Abstract
NPY has long been known to be involved in stress, centrally as an anxiolytic neuromodulator, and peripherally as a sympathetic nerve- and in some species, platelet-derived vasoconstrictor. The peptide is also a vascular mitogen, via Y1/Y5, and is angiogenic via Y2/Y5 receptors. Arterial injury activates platelet NPY and vascular Y1 receptors, inducing medial hypertrophy and neointima formation. Exogenous NPY, dipeptidyl peptidase IV (DPPIV, forming an Y2/Y5-selective agonist) and chronic stress augment these effects and occlude vessels with atherosclerotic-like lesions, containing thrombus and lipid-laden macrophages. Y1 antagonist blocks stress-induced vasoconstriction and post-angioplasty occlusions, and hence may be therapeutic in angina and atherosclerosis/restenosis. Conversely, tissue ischemia activates neuronal and platelet-derived NPY, Y2/Y5 and DPPIV, which stimulates angiogenesis/arteriogenesis. NPY-Y2-DPPIV agonists may be beneficial for ischemic revascularization, wound healing and fat augmentation, whereas antagonists may be therapeutic in retinopathy, tumors, and obesity. Since stress is an underestimated risk factor in many of these conditions, NPY-based drugs may offer new treatment possibilities.
Keywords: Stress, NPY, NPY receptor antagonists, norepinephrine, restenosis, atherosclerosis, angiogenesis, obesity
1.Introduction
Neuropeptide Y (NPY) is a ubiquitous hormone that has both central and peripheral effects that work to maintain homeostasis. Centrally, NPY exerts anxiolytic and anti-epileptic actions and is inhibitory for reproductive function, motor activity, and sympathetic activity, resulting in an overall drop in blood pressure, heart rate, and a decrease in metabolism[5, 12]. NPY is also a known orexigenic peptide and has been the target of recent appetite-suppressing drugs[14]. Peripherally, however, NPY’s actions are stimulatory, synergizing with glucocorticoids and catecholamines to potentiate the stress response. NPY peripherally induces vasoconstriction, vascular smooth muscle cell (VSMC) proliferation, stimulates hyperlipidemia, glucose intolerance and the release of adipokines in animal models with elevated levels of the peptide (ob/ob mice, mice given NPY slow-release pellets, and mice exposed to chronic cold stress). This review will focus on the peripheral actions of NPY in the vasculature.
NPY’s known actions are mediated by its receptors, Y1-y6, and are modified by the processing enzyme dipeptidyl peptidase IV (DPPIV, also known as CD26) which cleaves NPY1-36 to an Y2/Y5 receptor-preferring peptide, NPY3-36[26]. NPY often stimulates the up-regulation of its own receptors and has been shown to have actions in immune response and in the proliferation of various cell types from VSMCs to preadipocytes. The Y1 receptor post-synaptically mediates vasoconstriction (increasing the blood pressure) directly and also indirectly, by potentiating norepinephrine-induced contraction, and stimulates VSMC proliferation (Figure 1)[36]. The Y1 and Y5 receptors have been seen to be pro-atherogenic and will be further discussed in this review. The Y2 receptor acting on its own and also in conjunction with the Y5 receptor is potently angiogenic, stimulating proliferation, migration, and capillary tube formation of endothelial cells (Figure 2)[33]. In addition to Y2’s angiogenic actions, it also pre-synaptically inhibits norepinephrine (NE) release (Figure 1)[37].
Figure 1.
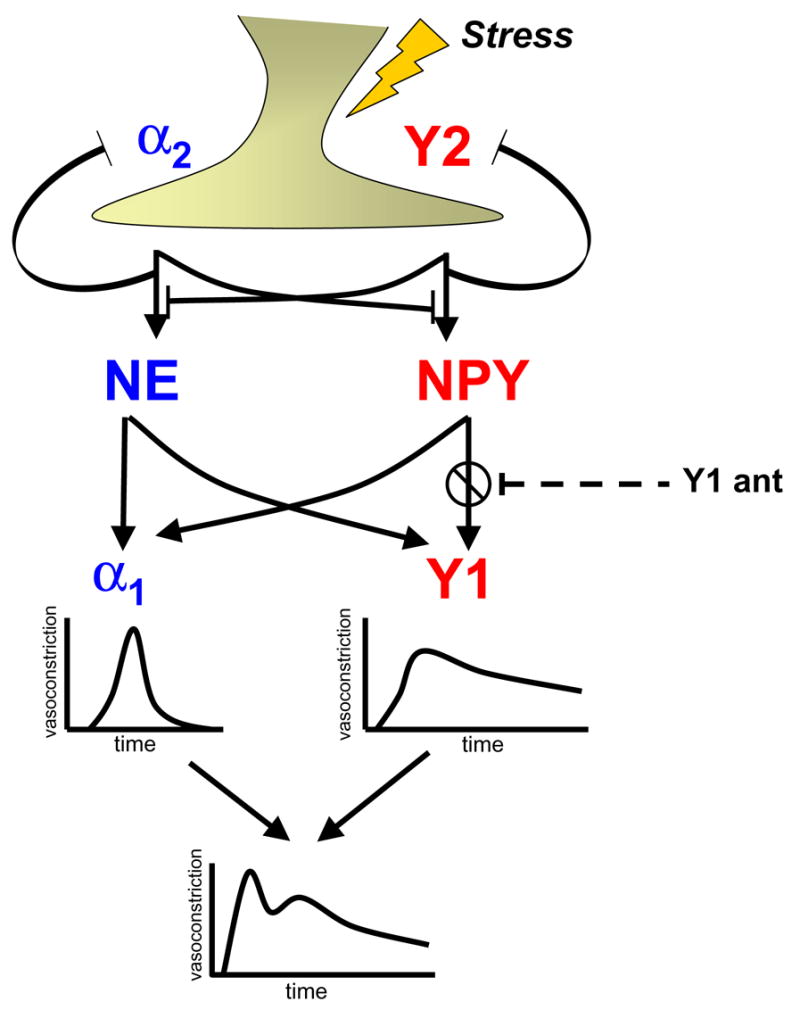
A schematic diagram of the NPY-NE cooperation in vasoconstriction during stress and the putative role of an Y1 receptor antagonist (Y1 ant) in inhibiting a stress-induced pressor response.
Figure 2.
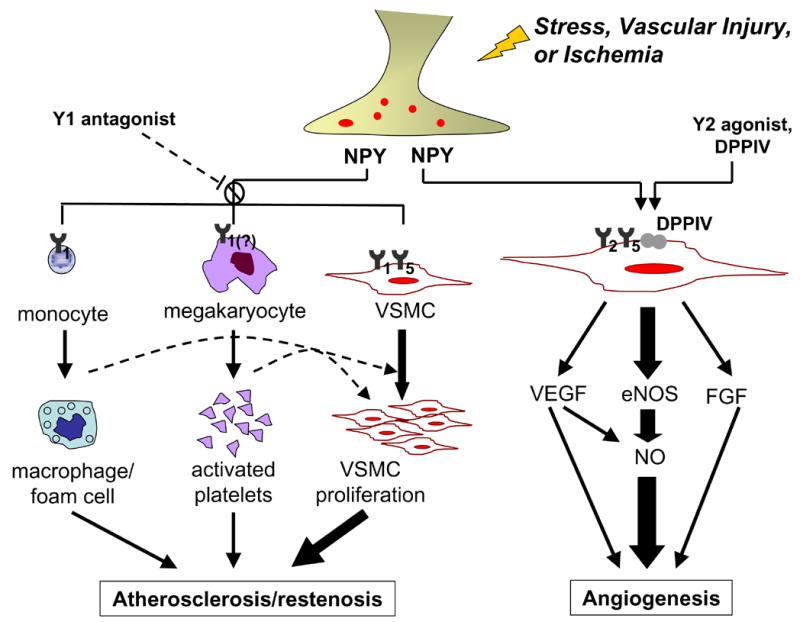
A schematic depicting the multiple roles of NPY during stress, vascular injury, and ischemia. The activation of Y1 or Y1/Y5 receptors on vascular smooth muscle cells (VSMC), megakaryocytes, and monocytes leads to cell proliferation and activation, promoting restenosis and atherosclerosis; Y1 antagonist inhibits this process. Activation of Y2/Y5 and DPPIV results in angiogenesis either directly via eNOS activation, or indirectly via VEGF and FGF; Y2 agonist or DPPIV stimulates this process.
2. NPY, Stress and Vasoconstriction
Although NPY and NE are often co-released and cooperate at the sympathetic neuro-effector junction, the ratio of the two neurotransmitters and their contribution to the regulation of vascular function differs in various conditions. Tonic sympathetic activation and acute stress preferentially releases NE, and adrenergic mechanisms are therefore primarily responsive for the maintenance of our arterial blood pressure, transient increases in vasoconstriction (Figure 1) and cardiac function as well as β-adrenergic lipolysis. The latter is considered the major mechanism of stress-induced weight loss in some individuals[11]. NPY, on the other hand, is preferentially released during prolonged and/or intense stress, such as exhaustive exercise, particularly when combined with hypoxia [10], vaginal delivery in newborns [32], panic attacks [8] or cold exposure [43]. It causes prolonged vasoconstriction (Figure 1) and vascular remodeling via VSMC proliferation (Figure 2), and in addition, exerts other actions, not shared by or opposite to those of NE. NPY stimulates monocyte migration and activation[1], exerts bimodal effects on immune T-cell function[38], activates platelets, and is angiogenic (Figure 2). These actions of NPY fit well into a more chronic modulatory function of the peptide, and make it a candidate for a mediator of chronic stress.
A model of stress that has been demonstrated to increase plasma NPY in humans and rodents is the cold-pressor test [24]. Rats exposed to 2 hours of cold-water (standing in 1 cm ice-cold water) exhibit an elevation of mean arterial pressure, increase their heart rate, decrease mesenteric blood flow, and increase mesenteric vascular resistance [40]. These vascular changes persist for an hour following a bout of cold-water stress. The Y1 antagonist, BIBP3226 (3mg/kg, iv) inhibits up to 80% of the vasoconstrictive effects brought on by cold stress [40]. Similarly, NPY administered into a human coronary artery caused severe vasoconstriction [4] which was mimicked by mental stress [31], both resulting in a severe reduction in blood flow. In both rats and in humans, stress-induced plasma NPY levels and vasoconstrictive responses are greater in males than in females, and this sex difference appears to be primarily due to a strong androgen-driven stimulation of NPY gene expression [23, 41]
3. Pro-atherogenic Actions of NPY and Stress
In addition to NPY’s potent vasoconstrictive effects, the peptide has been shown to accelerate vascular events such as restenosis (Figure 2). In vitro, primary vascular smooth muscle cells responded bimodally to NPY (10−14 to 10−8 M) by increasing proliferation, while an Y1 and Y5 receptor antagonist cocktail was effective in blocking NPY’s mitogenic effects [33]. In vivo, cold stress in rats accelerated angioplasty-induced occlusion of the carotid artery with an atherosclerotic-like lesions that were lipid laden, had microvessels and neointima formation [24]. NPY-slow release pellets (10μg/14 days) administered near the site of injury caused a similar dramatic athero-thrombotic event [25], while an Y1 receptor antagonist (0.02μmol/kg/min/ 14 days) completely prevented the occlusion caused by both stress-induced [24] and NPY-induced [25] occlusion (Figure 2).
DPPIV-inhibitors act as Y1 receptor agonists since they prevent the cleavage of NPY1-36 to Y2/Y5 receptor preferring NPY3-36. As predicted from such action, DPPIV-inhibitors augmented Y1-mediated effects of NPY and resulted in complete occlusion of the carotid artery following angioplasty, in the absence or presence of a slow release pellet delivering low doses of NPY[17], which by itself was not occlusive [25]. DPPIV is a peptidase that, in addition to cleaving NPY, also inactivates insulin-sensitizing hormone GLP-1(7–36) by cleaving it to form GLP-1(9–36). DPPIV/CD26 has therefore been the target of new anti-diabetic drugs which attempt to decrease clearance of GLP-1 and increase insulin stimulation and sensitivity[39]. From the previously mentioned studies on restenosis and the potential role of NPY/Y1 in the progression of atherosclerosis, this presents a potentially dangerous scenario for diabetic patients using DPPIV-inhibitors as an anti-diabetic drug.
4. NPY and Platelets
Another component of NPY-mediated vascular occlusion is platelets (Figure 2). This anucleated cell is packed with growth factors and is often found at the site of plaque or vascular injury, lending itself to be either directly or indirectly involved in vascular remodeling. Our and other previous studies [3, 7, 29] have shown that rats and some strains of mice express NPY in platelets and their nucleated precursors, megakaryocytes [28]. SV129/X1 mice expressed NPY in megakaryocytes and had NPY in their platelets (as measured in platelet-rich-plasma, PRP) whereas C57BL/6J mice lacked NPY mRNA in megakaryocytes and platelet NPY, although plasma peptide levels were similar between the two strains at rest. The differences between these two strains became dramatically apparent when their femoral arteries were denuded by angioplasty. The injured vessels of SV129/X1 mice had significant neointimal formation while platelet NPY-deficient C57BL/6J mice appeared to be protected from restenosis (Fig. 1).
Could this differential response to angioplasty seen in the two different background strains of mice be explained by their respective platelet NPY content? Current studies using NPY−/− mice transfused with SV129/X1’s platelets indicate that, indeed, platelet NPY content aided in VSMC proliferation, neointimal formation and monocyte/macrophage infiltration to the site of vascular injury. Immunohistochemistry confirmed the presence and abundance of the NPY system in human atherosclerotic vessels. Interestingly, while healthy humans do not express NPY in platelets [28], one report [12] suggests that some patients do, as elevated platelet NPY levels were found in depressed patients, and in a small sample of patients with peripheral vascular disease (unpublished observation from our laboratory).
5. NPY and Angiogenesis
NPY-mediated growth is not limited to vascular atherosclerotic-like remodeling. Recently, our lab has shown that NPY has potent angiogenic effects via the activation of non-Y1 receptors, primarily Y2 (Figure 2). In vitro, we have demonstrated that NPY stimulates endothelial cell (EC) activation, proliferation, migration, and tube formation [27]. In rat aortic rings embedded in collagen, NPY stimulated formation of long, thick sprouts resembling normal vessels[42]. This sprouting was blocked in eNOS−/ − mice, indicating that NPY-mediated angiogenesis is critically dependent on eNOS[22]. NPY also induces expression of other growth factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), which are, in part, downstream mediators of NPY’s effect (Figure 2)[22].
NPY plays a significant role in ischemic revascularization. Femoral artery occlusion-induced hindlimb ischemia in rats up-regulated NPY, Y2, Y5 and DPPIV expression, and elevated venous plasma NPY outflow from the ischemic muscles [21]. Exogenous, local NPY administered (via slow-release pellets) to the ischemic tissue induced capillary angiogenesis below the occlusion and formation of new muscular arteries around the occluded femoral artery, restoring blood flow and improving function in ischemic limbs [21]. Similar NPY-dependent angiogenesis was found to play a role in wound healing [6, 9]. In aging, when new vessel formation is generally impaired, NPY-mediated angiogenesis was reduced, along with a loss of Y2 receptors and DPPIV [18]. Thus, stimulation of the NPY/Y2/DPPIV angiogenic pathway (Figure 2) may afford a novel therapy for impaired vascularization in ischemia, wound healing, or aging. On the other hand, NPY is also involved in pathologic angiogenesis such as in tumors [16] and retinopathy [19], and, in these conditions, Y2 receptor antagonists could be therapeutic.
6. NPY and Obesity
One tissue that continues to be remodeled throughout the adult life is adipose tissue which is also highly vascularized and innervated. Rupnick et al, [34] and others [2] have seen that treatment with anti-angiogenic factors result in a dose-dependent, reversible weight reduction and adipose tissue loss, resulting in overall metabolic improvement. While these studies provided evidence that adipogenesis can be manipulated by starving the tissue of their blood supply, physiological mechanisms responsible for it have not been determined. We hypothesized that due to sympathetic innervation of white adipose tissue, nerve-released NPY may be a link between angiogenesis and adipogenesis.
In vivo, subcutaneous NPY slow-release pellets (1μg/14 days) increased fat pad weight gain, while Y2 antagonist (10μg/14 days) reduced fat pad weight gain in lean and obese mice, of genetic nature (leptin deficient ob/ob mice) or diet induced. The fat loss was accompanied by decreased vascularization of white adipose tissue at the site of Y2 antagonist injection, whereas increased staining of CD31-positive endothelial cells was found following the treatment with an NPY pellet. Y2 antagonist also caused increased apoptosis of endothelial cells and adipocytes indicating that its anti-angiogenic actions led to anti-adipogenic effects. Thus, Y2 receptor antagonist, locally administered into the fat, may be a new way for ‘melting’ the fat and a therapy for obesity, by its bi-modal effects of being anti-angiogenic as well as directly anti-adipogenic.
7. Relevance to Human Diseases and Conclusions
How relevant are these animal studies to humans? Are our studies of cold stress only applicable to that particular type of stress? Why is cold such a powerful stimulus for NPY release? These and other questions will require more research, but existing evidence already suggests that “NPY is not a rodent phenomenon”. In humans from Northern Europe, there is a Leu7Pro7 NPY polymorphism in its signal peptide gene which affects peptide turnover and leads to greater plasma NPY responses to stress and exercise[13]. It is also associated with increased incidence of cardiovascular disease[15], diabetic retinopathy, atherosclerosis, obesity, and overall “poor stress coping” states, such as alcoholism [20]. Interestingly, this genetic polymorphism follows a geographic gradient from north to south and appears to be more prevalent in colder-climate regions, but disappears in populations in warm climates of Asia, Africa and South America.
A hypothesis that we have put forth and which has some support from the literature[35] is that high NPY response to environmental stress provides an evolutionary advantage in cold climates, and in hibernating animals is a part of the hibernation response. In the pre-hibernation phase, raising NPY levels in the hypothalamus would stimulate food intake and motivate the animal to forage for food, while increased NPY release in the periphery would elevate blood pressure and adjust blood flow of exercising muscles. With the beginning of hibernation, continued increased NPY levels in the brain could slow down reproductive and locomotor activity, induce anxiolysis and hypotension and result in overall decreased metabolism and body temperature.
Cold-induced NPY release might have offered an evolutionary advantage for surviving cold climates and starvation in humans as well. However, this is no longer the case for modern-day humans who have changed their lifestyles into one of continuous stress and hyper-caloric diets, without the periods of starvation and slowing down, thus leading to protracted periods of high NPY release and its deleterious effects on blood vessels: vasoconstriction and atherosclerosis/restenosis. Why does stress not promote other beneficial actions of NPY, such as ischemic revascularization? Turning ‘bad’ stress into stress that is ‘good’ for the body would indeed be a major achievement, which is, however, not yet attainable. Meanwhile, NPY-based drugs are already offering several potential therapeutic uses: Y1 antagonists for the treatment of angina, hypertension and atherosclerosis and restenosis, Y2 agonists for ischemic revascularization and wound healing, and Y2 antagonists, with their anti-angiogenic, growth-inhibitory actions – as therapy for tumors and obesity.
Why these antagonists have not yet become drug targets for cardiovascular diseases may largely be due to the fact that ‘life without NPY’, as shown in the example of NPY knockout mice [30], seemed to be normal, questioning the peptide’s role in physiology. However, while NPY is not critical to survival, what our studies have demonstrated is that the peptide plays a major role in the adaptation to environmental stress. The quality of that adaptation and whether or not it becomes maladaptation is critical for the development or prevention of pathology. Thus, NPY-based drugs may be particularly useful in stress-related cardiovascular and metabolic diseases, and their inability to alter normal homeostasis an added beneficial feature.
Acknowledgments
This research was supported by grants HL067557 and HL55310 to Z. Zukowska, and was a joint effort of the research team at the Neurovascular Lab, whom the authors wish to thank.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bedoui S, Kawamura N, Straub RH, Pabst R, Yamamura T, von Horsten S. Relevance of neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol. 2003;134:1–11. doi: 10.1016/s0165-5728(02)00424-1. [DOI] [PubMed] [Google Scholar]
- 2.Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–88. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 3.Chen SH, Han QD. Increase of release of neuropeptide Y in vitro from platelets of spontaneously hypertensive rats. Zhongguo Yao Li Xue Bao. 1995;16:149–52. [PubMed] [Google Scholar]
- 4.Clarke JG, Davies GJ, Kerwin R, Hackett D, Larkin S, Dawbarn D, et al. Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet. 1987;1:1057–9. doi: 10.1016/s0140-6736(87)90483-1. [DOI] [PubMed] [Google Scholar]
- 5.Dumont Y, Martel JC, Fournier A, St-Pierre S, Quirion R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog Neurobiol. 1992;38:125–67. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- 6.Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, Jonsson-Rylander AC, Hassani H, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003;100:6033–8. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ericsson A, Hemsen A, Lundberg JM, Persson H. Detection of neuropeptide Y-like immunoreactivity and messenger RNA in rat platelets: the effects of vinblastine, reserpine, and dexamethasone on NPY expression in blood cells. Exp Cell Res. 1991;192:604–11. doi: 10.1016/0014-4827(91)90082-6. [DOI] [PubMed] [Google Scholar]
- 8.Esler M, Alvarenga M, Pier C, Richards J, El-Osta A, Barton D, et al. The neuronal noradrenaline transporter, anxiety and cardiovascular disease. J Psychopharmacol. 2006;20:60–6. doi: 10.1177/1359786806066055. [DOI] [PubMed] [Google Scholar]
- 9.Ghersi G, Dong H, Goldstein LA, Yeh Y, Hakkinen L, Larjava HS, et al. Regulation of fibroblast migration on collagenous matrix by a cell surface peptidase complex. J Biol Chem. 2002;277:29231–41. doi: 10.1074/jbc.M202770200. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DS. Stress-induced activation of the sympathetic nervous system. Baillieres Clin Endocrinol Metab. 1987;1:253–78. doi: 10.1016/s0950-351x(87)80063-0. [DOI] [PubMed] [Google Scholar]
- 11.Hagstrom-Toft E, Arner P, Wahrenberg H, Wennlund A, Ungerstedt U, Bolinder J. Adrenergic regulation of human adipose tissue metabolism in situ during mental stress. J Clin Endocrinol Metab. 1993;76:392–8. doi: 10.1210/jcem.76.2.8381801. [DOI] [PubMed] [Google Scholar]
- 12.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–24. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Kallio J, Pesonen U, Kaipio K, Karvonen MK, Jaakkola U, Heinonen OJ, et al. Altered intracellular processing and release of neuropeptide Y due to leucine 7 to proline 7 polymorphism in the signal peptide of preproneuropeptide Y in humans. Faseb J. 2001;15:1242–4. [PubMed] [Google Scholar]
- 14.Kalra SP, Kalra PS. NPY and cohorts in regulating appetite, obesity and metabolic syndrome: beneficial effects of gene therapy. Neuropeptides. 2004;38:201–11. doi: 10.1016/j.npep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Karvonen MK, Valkonen VP, Lakka TA, Salonen R, Koulu M, Pesonen U, et al. Leucine7 to proline7 polymorphism in the preproneuropeptide Y is associated with the progression of carotid atherosclerosis, blood pressure and serum lipids in Finnish men. Atherosclerosis. 2001;159:145–51. doi: 10.1016/s0021-9150(01)00468-3. [DOI] [PubMed] [Google Scholar]
- 16.Kitlinska J, Abe K, Kuo L, Pons J, Yu M, Li L, et al. Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Res. 2005;65:1719–28. doi: 10.1158/0008-5472.CAN-04-2192. [DOI] [PubMed] [Google Scholar]
- 17.Kitlinska J, Lee EW, Li L, Pons J, Estes L, Zukowska Z. Dual role of dipeptidyl peptidase IV (DPP IV) in angiogenesis and vascular remodeling. Adv Exp Med Biol. 2003;524:215–22. doi: 10.1007/0-306-47920-6_26. [DOI] [PubMed] [Google Scholar]
- 18.Kitlinska J, Lee EW, Movafagh S, Pons J, Zukowska Z. Neuropeptide Y-induced angiogenesis in aging. Peptides. 2002;23:71–7. doi: 10.1016/s0196-9781(01)00581-2. [DOI] [PubMed] [Google Scholar]
- 19.Koulu M, Movafagh S, Tuohimaa J, Jaakkola U, Kallio J, Pesonen U, et al. Neuropeptide Y and Y2-receptor are involved in development of diabetic retinopathy and retinal neovascularization. Ann Med. 2004;36:232–40. doi: 10.1080/07853890410031236. [DOI] [PubMed] [Google Scholar]
- 20.Lappalainen J, Kranzler HR, Malison R, Price LH, Van Dyck C, Rosenheck RA, et al. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–31. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- 21.Lee EW, Grant DS, Movafagh S, Zukowska Z. Impaired angiogenesis in neuropeptide Y (NPY)-Y2 receptor knockout mice. Peptides. 2003;24:99–106. doi: 10.1016/s0196-9781(02)00281-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee EW, Michalkiewicz M, Kitlinska J, Kalezic I, Switalska H, Yoo P, et al. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J Clin Invest. 2003;111:1853–62. doi: 10.1172/JCI16929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewandowski J, Pruszczyk P, Wocial B, Ignatowska-Switalska H, Romejko-Wolniewicz E, Madej-Firek A, et al. Sex Hormone Modulation of Neuropeptide Y and Cardiovascular Responses to Stress in Humans. In: McCarty RGA, Sabban E, Kvetnansky R, editors. Stress: Molecular Genetic and Neurobiological Advances. New York, NY: Gordon and Breach Science Publishers S.A.; 1996. pp. 569–78. [Google Scholar]
- 24.Li L, Jonsson-Rylander AC, Abe K, Zukowska Z. Chronic stress induces rapid occlusion of angioplasty-injured rat carotid artery by activating neuropeptide Y and its Y1 receptors. Arterioscler Thromb Vasc Biol. 2005;25:2075–80. doi: 10.1161/01.ATV.0000179601.19888.19. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Lee EW, Ji H, Zukowska Z. Neuropeptide Y-induced acceleration of postangioplasty occlusion of rat carotid artery. Arterioscler Thromb Vasc Biol. 2003;23:1204–10. doi: 10.1161/01.ATV.0000071349.30914.25. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig R, Lucius R, Mentlein R. A radioactive assay for the degradation of neuropeptide Y. Biochimie. 1995;77:739–43. doi: 10.1016/0300-9084(96)88191-0. [DOI] [PubMed] [Google Scholar]
- 27.Movafagh S, Hobson JP, Zukowska Z. Neuropeptide Y (NPY) induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. FASEB Journal. 2006 doi: 10.1096/fj.05-4770fje. [DOI] [PubMed] [Google Scholar]
- 28.Myers AK, Torres Duarte AP, Zukowska-Grojec Z. Immunoreactive neuropeptide Y (NPY) in plasma and platelets of rat and mouse strains and human volunteers. Regul Pept. 1993;47:239–45. doi: 10.1016/0167-0115(93)90391-k. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa T, Kitamura K, Kangawa K, Matsuo H, Eto T. Platelet neuropeptide Y in spontaneously hypertensive rats. J Hypertens. 1992;10:765–71. [PubMed] [Google Scholar]
- 30.Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog Horm Res. 1998;53:163–99. [PubMed] [Google Scholar]
- 31.Papademetriou V, Gottdiener JS, Kop WJ, Howell RH, Krantz DS. Transient coronary occlusion with mental stress. Am Heart J. 1996;132:1299–301. doi: 10.1016/s0002-8703(96)90485-8. [DOI] [PubMed] [Google Scholar]
- 32.Petraglia F, Coukos G, Battaglia C, Bartolotti A, Volpe A, Nappi C, et al. Plasma and amniotic fluid immunoreactive neuropeptide-Y level changes during pregnancy, labor, and at parturition. J Clin Endocrinol Metab. 1989;69:324–8. doi: 10.1210/jcem-69-2-324. [DOI] [PubMed] [Google Scholar]
- 33.Pons J, Kitlinska J, Ji H, Lee EW, Zukowska Z. Mitogenic actions of neuropeptide Y in vascular smooth muscle cells: synergetic interactions with the beta-adrenergic system. Can J Physiol Pharmacol. 2003;81:177–85. doi: 10.1139/y02-166. [DOI] [PubMed] [Google Scholar]
- 34.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–5. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saitongdee P, Milner P, Loesch A, Knight G, Burnstock G. Electron-immunocytochemical studies of perivascular nerves of mesenteric and renal arteries of golden hamsters during and after arousal from hibernation. J Anat. 1999;195 (Pt 1):121–30. doi: 10.1046/j.1469-7580.1999.19510121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahlestedt C, Hakanson R, Vaz CA, Zukowska-Grojec Z. Norepinephrine and neuropeptide Y: vasoconstrictor cooperation in vivo and in vitro. Am J Physiol. 1990;258:R736–42. doi: 10.1152/ajpregu.1990.258.3.R736. [DOI] [PubMed] [Google Scholar]
- 37.Westfall TC, Yang CL, Curfman-Falvey M. Neuropeptide-Y-ATP interactions at the vascular sympathetic neuroeffector junction. J Cardiovasc Pharmacol. 1995;26:682–7. doi: 10.1097/00005344-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, Herzog H, et al. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202:1527–38. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiedeman PE, Trevillyan JM. Dipeptidyl peptidase IV inhibitors for the treatment of impaired glucose tolerance and type 2 diabetes. Curr Opin Investig Drugs. 2003;4:412–20. [PubMed] [Google Scholar]
- 40.Zukowska-Grojec Z, Dayao EK, Karwatowska-Prokopczuk E, Hauser GJ, Doods HN. Stress-induced mesenteric vasoconstriction in rats is mediated by neuropeptide Y Y1 receptors. Am J Physiol. 1996;270:H796–800. doi: 10.1152/ajpheart.1996.270.2.H796. [DOI] [PubMed] [Google Scholar]
- 41.Zukowska-Grojec Z, Golczynska M, Lewandowski J, Pruszczyk P, Switalska H, Hiremagalur B, et al. Neuropeptide Y: A Major Regulator of Cardiovascular Responses to Stress. In: McCarty RGA, Sabban E, Kvetnansky R, editors. Stress: Molecular Genetic and Neurobiological Advances. New York, NY: Gordon and Breach Science Publishers S.A.; 1996. pp. 513–29. [Google Scholar]
- 42.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–95. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 43.Zukowska-Grojec Z, Vaz AC. Role of neuropeptide Y (NPY) in cardiovascular responses to stress. Synapse. 1988;2:293–8. doi: 10.1002/syn.890020319. [DOI] [PubMed] [Google Scholar]


