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschall PE, Komaki G, Arimura A. Increased circulating interleukin-1 and interleukin-6 after intracerebroventricular injection of lipopolysaccharide. Neuroendocrinology. 1992;56:935–938. doi: 10.1159/000126328. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd., Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J. Neuropathol. Exp. Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J. Leukoc. Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- Huberman M, Shalit F, Roth-Deri I, Gutman B, Brodie C, Kott E, Sredni B. Correlation of cytokine secretion by mononuclear cells of Alzheimer patients and their disease stage. J. Neuroimmunol. 1994;52:147–152. doi: 10.1016/0165-5728(94)90108-2. [DOI] [PubMed] [Google Scholar]
- t′ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. in. [DOI] [PubMed] [Google Scholar]
- Jiang S, Zhang M, Ren D, Tang G, Lin S, Qian Y, Zhang Y, Jiang K, Li F, Wang D. Enhanced production of amyloid precursor protein mRNA by peripheral mononuclear blood cell in Alzheimer’s disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2003;118:99–102. doi: 10.1002/ajmg.b.10067. [DOI] [PubMed] [Google Scholar]
- Lindberg C, Chromek M, Ahrengart L, Brauner A, Schultzberg M, Garlind A. Soluble interleukin-1 receptor type II, IL-18 and caspase-1 in mild cognitive impairment and severe Alzheimer’s disease. Neurochem. Int. 2005;46:551–557. doi: 10.1016/j.neuint.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Lombardi VR, Garcia M, Rey L, Cacabelos R. Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer’s Disease (AD) individuals. J. Neuroimmunol. 1999;97:163–171. doi: 10.1016/s0165-5728(99)00046-6. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp. Gerontol. 1998;33:371–378. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Meda L, Baron P, Prat E, Scarpini E, Scarlato G, Cassatella MA, Rossi F. Proinflammatory profile of cytokine production by human monocytes and murine microglia stimulated with beta-amyloid[25-35] J. Neuroimmunol. 1999;93:45–52. doi: 10.1016/s0165-5728(98)00188-x. [DOI] [PubMed] [Google Scholar]
- Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL. Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Nagai A, Nakagawa E, Hatori K, Choi HB, McLarnon JG, Lee MA, Kim SU. Generation and characterization of immortalized human microglial cell lines: expression of cytokines and chemokines. Neurobiol. Dis. 2001;8:1057–1068. doi: 10.1006/nbdi.2001.0437. [DOI] [PubMed] [Google Scholar]
- Neuroinflammation Working Group. Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Mrak RE, Graham DI, Stewart J, Wilcock G, MacGowan S, Esiri MM, Murray LS, Dewar D, Love S, Moss T, Griffin WS. Association of interleukin-1 gene polymorphisms with Alzheimer’s disease. Ann. Neurol. 2000;47:365–368. [PMC free article] [PubMed] [Google Scholar]
- Paganelli R, Di Iorio A, Patricelli L, Ripani F, Sparvieri E, Faricelli R, Iarlori C, Porreca E, Di Gioacchino M, Abate G. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer’s disease patients. Exp. Gerontol. 2002;37:257–263. doi: 10.1016/s0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Bagli M, Jessen F, Bayer TA, Maier W, Rao ML, Heun R. A genetic variation of the inflammatory cytokine interleukin-6 delays the initial onset and reduces the risk for sporadic Alzheimer’s disease. Ann. Neurol. 1999;45:666–668. doi: 10.1002/1531-8249(199905)45:5<666::aid-ana18>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Pompl PN. Cyclo-oxygenase inhibitors and Alzheimer’s: are we well ADAPTed? Lancet. Neurol. 2002;1:403–404. doi: 10.1016/s1474-4422(02)00214-4. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Gambi F, Lucci I, Salvatore M, Gambi D. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Exp. Gerontol. 2005;40:165–171. doi: 10.1016/j.exger.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Remarque EJ, Bollen EL, Weverling-Rijnsburger AW, Laterveer JC, Blauw GJ, Westendorp RG. Patients with Alzheimer’s disease display a pro-inflammatory phenotype. Exp. Gerontol. 2001;36:171–176. doi: 10.1016/s0531-5565(00)00176-5. [DOI] [PubMed] [Google Scholar]
- Richartz E, Batra A, Simon P, Wormstall H, Bartels M, Buchkremer G, Schott K. Diminished production of proinflammatory cytokines in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2005;19:184–188. doi: 10.1159/000083497. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Leiter LM, McPhee J, Cahill CM, Zhan SS, Potter H, Nilsson LN. Translation of the alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5′-untranslated region sequences. J. Biol. Chem. 1999;274:6421–6431. doi: 10.1074/jbc.274.10.6421. [DOI] [PubMed] [Google Scholar]
- Sala G, Galimberti G, Canevari C, Raggi ME, Isella V, Facheris M, Appollonio I, Ferrarese C. Peripheral cytokine release in Alzheimer patients: correlation with disease severity. Neurobiol. Aging. 2003;24:909–914. doi: 10.1016/s0197-4580(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Schlossmacher MG, Ostaszewski BL, Hecker LI, Celi A, Haass C, Chin D, Lieberburg I, Furie BC, Furie B, Selkoe DJ. Detection of distinct isoform patterns of the beta-amyloid precursor protein in human platelets and lymphocytes. Neurobiol. Aging. 1992;13:421–434. doi: 10.1016/0197-4580(92)90117-g. [DOI] [PubMed] [Google Scholar]
- Song DK, Im YB, Jung JS, Suh HW, Huh SO, Park SW, Wie MB, Kim YH. Differential involvement of central and peripheral norepinephrine in the central lipopolysaccharide-induced interleukin-6 responses in mice. J. Neurochem. 1999;72:1625–1633. doi: 10.1046/j.1471-4159.1999.721625.x. [DOI] [PubMed] [Google Scholar]
- Song DK, Im YB, Jung JS, Cho J, Suh HW, Kim YH. Central beta-amyloid peptide-induced peripheral interleukin-6 responses in mice. J. Neurochem. 2001;76:1326–35. doi: 10.1046/j.1471-4159.2001.00121.x. [DOI] [PubMed] [Google Scholar]
- Song M, Kellum JA. Interleukin-6. Crit. Care. Med. 2005;33:S463–465. doi: 10.1097/01.ccm.0000186784.62662.a1. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieb K, Ransmayr G, Sgonc R, Lassmann H, Grubeck-Loebenstein B. APP peptides stimulate lymphocyte proliferation in normals, but not in patients with Alzheimer’s disease. Neurobiol. Aging. 1996;17:541–547. doi: 10.1016/0197-4580(96)00068-1. [DOI] [PubMed] [Google Scholar]
- Tyrsted G, Munch-Petersen B. Early effects of phytohemagglutinin on induction of DNA polymerase, thymidine kinase, deoxyribonucleoside triphosphate pools and DNA synthesis in human lymphocytes. Nucleic. Acids. Res. 1977;4:2713–2723. doi: 10.1093/nar/4.8.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Downey JS, Gu J, Di Padova F, Gram H, Han J. Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J Immunol. 2000;164:6349–6358. doi: 10.4049/jimmunol.164.12.6349. [DOI] [PubMed] [Google Scholar]