Abstract
Formation of the neuromuscular junction requires the release of agrin from the presynaptic terminal of motor neurons. Clustering of acetylcholine receptors (AChRs) on the postsynaptic sarcolemma is initiated by agrin-dependent activation of the muscle-specific kinase. While the postsynaptic scaffolding protein rapsyn is vital for high density AChR aggregation, little is known about the mechanism through which AChRs are immobilized on the postsynaptic membrane. Ultrastructural and immunohistochemical studies have suggested that AChRs are anchored to a membrane-associated cytoskeleton that contains spectrin-like proteins and is thus similar to that of the human erythrocyte (Bloch et al., 1997). We are studying a protein of the spectrin superfamily, ACF7 (also known as MACF), as a postsynaptic cytoskeletal component of the neuromuscular junction. ACF7 has multiple cytoskeleton-binding domains, including an N-terminal actin-binding domain that, we postulate, may interact with rapsyn, the scaffolding protein that binds directly to AChRs. To test this hypothesis, we co-expressed fragments of these molecules in cultured fibroblasts and assessed their co-distribution and interaction using confocal microscopy and co-immunoprecipitation. We demonstrate that the actin-binding domain of ACF7 specifically interacts with the tetratricopeptide repeat domains of rapsyn. Furthermore, we show using surface plasmon resonance and blot overlay that the actin-binding domain of ACF7 binds directly to rapsyn. These results suggest that AChRs are immobilized in the membrane through rapsyn-mediated anchoring to an ACF7-containing network that in turn is linked to the actin cytoskeleton.
Keywords: spectrin superfamily, neuromuscular junction, acetylcholine receptor, postsynaptic, cytoskeleton
Fast synaptic transmission at the vertebrate neuromuscular junction (NMJ) requires the precise apposition of presynaptic active zones and high density aggregates of postsynaptic acetylcholine receptors (AChRs). The formation of AChR clusters and possibly their maintenance at the nascent NMJ is dependent upon the release of agrin from the presynaptic terminal, although agrin-independent formation of NMJs also occurs in the absence of neurotransmission (reviewed in Sanes and Lichtman, 2001; Kummer et al., 2006). Agrin activates the muscle-specific receptor tyrosine kinase, MuSK (Sanes and Lichtman, 2001). The postsynaptic scaffolding protein rapsyn, which binds to AChRs, is also required for high density AChR aggregation (Sanes and Lichtman, 2001). Despite the characterization of several molecules involved in the initial steps of AChR clustering, little is know about the mechanism through which AChRs are immobilized in the postsynaptic membrane.
Rapsyn is comprised of several modular domains, including an N-terminal myristoylation sequence, seven tetratricopeptide repeat (TPR) domains, an AChR-binding coiled coil domain, and a C-terminal zinc finger domain (Musil et al., 1988; Carr et al., 1989; Phillips et al., 1991; Maimone and Merlie, 1993; Ponting and Phillips, 1996; Bezakova and Bloch, 1998; Cartaud et al., 1998; Ramarao and Cohen, 1998; Bartoli et al., 2001; Ramarao et al., 2001; Huebsch and Maimone, 2003). We have previously shown that rapsyn is linked to the postsynaptic cytoskeleton through its interaction with ACF7, also known as MACF (Antolik et al., 2006a). Using electron microscopy and immunohistochemistry with novel antibodies, we have shown that ACF7 is the unusual β-spectrin-like protein present at the crests of the postsynaptic junctional folds and at clusters of AChRs in cultured myotubes (Bloch and Morrow, 1989; Antolik et al., 2006a), where it is arrayed in filamentous network resembling the spectrin network of the erythroid cell membrane (Pumplin, 1995; Antolik et al., 2006a). The zinc finger domain of rapsyn promotes this interaction with ACF7 (Antolik et al., 2006a). As the binding of the coiled coil domain of rapsyn to AChRs is unlikely to be affected by rapsyn’s association with ACF7, it seems likely that rapsyn links AChRs to the cytoskeleton through its ability to bind directly or indirectly to ACF7. However, the domains of ACF7 that mediate this interaction, as well as the possible roles of the other domains of rapsyn in this interaction, have not been defined.
Cell biological and biochemical studies of the functions and interactions of ACF7 are hindered because it is a gigantic protein (Byers et al., 1995; Leung et al., 1999). This limits the ability to express full length, recombinant constructs in mammalian or bacterial cells. Fortunately, ACF7 is also a modular protein, consisting of multiple domains involved in cytoskeletal cross-linking and protein-protein interaction, which makes it possible to study the activities of its individual domains (Karakesisoglou et al., 2000).
To begin to determine which domains are involved in its interaction with rapsyn, we co-expressed the N-terminal actin-binding domain (ABD) of ACF7 with full length rapsyn in COS-7 cells. We selected the ABD because preliminary studies suggested that it could alter the distribution of rapsyn in COS-7 cells, and yeast two-hybrid experiments with cloned fragments of ACF7 failed to identify other regions with potential rapsyn-binding activity (J. Ursitti, P. Lee, P. Reed and R.J. Bloch, unpublished results). COS-7 cells are also a useful system to study the protein-protein interactions of actin-binding domains from proteins expressed in skeletal muscle (Ursitti et al., 2004; Stone et al., 2005).
We assayed the ability of rapsyn deletion mutants to interact with the ABD of ACF7. We report that the ABD of ACF7, when expressed alone, incorporates into actin filaments. Upon co-expression, it specifically interacts with rapsyn, and has the ability to redistribute rapsyn from its characteristic membrane-associated aggregates into actin filaments. The TPR domains of rapsyn mediate its interaction with ACF7 in a promiscuous manner, similar to their interaction with MuSK (Antolik et al., 2006b). The binding of rapsyn and the ABD of ACF7 is direct, as determined by blot overlay and surface plasmon resonance experiments. Furthermore, the ABD and rapsyn associate in clusters of AChRs isolated from cultured myotubes, even when regions in the middle of the ACF7 molecule are removed by proteolysis. Finally, we show that rapsyn, the ABD of ACF7, and an intracellular sequence of MuSK co-distribute when co-expressed in COS-7 cells. Our results suggest that the ABD of ACF7 associates directly and specifically with rapsyn at the AChR-rich membrane of skeletal muscle.
EXPERIMENTAL PROCEDURES
Cell culture and transfection
COS-7 cells (American Type Culture Collection, Manassas, VA) were cultured and transfected as previously described (Stone et al., 2005; Antolik et al., 2006b).
Preparation of expression constructs
Expression constructs encoding green fluorescent protein (GFP) fused in frame to the COOH-termini of full length mouse rapsyn, rapsyn lacking the zinc finger domain (ΔZnF), rapsyn lacking both the coiled-coil and zinc finger domains (ΔCC-ZnF), and myristoylated TPR tandem pairs were prepared as previously described (Antolik et al., 2006b). The hemagglutinin (HA)-tagged expression construct containing amino acids 760–820 of mouse MuSK (MuSK760–820/HA) was generated as described (Antolik et al., 2006b). Plasmids encoding DsRed-tagged forms of the ABDs of β-spectrin, utrophin and dystrophin were prepared as described (Stone et al., 2005). Plasmids encoding DsRed-tagged ACF7 and filamin ABDs were a kind gift from Dr. Michele R. Stone (Aeras Global TB Vaccine Foundation, Bethesda, MD). A procaryotic expression construct consisting of the ABD of ACF7 fused to the COOH-terminus of maltose binding protein (MBP) was generated by amplifying the ABD using PCR primers that contained EcoRI and HindIII restriction sites. The ABD product was then digested with the restriction enzymes and ligated into the EcoRI and HindIII sites of pMAL-c2x (New England Biolabs, Ipswich, MA).
Immunofluorescence and microscopy
For transfection of plasmids encoding GFP and/or DsRed, non-permeabilized cells were fixed, washed with phosphate-buffered saline (PBS) and mounted. COS cells were processed for anti-HA immunolabeling as previously described (Stone et al., 2005; Antolik et al., 2006b). For triple labeling experiments, Alexa-633 Fluor-conjugated secondary antibodies were used to detect anti-HA primary antibodies. All Alexa-Fluor conjugated secondary antibodies in this study were from Invitrogen (Carlsbad, CA) and were diluted 1:200 in PBS containing 1 mg/ml bovine serum albumin (PBS/BSA). To label actin filaments, fixed cells were permeabilized for 10 min with 0.5% Triton X-100 in PBS, then incubated for 1 h with Alexa-488 Fluor-conjugated phalloidin (Invitrogen; diluted 1:200 in PBS/BSA), washed with PBS, and mounted. All fluorescent specimens were examined with Kr/Ar and He/Ne laser excitation on a Zeiss confocal microscope (LSM 410; Carl Zeiss, Inc., Tarrytown, NY), as described (Stone et al., 2005; Antolik et al., 2006b).
Co-distribution of the ABD of ACF7 with AChRs and rapsyn
We cultured muscle cells from neonatal rats (Bloch, 1979), and then isolated clusters of AChRs by shearing, as described (Avnur and Geiger, 1981). After isolation, clusters were either fixed with 2% paraformaldehyde in PBS, or treated with 2 M NaCl for 5 min at room temperature, to induce the formation of fine aggregates of AChRs and AChR-associated proteins (our unpublished results). These samples were either fixed as above or treated with chymotrypsin (500 ng/ml, in PBS) for 5 min at room temperature. All remaining samples were then fixed and processed for immunofluorescence with antibodies to the N-terminal actin binding domain of ACF7 (anti-ABD, 1 μg/ml), or affinity-purified rabbit antibodies to the region of ACF7 containing amino acids 2295-2413 (B5, 2 μg/ml), followed by goat anti-rabbit IgG coupled to tetramethylrhodamine (1:100, Jackson ImmunoResearch, West Chester, PA). Samples that were not pre-labeled with R-BTX were labeled for double immunofluorescence with monoclonal antibodies to rapsyn (mAbs 1234 and 1579, each at 100 nM [Bloch and Froehner, 1987]), followed by fluoresceinated secondary antibodies (Jackson ImmunoResearch). After washing, samples were mounted and imaged as above.
Generation of lysates, immunoblotting and immunoprecipitation
Lysates were generated from COS cells exactly as previously described (Antolik et al., 2006b). Briefly, cells were collected by scraping in ice-cold Lysis Buffer (150 mM NaCl, 20 mM Tris-Cl, pH 7.4) followed by centrifugation. Pellets were resuspended in Lysis Buffer plus 1% Triton X-100, triturated through a 28 gauge needle, rotated for 45 min, then subjected to centrifugation to remove insoluble cell debris. Protein concentrations were determined by the Bradford assay (Bio-Rad, Hercules, CA).
Co-immunoprecipitation was performed on ~500 μg of protein from lysates, as described (Antolik et al., 2006b). Briefly, paramagnetic DynaBeads (Invitrogen) coupled to rabbit anti-GFP antibodies (Invitrogen) were incubated with lysate overnight at 4°C. Immunoprecipitates were then extensively washed with PBS/0.5% Tween-20, and bound proteins were eluted by boiling for 10 min in sodium dodecylsulfate sample buffer.
Samples were immunoblotted as described (Antolik et al., 2006b). Membranes were probed overnight with monoclonal mouse antibodies to either GFP (1 μg/ml, MBL, Nagoya, Japan) or DsRed (1 μg/ml, BD Pharmingen, Palo Alto, CA). After incubation with alkaline phosphatase-conjugated secondary antibodies, bands were visualized with chemiluminescence (Tropix Laboratories, Bedford, MA).
Surface plasmon resonance and blot overlay
cDNAs encoding the ABD of ACF7 fused to MBP (ABD/MBP) and MBP alone were expressed in E. coli (BL21-AI cells, Invitrogen) following induction with 0.3 mM IPTG for 3 h. Recombinant proteins were affinity purified on an amylose resin (New England Biolabs) according to manufacturer’s instructions. Rapsyn/GFP and GFP alone were purified by immunoprecipitation from transfected COS cells, as described (Antolik et al., 2006b). For blot overlays, immunoprecipitates of rapsyn/GFP and GFP were separated by SDS-PAGE, transferred to nitrocellulose, blocked with PBS/5% nonfat dry milk/0.1% Tween-20, and incubated with either recombinant ABD/MBP or MBP alone (10 μg/ml; see Stone et al., 2005; Antolik et al., 2006b) overnight at 4°C. After extensive washing, bound MBP proteins were detected by incubation with anti-MBP antibodies (0.2 μg/ml, New England Biolabs) at 4°C for 6 h followed by alkaline phosphatase-conjugated secondary antibodies, as described above.
Surface plasmon resonance was detected with a Biacore 3000 (Biacore, Inc., Uppsala, Sweden) as previously described (Kontrogianni-Konstantopoulos et al., 2003; Stone et al., 2005; Antolik et al., 2006b). Briefly, polyclonal rabbit anti-GFP (Invitrogen) was coupled to an activated carboxymethyl-dextran sensor chip, according to the manufacturer’s instructions (Biacore, Inc.). Lysates from transfected COS cells were diluted 1:1 in Biacore buffer and either GFP or rapsyn/GFP was immobilized on the activated sensor chip. Recombinant ABD/MBP (0.5–2 μM in Biacore buffer) was then flowed over the activated chip surfaces. Association was measured for ~200 sec and dissociation was measured over 500–600 sec at a flow rate of 20 μl/min.
Data analysis
Confocal images were processed with MetaMorph software (Universal Imaging, Downingtown, PA). Quantitative comparison of the co-distribution of ABD and rapsyn constructs was performed as described previously (Antolik et al., 2006b). One-way analysis of variance and post hoc tests were done with InStat® (GraphPad Software Inc., San Diego, CA).
RESULTS
The ABD of ACF7 specifically co-distributes with rapsyn in COS cells
To determine whether the ABD plays a role in the interaction of ACF7 with rapsyn, we co-transfected COS cells with constructs encoding full length rapsyn/GFP and DsRed-tagged ABDs of the related proteins ACF7, β-spectrin, dystrophin, utrophin and filamin (Fig. 1A-E). Surprisingly, the ABD of ACF7 (Fig. 1A), but none of the other ABDs (Fig. 1B–E), co-distributed with rapsyn in intracellular filaments and at the cell periphery. Labeling with a fluorescent conjugate of the actin-binding fungal toxin, phalloidin, established that these structures were actin microfilaments (Fig. 1F). When it is expressed alone, rapsyn forms punctate rather than filamentous or continuous structures (Fig. 1G). In some cells, the ABD of ACF7 was also observed in membrane-associated rapsyn clusters upon co-expression (data not shown). Thus, the ABD of ACF7 can significantly alter the intracellular distribution of rapsyn. This activity is specific to ACF7, as other homologous ABDs do not have this effect.
Fig. 1.

The ABD of ACF7 specifically co-distributes with rapsyn in COS cells. Cells were co-transfected with plasmids encoding rapsyn/GFP (green) and DsRed-tagged constructs (red) encoding the ABDs of ACF7 (A), β-spectrin (B), dystrophin (C), utrophin (D), and filamin (E). Cells were also transfected with the ACF7 ABD construct alone and actin was labeled with phalloidin (green; F). As a control, rapsyn/GFP alone was expressed in COS cells (G). Cells were fixed 24 h later and fluorescent proteins and phalloidin were visualized with confocal microscopy. Rapsyn/GFP was recruited into actin filaments when co-expressed with the ACF7 ABD (A; areas of co-localization are shown in yellow in the overlay panel), but not the other ABDs (B–E).
The TPR domains of rapsyn mediate co-distribution with the ABD of ACF7
We next identified the domains of rapsyn that are required for co-distribution with the ACF7 ABD. COS cells were co-transfected with DsRed-tagged ACF7 ABD and either rapsyn ΔZnF/GFP (Fig. 2B) or ΔCC-ZnF/GFP (Fig. 2C). Co-transfection of the ACF7 ABD with full length rapsyn/GFP is shown for comparison (Fig. 2A). Each rapsyn construct co-distributed in filaments with the ABD of ACF7. Quantitative comparisons showed that the extent of co-localization of the ACF7 ABD with each rapsyn construct was statistically indistinguishable, and that the co-distribution of the control spectrin ABD with full length rapsyn was significantly lower (Fig. 2D). Thus, the TPR domains of rapsyn mediate its ability to co-distribute with ACF7.
Fig. 2.
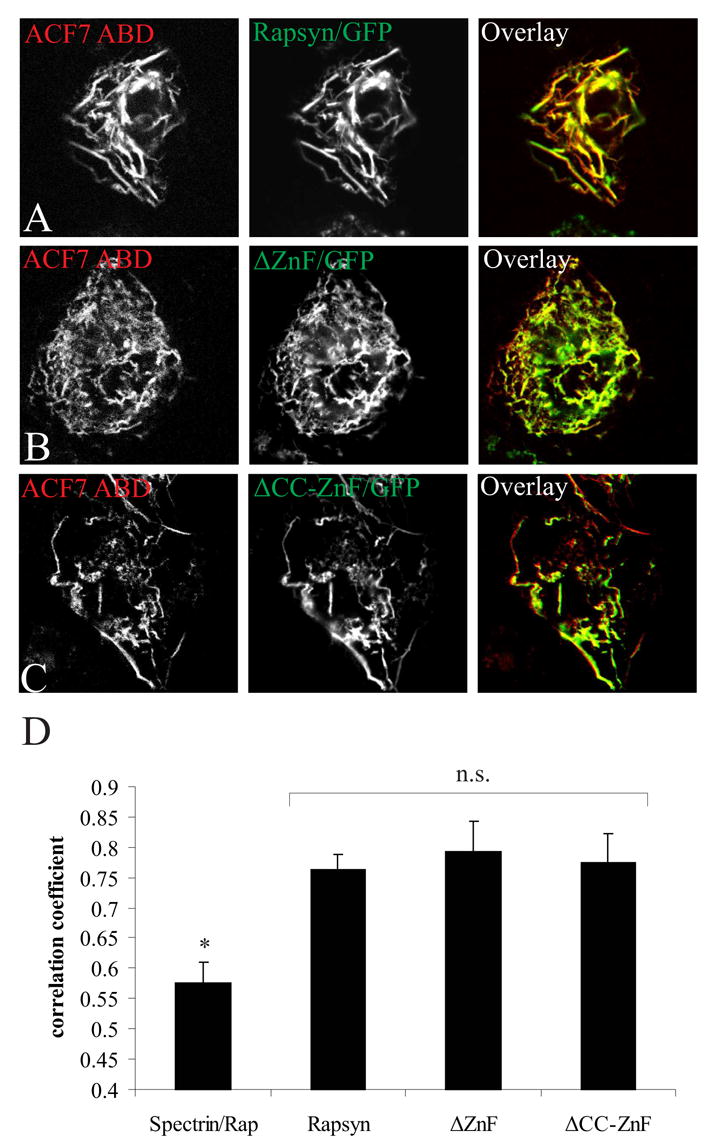
Rapsyn’s TPR domains mediate co-distribution with the ACF7 ABD in COS cells. Cells co-expressing the ABD of ACF7 and full length rapsyn/GFP (A), rapsyn ΔZnF/GFP (B) or rapsyn ΔCC-ZnF/GFP (C) were fixed and imaged as in Fig. 1. The ABD of ACF7 (red) and each rapsyn construct (green) co-distributed significantly in actin filaments (yellow in the overlay panel). (D) Quantitative analysis showed that there was no difference in co-localization with the ABD of ACF7 and each of the rapsyn constructs, but that all three constructs showed significantly higher co-distribution than the ABD of spectrin with full length rapsyn (mean ± S.E.M., n= 20 from 2–3 separate experiments). n.s., not significantly different (p > 0.1, one-way ANOVA followed by Tukey’s HSD post hoc test); *, spectrin ABD is significantly different from ACF7 ABD co-distribution with rapsyn constructs (p < 0.01); Rap, full length rapsyn.
The ACF7 ABD interacts biochemically with the TPR domains of rapsyn
We used co-immunoprecipitation to determine whether the ACF7 ABD formed a biochemical complex with the TPR domains of rapsyn. COS cells were co-transfected with DsRed-tagged ACF7 ABD and either full length rapsyn/GFP, rapsyn ΔZnF/GFP or ΔCC-ZnF/GFP. As a control, the spectrin ABD was co-transfected with full length rapsyn/GFP. Lysates were then prepared and equal amounts of protein were immunoprecipitated with antibodies to GFP. Immunoprecipitates were subjected to immunoblot analysis with antibodies to DsRed (Fig. 3). The ACF7 ABD co-immunoprecipitated with each rapsyn construct, whose expression levels were similar (Fig. 3, input), while the spectrin ABD did not co-immunoprecipitate with rapsyn. Each ABD construct was expressed equally in lysates of co-transfected cells (Fig. 3, input). Thus, the TPR domains of rapsyn mediate its specific interaction with the ABD of ACF7.
Fig. 3.

The ACF7 ABD interacts biochemically with the TPR domains of rapsyn. Lysates from cells co-transfected with plasmids encoding DsRed-tagged ACF7 ABD and either full length rapsyn/GFP, ΔZnF/GFP or ΔCC-ZnF/GFP were immunoprecipitated with anti-GFP antibodies. Following SDS-PAGE, precipitates were immunoblotted with anti-DsRed antibodies. The ACF7 ABD was specifically detected in immunoprecipitates from cells expressing rapsyn constructs, while the spectrin ABD was not detected in immunoprecipitates of cells co-expressing full length rapsyn/GFP. Immunoblots of lysates with anti-GFP and anti-DsRed showed that rapsyn and ABD constructs expressed at similar levels (inputs).
Interaction of the ABD of ACF7 with rapsyn TPR deletion constructs
To determine which TPR domains mediate interaction with the ACF7 ABD, we used GFP-tagged constructs of overlapping pairs of TPR domains that also retained the N-terminal myristoylation sequence for membrane localization (see Antolik et al., 2006b). Fluorescence of each TPR tandem construct and the DsRed-tagged ACF7 ABD in co-transfected cells showed that any two TPR domains tested can induce the rapsyn construct to assume a filamentous distribution in the presence of the ACF7 ABD (Fig. 4A–F). Quantitations confirmed that the ACF7 ABD co-distributes with each TPR tandem construct to a very similar extent, which is significantly higher than with GFP alone (Fig. 4G). Thus, the TPR domains of rapsyn interact with the ACF7 ABD in a promiscuous manner, similar to that reported for the binding of rapsyn to another vital postsynaptic protein, MuSK (Antolik et al., 2006b).
Fig. 4.

The ABD of ACF7 co-distributes with TPR domain pairs. COS cells were co-transfected with plasmids encoding DsRed-tagged ACF7 ABD and GFP constructs containing TPR domains 1–2 (A), 2–3 (B), 3–4 (C), 4–5 (D), 5–6 (E) and 6–7 (F). Cells were processed for fluorescence and imaged as in Fig. 1. The ACF7 ABD (red) and each rapsyn TPR pair (green) co-localized significantly (yellow in the overlay panel). (G) Quantitative analysis confirmed that there was no difference in the co-distribution of the ACF7 ABD with any of the TPR pairs, and that all of these combinations resulted in co-distributions that were significantly greater than with GFP alone (mean ± S.E.M., n= 20 from 2–3 separate experiments). n.s., not significantly different (p > 0.1, one-way ANOVA followed by Tukey’s HSD post hoc test); *, GFP control is significantly different from rapsyn constructs (p < 0.05).
The ABD of ACF7 binds directly to rapsyn
We next wanted to determine whether the ACF7 ABD binds directly to the TPR domains of rapsyn. Recombinant MBP and an MBP-tagged ABD fusion protein were purified from E. coli, separated by SDS-PAGE, and the purity of each protein was confirmed by staining with Coomassie blue (Fig. 5A). Immunoprecipitation with anti-GFP antibodies was used to purify rapsyn/GFP that had been over-expressed in COS cells (see Antolik et al., 2006b). Rapsyn precipitates were then electrophoretically separated, transferred to nitrocellulose and subjected to blot overlay with a recombinant MBP-tagged ACF7 ABD (10 μg/ml). Anti-MBP antibodies were used to detect bound recombinant protein. The recombinant ACF7 ABD specifically bound to rapsyn/GFP but not to GFP alone (Fig. 5B, left panel). This binding was specific, as recombinant MBP alone (10 μg/ml) did not bind to rapsyn (Fig. 5B, middle panel). Rapsyn/GFP and GFP expression was confirmed by probing the same immunoprecipitates used in the blot overlay with anti-GFP antibodies (Fig. 5B, right panel).
Fig. 5.

The ABD of ACF7 binds directly to rapsyn. (A) Recombinant MBP and MBP-tagged ABD proteins were purified on amylose resins, separated by SDS-PAGE (1.4 μg and 2 μg per lane, respectively) and stained with Coomassie Blue. (B) Rapsyn/GFP and GFP alone were immunoprecipitated with anti-GFP antibodies from extracts of transfected COS cells, separated by SDS-PAGE and transferred to nitrocellulose. Blots were then incubated with recombinant MBP-tagged ACF7 ABD (left panel) or MBP alone (middle panel). After washing, bound recombinant proteins were detected with anti-MBP antibodies and chemiluminescence. MBP-tagged ACF7 ABD bound directly to rapsyn/GFP but not GFP alone; MBP did not bind to rapsyn/GFP. Immunoblotting confirmed the presence of equivalent amounts of GFP-tagged protein in each sample (right panel). (C) Rapsyn/GFP and GFP alone were purified as above and immobilized with anti-GFP antibodies on Biacore sensor chips. Recombinant MBP-tagged ACF7 ABD, at concentrations from 0.5 to 2 μM, was used as soluble binding partner. The y-axis shows binding in Resonance Units (RU) as a function of time after injection of recombinant ABD. Each curve represents binding at a specific concentration of recombinant ABD. Non-specific binding was subtracted from the binding of ABD to rapsyn.
Surface plasmon resonance was used to confirm that the ABD of ACF7 binds directly to rapsyn (Johnsson et al., 1991; reviewed in Cooper, 2003). Rapsyn/GFP and GFP alone were immunoprecipitated from COS cell extracts and bound to anti-GFP antibodies conjugated to the surface of different channels of a sensor chip. Recombinant ABD/MBP (0.5–2 μM) was then flowed over the GFP- or rapsyn/GFP-bound chip surface. The ABD/MBP specifically bound to the immobilized rapsyn/GFP but not to GFP alone (Fig. 5C, n = 3). These results confirm that the ACF7 ABD and rapsyn bind directly.
The ABD of ACF7 associates selectively with rapsyn and AChRs in isolated clusters
The experiments described above suggest that the ABD of ACF7 can associate directly with rapsyn. We attempted several experiments, including transfection of primary cultures of rat myotubes with plasmids expressing the ABD, and intracellular injection of a recombinant form of this protein (e.g., Bezakova and Bloch, 1998) in an unsuccessful attempt to prove that the stability of AChR clusters in myotubes depends on the interaction of the ABD of ACF7 with rapsyn. We suspect that we were unable to introduce amounts of the ABD sufficient to saturate the endogenous binding sites and compete with the presumptive ACF7-rapsyn complex that, our evidence suggests, exists in situ. We therefore used a different approach which had as its aim the demonstration that the ABD of ACF7, located near the N-terminus of the protein (Karakesisoglou et al., 2000), and not more central portions of the molecule (we selected a region we termed B5, located between amino acids 2295 and 2413), associated selectively with rapsyn. We reasoned that if the ABD was closely associated with rapsyn but the middle of the ACF7 molecule was not, then very mild proteolysis should selectively remove epitopes in the middle, while leaving epitopes in the ABD associated with rapsyn at the membrane of isolated AChR clusters.
We generated antibodies specific to these portions of ACF7 and affinity purified them on columns that were covalently coupled to the fusion proteins used as antigens. The specificity of these antibodies, and their ability to label the postsynaptic membrane of the neuromuscular junction and to co-immunoprecipitate rapsyn with ACF7 from myotubes, has been reported (Antolik et al., 2006a). We then isolated AChR clusters from cultured rat myotubes by a shearing procedure that leaves intact the association of rapsyn and AChR with the membrane cytoskeleton (reviewed in Bloch et al., 1997). We treated these clusters briefly with high salt concentrations (2 M NaCl) to induce aggregation of the AChR and associated molecules, which makes them easier to visualize at later stages of the experiment (our unpublished results), and then digested them briefly with very low concentrations of chymotrypsin, which efficiently removes the receptor-associated cytoskeletal network from the cluster membrane without affecting rapsyn or AChR (Bloch et al., 1997). We then compared the distribution of the ABD of ACF7 and the middle of the ACF7 molecule, labeled with our region-specific affinity-purified antibodies, to the distribution of the AChR or rapsyn. Both the ABD and the B5 regions of ACF7 are retained at clusters through the shearing and high salt extractions, together with rapsyn and AChR (Fig. 6A–F and A′–F′, and data not shown). After digestion with chymotrypsin, however, the ABD of ACF7 is selectively retained at microaggregates of rapsyn (Fig. 6G–I) or AChR (data not shown), whereas the B5 epitopes in the sixth spectrin repeat, in the middle of the molecule, have been completely removed (Fig. 6G′–I′). ABD is also present in these preparations in non-AChR rich domains of clusters, perhaps because ACF7 associates with other proteins of the myotube membrane in addition to rapsyn. Some rapsyn also appears to be present without ACF7, suggesting that some ACF7 originally associated with rapsyn was degraded by the low concentrations of protease we used. Nonetheless, the ABD of ACF7 in situ is more stably associated with rapsyn and AChR than the middle of the molecule. These results are therefore consistent with the idea that the ABD of ACF7 associates with rapsyn in AChR clusters.
Fig. 6.

The ABD of ACF7, but not the spectrin repeat region, is closely associated with rapsyn in situ. Rat myotubes in culture were sheared open and fixed (A–C, A′–C′), treated with 2 M NaCl and fixed (D–F, D′–F′), or treated with 2 M NaCl followed by 500 ng/ml chymotrypsin and fixed (G–I, G′–I′). Samples were labeled for immunofluorescence with monoclonal antibodies to rapsyn (B,B′, E,E′, H,H′) and affinity purified antibodies to the actin binding domain (A, D, G) or to the sixth spectrin repeat (A′, D′, G′) of ACF7. Both antibodies to ACF7 labeled rapsyn-rich structures in freshly isolated and fixed samples (A–C, A′–C′), and in samples treated briefly with 2 M NaCl (D–F, D′–F′). Epitopes in the actin binding domain of ACF7 (G–I), but not the spectrin repeat region (G′–I′) are retained after mild proteolysis with chymotryspin. Bar, 5 μm.
Rapsyn, an intracellular sequence of MuSK, and the ABD of ACF7 co-distribute in COS cells
We have shown that the TPR domains of rapsyn bind directly to amino acids 760–820 of mouse MuSK (MuSK760–820) when co-expressed in COS cells (Antolik et al., 2006b), that rapsyn interacts with ACF7 in myotubes (Antolik et al., 2006a), and that the TPR domains of rapsyn bind directly to the ABD of ACF7 in COS cells (this report). We therefore hypothesized that rapsyn, MuSK760–820, and the ABD of ACF7 would co-distribute when co-expressed in COS cells. There was indeed co-distribution in filaments of full length rapsyn/GFP, MuSK760–820/HA and DsRed-tagged ACF7 ABD in COS cells (Fig. 7A). The filamentous structures in which these three proteins co-distributed appeared to be at the cell surface, rather than in more internal filamentous structures. MuSK760–820 and the ABD of ACF7 do not co-distribute when co-expressed in COS cells (Fig. 7B). This raises the possibility that the TPR domains of rapsyn can simultaneously bind to both the ABD of ACF7 and cytoplasmic sequences of MuSK.
Fig. 7.

The ACF7 ABD, rapsyn, and MuSK760–820 co-distribute in COS cells. (A) Cells were triple-transfected with plasmids encoding DsRed-tagged ACF7 ABD, full length rapsyn/GFP and MuSK760–820/HA. After 24 h, cells were fixed and processed for immunocytochemistry with anti-HA antibodies, followed by Alexa-633-conjugated secondary antibodies. Images were obtained with the Kr/Ar (red and green) and He/Ne (blue) laser excitation. DsRed-tagged ACF7 ABD (red), full length rapsyn/GFP (green) and MuSK760–820/HA (blue) co-distributed in filaments (yellow arrows; areas of white in overlay panel). (B) Cells were transfected with plasmids encoding DsRed-tagged ACF7 ABD and MuSK760–820/HA. After 24 h, cells were fixed and processed for immunocytochemistry with anti-HA antibodies, followed by Alexa-488-conjugated secondary antibodies. The ACF7 ABD and MuSK760–820/HA did not co-distribute in the absence of rapsyn co-expression.
DISCUSSION
The mechanism that immobilizes AChRs at the NMJ, and the postsynaptic cytoskeletal components involved, are poorly understood. Our previous studies have shown that rapsyn and the spectrin-like cytoskeletal protein, ACF7, interact biochemically in myotubes, and that the zinc finger domain of rapsyn promotes their efficient interaction (Antolik et al., 2006a). To begin to define the domains of ACF7 involved in this interaction, we have used co-transfection in COS cells, which have proven useful for similar studies (Ursitti et al., 2004; Stone et al., 2005; Antolik et al., 2006b). We show here that the ABD of ACF7 binds directly to rapsyn, and that the TPR domains of rapsyn mediate this binding in a promiscuous manner, similar to that observed for their interaction with MuSK (Antolik et al., 2006b). Furthermore, we demonstrate that rapsyn co-distributes with the ACF7 ABD in muscle cells, and that it can bind simultaneously to MuSK760–820, presumably also through its TPR domains. Our results further expand the number of postsynaptic proteins that bind to the TPR domains of rapsyn.
We have proposed that there may be other domains of rapsyn, in addition to the zinc finger domain, that interact with ACF7, with the zinc finger domain serving to increase the affinity or otherwise stabilize this interaction (Antolik et al., 2006a). The TPR region of rapsyn seemed to be an attractive candidate for a potential ACF7 binding site, as we have already shown that it binds to other postsynaptic proteins (Antolik et al., 2006b). The results presented in this study suggest that the TPR domains of rapsyn do indeed mediate interaction with the ABD of ACF7, which in turn suggests that the zinc finger domain may stabilize the interaction without being either necessary or sufficient for it to occur. As it is downstream of the superhelical TPR region, the zinc finger would presumably serve this function by interacting with sequences C-terminal to the ABD. Sequences C-terminal to the ABDs of calponin, utrophin and dystrophin increase the affinity for actin (Gimona et al., 2002; Rybakova et al., 2002; Sutherland-Smith et al., 2003), suggesting that these sequences can indeed have modulatory roles in protein-protein interaction. As we have expressed just the ABD in these experiments, the presence of the zinc finger would not be expected to affect the interaction of the ABD with rapsyn. This model could be tested by quantitatively comparing the interaction of rapsyn constructs with or without the zinc finger and ACF7 constructs of increasing size. In addition, in vitro experiments to detect whether rapsyn constructs and the ABD of ACF7 bind directly would help to clarify the mechanism of rapsyn-ACF7 interaction.
The ABDs of proteins in the spectrin superfamily consist of tandem, 100 amino acid calponin homology (CH) domains that are functionally distinct (Castresana and Saraste, 1995; reviewed in Stradal et al., 1998; Gimona et al., 2002). ABDs bind to actin with affinities ranging from 4–50 μM (Winder et al., 1995; Gimona and Winder, 1998), and this binding is mediated by the N-terminal CH domain (CH1). The C-terminal CH domain (CH2) increases the affinity of the whole ABD for actin (Way et al., 1992; Carugo et al., 1997; Gimona and Winder, 1998). As CH2 domains do not bind actin but appear to play a regulatory role in actin interaction, we speculate that the TPR domains of rapsyn bind to this motif within the ABD of ACF7, leaving the CH1 domain free to bind to actin. This would account for the ability of the ABD of ACF7 to concentrate rapsyn and its TPR domains along actin filaments. Co-transfection experiments could test this idea.
The crystal structures of the ABDs from several spectrin-like proteins have been solved (Carugo et al., 1997; Goldsmith et al., 1997; Keep et al., 1999; Norwood et al., 2000; Sevcik et al., 2004). ABDs are predominantly α-helical, with each CH domain comprised of four long α-helices in a roughly parallel orientation, and three small helices, which together form a compact globular structure (reviewed in Broderick and Winder, 2002). ABDs contain a number of invariant core residues that are conserved in different species and thought to contribute to the three-dimensional structure of the ABD (Gimona et al., 2002), including the three actin-binding sites which in a properly folded molecule are thought to form a “patch” on the surface of the ABD (Winder et al., 1995; Moores et al., 2000). Given the high level of sequence similarity among ABDs from spectrin-family proteins, it should be possible to screen for amino acids or motifs in ACF7 that, being divergent from close family members that do not interact with rapsyn, could contribute to rapsyn interaction. In addition to rapsyn, ABDs also bind to phosphatidylinositol (4,5)-bisphosphate (PIP2; Fukami et al., 1996), calmodulin (Jarrett and Foster, 1995; Winder et al., 1995; Winder and Kendrick-Jones, 1995), and cytokeratins (Stone et al., 2005), suggesting that ABDs can do much more than bind to actin alone.
Rapsyn, the ABD of ACF7, and MuSK760–820 co-distribute in COS cells, probably in a TPR domain-dependent manner. Rapsyn is the central molecule in this co-distribution, as MuSK760–820 and the ABD do not co-distribute (see Fig. 7). The TPR-ABD interaction appears to be promiscuous, and can be explained in a manner similar to that of the TPR-MuSK760–820 interaction (Antolik et al., 2006b). The co-distribution of these three proteins can occur through the interaction of the TPR domain superhelix of a single rapsyn molecule with both the ABD and MuSK at the same time, or alternatively, some rapsyn molecules in a cluster may interact with the ABD while others interact with MuSK. In either case, rapsyn appears to be vital for interaction with multiple postsynaptic proteins and, given the apparent promiscuity of TPR interactions, may be able to form a dense macromolecular scaffold at the NMJ, linking AChRs and MuSK to the membrane-associated cytoskeleton through interactions with the ABD of ACF7.
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO1 NS17282, to R.J.B) and a stipend from the Interdisciplinary Training Program in Muscle Biology (T32 AR007592, to C.A). We thank Dr. Amber Bowman for recombinant protein purification, Dr. Michele R. Stone for the gift of some of the actin-binding domain constructs, and Dr. Paul Luther for help with quantitative image analysis.
Abbreviations
- ABD
actin-binding domain
- NMJ
neuromuscular junction
- MBP
maltose binding protein
- AChR
acetylcholine receptor
- TPR
tetratricopepteide repeat
- GFP
green fluorescent protein
- ZnF
zinc finger domain
- CC
coiled-coil domain
- PCR
polymerase chain reaction
- PBS
phosphate-buffered saline
- BSA
bovine serum albumin
- HA
hemagglutinin
Footnotes
Section editor: Menahem Segal, Weizmann Institute of Science, Department of Neurobiology, Hertzl Street, Rehovot 76100, Israel
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antolik C, Ursitti JA, Resneck WG, O’Neill AM, Lee PC, Pumplin DW, Bloch RJ. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2006a. ACF7, a β-spectrin-like protein at clusters of acetylcholine receptors, associates with rapsyn. Program No. 30.2. 2006 Online. [Google Scholar]
- Antolik C, Catino DH, Resneck WG, Bloch RJ. The tetratricopeptide repeat domains of rapsyn bind directly to cytoplasmic sequences of the muscle-specific kinase. Neuroscience. 2006b;141:87–100. doi: 10.1016/j.neuroscience.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Avnur Z, Geiger B. Substrate-attached membranes of cultured cells isolation and characterization of ventral cell membranes and the associated cytoskeleton. J Mol Biol. 1981;153:361–379. doi: 10.1016/0022-2836(81)90283-7. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Ramarao MK, Cohen JB. Interactions of the rapsyn RING-H2 domain with dystroglycan. J Biol Chem. 2001;276:24911–24917. doi: 10.1074/jbc.M103258200. [DOI] [PubMed] [Google Scholar]
- Bezakova G, Bloch RJ. The zinc finger domain of the 43-kDa receptor-associated protein, rapsyn: role in acetylcholine receptor clustering. Mol Cell Neurosci. 1998;11:274–288. doi: 10.1006/mcne.1998.0688. [DOI] [PubMed] [Google Scholar]
- Bloch RJ. Dispersal and reformation of acetylcholine receptor clusters of cultured rat myotubes treated with inhibitors of energy metabolism. J Cell Biol. 1979;82:626–643. doi: 10.1083/jcb.82.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch RJ, Froehner SC. The relationship of the postsynaptic 43K protein to acetylcholine receptors in receptor clusters isolated from cultured rat myotubes. J Cell Biol. 1987;104:645–654. doi: 10.1083/jcb.104.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch RJ, Morrow JS. An unusual beta-spectrin associated with clustered acetylcholine receptors. J Cell Biol. 1989;108:481–493. doi: 10.1083/jcb.108.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch RJ, Bezakova G, Ursitti JA, Zhou D, Pumplin DW. A membrane skeleton that clusters nicotinic acetylcholine receptors in muscle. Soc Gen Physiol Ser. 1997;52:177–195. [PubMed] [Google Scholar]
- Broderick MJ, Winder SJ. Towards a complete atomic structure of spectrin family proteins. J Struct Biol. 2002;137:184–193. doi: 10.1006/jsbi.2002.4465. [DOI] [PubMed] [Google Scholar]
- Byers TJ, Beggs AH, McNally EM, Kunkel LM. Novel actin crosslinker superfamily member identified by a two step degenerate PCR procedure. FEBS Lett. 1995;368:500–504. doi: 10.1016/0014-5793(95)00722-l. [DOI] [PubMed] [Google Scholar]
- Carr C, Tyler AN, Cohen JB. Myristic acid is the NH2-terminal blocking group of the 43-kDa protein of Torpedo nicotinic post-synaptic membranes. FEBS Lett. 1989;243:65–69. doi: 10.1016/0014-5793(89)81219-0. [DOI] [PubMed] [Google Scholar]
- Cartaud A, Coutant S, Petrucci TC, Cartaud J. Evidence for in situ and in vitro association between beta-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J Biol Chem. 1998;273:11321–11326. doi: 10.1074/jbc.273.18.11321. [DOI] [PubMed] [Google Scholar]
- Carugo KD, Banuelos S, Saraste M. Crystal structure of a calponin homology domain. Nat Struct Biol. 1997;4:175–179. doi: 10.1038/nsb0397-175. [DOI] [PubMed] [Google Scholar]
- Castresana J, Saraste M. Does Vav bind to F-actin through a CH domain? FEBS Lett. 1995;374:149–151. doi: 10.1016/0014-5793(95)01098-y. [DOI] [PubMed] [Google Scholar]
- Cooper MA. Label-free screening of bio-molecular interactions. Anal Bioanal Chem. 2003;377:834–842. doi: 10.1007/s00216-003-2111-y. [DOI] [PubMed] [Google Scholar]
- Fukami K, Sawada N, Endo T, Takenawa T. Identification of a phosphatidylinositol 4,5-bisphosphate-binding site in chicken skeletal muscle alpha-actinin. J Biol Chem. 1996;271:2646–2650. doi: 10.1074/jbc.271.5.2646. [DOI] [PubMed] [Google Scholar]
- Gimona M, Winder SJ. Single calponin homology domains are not actin-binding domains. Curr Biol. 1998;8:R674–R675. doi: 10.1016/s0960-9822(98)70432-4. [DOI] [PubMed] [Google Scholar]
- Gimona M, Djinovic-Carugo K, Kranewitter WJ, Winder SJ. Functional plasticity of CH domains. FEBS Lett. 2002;513:98–106. doi: 10.1016/s0014-5793(01)03240-9. [DOI] [PubMed] [Google Scholar]
- Goldsmith SC, Pokala N, Shen W, Fedorov AA, Matsudaira P, Almo SC. The structure of an actin-crosslinking domain from human fimbrin. Nat Struct Biol. 1997;4:708–712. doi: 10.1038/nsb0997-708. [DOI] [PubMed] [Google Scholar]
- Huebsch KA, Maimone MM. Rapsyn-mediated clustering of acetylcholine receptor subunits requires the major cytoplasmic loop of the receptor subunits. J Neurobiol. 2003;54:486–501. doi: 10.1002/neu.10177. [DOI] [PubMed] [Google Scholar]
- Jarrett HW, Foster JL. Alternate binding of actin and calmodulin to multiple sites on dystrophin. J Biol Chem. 1995;270:5578–5586. doi: 10.1074/jbc.270.10.5578. [DOI] [PubMed] [Google Scholar]
- Johnsson B, Lofas S, Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991;198:268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- Karakesisoglou I, Yang Y, Fuchs E. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J Cell Biol. 2000;149:195–208. doi: 10.1083/jcb.149.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep NH, Winder SJ, Moores CA, Walke S, Norwood FL, Kendrick-Jones J. Crystal structure of the actin-binding region of utrophin reveals a head-to-tail dimer. Structure Fold Des. 1999;7:1539–1546. doi: 10.1016/s0969-2126(00)88344-6. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A, Jones EM, Van Rossum DB, Bloch RJ. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol Biol Cell. 2003;14:1138–1148. doi: 10.1091/mbc.E02-07-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Leung CL, Sun D, Zheng M, Knowles DR, Liem RK. Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J Cell Biol. 1999;147:1275–1286. doi: 10.1083/jcb.147.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimone MM, Merlie JP. Interaction of the 43 kd postsynaptic protein with all subunits of the muscle nicotinic acetylcholine receptor. Neuron. 1993;11:53–66. doi: 10.1016/0896-6273(93)90270-2. [DOI] [PubMed] [Google Scholar]
- Moores CA, Keep NH, Kendrick-Jones J. Structure of the utrophin actin-binding domain bound to F-actin reveals binding by an induced fit mechanism. J Mol Biol. 2000;297:465–480. doi: 10.1006/jmbi.2000.3583. [DOI] [PubMed] [Google Scholar]
- Musil LS, Carr C, Cohen JB, Merlie JP. Acetylcholine receptor-associated 43K protein contains covalently bound myristate. J Cell Biol. 1988;107:1113–1121. doi: 10.1083/jcb.107.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood FL, Sutherland-Smith AJ, Keep NH, Kendrick-Jones J. The structure of the N-terminal actin-binding domain of human dystrophin and how mutations in this domain may cause Duchenne or Becker muscular dystrophy. Structure Fold Des. 2000;8:481–491. doi: 10.1016/s0969-2126(00)00132-5. [DOI] [PubMed] [Google Scholar]
- Phillips WD, Maimone MM, Merlie JP. Mutagenesis of the 43-kD postsynaptic protein defines domains involved in plasma membrane targeting and AChR clustering. J Cell Biol. 1991;115:1713–1723. doi: 10.1083/jcb.115.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CC, Phillips C. Rapsyn’s knobs and holes: eight tetratrico peptide repeats. Biochem J. 1996;314 ( Pt 3):1053–1054. doi: 10.1042/bj3141053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin DW. The membrane skeleton of acetylcholine receptor domains in rat myotubes contains antiparallel homodimers of beta-spectrin in filaments quantitatively resembling those of erythrocytes. J Cell Sci. 1995;108:3145–3154. doi: 10.1242/jcs.108.9.3145. [DOI] [PubMed] [Google Scholar]
- Ramarao MK, Cohen JB. Mechanism of nicotinic acetylcholine receptor cluster formation by rapsyn. Proc Natl Acad Sci U S A. 1998;95:4007–4012. doi: 10.1073/pnas.95.7.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao MK, Bianchetta MJ, Lanken J, Cohen JB. Role of rapsyn tetratricopeptide repeat and coiled-coil domains in self-association and nicotinic acetylcholine receptor clustering. J Biol Chem. 2001;276:7475–7483. doi: 10.1074/jbc.M009888200. [DOI] [PubMed] [Google Scholar]
- Rybakova IN, Patel JR, Davies KE, Yurchenco PD, Ervasti JM. Utrophin binds laterally along actin filaments and can couple costameric actin with sarcolemma when overexpressed in dystrophin-deficient muscle. Mol Biol Cell. 2002;13:1512–1521. doi: 10.1091/mbc.01-09-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Sevcik J, Urbanikova L, Kost’an J, Janda L, Wiche G. Actin-binding domain of mouse plectin. Crystal structure and binding to vimentin. Eur J Biochem. 2004;271:1873–1884. doi: 10.1111/j.1432-1033.2004.04095.x. [DOI] [PubMed] [Google Scholar]
- Stone MR, O’Neill A, Catino D, Bloch RJ. Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol Biol Cell. 2005;16:4280–4293. doi: 10.1091/mbc.E05-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradal T, Kranewitter W, Winder SJ, Gimona M. CH domains revisited. FEBS Lett. 1998;431:134–137. doi: 10.1016/s0014-5793(98)00751-0. [DOI] [PubMed] [Google Scholar]
- Sutherland-Smith AJ, Moores CA, Norwood FL, Hatch V, Craig R, Kendrick-Jones J, Lehman W. An atomic model for actin binding by the CH domains and spectrin-repeat modules of utrophin and dystrophin. J Mol Biol. 2003;329:15–33. doi: 10.1016/s0022-2836(03)00422-4. [DOI] [PubMed] [Google Scholar]
- Ursitti JA, Lee PC, Resneck WG, McNally MM, Bowman AL, O’Neill A, Stone MR, Bloch RJ. Cloning and characterization of cytokeratins 8 and 19 in adult rat striated muscle. Interaction with the dystrophin glycoprotein complex. J Biol Chem. 2004;279:41830–41838. doi: 10.1074/jbc.M400128200. [DOI] [PubMed] [Google Scholar]
- Way M, Pope B, Weeds AG. Are the conserved sequences in segment 1 of gelsolin important for binding actin? J Cell Biol. 1992;116:1135–1143. doi: 10.1083/jcb.116.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder SJ, Kendrick-Jones J. Calcium/calmodulin-dependent regulation of the NH2-terminal F-actin binding domain of utrophin. FEBS Lett. 1995;357:125–128. doi: 10.1016/0014-5793(94)01347-4. [DOI] [PubMed] [Google Scholar]
- Winder SJ, Hemmings L, Maciver SK, Bolton SJ, Tinsley JM, Davies KE, Critchley DR, Kendrick-Jones J. Utrophin actin binding domain: analysis of actin binding and cellular targeting. J Cell Sci. 1995;108 (Pt 1):63–71. doi: 10.1242/jcs.108.1.63. [DOI] [PubMed] [Google Scholar]


