SUMMARY
Introduction
Depression has been hypothesized to be associated with metabolic abnormalities which increase the risk of cardiovascular disease and diabetes. Such a link could be due to increased HPA-axis activity. This study investigates the cross-sectional relationship between depression, urinary cortisol and metabolic syndrome in an older population.
Methods
Data are from 867 participants of the InChianti Study, aged ≥ 65 years. Depressive symptoms were assessed using the CES-D scale; cortisol levels were determined in 24-hour urine samples. Metabolic syndrome was defined as three or more of the following: abdominal obesity, high triglycerides, low HDL cholesterol, high blood pressure, and high fasting glucose.
Results
Clinically relevant depressed mood (CES-D≥20) was present in 20.6% of the sample, and 24.5% had the metabolic syndrome. After adjustment for sociodemographics and health indicators, depression score (per SD increase: OR=1.20, 95%CI=1.02–1.41) and urinary cortisol level (per SD increase: OR=1.23, 95%CI=1.01–1.51) were significantly associated with presence of metabolic syndrome. There was, however, a significant interaction (p=.003) between depressed mood and urinary cortisol in the probability of having metabolic syndrome. The odds of metabolic syndrome in persons with both depressed mood and urinary cortisol excretion in the highest tertile were 1.84 (95% CI=1.02–3.34) compared to persons with neither condition.
Discussion
This study suggests a synergistic relationship between depression, cortisol and metabolic syndrome. Hypercortisolemic depression may constitute a specific risk group for the metabolic syndrome.
Keywords: depression, cortisol, HPA-axis, metabolic syndrome, older persons
INTRODUCTION
There is growing evidence that depression may cause major life-threatening and disabling diseases, such as cardiovascular disease (CVD) and diabetes mellitus (Penninx et al., 1998b; Penninx et al., 2001; Brown et al., 2005; Whooley, 2006). Recent studies suggest that the link between depression and CVD and diabetes may operate through the metabolic syndrome (Vitaliano et al., 2002).The metabolic syndrome is a clustering of risk factors associated with a particularly high risk of cardiovascular events and diabetes, and includes at least three of the following conditions: abdominal obesity, high triglyceride levels, low high density lipoprotein (HDL) cholesterol, high blood pressure, and high levels of fasting glucose (National Cholesterol Education Program, 2002). The estimated prevalence of the metabolic syndrome is as high as 42% in adults aged 60 years and over (Ford et al., 2002).
Depression has been linked to metabolic abnormalities, such as abdominal obesity, high blood pressure and insulin resistance (Raikkonen et al., 1996; Raikkonen et al., 2001; Weber-Hamann et al., 2002). Several pathways for these links have been suggested. Vitaliano et al. (2002) proposed that chronic stress causes depression and successive poor health habits that can lead to the metabolic syndrome and subsequent coronary heart disease. On the other hand more biological mechanisms have been proposed (Rosmond, 2005). Probably one of the most important biological mechanisms is the dysregulation of the hypothalamic-pituitary-adrenal (HPA)-axis, of which cortisol is an essential component. Dysregulation of the HPA-axis is typically associated with chronic stress and a number of studies have described an association between depression and high cortisol levels (Deuschle et al., 1998; Holsboer, 2001) and, in turn, elevated levels of cortisol have been related to metabolic syndrome components such as abdominal obesity and glucose intolerance (Bjorntorp and Rosmond, 1999). Although a few studies have examined the link between depression and individual components of the metabolic syndrome, there is little research on the extent to which depressive symptoms correlate with the metabolic syndrome as a whole, and the role of cortisol in this link. A study by Weber-Hamann et al. (2002) among 45 older women found that hypercortisolemic depression, in contrast to normocortisolemic depression, was associated with increased visceral fat, indicating the importance of considering cortisol levels when examining the link between depression and metabolic abnormalities and suggesting that associations with metabolic abnormalities may be especially powerful for hypercortisolemic depression.
The present study uses data from a large sample of community-dwelling older persons to investigate the associations between depressive symptoms and urinary cortisol levels with metabolic syndrome and its components. We hypothesize that higher depressive symptoms and higher levels of cortisol are associated with metabolic syndrome and its components. In addition, the existence of an interaction between depressive symptoms and cortisol levels on the presence of metabolic syndrome will be explored, since we hypothesize that hypercortisolemic depression in particular will be linked to metabolic syndrome.
METHODS
Participants were part of the InChianti Study, a prospective population-based study of older persons. In 1998 and 1999, the study sample was randomly selected from the population registry of two sites in Italy: Greve in Chianti, and Bagno a Ripoli, using a multistage stratified sampling method. Data collection consisted of a home interview, a 24-hour urine collection and a medical evaluation at the study clinic, which took place within 21 days after the home interview. The Italian National Institute of Research and Care on Aging ethical committee approved the study protocol and all respondents signed informed consent. A more detailed description of the study design is given elsewhere (Ferrucci et al., 2000).
The InChianti Study included 1155 participants aged 65 and over, but because of missing data on depressive symptoms (N=76), metabolic syndrome (N=101), urinary cortisol (N=51), or incomplete (< 20 hours) urine collection (N=60), the present analysis included data from 867 participants. Excluded persons were significantly older (79.4 versus 74.1, p<.001), more often women (61.8% versus 55.0%, p=.04), and had less years of education (4.7 versus 5.5, p=.002).
Metabolic Syndrome
The metabolic syndrome was defined as the presence of three or more of the following criteria: 1) abdominal obesity (waist circumference >102 cm in men or >88 cm in women); 2) hypertriglyceridemia (triglyceride level ≥ 150 mg/dl); 3) low high density lipoprotein (HDL) cholesterol (<40 mg/dl in men or <50 mg/dl in women); 4) high blood pressure (systolic/diastolic blood pressure ≥160/90 mmHg, and/or currently using anti-hypertensive medication); 5) high fasting glucose (≥110 mg/dl and/or currently using anti-diabetic medication). These criteria are similar to those outlined by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (National Cholesterol Education Program, 2002), with a minor modification for hypertension. 92% of the InChianti respondents met the original blood pressure criteria (130/85 mmHg). In order to take into account the characteristics of the older study population and to only classify those participants who were definitively hypertensive the diagnostic cutoff was raised to 160/90 mmHg as done in other aging studies (Ferrucci et al., 1997).
During the medical evaluation, after a fasting period of at least 8 hours, a 60-ml blood sample was drawn, stored in cold glass tubes, and delivered within two hours to a central laboratory. Serum glucose, HDL cholesterol, and triglycerides were measured by standard laboratory methods. Waist circumference was measured by trained examiners at the largest mid-body point. Three blood pressure measurements were taken using a standard mercury sphygmomanometer with the respondent in a supine position; the average of the last two readings was used in this analysis. Drugs taken in the previous two weeks were identified and coded using the Anatomical Therapeutic Chemical (ATC) system to ascertain anti-diabetic and anti-hypertensive medication use. In addition to a dichotomous indicator of the metabolic syndrome, analyses were also conducted with continuous measures of metabolic syndrome components in order to investigate the consistency over and the importance of individual components. To incorporate medication use into the continuous metabolic syndrome component measures, 10 mmHg and 5 mmHg was added to systolic and diastolic blood pressure, respectively, for persons using antihypertensive medication since these values represent the average decline in blood pressure in antihypertensive medication trials (SHEP Cooperative Research Group, 1991; Tannen et al., 2006). Similarly, persons who used anti-diabetic medication and had a glucose level below 126 mg/dl, were given a value of 126 for fasting glucose.
Urinary Cortisol
The assessment of urinary cortisol over a 24-hour period provides a rather stable indicator of the total cortisol excretion by the adrenals and measures the biologically active (unbound) cortisol. Before the inclinic assessment, study participants were asked to collect all urine produced during a 24-hour period starting after the first voided urine following awakening and including the first voided urine on the following day. At assessment, 10 ml aliquots of urine were prepared and stored at −80°C for later assaying at the Clinical Chemistry Laboratory of the Careggi Hospital, Italy. Urinary cortisol was measured by an immunochemiluminescence method and an ADVIA-Centaur immunoassay system (Bayer Diagnostics). The intra-assay coefficient of variation was less then 2.0% and the inter-assay coefficient of variation was less then 3.0%. Urinary cortisol level was defined as micrograms of cortisol excreted over 24 hours. Both a continuous measure as well a tertile categorization of urinary cortisol was used in the present study.
Depressive Symptoms
Depressive symptoms were assessed using the original 20-item version of the Center for Epidemiological Studies-Depression Scale (CES-D) administered during the home interview (Radloff, 1977). The CES-D is a self-report scale, ranging from 0 to 60, which has been shown to be a valid instrument for identifying depressive symptoms in older community-dwelling adults (Beekman et al., 1997) also in an Italian sample (Fava, 1983). In our study, the internal consistency was high: Cronbach’s alpha = 0.82. Analyses were performed with both the continuous CES-D score (referred to as depressive symptoms) as well as a dichotomous indicator for clinically relevant depressed mood (CES-D≥20; referred to as depressed mood). Normally, a cutoff score of 16 on the CES-D is considered to represent clinically relevant depression; however, previous studies have demonstrated that a cutoff of 20 on the CES-D avoids overestimation in older adult populations (Penninx et al., 1998a; Penninx et al., 1998b; Penninx et al., 1998c).
Covariates
Covariates included sociodemographic variables (age, sex, and years of education), smoking status (non-former, or current smoker) and current alcohol use (yes or no 3 or more drinks a day). Number of chronic diseases (including cancer, liver disease, gastrointestinal disease, congestive heart failure, Parkinson’s disease, peripheral arterial disease, lung disease, hip fracture, herniated disc, arthritis, osteoporosis and dementia) was calculated as a global indicator of poor physical health. Serum creatinine, measured through a modified Jaffe method, was used to calculate creatinine clearance with the Cockcroft-Gault formula. Following K/DOQI guidelines (National Kidney Foundation, 2002), a creatinine clearance rate of 30 ml/min or lower was considered to indicate severe renal function impairment, which may profoundly disturb urinary cortisol levels. Identification of cardiovascular disease (including angina pectoris, myocardial infarction, stroke or transient ischemic attack) and diabetes was based on a standardized algorithm using information on self-reported history, pharmacological treatments, medical exam data, and hospital discharge records. Use of corticosteroids, antidepressants, and benzodiazepines was assessed and coded according to ATC-codes.
Statistical Analyses
Baseline characteristics were compared across depression status using χ2-and t-test statistics. Logistic regression analyses, adjusted for age, sex, education, smoking, alcohol use, number of chronic diseases, severe renal function impairment, and urine volume (analyses with cortisol only), were conducted to independently assess the association between depression and urinary cortisol variables with metabolic syndrome. To examine whether the relationship between depression and metabolic syndrome is (partially) mediated by urinary cortisol, analyses including both cortisol and depression variables were conducted as well. For the latter, both a linear variable and the squared term for urinary cortisol were used, because earlier findings in this study sample (Penninx et al., submitted) found a U-shaped association between depression and urinary cortisol, which was best described by a linear and squared term for urinary cortisol. In addition, since we hypothesized that especially hypercortisolemic depression could be associated with the metabolic syndrome, we tested for interaction between depressed mood and urinary cortisol by entering a depressed mood*cortisol interaction term.
Since sex differences in the relationship between depression and CVD have been observed before (Penninx et al., 1998b; Penninx et al., 1999), sex interactions were explored by entering sex*depression/cortisol interaction terms in adjusted models. In addition, it was explored whether associations were different for persons with and without already existing cardiovascular disease or diabetes by exploring CVD/diabetes*depression/cortisol interaction terms. Finally, in order to examine whether associations with depression and cortisol variables were consistent for individual metabolic syndrome components, adjusted linear regression analyses were conducted with continuous variables for all five components as the outcomes. For these analyses, triglycerides, HDL cholesterol, and fasting glucose were log-transformed in order to normalize distributions.
RESULTS
The mean age of the participants included in these analyses was 74.1 years (SD=6.6) and 55.0% of the participants were women. 20.6% were depressed (CES-D≥20) and 24.5% had the metabolic syndrome. The mean urinary cortisol level was 98.8 μg/24 hours (SD=48.1). As shown in Table 1, depressed persons were older, more often women, less likely to be a (former) smoker or heavy alcohol drinker, and had more chronic diseases than the non-depressed. Although cortisol levels appeared to be somewhat higher in the depressed group, this was not statistically significant. The prevalence of metabolic syndrome and some of its components (abdominal obesity, low HDL cholesterol, antihypertensive medication) was higher among depressed participants. Use of corticosteroids (p=.59), antidepressants (p=.50), and benzodiazepines (p=.33) was not associated with metabolic syndrome (data not shown).
Table 1.
Baseline Characteristics according to Depressed Mood
| Characteristic | Non-depressed N=688 | Depressed N=179 | P* |
|---|---|---|---|
| Age (years), mean (SD) | 73.7 (6.6) | 75.8 (6.3) | <.001 |
| Female, % | 50.1 | 73.7 | <.001 |
| Years of education, mean (SD) | 5.6 (3.1) | 5.1 (3.5) | .12 |
| Smoking | |||
| Nonsmoker, % | 53.8 | 72.6 | <.001 |
| Former smoker, % | 31.1 | 16.2 | |
| Current smoker, % | 15.1 | 11.2 | |
| Alcohol use (≥ 3 drinks a day), % | 11.9 | 5.0 | .007 |
| Number of chronic diseases, mean (SD) | 1.0 (0.9) | 1.3 (1.0) | <.001 |
| Severe renal function impairment, % | 1.6 | 1.7 | .94 |
| Corticosteroid use, % | 1.6 | 2.8 | .29 |
| Antidepressant use, % | 1.7 | 10.6 | <.001 |
| Benzodiazepine use, % | 15.0 | 36.3 | <.001 |
| Urine volume (ml), mean (SD) | 1522 (551) | 1521 (619) | .99 |
| Baseline CVD, % | 13.1 | 11.2 | .49 |
| Baseline diabetes, % | 12.4 | 10.6 | .52 |
| Cortisol | |||
| Urinary cortisol (μg), mean (SD) | 97.3 (43.2) | 104.6 (63.5) | .15 |
| Metabolic Syndrome | |||
| Waist circumference (cm), mean (SD) | 92.9 (10.0) | 92.0 (11.6) | .36 |
| Abdominal obesity, % | 38.1 | 48.3 | .01 |
| Triglycerides (mg/dl), mean (SD) | 128.7 (73.3) | 128.8 (66.3) | .99 |
| High triglycerides, % | 24.9 | 25.1 | .94 |
| HDL cholesterol (mg/dl), mean (SD) | 56.0 (15.3) | 56.2 (14.7) | .92 |
| Low HDL cholesterol, % | 20.1 | 27.9 | .02 |
| Systolic blood pressure (mmHg), mean (SD) | 150 (19.5) | 150 (19.2) | .81 |
| Diastolic blood pressure (mmHg), mean (SD) | 84 (8.5) | 84 (8.6) | .66 |
| Antihypertensive medication, % | 40.0 | 50.3 | .01 |
| High blood pressure, % | 65.5 | 67.8 | .56 |
| Fasting glucose (mg/dl), mean (SD) | 96.7 (25.8) | 92.9 (23.4) | .08 |
| Anti-diabetic medication, % | 6.7 | 6.7 | .99 |
| High fasting glucose, % | 16.9 | 12.8 | .19 |
| Metabolic syndrome, % | 22.8 | 30.7 | .03 |
| Number of metabolic components, mean (SD) | 1.7 (1.2) | 1.8 (1.2) | .12 |
based on chi-square for dichotomous variables and independent t-test for continuous variables
Table 2 shows that after adjustment for covariates there was a significant association between the severity of depressive symptoms (continuous CES-D score) and metabolic syndrome (OR per SD increase=1.20, 95%-CI=1.02–1.41). However, the association between depressed mood (CES-D≥20) and the metabolic syndrome was not statistically significant (OR=1.30, 95%CI=0.88–1.90). Level of urinary cortisol showed a significant linear association with metabolic syndrome (OR per SD increase=1.18, 95%CI=1.01–1.38). Additional adjustment for urinary cortisol and the squared term of urinary cortisol weakened the associations between depression and metabolic syndrome considerably: OR per SD increase in CES-D score became 1.11 (95%CI=0.94–1.32) and the OR for depressed mood became 1.13 (95%CI=0.76–1.68).
Table 2.
Associations between Depressed Mood, Urinary Cortisol and Metabolic Syndrome
| Unadjusted * | Adjusted † | Adjusted for cortisol2 ‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Total Sample (N=867) | |||||||||
| Depressive symptoms (per SD increase in CESD-score a) | 1.29 | 1.11–1.50 | .001 | 1.20 | 1.02–1.41 | .03 | 1.11 | 0.94–1.32 | .23 |
| Depressed mood (CESD ≥ 20) | 1.50 | 1.04–2.16 | .03 | 1.30 | 0.88–1.90 | .19 | 1.13 | 0.76–1.68 | .55 |
| Urinary cortisol (per SD increase b) | 1.12 | 0.96–1.30 | .14 | 1.18 | 1.01–1.38 | .04 | |||
| Depressed mood * urinary cortisol | .001 | .003 | |||||||
| Non-depressed (N=688) | |||||||||
| Urinary cortisol (per SD increase b) | 0.87 | 0.72–1.09 | .25 | 0.95 | 0.67–1.18 | .61 | |||
| Depressed (N=179) | |||||||||
| Urinary cortisol (per SD increase b) | 1.52 | 1.18–1.94 | .001 | 1.56 | 1.20–2.04 | .001 | |||
based on unadjusted logistic regression analyses
adjusted for age, sex, education, smoking, alcohol use, # of chronic diseases, severe renal function impairment, and urine volume (analyses with cortisol only)
additionally adjusted for cortisol2 and cortisol
Depressive symptoms: SD = 8.7
Urinary cortisol (μg): SD = 48
Figure 1 shows that the unadjusted prevalence of metabolic syndrome differed significantly (p=.008) across depressed mood and urinary cortisol tertile groups. Metabolic syndrome was more prevalent among the depressed in the highest cortisol tertile than in all other groups defined by tertile of cortisol and depression status. After adjustment, when persons had both depressed mood and urinary cortisol excretion in the highest tertile, the odds of metabolic syndrome was 1.84 (95% CI=1.02–3.34, p=.04) compared to persons without depression in the lowest tertile of urinary cortisol (reference group). Other depression/cortisol groups did not differ from the reference group (all p>.15). The interaction between depressed mood and urinary cortisol levels in predicting the odds of metabolic syndrome was significant (p interaction=.003). Consequently, additional analyses stratified for depressed mood were conducted. For those without depressed mood (N=688), urinary cortisol was not associated with the metabolic syndrome (OR per SD increase=0.95, 95%CI=0.67–1.18). In the depressed group (N=179), however, the odds of having the metabolic syndrome increased significantly with increasing levels of urinary cortisol (OR per SD increase=1.56, 95%CI=1.20–2.04).
Figure 1.
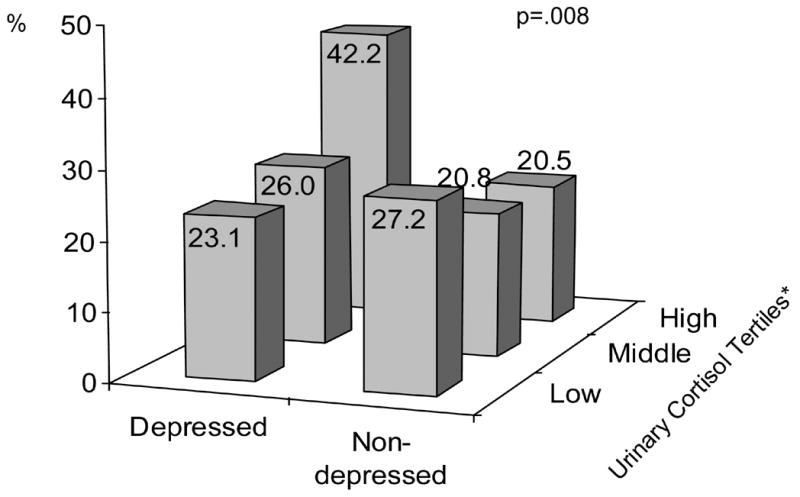
Prevalence of Metabolic Syndrome across Depression Status and Tertiles of Urinary Cortisol * Lowest Tertile: <77 μg; Middle Tertile: 77-111 μg; Highest Tertile: >111 μg
Next, logistic regression analyses were repeated including sex*depressed mood, sex*urinary cortisol, and sex*depressed mood*urinary cortisol interaction terms, respectively. No significant sex interactions were found (all p>.15; data not shown). Also, when testing for CVD/diabetes interactions with depressed mood, urinary cortisol and their interacting effect, no significant interactions in the odds of having the metabolic syndrome were found (all p>.15; data not shown), which suggests that the link between depression, cortisol and metabolic syndrome is consistent for persons with and without CVD or diabetes.
Finally, Table 3 shows that depressive symptoms tended to be associated with waist circumference (β=.064, p=.07) and were negatively associated with HDL cholesterol levels (β= −.070, p=.05). However, no associations between depressed mood (CES-D≥20) and any of the individual metabolic syndrome components were observed. Further, urinary cortisol levels showed a significant association with waist circumference (β=.081, p=.02) and fasting glucose levels (β=.082, p=.02). Moreover, there was a significant interaction between depressed mood and urinary cortisol levels in predicting levels of triglycerides (p<.001), HDL cholesterol (p=.02), and fasting glucose (p=.003), and a trend for an interaction for waist circumference (p=.08). Large waist circumference, high triglycerides levels and low HDL levels were significantly associated with higher cortisol levels in the depressed group only (β=.178, p=.02; β=.247, p=.001; β= −.156, p=.04, respectively). In the non-depressed group, higher levels of urinary cortisol were associated with lower triglycerides levels (β= −.103, p=.01) and with higher levels of fasting glucose (β=.156, p<.001).
Table 3.
Adjusted* Associations between Depressed Mood, Urinary Cortisol, and Continuous Metabolic Syndrome Measures
| Waist circumference | Triglycerides # | HDL cholesterol # | Systolic blood pressure | Diastolic blood pressure | Fasting glucose # | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | β | P | |
| Total Sample (N=867) | ||||||||||||
| Depressive symptoms | .064 | .07 | .006 | .88 | −.070 | .05 | −.049 | .17 | .024 | .52 | −.038 | .30 |
| Depressed mood (CESD ≥ 20) | .025 | .46 | .009 | .79 | −.048 | .15 | −.033 | .35 | .024 | .51 | −.053 | .13 |
| Urinary cortisol | .081 | .02 | −.003 | .92 | −.017 | .61 | .014 | .70 | .025 | .47 | .082 | .02 |
| Depressed mood * urinary cortisol | .08 | <.001 | .02 | .72 | .58 | .003 | ||||||
| Non-depressed (N=688) | ||||||||||||
| Urinary cortisol | .028 | .46 | −.103 | .01 | .051 | .17 | .006 | .87 | .011 | .78 | .156 | <.001 |
| Depressed (N=179) | ||||||||||||
| Urinary cortisol | .178 | .02 | .247 | .001 | −.156 | .04 | .048 | .53 | .059 | .46 | −.065 | .39 |
Based on linear regression analyses adjusted for age, sex, education, smoking, alcohol use, # of chronic diseases, severe renal function impairment, and urine volume (analyses with cortisol only)
Log-transformed values of triglycerides, HDL cholesterol and fasting glucose were used
DISCUSSION
This community-based study among a large sample of older adults examined the relationship between depression, urinary cortisol and the metabolic syndrome. Higher levels of depressive symptoms were significantly associated with an increased prevalence of the metabolic syndrome. This relationship might be partially mediated by urinary cortisol levels, since entering cortisol levels in the analyses reduced the association between depressive symptoms and metabolic syndrome. Moreover, we found an interaction between depressed mood and urinary cortisol in predicting metabolic syndrome. Among non-depressed participants, no association between urinary cortisol and metabolic syndrome was found. However, among depressed persons, urinary cortisol level did strongly predict the likelihood of having the metabolic syndrome. Persons with depressed mood and urinary cortisol levels in the highest tertile in particular had an increased prevalence of metabolic syndrome, which suggests that hypercortisolemic depression constitutes a specific risk factor for the metabolic syndrome.
Our results are consistent with a few other studies that investigated the relationship between depressive symptoms and the metabolic syndrome. McCaffery et al. (2003) found an association between depressive symptoms and metabolic risk in adult male twins, and Raikkonen et al. (2002) confirmed this association in middle-aged women. Furthermore, a study by Kinder et al. (2004) suggests that a history of depression increases the risk of metabolic syndrome in young women, but not men. Our results show that the relationship between depression and the metabolic syndrome extends to an older population. In addition, the involvement of the HPA-axis in the link between depression and the metabolic syndrome has been hypothesized by others (Rosmond, 2005), but no study has tested this directly. Our study provides evidence for the proposition that elevated cortisol levels increase the risk of the metabolic syndrome. An association between urinary cortisol and metabolic parameters was not observed in a study by Otte et al. (2004); however this study did not investigate the metabolic syndrome as a whole and did not examine depression*cortisol interactions. Most importantly, our study confirmed the hypothesis that when both depression and high cortisol levels are present, the odds of the metabolic syndrome is increased. This is consistent with a study by Weber-Hamann et al. (2002), who found among 45 older women that hypercortisolemic depression was associated with increased visceral fat and a larger accumulation of visceral fat over time (Weber-Hamann et al., 2006).
How can it be explained that hypercortisolemic depression in particular is associated with a higher prevalence of metabolic syndrome? Previous findings from this and other aging studies have suggested that late-life depression may be associated with high as well as low levels of cortisol (Morrison et al., 2000; Oldehinkel et al., 2001; Penninx et al., submitted). A number of studies provided some evidence that hypocortisolemic depression is associated with physical frailty and conditions characterized by fatigue and pain (Gur et al., 2004; Fries et al., 2005; Penninx et al., submitted), whereas hypercortisolemic depression is linked with more severe symptoms of depression (Nelson and Davis, 1997; Penninx et al., submitted). In addition, it has been suggested that melancholic depression is associated with a hyperactive HPA-axis, whereas a hypoactive HPA-axis may be linked with atypical depression (Gold and Chrousos, 2002). Therefore, it is possible that hyperactivity of the HPA-axis identifies a specific subtype of depression and it may be that only this subtype of depression is associated with the metabolic syndrome. What mechanisms, then, could underlie this association between hypercortisolemic depression and the metabolic syndrome? As described in a review by Björntorp (2001), cortisol binds to glucocorticoid receptors which have a high density in visceral fat depots; there it activates lipoprotein lipase and inhibits lipid mobilization, which leads to an accumulation of triglycerides in this area. This review also suggests that these effects are even more pronounced when combined with inhibition of sex steroids. Since low sex steroid hormones levels have also been associated with depression (Barrett-Connor et al., 1999) this could explain why especially the combination of depression and high levels of cortisol increase the prevalence of the metabolic syndrome. Additionally, both depression and hyperactivity of the HPA-axis have been associated with increased inflammation (Sternberg et al., 1992; Penninx et al., 2003), which in turn have been linked to metabolic abnormalities (Yudkin et al., 1999). Therefore, a combination of depression and hypercortisolemia could increase the risk for metabolic syndrome even more. Future studies should test these hypothesized explanations.
We evaluated whether depression and cortisol associations were consistent across the various metabolic syndrome components. Higher depressive symptoms were associated with larger waist circumference and lower HDL cholesterol levels, but for the other metabolic syndrome components no significant associations with depression were observed. Furthermore, in the depressed, we observed expected relationships between urinary cortisol and waist circumference, triglycerides and HDL cholesterol. This may indicate that hypercortisolemic depression is especially associated with the obesity-related components of the metabolic syndrome. However, Kopf et al. (2004) found that in depressed persons higher cortisol levels were associated with a better lipid profile, although only in overweight subjects. In contradiction to some studies (Weber-Hamann et al., 2002; Kopf et al., 2004; Weber-Hamann et al., 2006), we did not find an association between hypercortisolemic depression and fasting glucose. In contrast, we found an association between cortisol levels and high fasting glucose in the non-depressed only. We do not have a clear explanation for this finding and it may be a chance finding, since it is contrary to findings for the other metabolic syndrome components. Lastly, no associations were found between depression/cortisol and high blood pressure, which may be in line with other research showing that the blood pressure component contributes less strongly and consistently to the concept of ‘metabolic syndrome’ (Shen et al., 2003) compared to other components.
Our definition of the metabolic syndrome did not exclude persons who already had CVD or diabetes. In fact, in our sample 19.7% and 31.2% of those with metabolic syndrome had CVD and diabetes. Since previous studies reported a relationship between depression and CVD or diabetes (Penninx et al., 1998b; Penninx et al., 2001; Brown et al., 2005), it is possible that an observed link between depression and metabolic syndrome merely reflects the association between depression and existing CVD/diabetes. However, we observed no CVD/diabetes interactions, suggesting that the relationship between depression and the metabolic syndrome is independent of the presence of CVD/diabetes and also exists among persons without CVD or diabetes.
Some limitations of our study should be acknowledged. Because of the cross-sectional nature of our study, we cannot make inferences about causality. Longitudinal analyses are needed to address this issue. Further, we did not have access to psychiatric diagnoses of depression. However, the CES-D is a commonly-used scale to measure depressive symptoms and has been linked to CVD outcomes. When persons have psychiatric diagnoses of depression, the association with the metabolic syndrome could even be stronger. Lastly, the InChianti sample is comprised only of Italian older adults, who generally score rather high on depressive symptoms questionnaires compared to northern European countries and the US, which may be due to cultural but not per se clinical differences. Nevertheless, the Italian version of the CES-D has shown to be similarly predictive of major depression than other language versions, which suggests that its validity is comparable to that used in other international studies (Fava, 1983). Strengths of our study were the large, randomly selected sample, and the 24-hour urine measures of cortisol. This measure of cortisol is rather stable and insensitive to transient fluctuations in cortisol that lead to over- and underestimates in moment-by-moment sampling. On top, this assessment of the HPA-axis reflects biologically active, or unbound cortisol.
In conclusion, our results suggest a synergistic relationship between depression, cortisol, and the metabolic syndrome in an elderly population. Persons with hypercortisolemic depression in particular may be at risk for having the metabolic syndrome, and therefore have an increased risk for developing cardiovascular disease or diabetes. Further research should confirm our findings and investigate the causality of the relationships between depression, cortisol and the metabolic syndrome using prospective studies.
Acknowledgments
This study was supported in part by grants R01 HL72972-01 (Dr. Penninx) and IRTA 2300 320 7 from the National Institutes of Health, Bethesda, MD (Drs. Suthers, Ble, & Schrager). The Extramural Branch of the NIH responsible for funding of the InChianti study did not have any role in the design and conduct of the study, the collection, management, analysis and interpretation of the data nor the preparation, review or approval of the manuscript. Cortisol assays were supported by a professional services contract from the Laboratory of Epidemiology, Demography and Biometry, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, van Limbeek J, Braam AW, De Vries MZ, van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Hypothalamic origin of the metabolic syndrome X. Ann NY Acad Sci. 1999;892:297–307. doi: 10.1111/j.1749-6632.1999.tb07803.x. [DOI] [PubMed] [Google Scholar]
- Brown LC, Majumdar SR, Newman SC, Johnson JA. History of depression increases risk of type 2 diabetes in younger adults. Diabetes Care. 2005;28:1063–1067. doi: 10.2337/diacare.28.5.1063. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Weber B, Colla M, Depner M, Heuser I. Effects of major depression, aging and gender upon calculated diurnal free plasma cortisol concentrations: a re-evaluation study. Stress. 1998;2:281–287. doi: 10.3109/10253899809167292. [DOI] [PubMed] [Google Scholar]
- Fava GA. Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. J Clin Psychol. 1983;39:249–251. doi: 10.1002/1097-4679(198303)39:2<249::aid-jclp2270390218>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Guralnik JM, Pahor M, Harris T, Corti MC, Hyman BT, Wallace RB, Havlik RJ. Apolipoprotein E epsilon 2 allele and risk of stroke in the older population. Stroke. 1997;28:2410–2416. doi: 10.1161/01.str.28.12.2410. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gur A, Cevik R, Nas K, Colpan L, Sarac S. Cortisol and hypothalamic-pituitary-gonadal axis hormones in follicular-phase women with fibromyalgia and chronic fatigue syndrome and effect of depressive symptoms on these hormones. Arthritis ResTher. 2004;6:R232–R238. doi: 10.1186/ar1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Kinder LS, Carnethon MR, Palaniappan LP, King AC, Fortmann SP. Depression and the metabolic syndrome in young adults: findings from the Third National Health and Nutrition Examination Survey. Psychosom Med. 2004;66:316–322. doi: 10.1097/01.psy.0000124755.91880.f4. [DOI] [PubMed] [Google Scholar]
- Kopf D, Westphal S, Luley CW, Ritter S, Gilles M, Weber-Hamann B, Lederbogen F, Lehnert H, Henn FA, Heuser I, Deuschle M. Lipid metabolism and insulin resistance in depressed patients: significance of weight, hypercortisolism, and antidepressant treatment. J Clin Psychopharmacol. 2004;24:527–531. doi: 10.1097/01.jcp.0000138762.23482.63. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Niaura R, Todaro JF, Swan GE, Carmelli D. Depressive symptoms and metabolic risk in adult male twins enrolled in the National Heart, Lung, and Blood Institute twin study. Psychosom Med. 2003;65:490–497. doi: 10.1097/01.psy.0000041545.52924.82. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Redei E, TenHave T, Parmelee P, Boyce AA, Sinha PS, Katz IR. Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry. 2000;47:144–150. doi: 10.1016/s0006-3223(99)00099-2. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154:1497–1503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, van dB, Flentge F, Bouhuys AL, ter Horst GJ, Ormel J. Urinary free cortisol excretion in elderly persons with minor and major depression. Psychiatry Res. 2001;104:39–47. doi: 10.1016/s0165-1781(01)00300-6. [DOI] [PubMed] [Google Scholar]
- Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, Whooley MA. Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: The Heart and Soul Study. Biol Psychiatry. 2004;56:241–247. doi: 10.1016/j.biopsych.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Beekman AT, Bandinelli S, Corsi AM, Bremmer MA, Hoogendijk WJG, Guralnik JM, Ferrucci L. Late-life depression is associated with both hyper- and hypoactivity of the HPA-axis. (Submitted) [Google Scholar]
- Penninx BW, Beekman AT, Honig A, Deeg DJ, Schoevers RA, van Eijk JT, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Geerlings SW, Deeg DJ, van Eijk JT, van Tilburg W, Beekman AT. Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry. 1999;56:889–895. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998a;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Mendes de Leon CF, Pahor M, Visser M, Corti MC, Wallace RB. Cardiovascular events and mortality in newly and chronically depressed persons > 70 years of age. Am J Cardiol. 1998b;81:988–994. doi: 10.1016/s0002-9149(98)00077-0. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, Havlik RJ. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998c;90:1888–1893. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raikkonen K, Keltikangas-Jarvinen L, Adlercreutz H, Hautanen A. Psychosocial stress and the insulin resistance syndrome. Metabolism. 1996;45:1533–1538. doi: 10.1016/s0026-0495(96)90184-5. [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Matthews KA, Kuller LH. Trajectory of psychological risk and incident hypertension in middle-aged women. Hypertension. 2001;38:798–802. [PubMed] [Google Scholar]
- Raikkonen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism. 2002;51:1573–1577. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Shen BJ, Todaro JF, Niaura R, McCaffery JM, Zhang J, Spiro A, III, Ward KD. Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. Am J Epidemiol. 2003;157:701–711. doi: 10.1093/aje/kwg045. [DOI] [PubMed] [Google Scholar]
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992;117:854–866. doi: 10.7326/0003-4819-117-10-854. [DOI] [PubMed] [Google Scholar]
- Tannen RL, Weiner MG, Marcus SM. Simulation of the Syst-Eur randomized control trial using a primary care electronic medical record was feasible. J Clin Epidemiol. 2006;59:254–264. doi: 10.1016/j.jclinepi.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Weber-Hamann B, Hentschel F, Kniest A, Deuschle M, Colla M, Lederbogen F, Heuser I. Hypercortisolemic depression is associated with increased intra-abdominal fat. Psychosom Med. 2002;64:274–277. doi: 10.1097/00006842-200203000-00010. [DOI] [PubMed] [Google Scholar]
- Weber-Hamann B, Werner M, Hentschel F, Bindeballe N, Lederbogen F, Deuschle M, Heuser I. Metabolic changes in elderly patients with major depression: evidence for increased accumulation of visceral fat at follow-up. Psychoneuroendocrinology. 2006;31:347–354. doi: 10.1016/j.psyneuen.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Whooley MA. Depression and cardiovascular disease: healing the broken-hearted. JAMA. 2006;295:2874–2881. doi: 10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]


