Abstract
Objective
This paper describes the randomized clinical trial methodology for a population based study of oncology patients receiving cancer care in a public sector medical center. The primary goal is to test the effectiveness of socioculturally tailored collaborative care intervention in improving depression and quality of life outcomes among low-income ethnic minority patients with major depression and cancer.
Methods
The PHQ-9 depression scale was used to identify patients meeting criteria for major depression (1 cardinal depression symptom + a PHQ-9 score of ≥ 10). Study eligible patients were ≥90 days from cancer diagnosis who were receiving acute cancer treatment or follow-up care in oncology clinics. Patients with advanced disease limiting life expectancy to <6 months, acutely suicidal or on antipsychotic medication were excluded. Allowing for attrition due to death or loss to follow-up, the study was powered at the 80% level to detect a 20% difference between study arms in the proportion of patients with ≥50% reduction in PHQ-9 symptoms at 12 months.
Results
Of 2,330 patients screened, 23.2% met criteria. An 82.4% enrollment rate resulted in 447 primarily women being recruited and randomized to intervention or usual care.
Conclusion
The study applies methods used in primary care depression trials with adaptations for oncology care clinics and for low-income minority patients.
Keywords: Depression, Cancer, Randomized Clinical Trial, Collaborative Care, Low-Income
Introduction
Depressive disorders and symptoms are common in cancer patients (up to 38% having major depression) [1–3], worsen over the course of cancer treatment, persist long after cancer therapy [4], reoccur with the recurrence of cancer [5], and significantly impact quality of life [6–9]. Unfortunately, clinicians and patients often perceive depression as an expected and reasonable reaction to cancer, thus depression is frequently under-recognized and under-treated in oncology practice [10–16]. Low-income patients are particularly unlikely to receive mental health treatment [17–18]
Patient, provider, and health system barriers to care contribute to the failure to effectively manage depression symptoms. Patients may be reluctant to report symptoms or to see a mental health professional and if prescribed treatment may not adhere to prescribed treatment, citing concerns about side-effects and/or preoccupation with active cancer treatment. Providers may be reluctant to raise the issue, and be less aware of effective treatments, while organizational barriers reduce timely and integrated access to mental health professionals. Culturally based preferences for depression care can become a barrier to care if the preferred mode of care is not available [19], while culturally based explanations for depression symptoms may influence symptom expression and patient-provider communication [20–22; 23]. Perceived stigma, family perceptions, and practical barriers such as cost and transportation to therapy may also impede receipt of care among low-income populations [24–25].
Depression care quality improvement strategies are effective in reducing barriers to depression care - including among racial/ethnic minorities [26]. Organizational strategies [27] generally include multifaceted quality improvement disease management interventions that change the way depression care is delivered, such as the implementation of routine depression screening, systematic application of evidence-based practice guidelines, clinical decision-making protocols and algorithms (cancer specific available on the NCI and NCCN websites), follow-up through remission and maintenance, enhanced roles of nurses or social workers as depression care managers as well as integration between primary care and mental health specialists or service systems.
Depression care models that use collaboration between primary care physicians and mental health professionals, where expertise in psychopharmacology in treating depression is provided by a psychiatrist and psychotherapy and supportive care management is provided by depression specialist nurses or social workers, have been found to be effective in primary care [28]. An adapted model for oncology was found to be effective in a randomized pilot study of 55 low-income, predominantly Latina breast or cervical cancer patients who met criteria for major depression [29] suggesting that cancer patients in public sector oncology clinics can benefit from depression treatment. What was learned from this preliminary study led to further adaptations for low-income minority patients and the public sector that serves them. We present here the design of the Alleviating Depression Among Patients with Cancer (ADAPt-C) randomized clinical trial, sociocultural adaptations in the care management model and the baseline characteristics of the sample.
Methods
Study Site, Sample Recruitment and Randomization
Los Angeles County + University of Southern California Medical Center is a large public sector center that provides oncology care to a predominantly Hispanic population. The study was approved by the University of Southern California-Health Sciences Institutional Review Board. Trained bilingual study recruiters identified potentially eligible patients by reviewing daily oncology clinic charts. Patients were then assessed for language preference and asked to provide brief verbal consent to be screened for depressive symptoms. To recruit a representative sample, we included patients ≥90 days from cancer diagnosis who were receiving acute treatment or follow-up care in oncology clinics but did not have advanced cancer or another medical condition that limited remaining life expectancy to less than 6 months. The Patient Health Questionnaire depression scale (PHQ-9) was used because it provides both a dichotomous diagnosis of major depression as well as a continuous severity score [30] and measures a common concept of depression across racial and ethnic groups [31]. If patients met criteria for major depression (one of the 2 cardinal depression symptoms plus a PHQ-9 score of ≥ 10), an additional screening protocol was administered to exclude patients with severe mental illness (including schizophrenia or bipolar disorder), evidence of cognitive impairment or current suicidal ideation, a score of 8 or greater on the AUDIT alcohol assessment [32], recent use of lithium or antipsychotic medication and a self-reported Karnofsky Performance Status Scale score of 2 or less on a 10 point representing severe functional impairment in cancer patients [33].
After completion of the brief screener, eligible patients provided written informed consent to study participation. The baseline interview was conducted in the clinic or if patients preferred, via telephone. After completion of the baseline interview, patients were assigned to Intervention or modestly Enhanced Usual Care by selecting a sealed envelope containing the name of a study group that had been generated via computer algorithm. A total of 446 patients were recruited into the study over 29 months (Figure 2).
Figure 2.
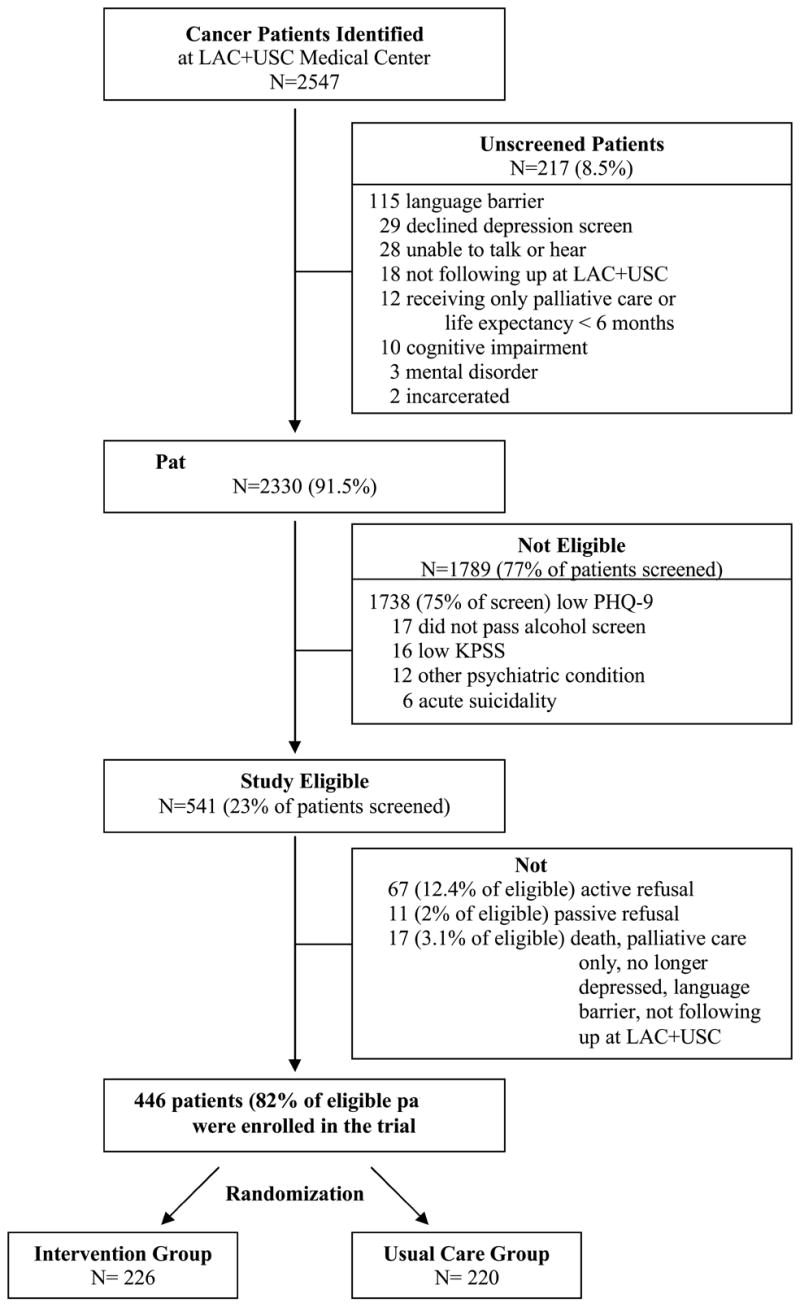
Study Recruitment
Socio-cultural Considerations
In view of known barriers to participation in cancer trials and to depression treatment retention in low-income minority populations, efforts were made to facilitate recruitment and acceptance of the intervention and to minimize study attrition [34–40]: 1) recruitment from a public sector system of care; 2) Spanish-speaking research staff and study and intervention materials in Spanish that are adapted for literacy and idiomatic content; 3) telephone data collection and intervention telephone option; 4) flexible outcome interview and intervention hours, including evening and weekend telephone visits and scheduling depression treatment visits to coincide with cancer treatment or follow-up appointments; 5) supportive patient navigation/case management intervention to address barriers to both cancer and depression treatment; 6) family education materials and attention to family member roles; and 7) a maintenance open-ended support group in both English and Spanish. Staff receive brief training in cultural competency via a self-administered cultural competence training manual developed in previous studies [41,42]. Independent study interviewers and the clinical team make multiple attempts to reach patients. Study participants are reimbursed for time in completing outcome interviews, for transportation when indicated, and if they are required to make additional personal co-pay for antidepressant medication.
Intervention Design
Quality Improvement Collaborative Care
The ADAPt-C intervention design was adapted from collaborative care quality improvement interventions for depression that effectively integrate mental health professionals into primary care [43–46], and are effective for low-income and minority patients [47–48,45,36,25]. The intervention is an individualized stepped care depression treatment program provided by a Cancer Depression Clinical Specialist (CDCS) in collaboration with a study psychiatrist. Bilingual social workers with a Masters degree were chosen to implement the CDCS role because our previous studies with the medical center population found that many patients need patient navigation and case management services to address barriers to engagement in depression care (while simultaneously managing their cancer treatment) as well as supportive assistance in addressing psychosocial and practical problems in their daily lives. In addition, medical and nursing oncology staff are comfortable with social workers working with cancer patients as this model is used throughout the medical center via the clinical social work department. The social workers carry out the majority of treatment, communicate with the oncologist and nursing staff as needed, act as translators during psychiatric evaluations and provide patient navigation/case management services. The initial CDCS visit includes extensive patient education, a semi-structured psychiatric and psychosocial history and assessment, consideration of initial treatment choice, provision of patient navigation assistance, and in some cases may include meeting with family members. Given that the majority of these patients are unfamiliar with depression as a concept or its treatment, patient education is implemented based on CDCS clinical judgment and includes discussion of the brochure provided all patients (adapted from the IMPACT study for this population), and optional use of a video in Spanish or English on depression. Depression education includes discussion of etiology (both biochemical and environmental factors), common depressive symptoms among cancer patients, and the advantages and concerns about counseling and/or antidepressant treatment. The clinical goal is to normalize depression by reducing perceptions of stigma and to empower patients in taking an active role with their CDCS, psychiatrist, or oncologist in understanding and deciding on first line treatment. The CDCS also interacts via written notes or verbally with the treating oncologist. We have found that a full time CDCS can effectively manage 35–40 patients in active treatment.
CDCS Training
The CDCS receives an initial orientation to the needs and psychiatric evaluation of patients with cancer, antidepressant medication use for cancer patients, and cultural issues in cancer care from the study psychiatrist, PI and self-study written materials. The CDCS receives an initial 2 weeks of formal Problem Solving Therapy (PST) training including: self-study of the PST and ADAPt-C manuals, video and in-person didactic sessions, observation of a skilled therapist doing an initial evaluation and 2 PST sessions with a cancer patient, and treatment of 3–5 patients under close supervision by a therapist experienced in PST. To monitor the ongoing quality and fidelity of the depression care management, the CDCS meets weekly via 1 hour telephone calls with the study psychiatrist and PI. Audio-taping of treatment sessions is conducted on a minimum of 5 patients; these are reviewed by a PST expert on a Likert scale of 1 to 5 (no review (1–2); some review or extensive review (3–5) on 12 session-specific items [49–50]. If there is evidence of inadequacy, further consultation and supervision is provided. Supervision of the CDCS by the study psychiatrist occurs weekly and more frequently on an as needed basis. Supervision is focused on new patients, patients in initial phase of treatment and in the maintenance phase. Patients on medication are initially evaluated by the study psychiatrist face to face and as frequently as requested by the CDCS or for medication management.
A clinical data tracking secure website was developed to facilitate CDCS and study psychiatrist patient care management and PHQ-9 symptom monitoring. The CDCS and psychiatrist log on to a secure server at the USC School of Social Work and enter a unique login ID and password. The CDCS completes a new enrollment form on each new intervention patient and then enters an initial assessment, follow-up assessments, treatment plans, or a relapse prevention plan as treatment proceeds. Data entry can occur ‘on line’ during a patient session, after a patient session, or at the end of the day. If data are not entered in ‘real time’, the CDCS make notes and enters the data as soon as possible, usually the same day.
Depression Care Management Stepped Care Algorithm
Patients with different severities of depression receive different levels of care according to their clinical presentations and responses to depression treatment. Some patients may have had previous psychotherapeutic and/or psychotropic medication treatments. Other patients may have comorbid psychiatric and medical conditions in addition to cancer that contribute to their depressed clinical presentation. These variations preclude the application of a single treatment algorithm that would adequately address all patients’ clinical needs. Based on our pilot and other previous studies suggesting that the low-income, predominantly Hispanic population might prefer to begin treatment using psychotherapy [29,51], we elected to give patient’s the choice of first-line treatment (PST alone, antidepressant medication alone, or both PST and antidepressant medication treatment). All patients are educated about the different treatment options.
The stepped care algorithm (Figure 3) is based on a number of consensus statements and treatment guidelines for depression in the primary care setting, as well as clinical recommendations and the National Comprehensive Cancer Network [52] guidelines for the treatment of depression in cancer patients. The goal of treatment is full symptomatic remission of depression.
Figure 3.
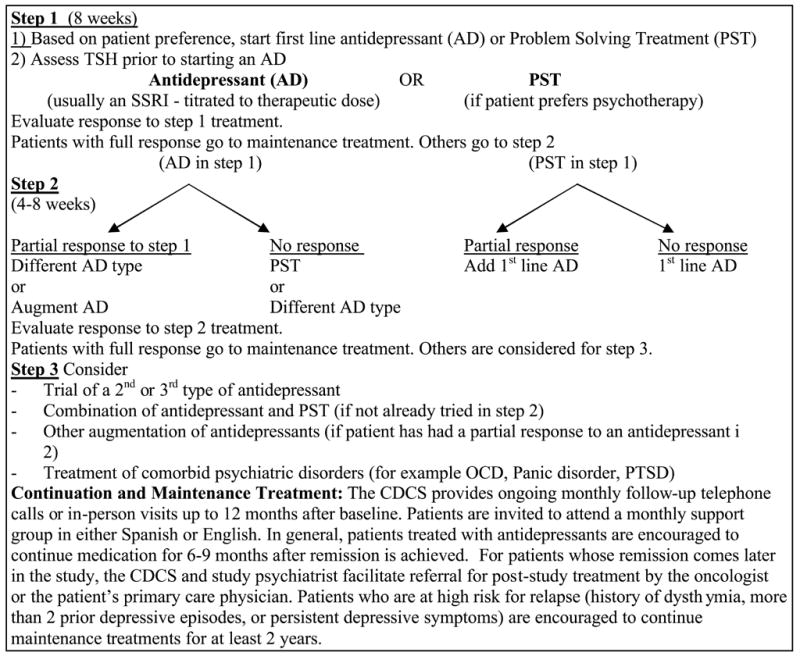
Stepped Care Treatment Algorithm
In Step 1 of treatment, the patient receives an initial evaluation by the CDCS and appropriate supervision of the case by the study psychiatrist. If the patient initially chooses only antidepressant medication treatment, the patient is evaluated by the study psychiatrist. In previous trials of collaborative care for depression in primary care settings [45,46,53], primary care providers have generally prescribed the initial antidepressant treatment, reserving consultation with the study psychiatrist for patients who do not respond to initial treatment. However, in our pilot study [54], we found that the oncologists at our study sites were reluctant to prescribe antidepressants and that antidepressant medication treatment was more effectively and efficiently managed by a study psychiatrist. If the patient is eligible to receive antidepressant medication treatment and agrees, the patient will start a first line antidepressant tailored to individual patient need. Additional psychotropic medications such as an anti-anxiety agent or sedative-hypnotic are used adjunctively if clinically indicated. The patient is evaluated at regular intervals or more frequently as needed by the study psychiatrist and the CDCS for treatment response and adverse medication effects. Treatment response is defined as: Full response/remission, fewer than 3/9 DSM IV depressive symptoms AND at least a 50% reduction in the PHQ-9 score; Partial response; a minimum 30% reduction in DSM IV depressive symptoms and PHQ-9 score; No response; 5 or more DSM IV depression symptoms OR greater than 15 on the PHQ-9.
For a patient who has not had full response to treatment by 4–8 weeks, Step 2 of the algorithm is employed. The patient who is on antidepressant medication treatment may require change or augmentation to the medication treatment or the addition of PST. The patient on PST alone is again educated about the option of antidepressant medication and is evaluated by the study psychiatrist for antidepressant medication treatment. The patient who has not had a full response by weeks 8–12 will proceed to Step 3 of the algorithm. The patient will be monitored by the study psychiatrist closely and adjustments to medication treatment, such as changing or adding an antidepressant medication, will be made. If indicated, the patient may be referred for specialty mental health care. The choice of treatment at this step depends on the clinical situation, the resources available, and the patient’s treatment preferences.
After completion of the acute phase of treatment, the patient is followed with a program to maximize maintenance treatment and prevent relapse. This includes the CDCS contacting the patient monthly up to 12 months after treatment initiation, behavioral activation support for engaging in pleasant activities, motivational support for ongoing use of PST skills and medication adherence and inviting patients to attend an open-ended PST support group. The telephone contacts include PHQ-9 monitoring of depressive symptoms [55–56] and education about the importance of continuing treatment. If the patient has been treated with antidepressants, maintenance prescriptions are continued by the patient’s oncologist in consultation with the study psychiatrist. If patients are experiencing a relapse of symptoms, the CDCS will arrange a visit and/or a visit with the study psychiatrist. If indicated, additional PST sessions will be provided and/or medication adjusted.
Problem Solving Therapy (PST) was chosen because it has been effective in primary care [28], our pilot study [29], and other studies with cancer patients [15, 57–59] PST’s brief psychoeducational characteristics make it feasible to provide and acceptable to patients with less education. PST uses the behavioral activation components of CBT, but with less emphasis on changing cognition and greater emphasis on patient assessment of personal contextual problems and skill-building to enhance self-management skills [60]. PST sessions ranging from 6–12 weeks are highly structured as described in published treatment manuals [49]. The perception of counselor as teacher or coach in PST is compatible with preferences of Hispanic patients [61], particularly when therapists are sensitive to the desire for warmth and sensitivity to family relationships [63, 63]
Cancer Specific and Socio-cultural Adaptations
In our extensive previous work with cancer patients, we have identified “competing stressors” that contribute to patient and family distress and can become barriers to optimal depression treatment engagement and adherence. These include system and patient-oncologist communication breakdown resulting in confusion about cancer treatment, medication management, and prognosis, cancer treatment side effects, the practical demands of keeping cancer treatment clinic appointments, hospitalizations, and a range of financial, day-to-day psychosocial stressors and family caregiving demands [64]. Therefore, supportive patient navigation and case management services are integrated throughout the ADAPt-C intervention.
The first stage of PST - problem orientation - may be the most critical for ethnic minorities and underserved populations due to the emphasis on personal control beliefs or self-efficacy [65, 66] and identifying negative thoughts [67, 68]. We find that successful advancement through PST stages is significantly dependent on pre-therapy clinical strategies: establishing a solid therapeutic alliance through rapport-building; application of motivational interviewing strategies [69]; providing assistance with practical needs (e.g., transportation, applying for disability insurance; facilitating receipt of financial assistance for cancer treatment; and childcare/caregiving issues); and addressing system barriers (i.e. patient-physician communication breakdown resulting in confusion about cancer treatment, medication management, and prognosis). Additionally, telephone-based counseling and regular telephone contact are found to reduce non-adherence or loss to follow-up, (i.e. “staying connected” via telephone calls when the patient feels too ill to come in as a result of cancer treatment side effects reinforces the therapeutic relationship and decreases attrition.). The problem orientation phase is used to review unfamiliar concepts and use of metaphors to explain unfamiliar skills. Strategies used to educate the patient about depression, its symptoms, effects, treatment options, medication management, and side-effects include: providing socio-cultural examples and role playing/modeling; allaying concerns, misconceptions, or stigma about depression and treatment; teaching time management and basic organizational skills; and discussing the usefulness of PST homework. As treatment proceeds to problem definition (because many patients present with complex psychosocial and health problems compounded by lack of resources), time is spent on helping the patient breakdown their complex problems into specific components and to concentrate on problems over which they have some control. The need for flexibility characterizes all care management, for example in the need to: reschedule appointments when the patient is not feeling well or misses appointments; arrange late afternoon and weekend appointments to accommodate patients or family members on whom the patient is dependent for transportation to the clinic who work during the day and cannot take off; and find a private available room within the busy, often noisy clinic setting. As a result, the majority of patients meet with the CDCS via telephone or in-person in addition to actual PST or psychiatric appointments. Initially, we asked a case manager to do some of the supportive navigation work, but found that this fragmented the critical relationship between patient and CDCS.
Enhanced Usual Care
EUC patients receive medical center standard oncology care and supportive services routinely provided to all patients with cancer. In addition, EUC patients are given a patient focused and a family focused educational pamphlet on depression and cancer and a listing of financial and community resources (e.g., mental health services and cancer support groups), and usual medical center services, such as social work department services, transportation services, patient financial services, and childcare resources (in Spanish for Spanish-speaking patients). With patient consent, as described in the informed written consent, the treating oncologist is informed via medical chart note if EUC patients screen positive for major depression. Treating oncology attending physicians, fellows and residents are invited to attend a didactic session led by the study psychiatrist on treating depression in cancer patients provided at the beginning of the study and repeated yearly thereafter. To date, participants are divided evenly between attending and fellows. At the end of the study, we will examine rates of antidepressant treatment among EUC patients at baseline and subsequent interviews and will include a question about the session in a survey of oncologists focused on satisfaction with the intervention model.
Evaluation
The analyses of outcome differences between intervention and EUC patients will use an intent-to-treat approach. The effectiveness of ADAPt-C intervention will be assessed by comparisons of PHQ-9 scores assessed at baseline versus 6, 12 and 18 months follow-up. A ≥50% reduction in PHQ-9 score at 12 months will be considered a substantial treatment response. In general, we will use logistic regression to estimate the effects of the intervention on the proportion of patients who show 50% or more improvement in PHQ-9 scores between intervention and EUC groups. Our power calculations and required sample size were based on prior quality improvement trials for depression [70, 36, 37, 45] and on expected attrition at 12 months due to patient deaths and inability to locate patients based on our previous studies [54, 41]. We assume a 45% attrition rate due to death and loss to follow-up at 12 months. Using that estimate, a reduced sample of N=320 would have 80% power to detect a 20% difference in the proportion of patients who show 50% or more reduction in PHQ-9 symptoms.
Health-related quality of life will be assessed using the Functional Assessment of Cancer Therapy Scale (FACT-G), a 27-item questionnaire (with Spanish translation) [71, 72], with subscale scores for physical, functional, social, and emotional well-being, as well as satisfaction with the treatment relationship. Because we previously found that usual care patients were more likely to have died during the intervention period and a trend towards improved cancer treatment adherence among intervention patients [54], we will compare survival and cancer treatment adherence rates between study groups. Adherence to external beam radiation will be defined as “completed as scheduled”, “completed but delayed” for those who missed at least one day of treatment, and “did not complete” for those who declined or did not complete the prescribed dose (unless the interruption was physician prescribed or resulted from machine breakdown). Adherence to IV-chemotherapy will be assessed as “completed as scheduled”, “completed but delayed due to toxicity”, “completed but delayed without a medical reason” and “did not complete or declined”. Univariate Logistic Regression and Polytomous Logistic Regression will be applied to evaluate the intervention effects on odds of cancer treatment adherence. Adherence to antidepressant medications will be assessed from: 1) patients’ report of adherence and side affects to the CDCS or study psychiatrist and recorded in the website clinical tracking data; 2) patient interview data at each outcome interview; and medical center pharmacy computer data (where the majority of patients obtain their medications) to examine refill rates and dosage. Adherence to PST treatment and psychiatric appointments will be based on website clinical tracking data. With the expected maximum sample size, we will not have statistical power to test hypotheses about the cost-effectiveness of intervention compared to usual care total cancer or health care costs. The direct cost of providing intervention will be calculated as the sum of the cost of the CDCS, the consulting time of the psychiatrist, antidepressant medication, and related intervention costs for training and administrative costs.
Baseline Characteristics of the ADAPt-C Study Sample
At the completion of recruitment, of 2,547 potentially eligible patients, 2,330 (91.5%) were screened; 1,682 (72.2%) females and 648 (27.8%) males. Of patients screened, 462 women and 130 men met criteria for major depression (27.5% vs 20.1%, p=0.0002, respectively). Of eligible patients, 28 women and 23 men met exclusion criteria (6.1% vs 17.7%, p<.0001). Of 541 study eligible patients, 375 women and 71 men were enrolled (86.4% vs 66.4%, p<.0001, respectively) and randomized to intervention or EUC. The majority of patients were Hispanic, foreign born, Spanish-speaking, and had lived in the US over 10 years. Sixty-four percent of patients had less than high school education, 81% of patients were unemployed and 58% of patients had comorbid medical conditions other than cancer. Of 446 enrolled patients, 48% also reported having symptoms consistent with dysthymia and 8% had a Brief Symptom Inventory Anxiety score of 14/24 or more. At baseline, 10% of patients reported taking antidepressant and/or anti-anxiety medication, 6.5% having received counseling from a social worker, psychologist, or doctor about their depression or anxiety, and 4% having attended a cancer support group.
Discussion
The ADAPt-C study has demonstrated the feasibility of recruiting a low-income, ethnic minority population-based oncology care sample of primarily female patients with depression and cancer in a randomized controlled trial. We believe that the use of bilingual, bicultural recruitment staff, approaching patients in the clinics, and offering patients a choice of treatment facilitated recruitment among women, but was less effective in overcoming reluctance to participate among men (a finding that may be in part attributable to perceptions about depression and helpseeking influenced by both gender and cultural preferences [73]. The intervention collaborative care program is modeled from the IMPACT study [28] and adapted for the low-income, predominantly Hispanic patients based on our previous studies of patients with cancer [29, 74, 17, 41]. The addition of patient navigation and case management services address needs encountered in public sector care systems. The stepped care model is flexible and thus responsive to the needs of patients with cancer. While we will not be able to analyze which components of this multifaceted intervention are most important in improving outcomes, earlier studies of collaborative care suggest that interventions aimed at multiple areas of care management are most effective.
Figure 1.
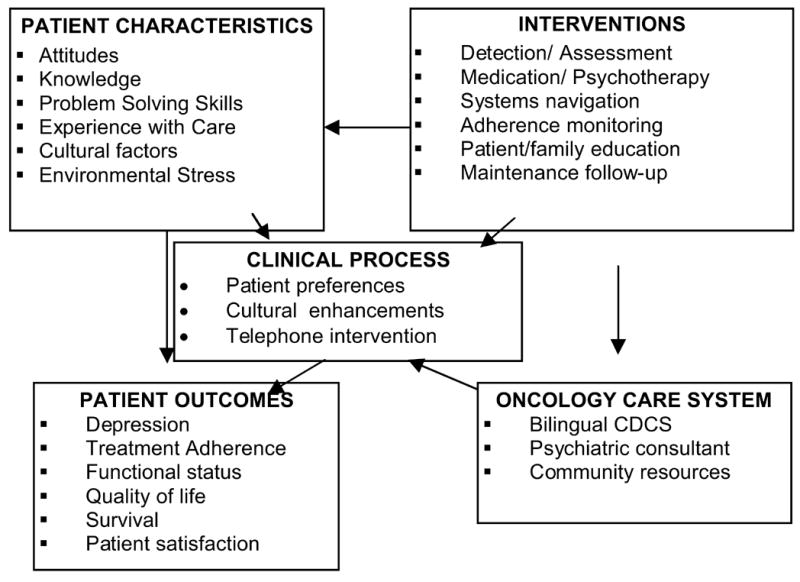
The ADAPt-C Model
Table 1.
Patient Demographic and Clinical Characteristics at Baseline
| EUC
N=220 |
INT
N=226 |
||
|---|---|---|---|
| n (%)
|
n (%)
|
p
|
|
| DEMOGRAPHIC CHARACTERISTICS | |||
| Female | 188 (85%) | 187 (83%) | 0.43 |
| 50yrs+ | 109 (50%) | 109 (48%) | 0.78 |
| Hispanic | 189 (86%) | 203 (90%) | 0.21 |
| Foreign Born | 187 (85%) | 203 (90%) | 0.12 |
| 10yrs+ | 171 (78%) | 160 (71%) | 0.09 |
| Spanish Only | 166 (76%) | 187 (84%) | 0.04 |
| Unmarried | 137 (62%) | 142 (63%) | 0.90 |
| 0–11 School Years | 139 (63%) | 145 (64%) | 0.83 |
| Unemployed | 188 (85%) | 174 (77%) | 0.02 |
| CLINICAL CHARACTERISTICS | |||
| PHQ-9 Score | 0.45 | ||
| Moderate 10–14 | 155 (70%) | 153 (68%) | |
| Major 15–19 | 57 (26%) | 59 (26%) | |
| Severe 20–27 | 8 (4%) | 14 (6%) | |
| Dysthymia | 97 (44%) | 117 (52%) | 0.10 |
| BSI Anxiety 14 or more | 17 (8%) | 19 (8%) | 0.79 |
| Taking medication for depression or anxiety | 24 (11%) | 21 (9%) | 0.57 |
| Receiving counseling for dep/anx | 13 (6%) | 16 (7%) | 0.62 |
| Attending a support group | 9 (4%) | 9 (4%) | 0.95 |
| Comorbidity | 132 (60%) | 128 (57%) | 0.47 |
| Brief pain inventory pain score 28+ | 23 (10%) | 22 (10%) | 0.80 |
| Cancer Site | 0.96 | ||
| Female Genital | 91 (41%) | 98 (43%) | |
| Breast | 48 (22%) | 49 (22%) | |
| Digestive System | 26 (12%) | 27 (12%) | |
| Other | 55 (25%) | 52 (23%) | |
| Cancer Stage | 0.72 | ||
| Stage 0,1,2 or unstaged | 161 (73%) | 162 (72%) | |
| Stage 3, 4 or recurrent | 59 (27%) | 64 (28%) | |
| Treatment Phase | 0.61 | ||
| Prior to Treatment | 28 (13%) | 23 (10%) | |
| Acute Treatment | 92 (42%) | 92 (41%) | |
| Follow-up Care | 100 (45%) | 111 (49%) | |
| Karnofsky Performance Status Scale | 6.01 (1.77) | 6.13 (1.76) | 0.49 |
| FACT-G (higher = better) | |||
| Physical Well-Being | 16.35 (5.77) | 16.68 (5.97) | 0.55 |
| Social/Family Well-Being | 14.34 (5.69) | 13.37 (6.42) | 0.09 |
| Emotional Well-Being | 13.43 (4.30) | 12.24 (4.00) | 0.003 |
| Functional Well-Being | 11.22 (4.86) | 11.19 (5.30) | 0.96 |
| Fatigue Scale | 24.66 (8.07) | 23.03 (8.38) | 0.06 |
| Spiritual Well-Being | 31.39 (7.13) | 30.59 (7.40) | 0.24 |
Acknowledgments
The study is supported by R01CA105269 from the National Cancer Institute, Office of Cancer Survivorship, Division of Cancer Control & Population Sciences, Bethesda, MD. (PI, Dr. Ell). Dr. Jurgen Unützer and Dr. Wayne Katon provided consultation on the study design.
Footnotes
Ell, K., Quon, B., Quinn, D., Dwight-Johnson, M.,Wells, A., Lee, P. J., Xie, B. (in press). Improving Treatment of Depression among Low-Income Patients with Cancer: The Design of the ADAPt-C Study. General Hospital Psychiatry.
References
- 1.Honda K, Goodwin RD. Cancer and mental disorders in a national community sample: findings from the national comorbidity survey. Psychother Psychosom. 2004;73(4):235–242. doi: 10.1159/000077742. [DOI] [PubMed] [Google Scholar]
- 2.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer I. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. Monographs. [DOI] [PubMed] [Google Scholar]
- 3.Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer I. 2004;32:32–39. doi: 10.1093/jncimonographs/lgh026. Monographs. [DOI] [PubMed] [Google Scholar]
- 4.Thompson DS, Shear MK. Psychiatric disorders and gynecological oncology: a review of the literature. Gen Hosp Psychiat. 1998;20(4):241–247. doi: 10.1016/s0163-8343(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 5.Okano Y, Okamura H, Watanabe T, et al. Mental adjustment to first recurrence and correlated factors in patients with breast cancer. Breast Cancer Res Tr. 2001;67(3):255–262. doi: 10.1023/a:1017942709369. [DOI] [PubMed] [Google Scholar]
- 6.Ahlberg K, Ekman T, Wallgren A, Gaston-Johansson F. Fatigue, psychological distress, coping and quality of life in patients with uterine cancer. J Adv Nurs. 2004;45(2):205–213. doi: 10.1046/j.1365-2648.2003.02882.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown KW, Levy AR, Rosberger Z, Edgar L. Psychological distress and cancer survival: a follow-up 10 years after diagnosis. Psychosom Med. 2003;65(4):636–643. doi: 10.1097/01.psy.0000077503.96903.a6. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52(1):106–111. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hjerl K, Andersen EW, Keiding N, Mouridsen HT, Mortensen PB, Jorgensen T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44(1):24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- 10.Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol. 1998;16(4):1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- 11.Ashbury FD, Madlensky L, Raich P, et al. Antidepressant prescribing in community cancer care. Support Care Cancer. 2003;11(5):278–285. doi: 10.1007/s00520-003-0446-8. [DOI] [PubMed] [Google Scholar]
- 12.Fallowfield L, Ratcliffe D, Jenkins V, Saul J. Psychiatric morbidity and its recognition by doctors in patients with cancer. Br J Cancer. 2001;84(8):1011–1015. doi: 10.1054/bjoc.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald MV, Passik SD, Dugan W, Rosenfeld B, Theobald DE, Edgerton S. Nurses’ recognition of depression in their patients with cancer. Oncol Nurs Forum. 1999;26(3):593–599. [PubMed] [Google Scholar]
- 14.Newell S, Sanson-Fisher RW, Girgis A, Bonaventura A. How well do medical oncologists’ perceptions reflect their patients’ reported physical and psychosocial problems? Data from a survey of five oncologists. Cancer. 1998;83(8):1640–1651. [PubMed] [Google Scholar]
- 15.Strong V, Sharpe M, Cull A, Maguire P, House A, Ramirez A. Can oncology nurses treat depression? A pilot project. J Adv Nurs. 2004;46(5):542–548. doi: 10.1111/j.1365-2648.2004.03028.x. [DOI] [PubMed] [Google Scholar]
- 16.Prieto JM, Atala J, Blanch J, et al. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol. 2005;23(25):6063–6071. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 17.Ell K, Sanchez K, Vourlekis B, et al. Depression, receipt of depression care, and correlates of depression among low-income women with breast or gynecological cancer. J Clin Oncol. 2005;23:3052–3060. doi: 10.1200/JCO.2005.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewitt M, Rowland JH. Mental health service use among adult cancer survivors: Analyses of the national health interview study. J Clin Oncol. 2002;20(23):4581–4590. doi: 10.1200/JCO.2002.03.077. [DOI] [PubMed] [Google Scholar]
- 19.Cooper-Patrick L, Powe NR, Jenckes MW, Gonzales JJ, Levine DM, Ford DE. Identification of patient attitudes and preferences regarding treatment of depression. J Gen Intern Med. 1997;12(7):431–438. doi: 10.1046/j.1525-1497.1997.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo JJ, Cooper-Patrick L, Lesikar S. Depressive symptoms of whites and African Americans aged 60 years and older. J Gerontol B Psychol Sci Soc Sci. 1998;53(5):277–286. doi: 10.1093/geronb/53b.5.p277. [DOI] [PubMed] [Google Scholar]
- 21.Marwaha S, Livingston G. Stigma, racism or choice. Why do depressed ethnic elders avoid psychiatrists? J Affect Disorders. 2002;72(3):257–265. doi: 10.1016/s0165-0327(01)00470-0. [DOI] [PubMed] [Google Scholar]
- 22.Mills TL, Alea NL, Cheong JA. Differences in the indicators of depressive symptoms among a community sample of African-American and Caucasian older adults. Community Ment Hlt J. 2004;40(4):309–331. doi: 10.1023/b:comh.0000035227.57576.46. [DOI] [PubMed] [Google Scholar]
- 23.Schraufnagel TJ, Wagner AW, Miranda J, Roy-Byrne PP. Treating minority patients with depression and anxiety: What does the evidence tell us? Gen Hosp Psychiat. 2006;28(1):27–36. doi: 10.1016/j.genhosppsych.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Johnson TK, Gilliland FD, Hoffman RM, et al. Racial/Ethnic differences in functional outcomes in the 5 years after diagnosis of localized prostate cancer. J Clin Oncol. 2004;22(20):4193–4201. doi: 10.1200/JCO.2004.09.127. [DOI] [PubMed] [Google Scholar]
- 25.Miranda J, Duan N, Sherbourne CD, Schoenbaum M, Lagomasino I, Wells KB. Improving care for minorities: Can quality improvement intervention improve care and outcomes for depressed minorities? Health Serv Res. 2003;38:613–630. doi: 10.1111/1475-6773.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells K, Sherbourne C, Schoenbaum M, et al. Five-year impact of quality improvement for depression: Results of a group-level randomized controlled trial. Arch Gen Psychiat. 2004;61(4):378–386. doi: 10.1001/archpsyc.61.4.378. [DOI] [PubMed] [Google Scholar]
- 27.Reuben DB. Organizational interventions to improve health outcomes of older persons. Med Care. 2002;40(5):416–428. doi: 10.1097/00005650-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 29.Dwight-Johnson M, Ell K, Jiuan-Lee P. Can collaborative care address the needs of low-income Latinas with comorbid depression and cancer? Results from a randomized pilot study. Psychosomatics. 2005;46(3):224–232. doi: 10.1176/appi.psy.46.3.224. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the patient health questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21(6):547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitz R, Lepore MF, Sullivan LM, Amaro H, Samet JH. Alcohol abuse and dependence in Latinos living in the United States: Validation of the CAGE (4M) questions. Arch Intern Med. 1999;159:718–724. doi: 10.1001/archinte.159.7.718. [DOI] [PubMed] [Google Scholar]
- 33.Mor V, Laliberte L, Morris JN, Wiermann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. J Clin Oncol. 1984;2:1170–1176. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Giuliano AR, Mokuau N, Hughes C, et al. Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Ann Epidemiol. 2000;10:S22–34. doi: 10.1016/s1047-2797(00)00195-2. [DOI] [PubMed] [Google Scholar]
- 35.Miranda J, Azocar F, Organista KC, Munoz RF, Lieberman A. Recruiting and retaining low-income Latinos in psychotherapy research. J Consult Clin Psych. 1996;64(5):868–874. doi: 10.1037//0022-006x.64.5.868. [DOI] [PubMed] [Google Scholar]
- 36.Miranda J, Chung JY, Green BL, et al. Treating depression in predominantly low-income young minority women: A randomized controlled trial. JAMA. 2003;290(1):57–65. doi: 10.1001/jama.290.1.57. [DOI] [PubMed] [Google Scholar]
- 37.Miranda J, Green BL, Krupnick JL, et al. One-year outcomes of a randomized clinical trial treating depression in low-income minority women. J Consult Clin Psych. 2006;74(1):99–111. doi: 10.1037/0022-006X.74.1.99. [DOI] [PubMed] [Google Scholar]
- 38.Skaff MM, Chesla CA, Mycue VD, Fisher L. Lessons in cultural competence: Adapting research methodology for Latino participants. J Community Psychol. 2002;30(3):305–323. [Google Scholar]
- 39.Swanson GM, Ward AJ. Recruiting minorities into clinical trials: Toward a participant-friendly system. J Natl Cancer I. 1995;87:1747–1766. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- 40.Wells A, Zebrack B. Psychosocial barriers contributing to the under-representation of racial/ethnic minorities in cancer clinical trials. Soc Work Health Care. doi: 10.1300/j010v46n02_01. in press. [DOI] [PubMed] [Google Scholar]
- 41.Ell K, Vourlekis B, Lee P, Xie B. Patient navigation and case management following an abnormal mammogram: A randomized clinical trial. Prev Med. doi: 10.1016/j.ypmed.2006.08.001. in press. [DOI] [PubMed] [Google Scholar]
- 42.Ell K, Vourlekis B, Nissly J, Padgett D, Pineda D, Walther V. Integrating mental health screening and abnormal cancer screening follow-up: An intervention to reach low-income women. Community Ment Hlt J. 2002;38:311–325c. doi: 10.1023/a:1015901409211. [DOI] [PubMed] [Google Scholar]
- 43.Hunkeler EM, Katon W, Tang L. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ. 2006;332(7536):259–263. doi: 10.1136/bmj.38683.710255.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katon W, Russo J, Sherbourne C, et al. Incremental cost-effectiveness of collaborative care intervention for panic disorder. Psychol Med. 2006;36(3):353–363. doi: 10.1017/S0033291705006896. [DOI] [PubMed] [Google Scholar]
- 45.Unutzer J, Katon W, Callahan CM, et al. IMPACT Investigators. Improving mood-promoting access to collaborative treatment Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 46.Wells KB, Sherbourne CD, Shoenbaum M, et al. Impact of disseminating quality improvement programs for depression in managed primary care: A randomized controlled trial. JAMA. 2000;283:212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 47.Areán PA, Unützer J. Inequities in depression management in low-income, minority, and old-old adults: A matter of access to preferred treatments? J Am Geriatr Soc. 2003;51:1808–1809. doi: 10.1046/j.1532-5415.2003.51569.x. [DOI] [PubMed] [Google Scholar]
- 48.Arean PA, Ayalon L, Hunkeler E, et al. Improving depression care for older, minority patients in primary care. Med Care. 2005;43(4):381–390. doi: 10.1097/01.mlr.0000156852.09920.b1. [DOI] [PubMed] [Google Scholar]
- 49.Nezu A, Nezu CM, Friedman SH, Faddis S, Houts PS. A problem-solving approach: Helping cancer patients cope. Washington, D.C.: American Psychological Association; 1998. [Google Scholar]
- 50.Mynors-Wallis L, Davies I, Gray A, Barbour F, Gath DH. A randomized controlled trial and cost analysis of problem-solving treatment for emotional disorders given by community nurses in primary care. Brit J Psychiat. 1997;170:113–119. doi: 10.1192/bjp.170.2.113. [DOI] [PubMed] [Google Scholar]
- 51.Dwight-Johnson M, Sherbourne CD, Liao D, Wells KB. Treatment preferences among depressed primary care patients. J Gen Intern Med. 2000;15:527–534. doi: 10.1046/j.1525-1497.2000.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Comprehensive Cancer Network. [Accessed July 15, 2006];Clinical Practice Guidelines in Oncology, Distress Management Assessment Tool. http://www.nccn.org/professionals/physician_gls/default.asp.
- 53.Katon W. Treatment trials in real world settings. Methodological issues and measurement of disability and costs. Gen Hosp Psychiat. 1999;21(4):237–238. doi: 10.1016/s0163-8343(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 54.Dwight-Johnson M, Ell K, Lee P. Can collaborative care address the needs of low-income Latinas with comorbid depression and cancer? Results from a randomized pilot study. Psychosomatics. 2005;46(3):224–232. doi: 10.1176/appi.psy.46.3.224. [DOI] [PubMed] [Google Scholar]
- 55.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Pinto-Meza A, Serrano-Blanco A, Penarrubia MT, Blanco E, Haro JM. Assessing depression in primary care with the PHQ-9: Can it be carried out over the telephone? J Gen Intern Med. 2005;20(8):738–742. doi: 10.1111/j.1525-1497.2005.0144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen SM, Shah AC, Nezu AM, et al. A problem-solving approach to stress reduction among younger women with breast carcinoma: A randomized controlled trial. Cancer. 2002;94(12):3089–3100. doi: 10.1002/cncr.10586. [DOI] [PubMed] [Google Scholar]
- 58.Nezu A, Nezu C, Houts P, et al. Cancer and psychological distress: Two investigations regarding the role of social problem-solving. J Psychosoc Oncol. 1999;16:27–40. [Google Scholar]
- 59.Nezu AM, Nezu CM, Houts PS, Friedman SH, Faddis S. Relevance of problem-solving therapy to psychosocial oncology. J Psychosoc Oncol. 1999;16(3):5–26. [Google Scholar]
- 60.Nezu A, Nezu C, Perri MG. Problem-solving therapy for depression: Theory, research and clinical guidelines. New York: Wiley; 1989. [Google Scholar]
- 61.Martinez C. Mexican-Americans. In: Comas-Dias L, Griffith EEH, editors. Clinical guidelines in cross-cultural Mental Health. New York: Wiley; 1988. pp. 182–203. [Google Scholar]
- 62.Bernal G, Bonilla J, Bellido C. Ecological validity and cultural sensitivity for outcome research: Issues for the cultural adaptation and development of psychosocial treatments with Hispanics. J Abnorm Psychol. 1995;23(1):67–82. doi: 10.1007/BF01447045. [DOI] [PubMed] [Google Scholar]
- 63.Mezzich JE, Ruiz P, Munoz RA. Mental health care for Hispanic Americans: A current perspective. Cultural Diversity & Ethnic Minority Psychology. 1999;5(2):91–102. doi: 10.1037/1099-9809.5.2.91. [DOI] [PubMed] [Google Scholar]
- 64.Nedjat-Haiem F, Palinkas LA, Cabassa LJ, Wells A, Ell K. Health-services utilization of low-income women with cancer: A qualitative study. J Health Care Poor U. Under review. [Google Scholar]
- 65.Maliski SL, Kwan L, Krupski T, Fink A, Orecklin JR, Litwin MS. Confidence in the ability to communicate with physicians among low-income patients with prostate cancer. Urology. 2004;64:329–334. doi: 10.1016/j.urology.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 66.Farrell K, Wicks MN, Martin JC. Chronic disease self-management improved with enhanced self-efficacy. Clin Nurs Res. 2004;13(4):289–308. doi: 10.1177/1054773804267878. [DOI] [PubMed] [Google Scholar]
- 67.Peden AR, Rayens MK, Hall LA, Grant E. Negative thinking and the mental health of low-income single mothers. J Nurs Scholarship. 2004;36(4):337–344. doi: 10.1111/j.1547-5069.2004.04061.x. [DOI] [PubMed] [Google Scholar]
- 68.Lucas MS. Problem-solving appraisal in counseling and with different populations. Couns Psychol. 2004;32(3):450–459. [Google Scholar]
- 69.Ell K, Wells A, Cabassa L, Hansen M. Problem-solving therapy: Socio-cultural adaptations for low-income, ethnic minority patients with depression. in progress. [Google Scholar]
- 70.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: A randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiat. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 71.Cella D, Hernandez L, Corona M, Vaquero M, Shimoto G, Baez L. Spanish language translation and intital validation of the functional assessment of cancer therapy quality-of-life instrument. Med Care. 1998;36(9):1407–1417. doi: 10.1097/00005650-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Cella DF, Tulsky G, Sarafian B, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 73.Rochlen AB, Hoyer WD. Marketing mental health to men: Theoretical and practical considerations. J Clin Psychol. 2005;61:675–684. doi: 10.1002/jclp.20102. [DOI] [PubMed] [Google Scholar]
- 74.Ell K, Sanchez K, Vourlekis B, et al. Depression, correlates of depression, and receipt of depression care among low-income women with breast or gynecologic cancer. J Clin Oncol. 2005;23:3052–3060. doi: 10.1200/JCO.2005.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


