Abstract
The prototypical form of the Ser/Thr phosphatase PP2A is a heterotrimeric complex consisting of catalytic subunit (C), A and B regulatory subunits. C-terminal methylation of PP2A-C influences holoenzyme assembly. Using late gestation development in the rat as an in vivo model of liver growth, we found that PP2A-C protein and activity levels were higher in fetal compared to adult liver extracts. However, unmethylated PP2A-C was much higher in the adult extracts. In MonoQ fractionation, unmethylated C eluted separately from methylated C, which was present predominantly in ABC heterotrimers. Gel filtration chromatography revealed that some unmethylated C was present as free catalytic subunit. In addition, a significant proportion of PP2A was in inactive forms that may involve novel regulatory subunits. Our results indicate that methylation of PP2A-C appears to be a primary determinant for the biogenesis of PP2A heterotrimers.
Keywords: Signal transduction, fetal development, liver, hepatocyte
Introduction
PP2A is a eukaryotic Ser/Thr phosphatase that plays an important role in numerous cellular processes, including metabolism, DNA replication, transcription, translation, apoptosis and cell cycle control [1]. Type 2 protein phosphatases, including PP2A, can be distinguished from other Ser/Thr phosphatases by their sensitivity to inhibition by nanomolar concentrations of okadaic acid [2]. PP2A is generally considered to exist as an obligate heterotrimeric complex consisting of a catalytic subunit (C), a scaffolding A subunit (A) and one of many distinct regulatory B subunits (B/B55/PR55, B’/B56/PR61, B’’/BR72/PR59/PR130, B’’’/PR93/PR110) [3]. Various regulatory subunits are expressed in a tissue-specific and developmental stage-specific manner, accounting for the existence of more than 70 different holoenzymes in mammalian cells [2;4]. The regulatory subunits are thought to contribute to the selectivity and specificity of PP2A for its substrates, thereby defining the signaling function of the individual holoenzyme complexes [5;6].
The PP2A catalytic subunit is regulated at many levels: transcription and translation of its mRNA, post-translational modification, and protein-protein interactions with the aforementioned spectrum of regulatory subunits. Post-translational modifications include phosphorylation of Tyr307 and carboxymethylation of the C-terminal Leu309 residue. Phosphorylation of Tyr307 inhibits PP2A activity, in part by inhibiting regulatory subunit binding [7;8]. The reversible methylation of Leu309 can be catalyzed by a specific PP2A-methyltransferase (PPMT) that associates directly with the phosphatase [9–11]. Similarly, demethylation is catalyzed by a specific protein phosphatase methylesterase (PME-1) that is conserved from yeast to human [12]. Methylation triggers dimer-to-trimer conversion by inducing the binding of regulatory B subunits to the AC core enzyme and by stabilizing the heterotrimeric holoenzyme [11;13–15]. Through effects on complex formation, the reversible methylation of C appears to play an important role in regulating the PP2A substrate specificity and phosphatase activity. In addition to the mechanisms for post-translational modification, PP2A activity is also modulated by phosphotyrosyl phosphatase activator of PP2A (PTPA), a prolyl isomerase [16] that can stimulate the Ser/Thr phosphatase activity of the inactive pool of PP2A complexed with PME-1 [9;17]. Thus, PME-1 also functions as an enzyme that stabilizes an inactive pool of PP2A that can be reactivated by PTPA.
Monomeric PP2A catalytic subunit is unstable in Drosophila melanogaster [18] but is stable in yeast [19]. In mammalian cells, there is evidence that PP2A is present in both dimeric and trimeric forms of different compositions [20;21]. Some results derived from immunoprecipitation using different antibodies suggest that the dimeric (AC) form of PP2A may constitute up to a third of the total PP2A pool [22]. Previous studies on the expression of hepatic PP2A components in the adult rat [23–25] have identified both dimeric and heterotrimeric PP2A complexes by using affinity purified antibodies specific for PP2A subunits [26]. Limited data are available on the forms of PP2A in fetal liver [27]. Free catalytic subunit has not been identified in hepatic cells, liver tissue or other mammalian cells. However, most purifications and chromatographic analyses have relied exclusively on activity to follow the PP2A and have not used immunoblotting, which could detect inactive forms. In the present study, we used immunoblotting with multiple antibodies to examine the composition of hepatic PP2A holoenzyme complexes in vivo and the relationship to Leu309 methylation using late gestation liver development as a model of normal liver growth in the rat [28;29]. Our choice of this model is based on our previous studies demonstrating a high rate of hepatocyte proliferation and cell cycle activity in the late gestation rat fetus relative to the quiescent state of adult hepatocytes [29;30;32]. Its use led to unexpected findings regarding the physiological role and regulation of PP2A methylation and the presence of free catalytic subunit and inactive forms.
Materials and Methods
Materials
Reagents for the measurement of PP2A activity were obtained from Promega Corporation (Madison, WI). Primary antibodies to Aα/β, Bα/β/γ and full-length catalytic subunit (C) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). C-terminal, demethyl- and methyl-C antibodies were obtained from Upstate USA, Inc. (Charlottesville, VA). Anti-phosphotyrosine (4G10), anti-PP2A-methyltrasferase (PPMT), anti-PP2A-methylesterase (PME-1) and anti-PTPA antibodies were also purchased from Upstate USA, Inc. Mid-portion antibody was prepared as described previously [31]. MonoQ HR5/5 and Sephacryl S-200 columns were purchased from GE Healthcare (Piscataway, NJ). Okadaic acid was purchased from Sigma-Aldrich (St. Louis, MO).
Animals
Adult Sprague-Dawley male rats and pregnant rats of known gestational age (term being 21 days) were used for all studies (Charles River Laboratories, Wilmington, MA). Adult male rats (125–175 g) were anesthetized with pentobarbital (50 mg/kg body weight, administered by intraperitoneal injection) and exsanguinated prior to removal of the liver. For pregnant rats, cesarean sections were performed under pentobarbital anesthesia and fetal (embryonic day 19) livers were harvested before sacrifice. Two-thirds partial hepatectomy was performed on adult male rats (125–175 g) under isofluorane anesthesia as previously described [32]. Except where noted, livers were flash-frozen in liquid nitrogen and stored at –70°C until use. Wherever replicate analyses are referred to, samples were prepared from separate adult livers or from separate pools of fetal livers, each pool being derived from a single pregnant dam. All animal studies complied with guidelines set by the Rhode Island Hospital Institutional Animal Care and Use Committee.
Preparation of Liver Extracts and Western Immunoblotting
Rat liver extracts were prepared using 15 ml/g liver of PP2A homogenization buffer (20 mM Tris-HCl, pH 7.5, 5 mM EGTA, 2 mM EDTA, 1 mM DTT and 10% glycerol) containing protease inhibitors (10 μg/ml leupeptin, 10 μg/ml aprotinin and 34.4 μg/ml 4-(2-aminoethyl)-benzenesulfonyl fluoride). Following homogenization, Nonidet P-40 (NP-40) was added to a final concentration of 0.1%, following which the samples were incubated on ice for 30 min. The detergent-extracted homogenates were centrifuged at 1000 x g for 15 min. The resulting supernatant was centrifuged at 40,000 x g for 20 min. For MonoQ and gel filtration fractionation, the supernatant was ultra-centrifuged at 100,000 x g for 1 hr. For Western immunoblotting, 80 μg of total homogenate protein in Laemmli sample buffer were separated using a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). Analyses with antibodies directed toward PP2A subunits included blocking for 1 hr in 3 % milk-TBST at 37°C. For alkali-induced demethylation, the membranes were incubated in 0.2 M NaOH for 20 min at 37°C prior to blocking. In selected experiments, the p85 subunit of phosphatidylinositol 3-kinase (PI3K) was used as a control for equal loading of samples. For all immunoblots, detection employed an enhanced chemiluminescence method (GE Healthcare, Piscataway, NJ).
Phosphatase Activity Measurements and Column Fractionation
For activity determinations, liver extracts (5 μg protein) or column fractions (1.5 μl) were added to a preincubation mixture that included the phosphopeptide substrate (RRApTVA; Promega Corporation, Madison, WI). The assay followed the supplier’s recommendations. Reactions were terminated by the addition of a molybdate dye mixture. Phosphatase activity was measured as the amount of free phosphate released from this phosphopeptide substrate by determining the absorbance of a molybdate-malachite green-phosphate complex.
Where indicated, 20 mg of fresh (non-frozen) liver extract protein was fractionated using MonoQ fast protein liquid chromatography. Proteins were adsorbed to the column and washed with 3 ml of homogenization buffer containing no salt. Except where noted, the column was developed with a linear, 24 ml gradient from 0 to 0.6 M salt. The column was eluted at 0.5 ml per min and 0.5 ml fractions were collected. PP2A activity was measured using 1.5 μl of each fraction.
Size fractionation was accomplished using a Sephacryl S-200 column. A 1 ml sample of fresh liver extract, prepared as above, was applied to the column, which was equilibrated in the homogenization buffer containing 150 mM NaCl. Flow rate was 0.5 ml per min and 1 mL fractions were collected.
Results
PP2A Catalytic Subunit in E19 Fetal and Adult Liver
In our initial experiments, we analyzed fetal and adult liver extracts for PP2A activity and catalytic subunit content. Activity was measured using a phosphopeptide substrate (RRApTVA) specific for PP2A in an assay linear with time for up to 30 min reaction time (Fig. 1A). Addition of 0.5 nM okadaic acid to the reaction mixture reduced activity to near blank, confirming the specificity of the assay for PP2A. Results showed significantly higher PP2A specific activity in fetal relative to adult liver (Fig. 1B). We analyzed the fetal and adult liver extracts by Western immunoblotting with antibodies made against the full length catalytic subunit (FL-C) as well as antibodies directed towards peptides corresponding to the mid-portion (MD-C) and carboxy-terminus (CT-C) of the catalytic subunit. Analysis of several fetal and adult liver fractions showed a significantly higher level of catalytic subunit in fetal liver compared to adult liver (Fig. 1B). The difference in catalytic subunit content was roughly proportional to the difference in PP2A activity. However, analyses using additional antibodies directed against PP2A-C gave unexpected results (Fig. 1C). Like the FL-C antibody, results obtained using the MD-C antibody showed more catalytic subunit in fetal compared to adult liver. However, results with the CT-C antibody showed the reverse, a much more intense signal in adult liver relative to fetal. This result was obtained in several replicate experiments. These findings were interpreted as indicating a profound difference in the modification of the carboxy terminus between fetal and adult liver PP2A-C.
Fig. 1. PP2A activity and content in fetal and adult rat liver.

Panel A: A liver cytosolic extract prepared from an adult male rat (5 μg homogenate protein per sample) was analyzed for PP2A activity in a time course experiment. The activity represents picomoles of free phosphate released from substrate at each time point. The unfilled square represents activity in the presence of 0.5 nM okadaic acid. Panel B: Fetal and adult liver extracts (n = 3 per group) were analyzed for PP2A activity (left). The reaction time was 15 min. *, p<0.02 by unpaired T-test. The same samples were analyzed for the intensity of the immunoreactive PP2A-C signal at 39 kDa as detected by the FL-C antibody (right). **, p<0.005 by unpaired T-test. Data in both graphs are shown as the mean plus 1 standard deviation. Panel C: Liver extracts prepared from fetal and adult liver were examined by Western immunoblotting using anti-PP2A-C antibodies directed towards full-length catalytic subunit (FL-C), the mid-portion of the protein (MD-C) and the carboxy-terminus (CT-C). The p85 subunit of PI3K was used as a control for quality and quantity of protein in each sample. The numbers to the right of the immunoblots indicate apparent molecular mass in kilodaltons.
To validate the antibody specificity, fetal and adult liver homogenates were subjected to immunoprecipitation and immunoblotting using the FL-C and CT-C antibodies. Results (not shown) demonstrated that both antibodies immunoprecipitated a 39 kDa protein that was detected by the other antibody by immunoblotting, confirming that both antibodies were detecting the same PP2A protein. The CT-C antibody also cross-reacted with a 42 kDa protein that was not detected by the FL-C or MD-C antibodies and was considered to be non-specific (see Fig. 1C).
Carboxy-Terminal Modification of PP2A-C in Fetal and Adult Liver
Because the CT-C antibody detected differences not seen with other anti-PP2A antibodies, we examined the status of the PP2A-C carboxy-terminus in fetal versus adult liver. Fetal and adult liver extracts were resolved by SDS-PAGE, with two identical sets of on either half of the gel. After transfer to PVDF, the membrane was cut in half lengthwise. One half was left untreated while the other half was incubated with NaOH to hydrolyze the methyl ester at the carboxy-terminus of PP2A. Immunoblotting of the treated and untreated membranes (Fig. 2) revealed that antibodies specific for the methylated form of PP2A-C (L309-OMe) reacted with PP2A in fetal but not adult liver (bottom panel). This staining was abolished by alkali hydrolysis, confirming that the methylated form of PP2A was being detected and was more prevalent in fetal liver. Antibodies specific for the unmethylated PP2A (L309-OH) gave the converse result. That is, they reacted well with PP2A in adult liver, but not with PP2A from fetal liver. Staining intensity in fetal liver was greatly increased upon alkali hydrolysis, consistent with the specificity of this antibody for unmethylated PP2A.
Fig. 2. C-terminal modification of PP2A-C in fetal and adult liver.

Triplicate fetal and adult liver extracts (80 μg of protein per lane) were electrophoresed in duplicate and transferred to PVDF. The membrane was divided and one half was subjected to chemical demethylation. Both membranes were analyzed using antibodies directed towards the full-length catalytic subunit (FL-C), the carboxy-terminus (CT-C), methylated C (L309-OMe) and unmethylated C (L309-OH). The blot was stripped and reprobed for the p85 subunit of PI3K for use as a control.
Phosphotyrosine immunoprecipitation followed by immunoblotting for PP2A-C (data not shown) revealed no evidence of PP2A-C Tyr-phosphorylation. Antibodies specific for phospho-Tyr307 did not yield a signal in direct immunoblotting (data not shown). This reduced the likelihood that differences in immunoblotting were due to Tyr307 phosphorylation. CT-C antibodies gave results that paralleled those with the L309-OH antibodies, showing that the carboxy-terminus PP2A-C antibodies (CT-C) preferentially detect the unmethylated form of C. The relative intensity of staining of PP2A in fetal and adult liver with FL-C antibodies was unaffected by alkali hydrolysis, demonstrating that this antibody detects both methylated and unmethylated forms of PP2A. We concluded that the difference in modification of the PP2A carboxy terminus between fetal and adult liver resulting in differential reactivity with the CT-C antibody could be accounted for by methylation. Our results also indicated that methylated PP2A predominates in fetal liver while a significant proportion of the catalytic subunit in adult liver is in the unmethylated state.
MonoQ Fractionation of PP2A Complexes in E19 and Adult Liver
We examined different subcellular fractions from liver and found PP2A predominantly in the cytosol (>90%), with only low levels in nuclei and membrane preparations. We went on to fractionate fetal and adult liver cytosolic extracts using MonoQ anionic exchange chromatography (Figure 3). Column fractions were analyzed for PP2A activity. We focused on the latter half of the gradient, which was found to contain PP2A activity from fetal and adult liver in two major peaks resolved by the gradient elution. These peaks were centered at fraction 41 and fractions 45/46. In several independent experiments, the major peak centered at fraction 41 was substantially larger for fetal liver compared to adult liver.
Fig. 3. MonoQ chromatography of liver extracts.
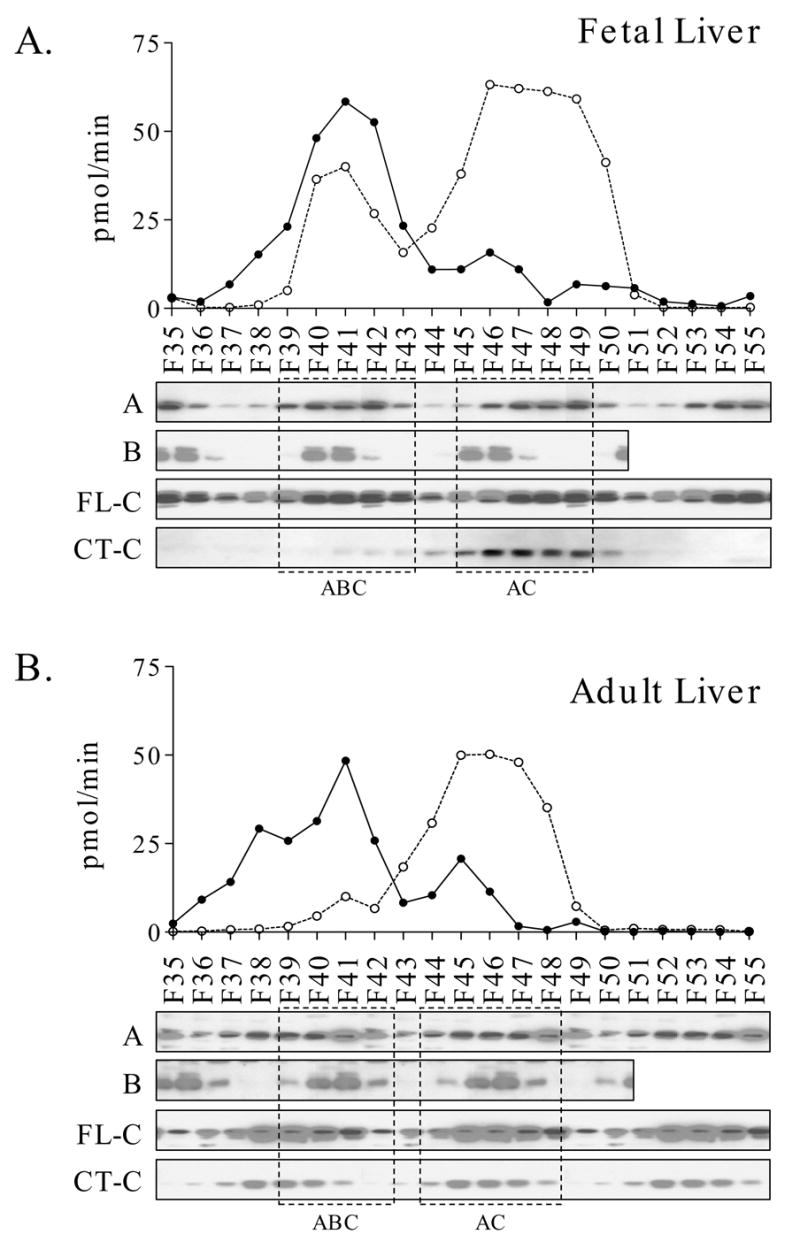
Parallel analyses of fetal (Panel A) and adult (Panel B) liver extracts are shown. Extract protein (20 mg) was applied to a MonoQ ion-exchange column and eluted with a linear gradient from 0 to 0.6 M NaCl. PP2A activity was measured in individual fractions (solid line). Fractions were also analyzed by immunoblotting with antibodies directed towards the A and B regulatory subunits and C (FL-C and CT-C antibodies). Densitometry values were derived from immunoblotting with the FL-C (dotted lines). The locations of the ABC heterotrimer and AC heterodimer are shown by the dashed boxes. It should be noted that the immunoblots in Panels A and B were exposed to optimize detection of the position of the various subunits, so comparisons between fetal and adult results should not be made. The results shown in this figure are representative of those obtained in a total of five independent experiments.
Fractions from MonoQ were analyzed by immunoblotting for PP2A catalytic subunit content using the FL-C and CT-C antibodies (Fig. 3). Immunoblots were optimized for exposure of the individual elution profiles, so comparisons between fetal and adult using the immunoblotting results in Figure 3 should not be made (see below). Results using the FL-C antibody showed that most of the PP2A eluted late in the gradient, overlapping and trailing the second peak of PP2A activity. Immunoblotting with the CT-C antibodies that are selectively reactive with unmethylated PP2A showed elution in the second peak. A comparison of PP2A activity with the abundance of PP2A-C in individual fractions indicated that the first peak had considerably higher specific activity than the second by approximately 4-fold and 2.5-fold in fetal and adult livers, respectively.
Immunoblotting of MonoQ fractions for the A (α/β) and B (α/β/γ) regulatory subunits of PP2A showed that the major peak of activity for both fetal and adult liver coincided with the position of heterotrimeric ABC complexes. The second activity peak coincided with the elution of AC heterodimer. Taken together, immunoblotting for the catalytic and regulatory PP2A components indicates the preferential localization of methylated PP2A-C to the ABC form and unmethylated PP2A-C to the later-eluting AC heterodimer.
While the A subunit of PP2A localized to both peaks of PP2A activity, the ratio of C to A subunit in these two peaks was very different, suggesting different subunit compositions. For both fetal and adult liver analyses, the ratio of C to A was much higher in fractions 45–60 compared to the ratio in fractions 39–42. If one assumes a 1:1 ratio of C to A in the heterotrimer (fraction 41), then the higher ratio of C to A in later fractions may indicate that C is bound to unidentified proteins other than the A subunit.
In order to allow direct comparisons of fetal and adult liver MonoQ profiles, pooled fractions were analyzed together on a single blot for PP2A immunoreactivity (Fig. 4A). Results showed that the higher levels of PP2A-C detected with the FL-C antibody in fetal liver extracts coincided with a higher signal in fractions containing both the ABC and AC forms. Immunoblotting of pooled fractions with the CT-C antibody showed that the immunoreactivity in the total adult liver extract could not be accounted for by the AC form in adult liver. This indicated to us that some unmethylated catalytic subunit in adult liver extracts must have been eluted from MonoQ in other fractions, presumably those containing minimal PP2A activity. Immunoblotting for A-subunit (Fig. 4A) showed a higher level in fractions corresponding to the ABC heterotrimer from the fetal analysis compared to the adult analysis. This result was consistent with other results indicating a greater abundance of the ABC form in fetal compared to adult liver. Immunoblotting for B-subunit (Fig. 4B) showed similar levels in total extracts and, as expected, a higher level in the fractions corresponding to ABC from the fetal liver analysis compared to the adult liver analysis. The larger ABC peak in fetal liver despite equal levels of B suggests that fetal liver may express other B subunits that were not detected with the antibodies used here.
Fig. 4. A comparison of PP2A subunit content in MonoQ pools derived from fetal versus adult liver.
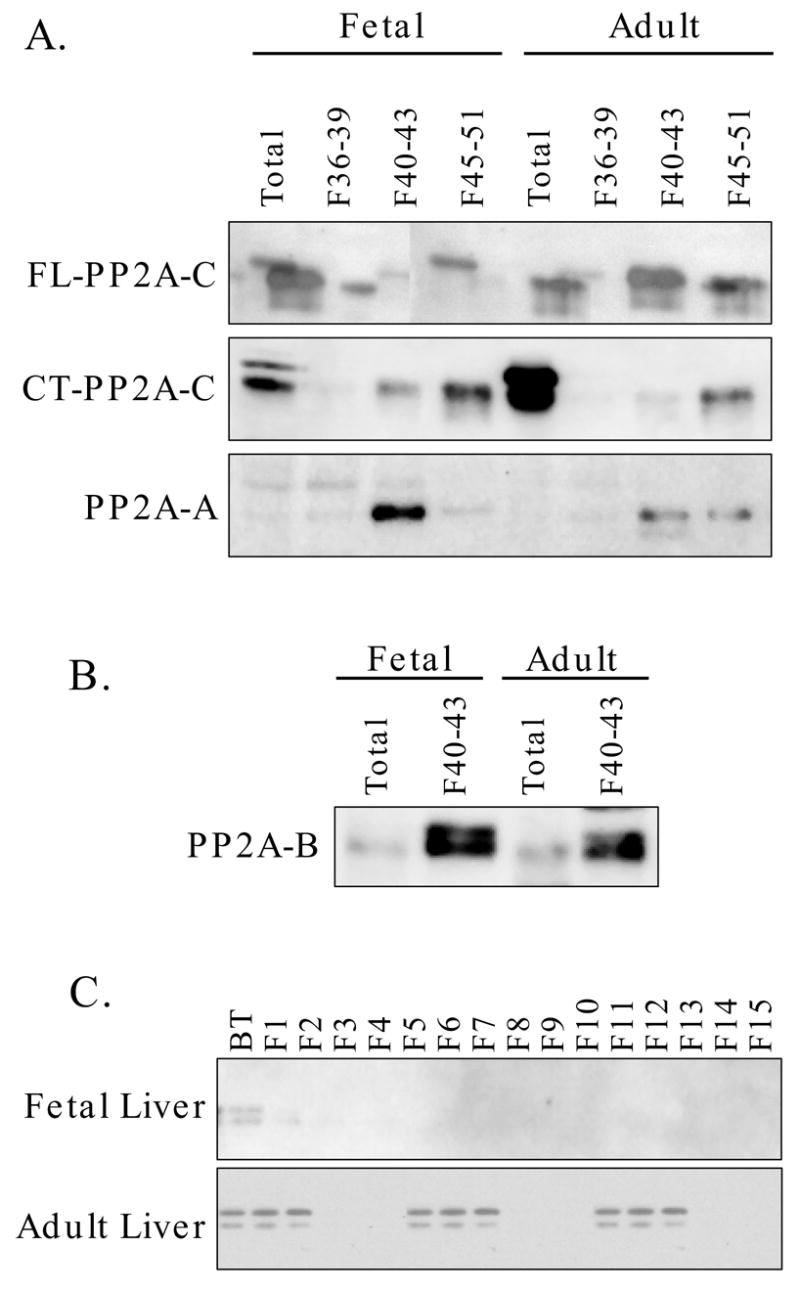
Fractions derived from the analyses shown in Figure 3 were pooled to allow for direct comparisons of subunit content in fractions containing catalytic subunit without A- or B-subunits (36-39), fractions containing ABC heterotrimer (40-43) and fractions containing AC heterodimer (45-51). Panel A: Extracts and all three pools were analyzed by immunoblotting for PP2A-C using the FL-C and CT-C antibodies and for A-subunit. Panel B: Immunoblotting of total extract and fraction pools for B-subunit. Panel C: Fetal and adult liver extract protein (25 mg) was eluted with a linear gradient from 0 to 0.5 M NaCl. The unbound (breakthrough; BT) fraction and fractions 1 through 15 were analyzed by immunoblotting for catalytic subunit using the CT-C antibody.
We repeated the MonoQ chromatography using a linear gradient of 0 to 0.5 M NaCl. The unbound and early eluting fractions were analyzed by immunoblotting (Fig. 4C). Results from an adult liver extract showed the presence of immunoreactive PP2A-C that was reactive with the CT-C antibody. Minimal immunoreactivity was detected in the breakthrough or early-eluting fractions from fetal liver. Given the volume of the breakthrough (approximately 5 ml), this unbound fraction could account for the higher level of unmethylated PP2A-C in adult liver relative to fetal liver.
Gel Filtration Analysis of Liver Extracts
The results of MonoQ chromatography indicated that the majority of unmethylated PP2A-C in adult liver was present in fractions that did not contain the heterotrimeric or heterodimeric forms. We hypothesized that a pool of unmethylated PP2A-C might be monomeric. To examine this possibility, an adult liver extract was analyzed by gel filtration chromatography using a Sephacryl S-200 column (Fig. 5A). Immunoblotting of fractions using antibodies directed towards the A and B subunits, as well as the FL-C and CT-C antibodies, revealed the presence of PP2A-C in two major peaks. One, which eluted at an apparent size of 140 to 180 kDa, corresponded to the unresolved AC and ABC forms. A second peak, centered at approximately 40 kDa, contained the CT-C immunoreactivity. These data were interpreted as indicating that monomeric catalytic subunit, immunoreactive with the CT-C antibody and, therefore, in the unmethylated state, was present in adult liver extracts. Fetal liver was also analyzed and key fractions were compared with corresponding fractions from adult liver (Fig. 5B). Results showed that fetal liver contained minimal monomeric, unmethylated PP2A-C relative to adult liver.
Fig. 5. Gel filtration analysis of fetal and adult liver extracts.
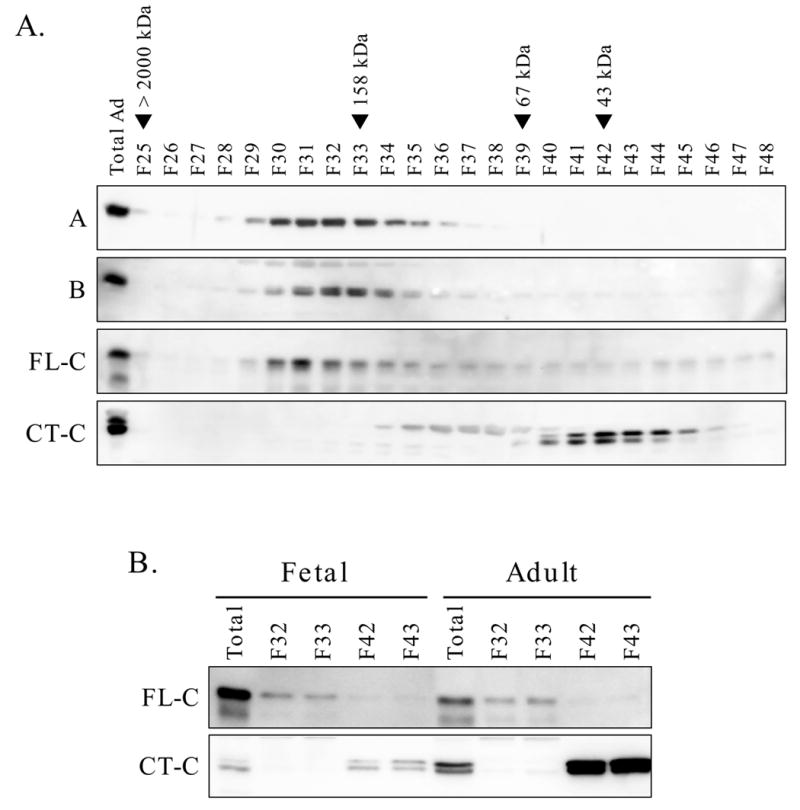
Panel A: An adult liver extract (1 ml containing 6 mg protein) was subjected to Sephacryl S-200 gel filtration analysis. The unfractionated sample (20 μl containing 120 μg protein per lane) and fractions (40 μl per lane) were analyzed by immunoblotting for A subunit, B subunit and catalytic subunit (FL-C and CT-C antibodies). The elution positions for blue dextran (>2000 kDa), aldolase (158 kDa), albumin (67 kDa) and ovalbumin (43 kDa) are shown above the immunoblot. Panel B: Fetal and adult liver extracts containing equal amounts of protein (6 mg) were analyzed in parallel by Sephacryl S-200 chromatography. In addition to the unfractionated extracts, fractions 32 and 33 and fractions 42 and 43 were analyzed on a single immunoblot using the FL-C and CT-C antibodies.
The Regulation of PP2A Catalytic Subunit Methylation in Liver
In order to assess the relationship between hepatocyte proliferation and PP2A-C methylation, adult rats were subjected to 2/3 hepatectomy. The regenerating liver remnants were obtained at times varying from 6 to 48 hr and used for preparation of homogenates. Western immunoblotting of these homogenates (data not shown) demonstrated no change in the immunoreactivity with FL-C and CT-C antibodies. The absence of a change in methylation status was confirmed by Western immunoblotting with the antibodies specific for the demethylated form of PP2A-C. Immunoblotting for the A and B subunits also showed no change in their content during the early stages of liver regeneration (data not shown).
Triplicate fetal and adult liver extracts were analyzed by immunoblotting for content of the enzymes responsible for PP2A-C methylation and demethylation, protein phosphatase methyl transferase (PPMT) and protein methyl esterase (PME-1) (Fig. 6). PPMT levels were similar in fetal and adult livers. The levels of PME-1 were much higher in fetal liver than adult liver, a result opposite from that predicted by PP2A-C methylation status. The fetal and adult liver extracts were also blotted for the phosphotyrosyl phosphatase activator of PP2A (PTPA). PTPA was slightly more abundant in fetal liver, perhaps consistent with the higher phosphatase activity in these extracts.
Fig. 6. PPMT, PME-1 and PTPA in fetal and adult liver.

Triplicate fetal and adult liver extracts were analyzed by Western immunoblotting. The p85 subunit of PI3K was used as a control for quality and quantity of protein in each lane.
Discussion
Previous studies aimed at characterizing hepatic PP2A have mainly focused on determining the phosphatase activity of different PP2A complexes and identifying their regulatory subunit composition. These studies led to the conclusion that the heterodimeric and heterotrimeric forms of PP2A predominate in mammalian cells and that there is little if any free catalytic subunit [33]. Most of these studies were performed prior to the availability of antibody reagents. Thus, they depended on purification of catalytic activity. For a long time, PP2A has been thought to exist in the cell predominantly as a trimer. This concept has been challenged by some based on results indicating that AC dimers and/or C subunits associated with other cellular regulatory proteins play a significant role in vivo [13;22;34–36].
We have observed that late gestation rat liver shows a high rate of hepatocyte proliferation and that this proliferation is resistant to the growth inhibitory effects of rapamycin. There is a complex functional relationship between signaling by the mammalian target of rapamycin, mTOR, and PP2A [37;38]. Therefore, we hypothesized that the expression, subunit composition and/or regulation of PP2A in fetal versus adult liver would differ. Western immunoblotting aimed at quantifying the abundance of PP2A catalytic subunit in fetal and adult liver homogenates showed that there is more PP2A-C in fetal than in adult liver. However, there were even more marked differences in the immunoreactivity with antibodies directed towards the full-length catalytic subunit versus the carboxy terminus. These experiments led to the observation that the catalytic subunit of fetal liver is mostly in the methylated form while the unmethylated form is more highly abundant in adult liver. Carboxy-terminal methylation abolished reactivity with the CT-C antibody, thus explaining the differential reactivity.
Methylation of PP2A catalytic subunit is thought to play a role in the biogenesis of PP2A, regulating the formation of PP2A heterotrimers by promoting B subunit binding to AC dimers [11;13;14;39;40]. Our observation that demethylated catalytic subunit preferentially eluted in ion exchange chromatography fractions containing AC dimer is consistent with this mechanism. The fetal versus adult difference in methylation status also predicted that fetal liver would contain more PP2A in the heterotrimeric form relative to the heterodimeric form, which was indeed the case. Contradictory data have been published with regard to the effect of catalytic subunit methylation on catalytic activity, with one group observing a moderate increase in phosphatase activity [41], another seeing no direct effect [42], and a third demonstrating decreased activity [43]. Our results showed that in liver methylation status correlates with higher phosphatase specific activity, although this effect can not be separated from the effect of methylation in promoting formation of the heterotrimeric complex.
Analysis of MonoQ fractions revealed the trimeric and dimeric forms of PP2A but did not account for all of the unmethylated catalytic subunit in adult liver extracts. This form was detected instead in the unbound fraction as catalytic subunit unassociated with A and B subunits. This led us to hypothesize that adult liver contains some unmethylated PP2A as free monomer, a conclusion supported by the results of gel filtration analyses. While this finding was unexpected, the migration position of unmethylated catalytic subunit at an apparent molecular size of 40 kDa and the fact that we analyzed fresh extracts made under mild conditions point to the presence of inactive catalytic subunit in the absence of regulatory components in these fractions. Also consistent with this conclusion was the relative absence of activity in the unbound fractions from MonoQ. The higher ratios of C to A subunits in the second PP2A activity peak from MonoQ relative to the ratio in the ABC peak suggests that the former may include unmethylated C bound to subunits other than A. These other subunits were not identified, but they did not appear to account for differences between fetal and adult liver PP2A.
It has been reported that PP2A catalytic subunit methylation is cell cycle dependent. Methylation state has been shown to oscillate in the cytoplasm during the G0/G1 transition, and in the nucleus at the G1/S transition [41;44]. The hepatocytes in late gestation fetal liver are proliferating asynchronously [28]. These data showing a higher level of methylation in proliferating fetal hepatocytes relative to quiescent adult hepatocytes are consistent with the cell cycle-dependence of PP2A methylation as described by others. However, we examined catalytic subunit methylation in homogenates from regenerating liver and found no changes at times up to 48 hr after partial hepatectomy. We conclude that the differences between fetal and adult liver with regard to PP2A catalytic subunit methylation cannot be explained solely on the basis of cell cycle activity. The levels of PPMT, PME-1 and PTPA did not demonstrate a simple correlation accounting for the high level of catalytic subunit methylation in fetal liver. The lack of association between the levels of these components and PP2A methylation suggests the existence of alternative mechanisms for modulating catalytic subunit methylation.
Unlike most previous studies [9;20;26;45], we took advantage of the ability to measure PP2A activity and to detect PP2A subunits by immunoblotting to examine the relative abundance of PP2A components in vivo under conditions in which hepatocytes are actively dividing (the late gestation fetus) or in which hepatocytes are quiescent (adult liver). Our initial observation that PP2A is more abundant in fetal liver was accompanied by the unexpected results showing a marked difference in the methylation of PP2A. The higher methylation of PP2A-C in fetal liver was correlated with a greater abundance of the active, heterotrimeric form of the enzyme. Thus, we were able to provide support for the physiological relevance of previous observations that PP2A methylation is associated with ABC heterotrimer formation. We also observed a significant pool of free unmethylated catalytic subunit in adult liver. This represents an observation not previously made in analyses of mammalian cells. These results are consistent with an important role for PP2A catalytic subunit methylation in modulating PP2A biogenesis.
Acknowledgments
We thank Jennifer Sanders for her assistance in the performance of animal studies and for helpful discussions. These studies were supported by USPHS grants HD24455 and HD35831 (to P.G.) and CA77584 (to D.B.).
References
- 1.Zolnierowicz S. Biochem Pharmacol. 2000;60:1225–1235. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]
- 2.Mumby MC, Walter G. Physiol Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- 3.Honkanen RE, Golden T. Curr Med Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Pham HT, Walter G. J Biol Chem. 2003;278:8617–8622. doi: 10.1074/jbc.M211181200. [DOI] [PubMed] [Google Scholar]
- 5.Van Hoof C, Goris J. Biochim Biophys Acta. 2003;1640:97–104. doi: 10.1016/s0167-4889(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 6.Strack S, Ruediger R, Walter G, Dagda RK, Barwacz CA, Cribbs JT. J Biol Chem. 2002;277:20750–20755. doi: 10.1074/jbc.M202992200. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Martin BL, Brautigan DL. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 8.Guo H, Damuni Z. Proc Natl Acad Sci USA. 1993;90:2500–2504. doi: 10.1073/pnas.90.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longin S, Jordens J, Martens E, Stevens I, Janssens V, Rondelez E, De Baere I, Derua R, Waelkens E, Goris J, Van Hoof C. Biochem J. 2004;380:111–119. doi: 10.1042/BJ20031643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalhor HR, Luk K, Ramos A, Zobel-Thropp P, Clarke S. Arch Biochem Biophys. 2001;395:239–245. doi: 10.1006/abbi.2001.2558. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Tolstykh T, Lee J, Boyd K, Stock JB, Broach JR. EMBO J. 2000;19:5672–5681. doi: 10.1093/emboj/19.21.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogris E, Du X, Nelson KC, Mak EK, Yu XX, Lane WS, Pallas DC. J Biol Chem. 1999;274:14382–14391. doi: 10.1074/jbc.274.20.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolstykh T, Lee J, Safai S, Stock JB. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant JC, Westphal RS, Wadzinski BE. BiochemJ. 1999;339:241–246. [PMC free article] [PubMed] [Google Scholar]
- 15.Mumby M. Sci STKE. 2001;79:PE1. doi: 10.1126/stke.2001.79.pe1. [DOI] [PubMed] [Google Scholar]
- 16.Leulliot N, Vicentini G, Jordens J, Quevillon-Cheruel S, Schiltz M, Barford D, van Tilbeurgh H, Goris J. Mol Cell. 2006;23:413–424. doi: 10.1016/j.molcel.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Fellner T, Lackner DH, Hombauer H, Piribauer P, Mudrak I, Zaragoza K, Juno C, Ogris E. Genes Dev. 2003;17:2138–2150. doi: 10.1101/gad.259903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Scuderi A, Letsou A, Virshup DM. Mol Cell Biol. 2002;22:3674–3684. doi: 10.1128/MCB.22.11.3674-3684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koren R, Rainis L, Kleinberger T. J Biol Chem. 2004;279:48598–48606. doi: 10.1074/jbc.M409359200. [DOI] [PubMed] [Google Scholar]
- 20.Tung HY, Alemany S, Cohen P. Eur J Biochem. 1985;148:253–263. doi: 10.1111/j.1432-1033.1985.tb08833.x. [DOI] [PubMed] [Google Scholar]
- 21.Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Proc Natl Acad Sci USA. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kremmer E, Ohst K, Kiefer J, Brewis N, Walter G. Mol Cell Biol. 1997;17:1692–1701. doi: 10.1128/mcb.17.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloeker S, Reed R, McConnell JL, Chang D, Tran K, Westphal RS, Law BK, Colbran RJ, Kamoun M, Campbell KS, Wadzinski BE. Protein Expr Purif. 2003;31:19–33. doi: 10.1016/s1046-5928(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 24.Khew-Goodall Y, Hemmings BA. FEBS Lett. 1988;238:265–268. doi: 10.1016/0014-5793(88)80493-9. [DOI] [PubMed] [Google Scholar]
- 25.Khew-Goodall Y, Mayer RE, Maurer F, Stone SR, Hemmings BA. Biochemistry. 1991;30:89–97. doi: 10.1021/bi00215a014. [DOI] [PubMed] [Google Scholar]
- 26.Jaspers SR, Miller TB., Jr Mol Cell Biochem. 1991;101:167–174. doi: 10.1007/BF00229533. [DOI] [PubMed] [Google Scholar]
- 27.Gruppuso PA. Pediatr Res. 1990;27:599–603. doi: 10.1203/00006450-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Curran TR, Jr, Bahner RI, Jr, Oh W, Gruppuso PA. Exp Cell Res. 1993;209:53–57. doi: 10.1006/excr.1993.1284. [DOI] [PubMed] [Google Scholar]
- 29.Boylan JM, Anand P, Gruppuso PA. J Biol Chem. 2001;276:44457–44463. doi: 10.1074/jbc.M103457200. [DOI] [PubMed] [Google Scholar]
- 30.Gruppuso PA, Awad M, Bienieki T, Boylan J, Fernando S, Faris R. In Vitro Cell Dev Biol. 1997;33:562–568. doi: 10.1007/s11626-997-0099-x. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Parsons S, Brautigan DL. J Biol Chem. 1994;269:7957–7962. [PubMed] [Google Scholar]
- 32.Awad MM, Gruppuso PA. Cell Growth Differ. 2000;11:325–334. [PubMed] [Google Scholar]
- 33.Goldberg Y. Biochem Pharmacol. 1999;57:321–328. doi: 10.1016/s0006-2952(98)00245-7. [DOI] [PubMed] [Google Scholar]
- 34.Murata K, Wu J, Brautigan DL. Proc Natl Acad Sci USA. 1997;94:10624–10629. doi: 10.1073/pnas.94.20.10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruediger R, Brewis N, Ohst K, Walter G. Virology. 1997;238:432–443. doi: 10.1006/viro.1997.8873. [DOI] [PubMed] [Google Scholar]
- 36.Chung H, Nairn AC, Murata K, Brautigan DL. Biochemistry. 1999;38:10371–10376. doi: 10.1021/bi990902g. [DOI] [PubMed] [Google Scholar]
- 37.Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Proc Natl Acad Sci USA. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Houghton PJ. Drug ResistUpdat. 2001;4:378–391. doi: 10.1054/drup.2002.0227. [DOI] [PubMed] [Google Scholar]
- 39.Yu XX, Du X, Moreno CS, Green RE, Ogris E, Feng Q, Chou L, McQuoid MJ, Pallas MDC. Mol Biol Cell. 2001;12:185–199. doi: 10.1091/mbc.12.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei H, Ashby DG, Moreno CS, Ogris E, Yeong FM, Corbett AH, Pallas DC. J Biol Chem. 2001;276:1570–1577. doi: 10.1074/jbc.M008694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Favre B, Zolnierowicz S, Turowski P, Hemmings BA. J Biol Chem. 1994;269:16311–16317. [PubMed] [Google Scholar]
- 42.De Baere I, Derua R, Janssens V, Van Hoof C, Waelkens E, Merlevede W, Goris J. Biochemistry. 1999;38:16539–16547. doi: 10.1021/bi991646a. [DOI] [PubMed] [Google Scholar]
- 43.Zhu T, Matsuzawa S, Mizuno Y, Kamibayashi C, Mumby MC, Andjelkovic N, Hemmings BA, Onoe K, Kikuchi K. Arch Biochem Biophys. 1997;339:210–217. doi: 10.1006/abbi.1996.9835. [DOI] [PubMed] [Google Scholar]
- 44.Turowski P, Fernandez A, Favre B, Lamb NJ, Hemmings BA. J Cell Biol. 1995;129:397–410. doi: 10.1083/jcb.129.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikuchi K, Shima H, Mitsuhashi S, Suzuki M, Oikawa H. Int J Mol Med. 1999;4:395–401. doi: 10.3892/ijmm.4.4.395. [DOI] [PubMed] [Google Scholar]


