Abstract
Memory T cell (TM) populations specific for transplantation Ags may arise from sensitization due to blood transfusions, tissue transplants or in multiparous females. In each of these scenarios, memory T cells (TM) are likely generated and have been shown to persist in such individuals for extended time periods. Heightened resistance to allogeneic marrow engraftment in certain individuals is therefore consistent with the presence of anti-donor TM. CD8 TM were generated against a single minor H Ag (MiHA) epitope to determine if such cells could inhibit allogeneic marrow engraftment. The present results demonstrate that B6 mice sensitized to a single immunodominant (H60) epitope efficiently reject donor marrow allografts expressing this MiHA alone or together with multiple minor transplantation antigens, even following ablative TBI conditioning. To further address the survival and function of these CD8 TM, sensitized mice were ablatively conditioned and administered a syngeneic HCT. CD8+H60TCR+ TM were clearly detected up to two weeks later in such recipients. Additionally, the memory cells present were capable of mediating effector responses as evidenced by their ability to resist a second, allogeneic HCT. In summary, these observations highlight the increased risk of resistance in the presence of anti-donor antigen specific CD8 TM due to their ability to survive and function even following rigorous conditioning and HCT.
Keywords: Bone Marrow, Spleen, MiHA-specific Memory T Cells, Marrow Transplantation
Introduction
Recipient resistance to donor allogeneic hematopoietic progenitor and stem cells constitutes a major barrier to successful engraftment. Experimental hematopoietic cell transplants (HCT) have demonstrated that immune resistance can involve CD4, CD8 and NK populations.1–4 Resistance to MHC-matched MiHA-mismatched allogeneic marrow engraftment is efficiently and rapidly mediated primarily by MiHA-reactive T cells. Experimental and clinical studies have reported that both CD8 and CD4 T cells can be identified in recipients during rejection of allogeneic HCT.5–8 Because of their potent immunological function, long-lived host memory T cells (TM) could be particularly problematic with respect to donor allogeneic hematopoietic engraftment. TM specific for transplantation antigens have been demonstrated after blood transfusions, tissue transplants or pregnancy.9–11 Moreover, heterologous immune responses, specifically those elicited by virus have been shown to induce the generation of alloantigen reactive cells.12–14 Indeed, such viral immunity has been shown to augment the T cell immune barrier to tissue transplants and CD8 TM cell populations were identified to be responsible for promoting this allograft rejection.14 Therefore, multiple alloreactive CD8 TM populations capable of mediating resistance may be present in many transplant recipients. It remains to be determined how conditioning and transplant affect the survival and subsequent function of such CD8 TM. To address this question, we utilized an established pre-clinical MiHA-mismatched allograft transplant model15–17 taking advantage of a well defined donor MiHA, i.e. H60 to monitor antigen-specific CD8 TM in recipients following HCT.18,19 Our findings demonstrate that antigen-specific host CD8 TM were capable of surviving and effecting resistance despite ablative conditioning, against donor hematopoietic grafts expressing H60 alone or together with multiple minor transplantation antigens. Moreover, the present results demonstrated that CD8 TM persist at least 2 – 3 weeks following rigorous conditioning with the ability to mediate effector responses. Thus, these observations suggest that patients naturally or iatrogenically sensitized to donor antigens could be at high risk of rejection of allogeneic HCT.
Materials and Methods
Mice
Female C57BL/6 (B6) Ly5.1 and Ly5.2 mice (10 – 12 weeks old) were purchased from The Jackson Laboratory. B6-Tg(ACTB-EGFP)1/Osb (B6-gfp) mice originally a gift from Dr. M. Okabe (Osaka University, Suita, Japan) were bred at the UM Animal Facility. BALB.B mice were bred and maintained at the UM Animal Facility. H60 congenic mice B6.C-H60c/Dcr were generated after 10 backcross generations as described.18
H60 sensitization of recipients prior to HCT
Dendritic cells were generated ex vivo as described 20. Briefly, B6 marrow cells were cultured in RPMI 1640 supplemented with 5% fetal bovine serum containing 20ng/ml rmGM-CSF and 20ng/ml rmIL-4 for 5 days and then the cultures replenished with fresh media containing these cytokines for an additional 3 days (GM-DC). The cells were washed, pulsed for 4hrs with 0.5μ H60 peptide (LTFNYRNL) and 1 – 2 × 106 pulsed GM-DC were injected ip to sensitize naïve female B6 Ly5.1 mice. Three weeks after the second GM-DC injection, sensitized and naïve control female mice were ablatively conditioned (9.0 Gy) and 24 hours later transplanted with sex-matched allogeneic BALB.B (H-2b), B6 H60 congenic or syngeneic B6 Ly5.2 T cell depleted bone marrow (TCD-BM).
Assessment of peripheral chimerism and progenitor cell assay
The presence of H60-specific CD8 T cells (H60+ CD8TCR+) was examined using H60 tetramer staining in mice which had received H60-pulsed GM-DC. At 1 week post-transplant, recipient PBMCs were analyzed for donor chimerism by routine cytometric analysis using PE-anti-Ly9.1 (clone 30C7, Pharmingen), PE-anti-CD45 (clone 104, Pharmingen) and gfp expression to detect BALB.B, B6 wild type or B6-gfp donor cells respectively. CFU-IL3 assay was then performed as previously described15 using recipient spleen cells to assess for the presence of committed hematopoietic progenitors. Progenitor colonies were enumerated (>25 cells) and numbers expressed as CFU/2×106 spleen cells. The student’s t test was used for statistical analysis.
Results and Discussion
Tetramer-binding (H60+) CD8 T cells were readily detectable in B6 mice sensitized with H60-pulsed B6 GM-DC (Fig. 1A). The frequency of these circulating CD8+H60TCR+ cells was ~1% amongst peripheral blood mononuclear cells (3–5% of CD8+ T cells) consistent with that observed previously in B6 mice sensitized against BALB.B alloantigens 18. To document the presence of CD8 TM, circulating CD8+H60TCR+ T cells in these mice were analyzed for the expression of markers characteristic of CD8 TM (Fig. 1A and 21). The tetramer positive CD8 T cells expressed both CD44 and Ly 6C indicating a CD8 TM phenotype. Thus, in B6 mice peptide-pulsed GM-DC generated CD8 TM specific for the immunodominant epitope H60. Since we wanted to monitor this population in HCT recipients, sensitized mice were conditioned with varying doses of TBI and assessed for CD8 TM survival. Results (Fig. 1B) demonstrated that not only were these cells readily detectable in host spleen and BM early post-conditioning and HCT, but appeared to exhibit selective survival since they demonstrated an increasing frequency amongst the CD8 T cell compartment with increasing doses of TBI. To determine if these H60-specific CD8 TM could mediate resistance to engraftment of MHC-matched allogeneic marrow differing genetically only by H60, ablatively conditioned naïve or sensitized B6 mice were transplanted with B6-H60 congenic marrow grafts. Seven days post-HCT, the level of donor (H60+) congenic (Ly 5.2) cells was 0.2% in these sensitized recipients compared to 4% in the naïve mice, indicating a 20-fold decrease in the presence of donor cells in sensitized recipients (Fig. 1C). Resistance was assessed in sensitized recipients following HCT from BALB.B that express H60 as well as several subdominant epitopes. Furthermore, splenic progenitor CFU-IL3 levels indicated that H60 sensitized recipients of congenic BM allografts contained significantly fewer colonies compared to recipients of syngeneic marrow grafts (Fig. 2B). These observations support the notion that recipients with CD8 TM specific for a single MiHA epitope mediate resistance to allogeneic donor marrow grafts.
Figure 1. Recipients containing CD8 TM specific for the single MiHA epitope resist engraftment.
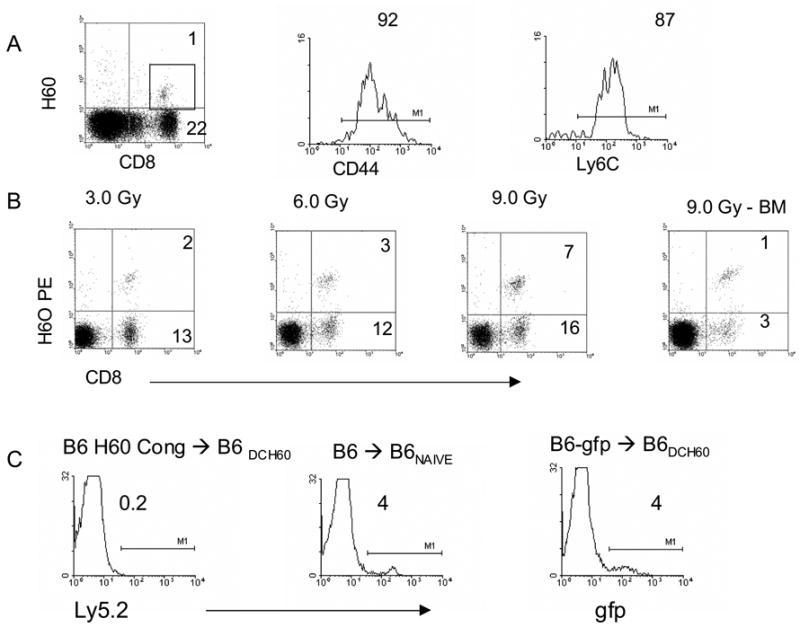
A) Generation of anti-H60 CD8 TM. B6 mice sensitized 2X with B6 marrow-derived DC pulsed with H60 were analyzed for the presence of circulating CD8+ H60+ T cells 23 days following a booster injection. Gated CD8+H60TCR+ cells were analyzed for the expression of the TM markers CD44 and Ly 6C (A, histograms). B) Sensitized mice were irradiated at the indicated doses of TBI and 1 day later transplanted with syngeneic marrow. One day post-HCT, host spleen (left 3 panels) and marrow (right) were examined for the presence of CD8+ H60TCR+ cells. Numbers in dot plot reflect % of mononuclear cells; % CD8+H60TCR+ cells in spleen = 13.3 (3.0 Gy), 20 (6.0 Gy), 30.4 (9.0 Gy; BM = 25% at 9.0 Gy). C) One week post-HCT, peripheral donor chimerism (Ly 5.2, B6 gfp) was analyzed in DC-H60 sensitized B6 (CD45.1) mice ablatively conditioned (9.0 Gy) and transplanted with B6 H60 congenic 5 × 106 TCD-BM.
Figure 2. Resistance by recipients with CD8 TM specific for a single epitope against MiHA-mismatched marrow allografts.
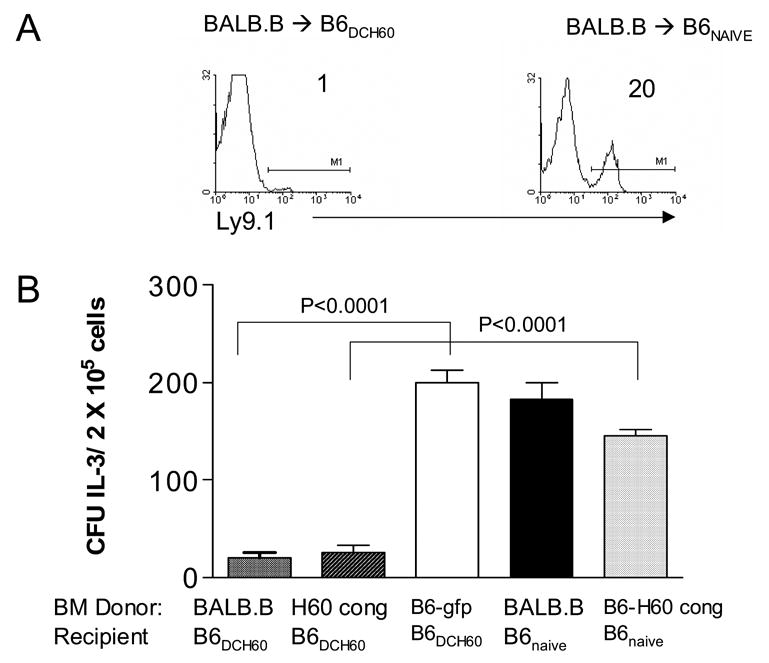
A: B6 naïve mice or mice containing CD8+H60TCR+ TM (B6DCH60) were transplanted with 5 × 106 BALB.B BMC. Peripheral blood donor chimerism (Ly 9.1) was analyzed at 1 week post-HCT. B: Recipient spleen cells were assayed for the presence of committed progenitors (CFU-IL3) one week post-HCT (2 independent experiments).
Since clinically it is unlikely that only a single MiHA disparity will be extant between donor and recipient, B6 recipients containing CD8 TM were administered transplants with marrow disparate for multiple MiHA including the H60 locus. Peripheral donor chimerism analyzed at 1 and 2 weeks post-HCT indicated that the frequency of circulating donor (Ly9.1+) cells in H60-sensitized recipients of BALB.B BM was 20X less compared to that present in naïve recipients (Fig. 2A and 3). Similar to results in recipients of B6 H60 congenic marrow allografts, there was a marked reduction in splenic CFU-IL3 levels one week post-HCT (Fig. 2B). Therefore, although BALB.B cells expressed H60 antigen as well as additional MiHA epitopes18, the response of recipient CD8 T cells to the single H60 epitope was sufficient to mediate resistance against transplanted donor MHC-matched progenitor cells. These results indicate that host CD8 TM specific for a single MiHA epitope are not only able to survive ablative conditioning and marrow transplant, but that these cells remain functional as demonstrated by their ability to resist donor allografts.
Figure 3. Resistance to MHC-matched allogeneic marrow in DC-H60 sensitized B6 mice is evident two weeks post-HCT.
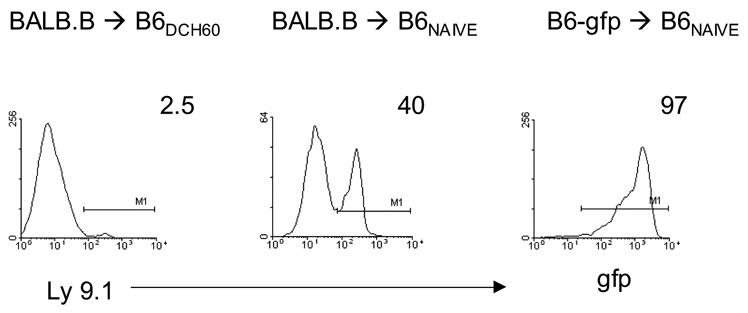
Naïve or B6 mice sensitized with marrow-derived DC pulsed with the H60 peptide were irradiated and transplanted as described in Fig. 2. Peripheral blood donor chimerism (Ly9.1+ or gfp+) was analyzed 2 weeks post-HCT. Numbers represent % of total cells gated by forward and side angle scatter from pooled mice (n = 3–4/group).
Longer term survival of circulating host CD8+H60TCR+ TM post-HCT was then examined. Sensitized mice were ablatively conditioned and administered a syngeneic HCT (Ly 5.1) to assess CD8 TM persistence in the absence of specific antigen. Two weeks post-HCT, CD8+H60TCR+ cells were clearly detectable in the PBL of these recipients (Fig. 4) and still comprised a significant percentage of the host CD8 compartment. To assess functional capacity of the surviving CD8 TM, these mice were administered a second round of conditioning (non-ablative, 5.5 Gy) and then received an allogeneic HCT (Fig. 5). Sensitized recipients of BALB.B bone marrow exhibited significantly decreased CFU-IL3 numbers compared to naïve recipients of these grafts or to sensitized mice receiving syngeneic B6-gfp marrow. These findings demonstrate that long term, antigen-specific CD8+H60TCR+ TM were not only readily detectable in the absence of antigen 2 weeks following ablative conditioning and HCT, but were capable of surviving a second round of non-ablative conditioning Moreover, these cells could also respond to antigen encounter and effect an immune response as evidenced by resistance to allogeneic hematopoietic engraftment. Thus, the present findings indicate that prior alloantigenic encounter confers considerable risk for the subsequent success of allogeneic bone marrow engraftment. First, memory cells would be typically generated, resulting in the presence of a more easily activated T cell population. Moreover, the present results monitoring antigen-specific host CD8 T cells clearly demonstrate that the memory subset effectively survives and functions at least several weeks following significant levels of conditioning. With respect to clinical HCT, the present findings suggest that prior antigenic encounters which elicit the generation of alloantigen reactive TM may complicate hematopoietic grafts dependent on the donor HLA/non-HLA disparities.
Figure 4. CD8+H60TCR+ cell presence following ablative TBI conditioning and syngeneic BMT.
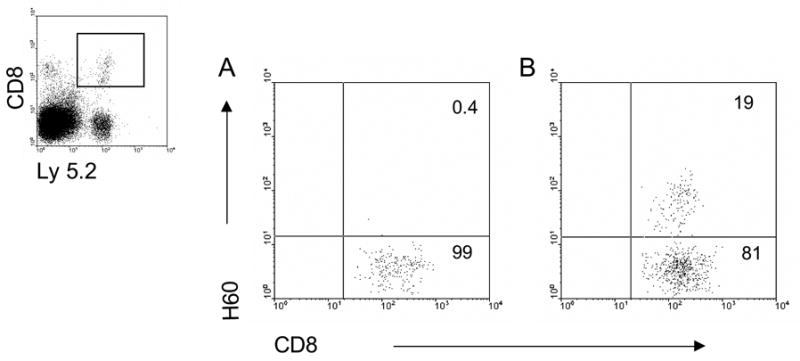
Naïve (A) or B6 mice sensitized (B) with BALB.B cells (2X) were irradiated with 9.0 Gy TBI and 24 h later transplanted with 2 × 106 syngeneic B6 Ly 5.1 BM-TCD. Fifteen days post-BMT, peripheral blood was analyzed for recipient (Ly 5.2) CD8+H60TCR+ cells. Ly 5.2+ cells were gated (inset) and analyzed for the presence of CD8 and H60 staining in naïve (A) or primed recipients (B). Numbers represent % CD8+H60TCR+ in gated cells.
Figure 5. Memory cells present in sensitized recipients following ablative conditioning and HCT are capable of mediating effector responses.
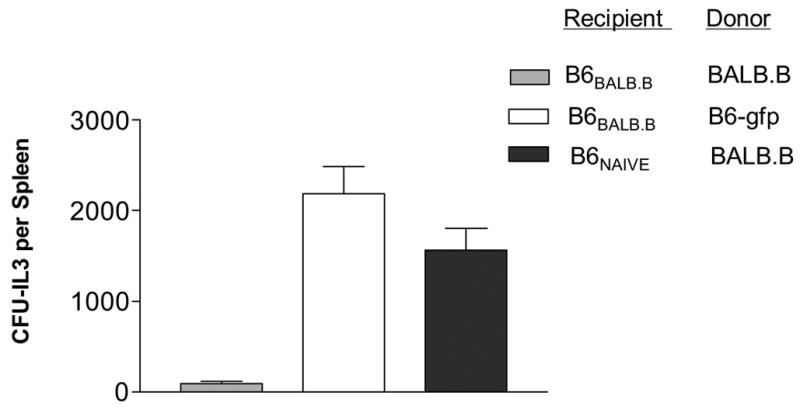
Sensitized or unsensitized B6 mice were first lethally (9.0 Gy) irradiated and 24 h later transplanted with 5 ×106 syngeneic TCD-BM. Twelve days following this first HCT, the mice were irradiated with 5.5 Gy and transplanted 1 day later with 1 × 107 BALB.B or B6-gfp TCD-BM. Ten days following this second transplant, mice were sacrificed and splenic CFU-IL3 analyzed. Legend represents donor-recipient combinations in 2nd transplant.
H60 is an unusual MiHA in that the H60 protein is a potent ligand for the activating NK receptor NKG2D22,23 along with it encoding an immunodominant CD8 T cell epitope.24 However, it is very unlikely that the observed resistance to donor allografts was a consequence of NK function of the H60 protein because our immunization procedure only utilized the immunodominant H60 CD8 T-cell peptide epitope and anti-NK1.1 mab failed to diminish resistance in B6 mice transplanted with BALB.B marrow cells.8 Notably, the present findings also highlight differences post-allogeneic HCT between HVG and GVHD. Whereas recognition of a single hematopoietic epitope by host CD8 TM is apparently sufficient to elicit resistance to MHC-matched allogeneic marrow engraftment, this same epitope is insufficient to induce clinical GVHD28 (and Roopenian, et. al. manuscript in preparation). This difference likely reflects the limited expression of H60 to hematopoietic but not GVHD target tissues such as skin, liver, and the intestinal tract.
CD8 TM to specific MiHAs can be generated as a consequence of transfusion, tissue transplantation or pregnancy and subsequently persist lifelong.9–11 Moreover, CD8 TM to immunodominant epitopes, such as H60, are readily generated as a consequence of indirect presentation,24 increasing the probability that priming for the generation of TM will occur regardless of whether sensitizing allogeneic cells were or were not matched with donor at the MHC. Finally, TM generated as the result of viral/microbial encounter expressing TCRs cross-reactive with MHC alloantigens would mediate increased resistance to allogeneic marrow engraftment via such heterologous immunity.14,27 It is therefore likely that greater donor-recipient MiHA mismatches would result in a higher probability of encountering endogenous host CD8 TM bearing anti-donor allo-specific TCRs. Survival of CD8 TM even after ablative conditioning could be a result of their increased expression of BCL-225 compared to naïve or effector cells rendering CD8 TM more resistant to apoptosis.26 Considering there are increasing numbers of reduced intensity conditioning HCT, there will likely be higher frequencies of surviving recipient CD8 TM that will constitute an even more formidable barrier to allogeneic marrow engraftment. Thus, strategies aimed at improving engraftment of MiHA-matched allogeneic marrow should consider the risks posed by recipient CD8 TM populations.
Acknowledgments
We gratefully acknowledge the NIH Tetramer Facility for producing the H60 tetramer and the Flow Cytometry Facility supported by the Sylvester Cancer Center. This work was supported by NIH grants CA120776-01 and AI46689-5A1 (RBL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raff RF, Deeg HJ, Loughran TJ, et al. Characterization of host cells involved in resistance to marrow grafts in dogs transplanted from unrelated DLA-nonidentical donors. Blood. 1986;68:861–8. [PubMed] [Google Scholar]
- 2.Murphy WJ, Kumar V, Bennett M. Acute rejection of murine bone marrow allografts by natural killer cells and T cells: differences in kinetics and target antigens recognized. J Exp Med. 1987;166:1499–550. doi: 10.1084/jem.166.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yankelevich B, Knobloch C, Nowicki M, et al. A novel cell type responsible for marrow graft rejection in mice. T cells with NK phenotype cause acute rejection of marrow grafts. J Immunol. 1989;142:3423–30. [PubMed] [Google Scholar]
- 4.Lee LA, Sergio JJ, Sykes M. Natural killer cells weakly resist engraftment of allogeneic, long-term, multilineage-repopulating hematopoietic stem cells. Transplantation. 1996;61:125–32. doi: 10.1097/00007890-199601150-00024. [DOI] [PubMed] [Google Scholar]
- 5.Kernan NA, Flomenberg N, Dupont B, et al. Graft rejection in recipients of T-cell-depleted HLA-nonidentical marrow transplants for leukemia. Identification of host-derived antidonor allocytotoxic T lymphocytes. Transplantation. 1987;43:842–7. [PubMed] [Google Scholar]
- 6.Marijt WAF, Kernan NA, Diazbarrientos T, et al. Multiple Minor Histocompatibility Antigen-Specific Cytotoxic T-Lymphocyte Clones Can be Generated During Graft-Rejection After Hla-Identical Bone-Marrow Transplantation. Bone Marrow Transplantation. 1995;16:125–32. [PubMed] [Google Scholar]
- 7.Fleischhauer K, Zino E, Mazzi B, et al. Peripheral blood stem cell allograft rejection mediated by CD4+ T lymphocytes recognizing a single mismatch at HLA-DPβ*0901. Blood. 2001;98:1122–6. doi: 10.1182/blood.v98.4.1122. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman ZF, Levy RB. MiHA Reactive CD4 and CD8 T-Cells Effect Resistance to Hematopoietic Engraftment Following Reduced Intensity Conditioning. Am J Transplant. 2006;6:2089–98. doi: 10.1111/j.1600-6143.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 9.Verdijk RM, Kloosterman A, Pool J, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–4. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 10.James E, Chai JG, Dewchand H, et al. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102:388–93. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 11.Zimring JC, Hair GA, Deshpande SS, et al. Immunization to minor histocompatibility antigens on transfused RBCs through crosspriming into recipient MHC class I pathways. Blood. 2006;107:187–9. doi: 10.1182/blood-2005-07-3059. [DOI] [PubMed] [Google Scholar]
- 12.Welsh RM, Markees TG, Woda BA, et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210–8. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams MA, Tan JT, Adams AB, et al. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J Immunol. 2001;167:4987–95. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 14.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–95. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones M, Komatsu M, Levy RB. Cytotoxically impaired transplant recipients can efficiently resist major histocompatibility complex-matched bone marrow allografts. Biol Blood Marrow Transplant. 2000;6:456–64. doi: 10.1016/s1083-8791(00)70038-3. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu M, Mammolenti M, Jones M, et al. Antigen-primed CD8+ T cells can mediate resistance, preventing allogeneic marrow engraftment in the simultaneous absence of perforin-, CD95L-, TNFR1-, and TRAIL-dependent killing. Blood. 2003;101:3991–9. doi: 10.1182/blood-2002-09-2859. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman ZF, Jones M, Shatry A, et al. Cytolytic patways utilized by effector cells derived from recipient Tnaive, Tmemory and NK cells in resistance to allogeneic hematopoietic cell transplants. Biol Blood Marrow Transplant. 2005;11:957–71. doi: 10.1016/j.bbmt.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Choi EY, Yoshimura Y, Christianson GJ, et al. Quantitative analysis of the immune response to mouse non-MHC transplantation antigens in vivo: The H60 histocompatibility antigen dominates over all others. Journal of Immunology. 2001;166:4370–9. doi: 10.4049/jimmunol.166.7.4370. [DOI] [PubMed] [Google Scholar]
- 19.Malarkannan S, Shih PP, Eden PA, et al. The Molecular and Functional Characterization of a Dominant Minor H Antigen, H60. J Immunol. 1998;161:3501–9. [PubMed] [Google Scholar]
- 20.Son YI, Egawa S, Tatsumi T, et al. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–57. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman Z, Shatry A, Deyev V, et al. Effector cells derived from host CD8 memory T cells mediate rapid resistance against MiHA-mismatched allogeneic marrow grafts without participation of perforin, FasL and the simultaneous inhibition of three TNF family effector pathways. Biol Blood Marrow Transplant. 2005;11:576–86. doi: 10.1016/j.bbmt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Diefenbach A, Jensen ER, Jamieson AM, et al. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogasawara K, Benjamin J, Takaki R, et al. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6:938–45. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi EY, Christianson GJ, Yoshimura Y, et al. Immunodominance of H60 is caused by an abnormally high precursor T cell pool directed against its unique minor histocompatibility antigen peptide. Immunity. 2002;17:593–603. doi: 10.1016/s1074-7613(02)00428-4. [DOI] [PubMed] [Google Scholar]
- 25.Grayson JM, Zajac AJ, Altman JD, et al. Cutting Edge: Increased Expression of Bcl-2 in Antigen-Specific Memory CD8+ T Cells. J Immunol. 2000;164:3950–4. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 26.Grayson JM, Harrington LE, Lanier JG, et al. Differential Sensitivity of Naive and Memory CD8+ T Cells to Apoptosis in Vivo. J Immunol. 2002;169:3760–70. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 27.Taylor DK, Neujahr D, Turka LA. Heterologous immunity and homeostatic proliferation as barriers to tolerance. Curr Opin Immunol. 2004;16:558–64. doi: 10.1016/j.coi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Berger M, Wettstein PJ, Korngold R. T cell subsets involved in lethal graft-versus-host disease directed to immunodominant minor histocompatibility antigens. Transplantation. 1994;57:1095–102. [PubMed] [Google Scholar]


