Introduction
Allergic responses are strongly associated with Th2 type immune responses. Modulation of the skewed Th2 response toward a more balanced response is the major goal of the allergen immunotherapy (IT) in allergic disorders. To this end, several approaches, including allergen protein-, peptide-, modified allergen protein-, and allergy gene-based IT have been tested (1–3). Traditionally, allergen IT has relied upon the frequent injection of gradually escalated amounts of the extracted allergen protein(s). However, even when given in a cautious and protracted schedule, standard allergen IT gives rise to local and systemic allergic reactions and carries the risk of eliciting rare but life-threatening reactions (4, 5). Thus, there is great interest in the development of novel forms of allergen IT in terms of safety and efficacy. As we had shown that a human Ig Fcγ-Fcε fusion protein (GE2) that directly cross-links FcεRI and FcγRIIb on human mast cells and basophils was able to inhibit degranulation (6), we reasoned that human gamma – allergen fusion protein would achieve a similar inhibitory effect in an allergen specific fashion while preserving the immunogenicity of the allergen component. Thus we have now constructed and developed a human-cat chimeric fusion protein composed of the human Fcγ1 and the cat allergen Fel d1 (Felis domesticus) for cat allergen specific IT (7, 8). In this paper, we summarize the therapeutic features and potential of this novel fusion protein for allergic IT.
Role of FcγRIIb in inhibition of allergic response
The cross-linking of FcεRI activates tyrosine phosphorylation of immunoreceptor tyrosine based activation motifs (ITAMs) in the β- and γ-FcεRI subunits in the cytoplasmic tails and leads to cell activation and degranulation in basophils and mast cells (4, 5). This leads to the classic immediate hypersensitivity reaction. Such an activation signal is balanced by the inhibitory receptors on these cells (9). Human mast cells and basophils express FcγRIIb, which contains an immunoreceptor tyrosine-based inhibitory motif (ITIMs) within its cytoplasmic tail (9). Co-aggregation of FcεRI to FcγRIIb has been shown to block in vitro and in vivo human basophil and mast cell function (10–13). This inhibition is mediated via the reduction in the tyrosine phosphorylation of Syk, ERK and several other cellular substrates and increased tyrosine phosphorylation of the adapter protein downstream of kinase (Dok) growth factor receptor-bound protein 2 (Grb2) and SH2 domain containing inositol 5-phosphatase (SHIP) (8, 14). A previously developed Fcγ-Fcε fusion protein GE2 (6) exhibited its inhibitory role in allergic responses with an allergen non-specific manner via directly cross-linking of the FcεRI and FcγRIIb on mast cells/basophils. A more detailed review on this issue has been published elsewhere (15).
Fcγ-Fel d1 fusion protein (GFD) inhibits Fel d1-mediated allergic degranulation
To explore whether allergic responses can be modified in an allergen-specific manner via “indirect” co-crosslinking of the FcγRIIb and FcεRI bound the allergen specific IgE, we decided to genetically link an an allergen molecule via a flexible linker to a human Fcγ region. As Fel d1 is the dominant allergen for cat allergy and cat allergy is a major clinical problem, we constructed a chimeric human-cat protein composed of the hinge through CH3 portion of human IgGγ1 Fc region fused to Fel d1 (7).
To test the efficacy of GFD on degranulation, freshly purified human basophils were purified from cat allergic patients and were cultured along with various doses of GFD ranging from 1 ng/ml to 1 μg/ml, followed by the challenge with an optimal dose of purified Fel d1. GFD at 10 ng/ml inhibited histamine release by more than 75% (P<0.002) while at 100 ng/ml, inhibition reached greater than 90% (P<0.001) (Figure 1A). Similar inhibition was observed in cord blood-derived mast cells sensitized with cat allergic serum. At 10 μg/ml, GFD reduced FcεRI-mediated release by an average of 77% (P< 0.05) in a dose-dependent fashion (Figure 1B). Critically, these results also showed that GFD does not function as an allergen since “pre-release” of mediators was not observed when Fel d1 sensitized basophils were incubated with GFD. These results indicated that GFD was able to block the Fel d1-induced degranulation of human mast cells/basophils, and the fusion of Fel d1 to the Fcγ altered the allergen nature of the Fel d1.
Figure 1.
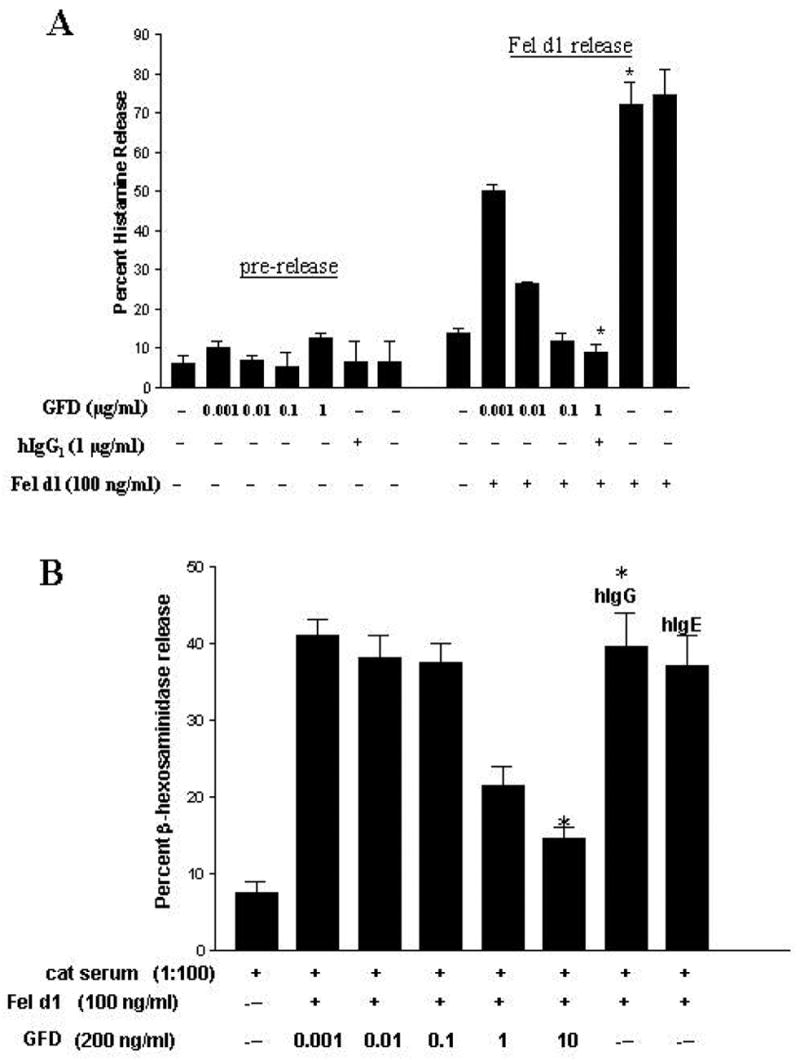
GFD inhibits fresh human basophil and mast cell degranulation. A: Dose-dependent inhibition of basophils histamine release by GFD. Basophils from an atopic donor were incubated for 2 hours with GFD and the supernatant assayed for histamine (pre-release). Cells were washed and then challenged with Fel d1 and histamine was measured in the supernatant (Fel d1 release). B: Dose-dependent inhibition of fresh cord blood-derived mast cell β-hexosaminidase release using GFD. The results from one experiment, done in duplicate, are representative of 3 separate donors. The asterisk indicates a statistically significant difference when comparing the two conditions. Human IgG and human IgG are represented as hIgG and hIgE, respectively. (Adapted from Zhu D, Kepley CL, Zhang K, et al. A Chimeric Human –Cat Fusion Protein Blocks Cat-induced Allergy, Nat Med. 2005; 11:446–9, with permission).
GFD inhibits signal events associated with degranulation
Tyrosine phosphorylation is a key event connecting FcεRI crosslinking to downstream signaling in human mast cells and basophils. Previous investigations have shown that the MAP kinases ERK1/2 and Syk are quickly phosphorylated in IgE-stimulated human FcεRI-positive cells (16, 17). To determine whether GFD is able to alter these critical early signaling events responsible for the early activation of mast cells/basophils, we investigated the role of GFD in IgE-dependent, FcεRI-mediated kinase phosphorylations. Cross-linking FcεRI on cord blood mast cells with IgE directed to Fel d1 induces substantial tyrosine phosphorylation of Syk and ERK, which was markedly reduced in cells pre-incubated with GFD (Figure 2). Inhibition was observed 2 min. after antigen stimulation and persisted as long as 15 min. Thus, GFD co-aggregation of FcεRI and FcγRII through formation of a Fcγ-Fel d1-IgE complex inhibits IgE-mediated Syk and ERK phosphorylation and likely is responsible for the inhibition of basophil/mast cell function.
Figure 2.
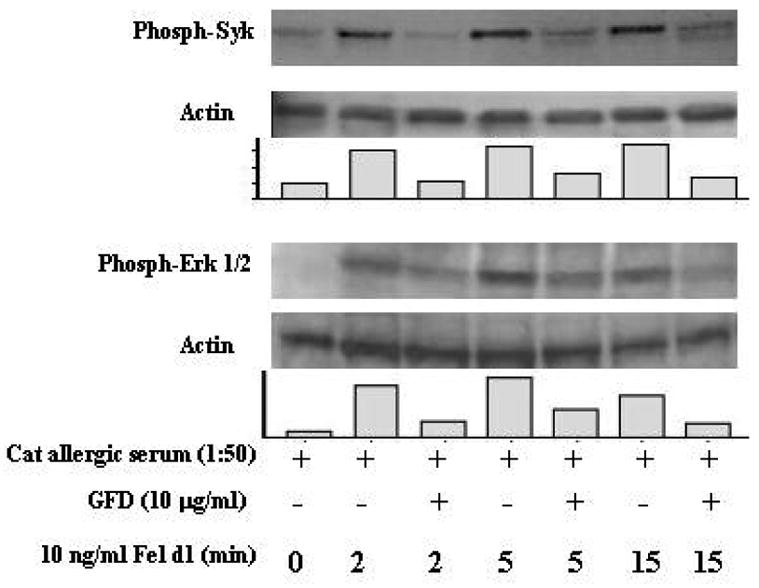
GFD inhibits FcεRI-mediated Syk and ERK phosphorylation. Cord blood-derived mast cells were sensitized with cat allergic serum, washed, and Western blotted with the indicated antibodies. Results are representative of three separate experiments. (Adapted from Zhu D, Kepley CL, Zhang K, et al. A Chimeric Human –Cat Fusion Protein Blocks Cat-induced Allergy, Nat Med. 2005; 11:446–9, with permission).
GFD blocks passive anaphylaxis reaction (PCA) in FcεRIα Transgenic (Tg) mice
Allergic degranulation of mast cells in vivo can be determined by a PCA assay in human FcεRIα Tg mouse (6, 7). Following passive sensitization of the back skin of the FcεRIα Tg mice with human IgE from cat allergic patient, and subsequent challenge with the appropriate antigen, the FcεRIα Tg mice will show positive PCA. As the mast cells in these transgenic mice also express the murine FcγRIIb that binds to human IgG (6), the inhibitory effects of GFD via co-crosslinking the of humanized FcεRI and the murine FcγRIIb can be tested using this PCA model.
As shown in the Figure 3A, GFD inhibited Cat-allergic patient’s IgE-mediated PCA in a dose-dependent manner (panel I-IV), with GFD at 100 ng/spot completely blocked the PCA (panel IV). Analogous inhibition was also observed by using GE2 fusion protein (Figure 3B). However, GFD exhibited higher efficacy for blocking PCA reactivity compared to GE2 (Figure 3B) with at least 10 fold less amounts of GFD required for complete blocking of PCA (Figure. 3B, panels c vs d). GFD blocked PCA reactivity equally well when injected 4 hours after or simultaneously with cat allergic patient’s serum (Figure 3B, panel c vs b). The purified cat allergic patient’s IgE-dependent PCA, as demonstrated by the inactivation of the PCA activity by heating the IgE with 56°C for 30 min (18), was also completely blocked by GFD. As a specificity control, GFD did not inhibit PCA reactivity to the human anti-NP (4-hydroxy-3-nitrophenylacetyl) IgE (data not shown). GFD itself was not inducing mast cell release at sensitized sites (data not shown). These data demonstrated that GFD is able to block the Fel d1 allergen specific allergic response in vivo.
Figure 3.
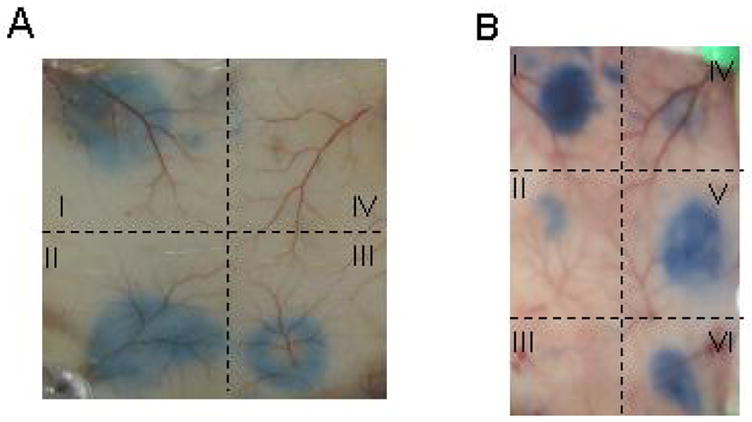
In vivo GFD inhibits IgE-mediated degranulation in transgenic mice expressing human FcεRIα. A. Dose-dependent inhibition of PCA by GFD. The labeled back skin sites were sensitized with cat allergic serum (1:5 dilution) for 4 hours, followed by the administration of: I, saline; II, 1 ng of GFD in 50 μl saline; III, 10 ng of GFD; and IV, 100 ng of GFD. B. Comparison of GFD and GE2 for their ability to inhibit PCA reactivity to human anti-Fel d1 IgE. The skin sites were sensitized with 1:5 diluted cat allergic serum, with the following treatment: I, saline injection 4 h later; II, 100 ng GFD 4 h later; III, 100 ng GFD simultaneously with serum; IV, 1 μg GE2 simultaneously with serum; V, 100 ng GE2 4 h later; VI, 100 ng GE2 simultaneously with serum. (Adapted from Zhu D, Kepley CL, Zhang K, et al. A Chimeric Human –Cat Fusion Protein Blocks Cat-induced Allergy, Nat Med. 2005; 11:446–9, with permission).
GFD blocks the allergic responses in a mouse model
A Balb/c mouse model of systemic reactivity to Fel d1 in actively sensitized mice was used in order to test the immunotherapeutic ability of GFD. The rationale for such a murine model to test the effects of human IgG Fc-Fel d1 fusion protein GFD is based on the fact that the murine Fc receptors for IgG (FcγRs) will bind human IgG Fc (19). Thus, the Fc portion of GFD is expected to bind murine FcγRs, including FcγRIIb, which contains the ITIM that drives inhibitory signaling. At the same time, the Fel d1 portion of GFD will bind to murine Fel d1 specific IgE and/or IgG1 on the surface of sensitized mast cells/basophils.
Balb/c mice were sensitized with Fed d1 and treated according to the protocol diagrammed in Figure 4A. The core body temperature, which was used as the indicator for systemic anaphylaxis, dropped by an average of 1.7 ±0.2° C starting at 5 minutes after the challenge (Figure 4D). This decrease of body temperature was completely blocked by the GFD treatment (P<.001). GFD treatment also completely blocked Fel d1-induced airway hyper-responsiveness (AHR) (Figure 4B) as assessed by pulmonary resistance following methacholine challenge. Similarly, the eosinophilic airway inflammation in Fel d1 sensitized and intratracheally challenged mice, which was evident as an increase of eosinophils in bronchoalveolar lavage (BAL) fluid, was blunted by GFD treatment (Figure 4C). These results indicate that allergic responses to Fel d1 could be ameliorated by GFD treatment and these beneficial immunomodulatory effects occurred when GFD was administrated in a regimen similar to allergen immunotherapy after initial allergen sensitization. Anti-Fel d1 IgG1, IgG2a, and IgE responses induced by Fel d1 immunization showed variable changes in response to GFD treatment but these changes did not reach statistical significance (data not shown). These results indicated that allergic response to Fel d1 in a mouse model was significantly inhibited through a protocol of allergen immunotherapy.
Figure 4.
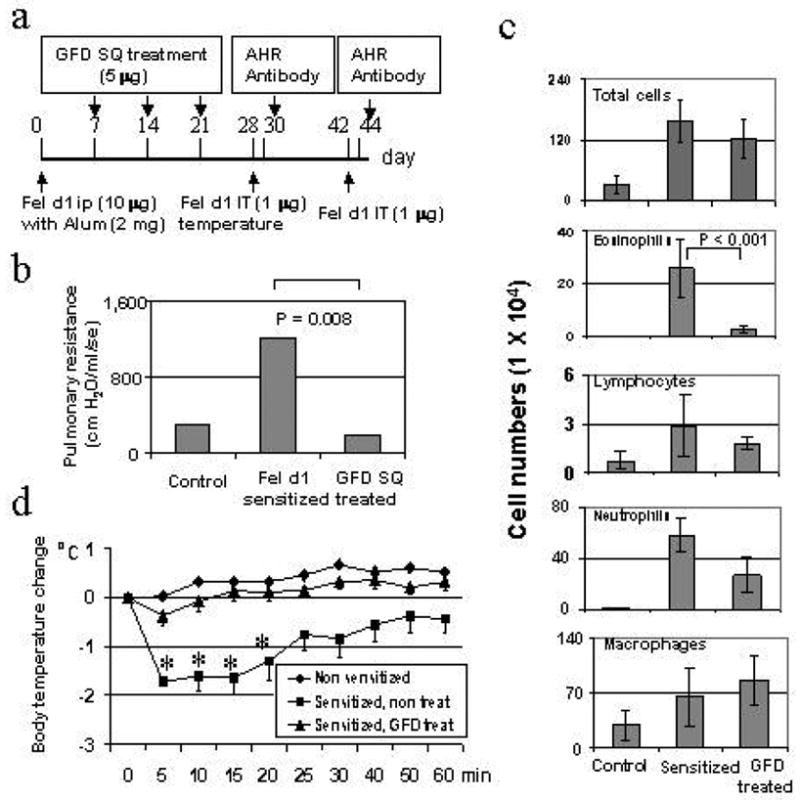
Subcutaneous (SQ) administration of GFD blocked Fel d1-induced allergic response in mouse model. A. Schematic diagram of the experimental protocol. B. Effect of GFD on blocking Fel d1-induced systemic allergic reactivity. The body temperature changes were assessed immediately after Fel d1 IT challenge (1 μg) with 5 minute intervals. The asterisk indicates a statistically significant difference between the two conditions. C. Effect of GFD on Fel d1-induced AHR. The airway resistance to methacholine challenge was assessed by a computer-controlled small animal FlexiVent® ventilator two days after IT Fel d1 challenge. The numbers represent the average values from three measurements of the airway resistance from a group of mice for each condition. D. Effect of GFD on Fel d1-induced pulmonary eosinophilic inflammation. Total and differential numbers of BAL fluid cells were counted. (Adapted from Zhu D, Kepley CL, Zhang K, et al. A Chimeric Human –Cat Fusion Protein Blocks Cat-induced Allergy, Nat Med. 2005; 11:446–9, with permission).
GFD fails to induce local or systemic reactivity upon administration to Fel d1 sensitized animals
Since GFD is a fusion protein containing Fel d1, it was important to determine whether GFD itself would function as Fel d1 to induce allergic reactivity. We undertook several approaches to examine this issue. As shown in Figure 1A, GFD by itself was not able to mediate histamine release from human basophils of cat-allergic patient. In Fel d1 sensitized mice, Fel d1 induced a systemic allergic reactivity as evidenced by significant body temperature drop (2.58 ± 0.4°C). In contrast, an equimolar amount of GFD did not induce a significant temperature drop in sensitized animals. In addition, IT administration of GFD did not induce AHR as was seen with Fel d1. Intradermal injection of Fel d1 induced mast cell degranulation in the skin of Fel d1 sensitized Balb/c mice, but GFD did not induce the skin reactivity in sensitized animals. These data strongly indicated that GFD failed to elicit allergic reactivity systemically in the skin or the airways of Fel d1 sensitized animals.
GFD blocks Fel d1-induced allergic reactivity in rush IT settings
With the experimental regimen capable of blocking Fel d1-mediated allergic responses in a Balb/c mice model as describe above, we further sought to test whether GFD, when administered in a protocol to mimic rush IT (e.g., high dose GFD administrated in a short period), was able to inhibit Fel d1-dependent allergic responses in already highly sensitized animals, and whether a single administration of GFD is sufficient to acutely block reactivity in animals with established Fel d1-induced allergic responses.
The Fel d1-induced systemic reaction, measured by the core temperature changes, was inhibited for a longer duration in the rush IT setting with three time of administration of GFD, whereas only acute, but not delayed, systemic reaction was inhibited by the single administration of GFD (8), with a similar manner as showed in the Figure 4D. Administration of GFD as the rush IT also blocked the skin mast cell degranulation, assayed with active skin test, of the sensitized mice (8).
Fel-d1 induced airway responsiveness and allergic lung inflammation were blocked by the rush IT protocols. Treatment with GFD completely reversed the Fel d1-induced airway hyper-responsiveness to methacholine, and blunted the airway allergic inflammation as demonstrated by the significantly decrease either in the percentage, or the absolute numbers, of eosinophils in the BAL fluid (P < 0.001). Histological examination revealed that Fel d1 induced pulmonary inflammation (Figure 5A) was significantly inhibited by GFD administration (Figure 5B, P< 0.001). Marked goblet cell metaplasia was observed in the large airways of sensitized mice and Fel d1 treated mice, while GFD treatment inhibited this goblet cell metaplasia (Figure 5C). These results indicted that Fel d1-induced allergic responses was significantly blocked by the rush IT protocol with higher dosage of GFD administration.
Figure 5.
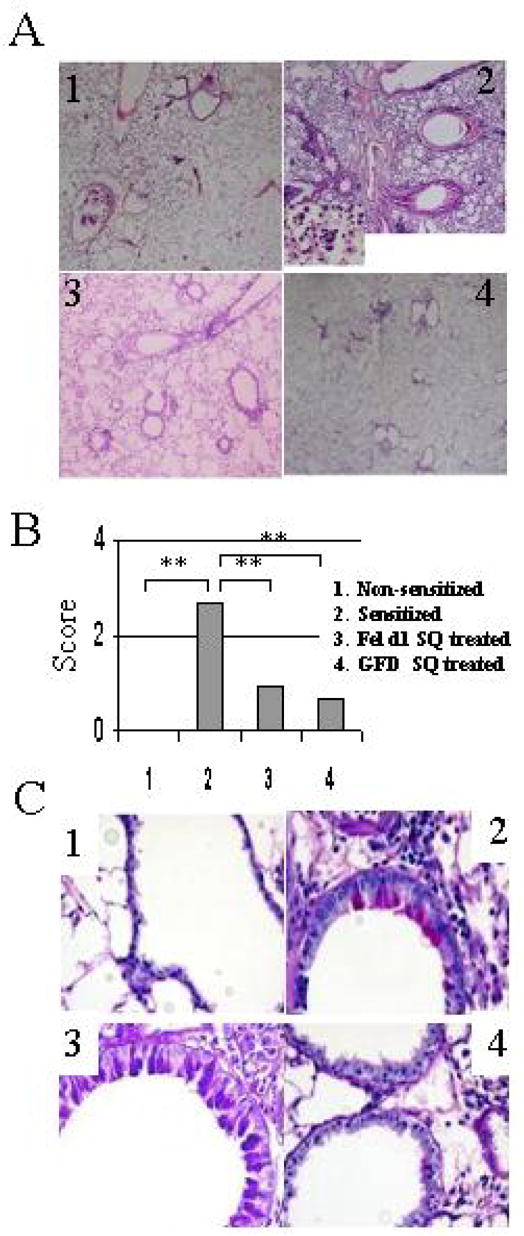
Lung histological changes in GFD treated mice. A. Light photomicrographs of H&E-stained sections of lung tissue from the different treatments. Few leukocytes were observed in the lung of non-sensitized mice (panel 1). In contrast, numerous eosinophils were present after Fel d1 challenge in the control mice (panel 2). Both Fel d1 and GFD treatments (panel 3 and 4) lead to markedly decreased eosinophil accumulation in the lung. B) Semi-quantitative analysis of the eosinophil accumulation in the lung of animals challenged IT with Fel d1 at day 54. Results of each group are expressed as mean ± SD. The asterisk (***) indicates P< 0.001 between the groups. C. Light photomicrographs of PAS-stained sections of lung tissue from non-sensitized mice (panel 1) and mice receiving the different treatments. Marked goblet cell metaplasia was observed in large airways in the sensitized - challenged animals (panel 2). GFD treatment (panel 4) but not Fel d1 (panel 3) clearly inhibited this goblet cell metaplasia (Adapted from Terada T, Zhang K, Belperio J et al. A chimeric human-cat Fcgamma-Fel d1 fusion protein inhibits systemic, pulmonary, and cutaneous allergic reactivity to intratracheal challenge in mice sensitized to Fel d1, the major cat allergen. Clin Immunol. 2006; 120:45–56; with permission).
GFD immunotherapy modulates the antibody response to Fel d1
To examine whether the administration of GFD with rush IT protocol was able to modulate the antibody responses to Fel d1, the Fel d1 specific IgG1, IgG2a, and IgE was analyzed. The Fel d1 sensitization and challenge induced significant increases in serum Fel d1 antibodies, with levels going from undetectable (<1.0 U/ml) for non-sensitized animals to geometric means of 57,859, 2, and 113 U/ml for IgG1, IgE, and IgG2, respectively (P<0.01 for all), reflecting the Th2 dominant allergic antibody responses. GFD treatment led to an increase in IgG1 antibodies to Fel d1 as compared to untreated (geometric means of 186,167 vs 57,859 U/ml, P<0.05) and Fel d1 treated animals (186,167 vs 33,157 U/ml, P<0.02,). GFD did not alter IgE or IgG2 antibodies (geometric means of 34.4 and 166 U/ml respectively) and Fel d1 treatment did not significantly alter any antibodies levels (geometric means of 33,157, 14.2 and 140 U/ml for IgG1, IgE or IgG2a) compared to untreated animals.
Potential use of GFD for allergic blockade and IT in cat-allergy
The data presented provides evidence that the chimeric GFD protein holds great promise as both a model for a new form of immunotherapy in allergy and a specific intervention against cat allergy. The advantages of this approach are that the allergen carries with it’s own negative signal, the Fcγ portion that has been shown to drive inhibitory signaling in human mast cells and basophils. GFD’s indirect cross linking of FcγRIIb and FcεRI via naturally occurring IgE to Fel d1 results in an acute antigen specific inhibition of mediator release. As a result, from a safety perspective, GFD should be able to be safely given in high doses and much briefer time frame than conventional immunotherapy with the only limitation being the time and dose necessary to induce the desired beneficial long-term modulation of the individuals allergic response to cats. If successful, a similar approach could be undertaken in severe food allergy where many of the specific allergens are known and therapeutic options are severely limited.
Footnotes
Supported by an USPHS-NIH grant AI-15251 to A.S.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer K, Burks W. Current developments in peanut allergy. Current Opinion in Allergy and Clinical Immunology. 2006;6:202–6. doi: 10.1097/01.all.0000225161.60274.31. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira f, Briza p, Infuhr D, et al. Modified recombinant allergens for safer immunotherapy. Inflamm Allergy Drug Targets. 2006;5:5–14. doi: 10.2174/187152806775269295. [DOI] [PubMed] [Google Scholar]
- 3.Raz E, Tighe H, Sato Y, et al. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver JM, Kepley CL, Ortega E, et al. Immunologically mediated signaling in basophils and mast cells: finding therapeutic targets for allergic diseases in the human FcεRI signaling pathway. Immunopharmacology. 2000;48:269–81. doi: 10.1016/s0162-3109(00)00224-1. [DOI] [PubMed] [Google Scholar]
- 5.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 6.Zhu D, Kepley CL, Zhang M, et al. A novel human immunoglobulin Fcγ-Fcε bifunctional fusion protein inhibits FcεRI-mediated degranulation. Nat Med. 2002;8:518–21. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu D, Kepley CL, Zhang K, Terada T, Yamada T, Saxon A. A Chimeric Human –Cat Fusion Protein Blocks Cat-induced Allergy. Nature Medicine, Nat Med. 2005;11:446–449. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
- 8.Terada T, Zhang K, Belperio J, et al. A chimeric human-cat Fcgamma-Fel d1 fusion protein inhibits systemic, pulmonary, and cutaneous allergic reactivity to intratracheal challenge in mice sensitized to Fel d1, the major cat allergen. Clin Immunol. 2006;120:45–56. doi: 10.1016/j.clim.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Malbec O, Fong DC, Turner M, et al. Fcε Receptor I-associated lyn-dependent phosphorylation of Fcγ Receptor IIB during negative regulation of mast cell activation. J Immunol. 1998;160:1647–58. [PubMed] [Google Scholar]
- 10.Fong DC, Malbec O, Arock M, et al. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated FcγR IIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol Lett. 1996;54:83–91. doi: 10.1016/s0165-2478(96)02654-5. [DOI] [PubMed] [Google Scholar]
- 11.Ott VL, Cambier JC. Activating and inhibitory signaling in mast cells: New opportunities for therapeutic intervention? J Allergy Clin Immunol. 2000;106:429–40. doi: 10.1067/mai.2000.109428. [DOI] [PubMed] [Google Scholar]
- 12.Ono M, Bolland S, Tempst P, et al. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature. 1996;383:263–6. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 13.Tam SW, Demissie S, Thomas D, et al. A bispecific antibody against human IgE and human FcgammaRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy. 2004;59:772–80. doi: 10.1111/j.1398-9995.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 14.Kepley CL, Taghavi S, Mackay G, et al. Co-aggregation of FcγRII With FcεRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J Biol Chem. 2004;279:35139–49. doi: 10.1074/jbc.M404318200. [DOI] [PubMed] [Google Scholar]
- 15.Saxon A, Zhu D, Zhang K, et al. Genetically engineered negative signaling molecules in the immunomodulation of allergic diseases. Curr Opin Allergy Clin Immunol. 2004;4:563–8. doi: 10.1097/00130832-200412000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Daeron M, Malbec O, Latour S, et al. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995;95:577–85. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H, Takei M, Yanagida M, et al. Early and late events in Fc epsilon RI signal transduction in human cultured mast cells. J Immunol. 1997;159:5881–8. [PubMed] [Google Scholar]
- 18.Zhang K, Kepley CL, Terada T, et al. Inhibition of allergen-specific IgE reactivity by a human Ig Fcγ-Fcε bifunctional protein. J Allergy Clin Immunol. 2004;114:321–7. doi: 10.1016/j.jaci.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 19.Tam SW, Demissie S, Thomas D, et al. A bispecific antibody against human IgE and human FcgammaRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy. 2004;59:772–80. doi: 10.1111/j.1398-9995.2004.00332.x. [DOI] [PubMed] [Google Scholar]


