Abstract
DNA methylation is an epigenetic feature that is associated with X chromosome inactivation, genomic imprinting, transcriptional silencing of genes and genomic stability. Folate provides a labile source of methyl groups which may be used for cellular methylation reactions including DNA methylation. The methylenetetrahydrofolate reductase (MTHFR) 677C→T variant is an important determinant of folate nutriture and may influence DNA methylation. This study sought to assess the influence of the MTHFR C677T genotype on global leukocyte DNA methylation in young (18–45y) Mexican American women (n=43; 14 CC, 12 CT and 17 TT). Subjects consumed a folate restricted diet (135 μg DFE/d) for 7 wk followed by folate treatment with 400 or 800 μg DFE/d for 7 wk. Global leukocyte DNA methylation was assessed via the cytosine extension assay at week 0, week 7 (after folate restriction) and week 14 (after folate treatment). No main effects of MTHFR C677T genotype or folate intake were detected at any time point during the study. However, at the end of folate treatment (wk 14), DNA methylation was lower (P<0.05) in women with the MTHFR 677TT genotype relative to the CT or CC genotype. Because it is unlikely that folate treatment would result in methyl group loss, we suggest that there was a delay in DNA methylation response to folate intake. Overall, these data suggest that the MTHFR 677TT genotype and folate interact to lower global leukocyte DNA methylation patterns in young Mexican American women.
Keywords: MTHFR, Folate, Folic acid, Women, DNA methylation, Human
1. Introduction
DNA methylation is an epigenetic feature that is associated with X chromosome inactivation, genomic imprinting, transcriptional silencing of genes and genomic stability [1]. Aberrations in DNA methylation are associated with numerous pathologies, including cancer. Cytosine DNA methylation occurs primarily on CpG dinucleotides and is catalyzed by a family of DNA methyltransferases (DNMTs) that transfer methyl groups from S-adenosylmethionine (SAM) to cytosine residues. SAM is formed in the methionine cycle where folate serves as an important source of methyl groups utlilized by SAM.
Folate, in a variety of forms, functions to donate and accept one carbon units in a metabolic system referred to as one-carbon metabolism. 5,10-methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in one-carbon metabolism because it diverts one-carbon units towards methylation reactions (5,10-methylenetetrahydrofolate → 5-methyltetrahydrofolate) at the expense of nucleotide synthesis. A common single nucleotide polymorphism in the MTHFR gene involving a cytosine (C) to thymine (T) transition at nucleotide 677 is associated with reduced enzyme activity [2], higher plasma total homocysteine [3] and altered risk for chronic diseases and congenital anomalies [2,4].
To date, only a few studies have assessed the influence of folate intake and/or the MTHFR 677C→T polymorphism on DNA methylation under controlled conditions [5–7]. Because of the relationship between aberrations in DNA methylation and disease risk, more studies are needed to more fully delineate the effect of folate intake and relevant common genetic variants on DNA methylation. The present study sought to investigate the influence of the MTHFR 677C→T variant on global leukocyte DNA methylation in young Mexican American women consuming controlled folate intakes. This study is the first to report that the MTHFR 677TT genotype interacts with folate intake to lower global leukocyte DNA methylation in young women consuming controlled folate intakes.
2. Methods
2.1 Subjects
From 1999–2002, self-reported Mexican American women defined as having two parents possessing the same race/ethnicity, were recruited among staff and students at Cal Poly Pomona University and from the surrounding Southern California communities. Additional inclusion criteria were appropriate MTHFR C677T genotype, non-smoker, non-anemic, non-supplement user (within past 3 mo), no chronic drug use, no anti-folate medication use, no history of chronic disease, non-pregnant and not planning a pregnancy; non-lactating and normal blood chemistry profile. The screening and experimental procedures were reviewed and approved by the Institutional Review Board of Cal Poly Pomona University for human subject use and informed consent was obtained from each participant.
2.2 Experimental Design
This was a 14-wk controlled feeding study consisting of 7 weeks of folate restriction followed by 7 weeks of folate treatment with 400 or 800 μg/d as dietary folate equivalents (DFE) and has been described in detail elsewhere [3]. During the period of folate restriction (wk 0–7), the subjects consumed a low folate diet providing 135 μg DFE/d. During the period of folate treatment (wk 8–14), the subjects continued to consume the folate restricted diet providing 135 μg DFE/d plus 156 or 391 μg/d supplemental folic acid (Sigma Chemical, St. Louis, MO) for total folate intakes of 400 or 800 μg DFE/d, respectively. The folic acid supplement was prepared as a solution [3] and consumed with the meals. The RDA or adequate intake (AI) for all other essential nutrients was provided as a combination of diet and supplements in the form of pills [3].
2.3 Analytical methods
2.3.1. Global DNA Methylation
The cytosine extension assay [8] with minor modifications [9] was used to assess global DNA methylation. DNA was extracted from mononuclear cells [10] and genomic DNA (0.75 μg) was digested with an excess of methylation-sensitive HpaII restriction endonuclease (New England Biolabs, Beverly, MA) according to the manufacturer’s protocol. A second DNA aliquot (0.75 μg) was digested simultaneously with methylation-insensitive isoschizomer MspI (New England Biolabs) and a third DNA aliquot (0.75 μg) was incubated without restriction enzyme and served as a background control. The single nucleotide extension reaction was performed in a 40 μL PCR mixture containing 0.75μg genomic DNA, 1x NEBuffer 2 (New England Biolabs), 10 units of Klenow Fragment/exo- (New England Biolabs) and 4μM [3H]dCTP (American Radiolabeled Chemicals, Inc., St. Louis, MO). After incubating, the sample (35 μL) was applied to Whatman DE-81 ion-exchange filter paper which was washed, air dried and processed for scintillation counting. Each sample was run in duplicate. The [3H]dCTP incorporation into DNA was expressed as mean disintegrations per minute (dpm) per 0.75μg of DNA. The absolute percent of double stranded unmethylated CCGG sites was calculated as: (HpaII induced dpm/MspI induced dpm)(100). In addition to running the undigested control, we included an aliquot of lambda DNA as a positive control suitable for high levels of [3H]dCTP incorporation, an aliquot of lambda DNA that had been methylated completely in vitro by the action of M·SssI as a negative control suitable for low levels of [3H]dCTP incorporation, and an aliquot of human pooled DNA as an intermediate control. Because of the large sample number, the samples were run in batches. All batches contained the controls described above and were blinded to the investigator performing biochemical tests. In addition, each batch (n= 14) contained one and occasionally two subjects with the CC, CT and TT genotype. For each subject within a batch, week 0, 7 and 14 were analyzed simultaneously in duplicate. The intra-assay coefficient of variation (CV) was 4% based on the sample duplicates. The inter-assay CVs were 11.6%, 14.8% and 8.3% based on the negative control, pooled plasma and the positive control, respectively.
2.3.2. MTHFR C677T genotype
DNA for genotyping was extracted from leukocytes by use of a commercially available kit (QIAmp blood kit; Qiagen, Santa Clarita, CA) and determination of the C677T MTHFR genotype was performed by the method described by Frosst et al. [2].
2.3.3. Statistical analysis
Baseline differences among genotypes in leukocyte DNA methylation were analyzed by 1-way ANOVA. This model utilized DNA methylation values at week 0 as the dependent variable, and MTHFR C677T genotype as the predictor (3 levels: CC, CT, TT). The relationship between leukocyte DNA methylation and genotype throughout the 14 wk study was analyzed by a repeated-measures ANOVA. This model utilized DNA methylation values as the dependent variable. Predictors were one within subjects factor (weeks 0, 7 and 14), and two between subjects factors, MTHFR C677T genotype (three levels: CC, CT, TT) and folate intake (two levels: 400 and 800 μg DFE/d). Where a significant within-subjects effect was detected, profile contrasts were performed to examine the influence of folate restriction (wk 7 versus 0) and folate treatment (wk 14 versus 7). Where a significant result based on the profile contrasts was detected, a 1-way ANOVA was performed for wk 7 and/or wk 14 to further explore this relationship. DNA methylation at the particular week was the dependent variable, and MTHFR C677T genotype (three levels: CC, CT, TT) was the predictor. Analyses follow Zar [11]. Data are presented as means ± SEM. All data summaries and analyses were performed using SPSS 11.5 for Windows (SPSS, Chicago, IL) and differences were determined at P < .05.
3. Results
Forty-three Mexican American women participated in this study pre-selected for the following MTHFR C677T genotypes: 14 wild type (CC), 12 heterozygous (CT) and 17 homozygous for the T variant (TT). The women were 25 y of age (range 18–44y) with a body mass index (kg/m2) of 25.2 (range = 19.5–32). No differences (P>0.05) in weight or age were detected among the MTHFR C677T genotypes. Body weights were maintained within 5% of baseline in all but nine subjects (3 CC, 3 CT and 3 TT) who lost ~ 8% (range: 6.5 – 10.7%) of baseline weight. Leukocyte global DNA methylation did not differ (P>0.05) between the MTHFR C677T genotypes at baseline. No differences existed in serum folate or plasma total homocysteine (tHcy) concentrations between the MTHFR C677T genotypes at baseline although RBC folate was lower in the TT compared to the CC genotype [3].
No week (i.e., folate restriction and/or folate treatment) or MTHFR C677T genotype main effect was detected on global leukocyte CpG dinucleotide methylation. In addition, no differences (P> .05) in the percent unmethylated CCGG sites were detected between the 400 or 800 μg DFE/d treatment groups (24.3 ± 0.56 % and 25.3 ± 1.0 %, respectively), nor was there an interaction (P> .05) between treatment level and MTHFR C677T genotype. However, a week by MTHFR C677T genotype interaction (P = .001) was detected, an effect that occurred primarily during folate treatment (400 + 800 μg DFE/d; P = .007; Fig. 1). At week 14, the percent unmethylated CCGG sites in DNA was higher in women with the MTHFR 677TT genotype relative to women with the CC (P = .015) or CT (P = .033) genotype.
Fig. 1.
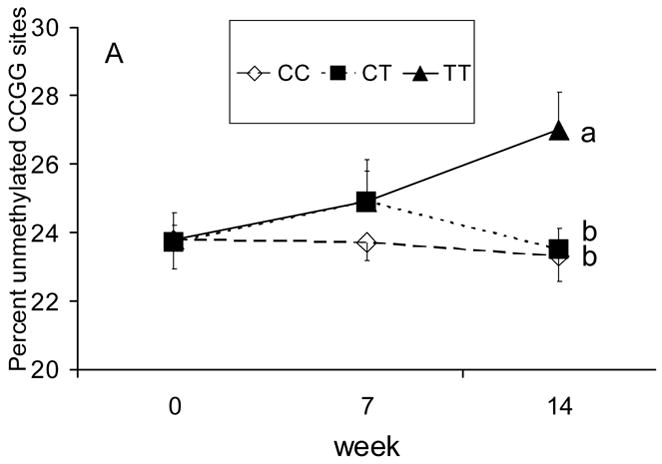
The response of global leukocyte DNA methylation to folate restriction (wk 0 – wk 7; 135 μg/d as dietary folate equivalents, DFE) and folate treatment (wk 7 – wk 14; 400 and 800 μg DFE/d groups combined) in young Mexican American women differing in the methylenetetrahydrofolate reductase (MTHFR) 677 Cytosine (C) → Thymine (T) genotype (n=14 CC, 12 CT and 17 TT). Values are means ± SEM. Lines with different superscript letters indicate differences (P < .05) in the measured variable at wk 14 between MTHFR C677T genotypes (1-way ANOVA).
Global leukocyte CpG dinucleotide methylation did not correlate with serum folate or plasma total homocysteine concentrations which were published separately [3]. Briefly, serum folate decreased (P< .001) after folate restriction and increased (P< .001) after folate treatment particularly in the 800 versus 400 μg DFE/d group (folate level interaction=P< .001). Plasma tHcy increased (P< .001) after folate restriction and decreased (P< .001) after folate treatment. The response to folate treatment with 400 versus 800 μg DFE/d was similar (P> .05).
4. Discussion
The most important finding of this study is that the response of DNA methylation to folate restriction/treatment is a function of MTHFR C677T genotype (week x genotype interaction, P = .001). At the end of the fourteen week study, leukocyte CpG methylation was significantly lower in women with the MTHFR 677 TT genotype relative to women with the 677 CC or CT genotype. Because it is unlikely that folate treatment resulted in methyl group loss, we suggest that there was a delay in DNA methylation response to folate intake. This hypothesis is consistent with findings from other controlled feeding protocols involving 7 weeks of folate depletion followed by 7 weeks of folate repletion in human subjects. Rampersaud et al. [6] reported that a significant proportion of elderly women continued to respond during folate repletion with increasing [3H]methyl incorporation (i.e., decreases in DNA methylation) even though significant improvements were detected in blood folate indexes. Further, Shellnut et al. [7] reported that DNA methylation declined significantly during folate treatment with 400 μg DFE/d in young women with the MTHFR 677CC genotype. The delay in DNA methylation response to folate intake may be related, in part, to the very slow in vivo turnover of whole-body folate pools [12].
Our finding that the MTHFR 677TT genotype affects genomic DNA methylation through an interaction with folate intake/status is consistent with previous work. Friso et al. [13] reported that global leukocyte DNA methylation was lower in subjects with the MTHFR 677TT genotype relative to the CC genotype only when folate levels were low. Quinlivan et al. [14] reported the diversion of one-carbon units towards nucleotide synthesis at the expense of DNA methylation in young women with the MTHFR 677TT genotype after 7 weeks of folate restriction. Our findings however are less consistent with the results from another controlled folate feeding study [7]. In this 14 wk study, global leukocyte DNA methylation was assessed in young Caucasian women after 7 weeks of folate restriction (~115 μg DFE/d) and after 7 weeks of folate treatment with 400 μg DFE/d. While no differences were detected between women possessing the MTHFR 677 CC or TT genotypes at any study time point, DNA methylation as measured by the methyl acceptance assay declined significantly during folate treatment in women with the CC genotype and increased significantly during folate treatment in women with the MTHFR 677 TT genotype [7]. The major difference between the two studies is the ethnic group in which the study was conducted. It is possible that differences exist between Mexican American and Caucasian women in one-carbon flux and the prevalence of other genetic variants in one-carbon metabolic genes.
The results of the present study and others [7,15,16] do not support the use of leukocyte DNA methylation as a sensitive biomarker of folate status in young women or men. Specifically folate intake, itself, was not enough to modify DNA methylation within the time constraints of the study. These results differ from previous work conducted in elderly women whereby folate restriction was associated with a significant reduction in global leukocyte DNA methylation [5,6]. Aging, itself, is associated with a progressive loss of 5-methylcytosine content, primarily within DNA repeated sequences [17], and may contribute, via an unknown mechanism, to the apparent enhanced sensitivity to folate restriction observed in older women.
Taken together with the results obtained from other studies [5–7,13,15–17], it is evident that numerous factors including age and gender, as well as monogenic and complex polygenic mechanisms, interact with the availability of methyl groups from dietary sources to affect DNA methylation.
Acknowledgments
This study was supported by the National Institutes of Health grant S06GM53933 and funds from the California Agricultural Research Initiative. We thank all the subjects who participated in the study.
Footnotes
Supported by the National Institutes of Health grant S06GM53933 and funds from the California Agricultural Research Initiative.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Juan Axume, Human Nutrition and Food Science Department, Cal Poly Pomona University, 3801 W Temple Avenue, Pomona, CA 91768, USA.
Steven S Smith, City of Hope National Medical Center and Beckman Research Institute, 1500 E. Duarte Road, Duarte, CA 91010, USA.
Igor P Pogribny, Division of Biochemical Toxicology, FDA-National Center for Toxicological Research, Jefferson, AR 72079.
David J. Moriarty, Biological Sciences Department, Cal Poly Pomona University, 3801 W Temple Avenue, Pomona, CA 91768, USA
Marie A. Caudill., Human Nutrition and Food Science Department, Cal Poly Pomona University, 3801 W Temple Avenue, Pomona, CA 91768, USA
References
- 1.McCabe D, Caudill MA. DNA methylation, genomic silencing, and links to nutrition and cancer. Nutr Rev. 2005;63:183–95. doi: 10.1301/nr.2005.jun.183-195. [DOI] [PubMed] [Google Scholar]
- 2.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LAJ, van den Heuve LP, Rozen R. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–13. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 3.Guinotte CL, Burns MG, Axume JA, Hata H, Urrutia TF, Alamilla A, McCabe D, Singgih A, Cogger EA, Caudill MA. Methylenetetrahydrofolate reductase 677C-->T variant modulates folate status response to controlled folate intakes in young women. J Nutr. 2003;133:1272–80. doi: 10.1093/jn/133.5.1272. [DOI] [PubMed] [Google Scholar]
- 4.Rozen R. Folate and genetics. J Food Sc. 2004;69:SNQ65–7. [Google Scholar]
- 5.Jacob RA, Gretz DM, Taylor PC, James SJ, Pogribny IP, Miller BJ, Henning SM, Swendseid ME. Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J Nutr. 1998;128:1204–12. doi: 10.1093/jn/128.7.1204. [DOI] [PubMed] [Google Scholar]
- 6.Rampersaud GC, Kauwell GP, Hutson AD, Cerda JJ, Bailey LB. Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. Am J Clin Nutr. 2000;72:998–1003. doi: 10.1093/ajcn/72.4.998. [DOI] [PubMed] [Google Scholar]
- 7.Shelnutt KP, Kauwell GP, Gregory JF, Maneval DR, Quinlivan EP, Theriaque DW, Henderson GN, Bailey LB. Methylenetetrahydrofolate reductase 677C-->T polymorphism affects DNA methylation in response to controlled folate intake in young women. J Nutr Biochem. 2004;15:554–60. doi: 10.1016/j.jnutbio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Pogribny IP, James SJ, Jernigan S, Pogribna M. Genomic hypomethylation is specific for preneoplastic liver in folate/methyl deficient rats and does not occur in non-target tissues. Mutat Res. 2004;548:53–9. doi: 10.1016/j.mrfmmm.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Shevchuk T, Kretzner L, Munson K, Axume J, Clark J, Dyachenko OV, Caudill M, Buryanov Y, Smith SS. Transgene-induced CCWGG methylation does not alter CG methylation patterning in human kidney cells. Nucleic Acids Res. 2005;33:6124–36. doi: 10.1093/nar/gki920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambrook J, Russell DW. Molecular cloning: A laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. Isolation of high-molecular weight DNA from mammalian cells using proteinase K and phenol; pp. 6.8–6.11. [Google Scholar]
- 11.Zar JH. Biostatistical Analysis. 4. Upper Saddle River, NJ: Prentice Hall; 1999. p. 929. [Google Scholar]
- 12.Gregory JF, Williamson J, Liao JF, Bailey LB, Toth JP. Kinetic model of folate metabolism in nonpregnant women consuming [2H2]folic acid: isotopic labeling of urinary folate and the catabolite para-acetamidobenzoylglutamate indicates slow, intake-dependent, turnover of folate pools. J Nutr. 1998;128:1896–906. doi: 10.1093/jn/128.11.1896. [DOI] [PubMed] [Google Scholar]
- 13.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, Olivieri O, Jacques PF, Rosenberg IH, Corrocher R, Selhub J. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA. 2002;99:5606–11. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinlivan EP, Davis SR, Shelnutt KP, Henderson GN, Ghandour H, Shane B, Selhub J, Bailey LB, Stacpoole PW, Gregory JF. Methylenetetrahydrofolate reductase 677C->T polymorphism and folate status affect one-carbon incorporation into human DNA deoxynucleosides. J Nutr. 2005;135:389–96. doi: 10.1093/jn/135.3.389. [DOI] [PubMed] [Google Scholar]
- 15.Jacob RA, Pianalto FS, Henning SM, Zhang JZ, Swendseid ME. In vivo methylation capacity is not impaired in healthy men during short-term dietary folate and methyl group restriction. J Nutr. 1995;125:1495–502. doi: 10.1093/jn/125.6.1495. [DOI] [PubMed] [Google Scholar]
- 16.Fenech M, Aitken C, Rinaldi J. Folate, vitamin B12, homocysteine status and DNA damage in young Australian adults. Carcinogenesis. 1998;19:1163–71. doi: 10.1093/carcin/19.7.1163. [DOI] [PubMed] [Google Scholar]
- 17.Issa JP. Age-related epigenetic changes and the immune system. Clin Immunol. 2003;109:103–8. doi: 10.1016/s1521-6616(03)00203-1. [DOI] [PubMed] [Google Scholar]


