Abstract
Extraversion and neuroticism are two important and frequently studied dimensions of human personality. They describe individual differences in emotional responding that are quite stable across the adult lifespan. Neuroimaging research has begun to provide evidence that neuroticism and extraversion have specific neuroanatomical correlates within the cerebral cortex and amygdala of young adults. However, these brain areas undergo alterations in size with aging, which may influence the nature of these personality factor-brain structure associations in the elderly. One study in the elderly demonstrated associations between perisylvian cortex structure and measures of self transcendence (Kaasinen et al., 2005), but the neuroanatomical correlates of extraversion and neuroticism, or other measures of the Five Factor Model of personality have not been explored. The purpose of the present study was to investigate the structural correlates of neuroticism and extraversion in healthy elderly subjects (n=29) using neuroanatomic measures of the cerebral cortex and amygdala. We observed that the thickness of specific lateral prefrontal cortex (PFC) regions, but not amygdala volume, correlates with measures of extraversion and neuroticism. The results suggest differences in the regional neuroanatomic correlates of specific personality traits with aging. We speculate that this relates to the influences of age-related structural changes in the PFC.
Keywords: amygdala, emotion, aging, magnetic resonance imaging, personality, prefrontal cortex
Introduction
Personality characteristics describe distinctive and recurrent patterns of thoughts, feelings and actions that occur in response to particular situational demands (Mischel, 2004). The Five Factor Model (FFM) is an influential model of human personality, reflecting several decades of psychological research (John, 1990; McCrae and Costa, 1990; McCrae and John, 1992; Costa and McCrae, 2000). This model provides a convenient method for summarizing a wide range of human behaviors and describes personality along the dimensions of extraversion, neuroticism, conscientiousness, agreeableness, and openness to experience. These factors are notably stable across the life span from early adulthood well into late-life, with no change or small decreases in neuroticism, extraversion, and openness; and variable small changes in agreeableness and conscientiousness primarily between college age and middle adulthood (Costa and McCrae, 1977, 1986, 1997; McCrae et al., 1999; Costa et al., 2000).
Two of the dimensions, extraversion and neuroticism, are of particular interest since their brain bases might contribute to mood and anxiety disorder predisposition throughout the lifespan (Rosenbaum et al., 1991; Watson and Clark, 1992; Costa and McCrae, 1996; Biederman et al., 2001; Widiger and Seidlitz, 2002; Duberstein et al., 2003; Khan et al., 2005). The introversion/extraversion factor captures the social dimension of personality. Extraverts have a preference for seeking and engaging in social interactions, whereas introverts prefer to avoid social situations and tend to be reserved or socially awkward. Extraversion may also be characterized as a sensitivity to positive or pleasure cues in the environment (Larsen and Ketelaar, 1991; McCrae and Costa, 1991; Costa and McCrae, 1992; Watson and Clark, 1992; Izard et al., 1993; Barrett and Pietromonaco, 1997; Lucas et al., 2000; Lucas and Diener, 2001; Pickering and Gray, 2001).
Neuroticism is characterized as a pervasive sensitivity to negative or punishment cues in the environment, like those present in social situations (Bolger and Schilling, 1991; Larsen and Ketelaar, 1991; McCrae and Costa, 1991; Costa and McCrae, 1992; Watson and Clark, 1992; Izard et al., 1993; Bolger and Zuckerman, 1995; Pickering and Gray, 2001). Individuals high in neuroticism also tend to automatically orient to novel situational cues (Wallace et al., 1991; Wallace and Newman, 1997, 1998) and more readily assess situations as threatening than those who are low in neuroticism (Schneider, 2004).
Studies using magnetic resonance imaging (MRI) combined with different quantitative techniques for structural analysis have begun to map the neuroanatomic relationships of extraversion and neuroticism in healthy young adults (Omura et al., 2005; Rauch et al., 2005; Wright et al., 2006b). These studies primarily point to associations between specific regions of the PFC - including orbitofrontal and dorsolateral regions - or amygdala, and extraversion and neuroticism. This is consistent with earlier functional imaging studies showing associations between extraversion, neuroticism or related measures and the activities of the PFC and amygdala during resting states (Johnson et al., 1999), or in response to specific activation procedures (Canli et al., 2001; Canli et al., 2002; Zald et al., 2002; Gusnard et al., 2003; Schwartz et al., 2003; Canli et al., 2004; Gray et al., 2005). However, the structure (and function) of the PFC and amygdala are altered with aging, and it remains uncertain how this effect may influence the relationships between these structures with extraversion and neuroticism. Specifically, lateral regions of the PFC diminish in size with age, while those on the medial and inferior surfaces are stable or increase across the adult life span (Salat et al., 2001; Salat et al., 2004; Grieve et al., 2005). These anatomic changes in the PFC may respectively relate to diminished executive functioning and working memory (Raz et al., 1999), but enhanced emotional control with aging (Carstensen et al., 2000). The amygdala is also known to exhibit mild atrophy with aging, and functional imaging studies suggest an altered profile of activation in this structure in young vs. elderly subjects (Iidaka et al., 2002; Gunning-Dixon et al., 2003; Mather et al., 2004; Fischer et al., 2005; Tessitore et al., 2005; Wedig et al., 2005; Wright et al., 2006a).
The main purpose of the present study is to examine the structural correlates of neuroticism and extraversion in healthy elderly subjects, given the known structural and functional changes in the PFC and amygdala with aging. As in earlier work in healthy young subjects (Wright et al., 2006b), we employed two quantitative techniques to analyze high-resolution MRI data for anatomic correlates of extraversion and neuroticism. PFC thickness was studied using the technique of cortical surface analysis (Fischl and Dale, 2000; Salat et al., 2004; Rauch et al., 2005; Wright et al., 2006b), and amygdala volume was measured using manual anatomic methods (Wedig et al., 2005; Wright et al., 2006a; Wright et al., 2006b).
Materials and Methods
Subjects
29 right-handed elderly subjects (17 female, 12 male) were studied (age in years: m=70.3, sd=6.6, range=61–84). All subjects underwent cortical thickness analyses and anatomic region of interest analysis of the amygdala (see below). Subjects were recruited from the local area via word of mouth and using advertisements. Written informed consent was obtained from each subject. The study was approved and conducted in accordance with guidelines established by the Partners Human Research Committee. Routine screening assessments were used to rule out the presence of neurological, medical, psychiatric conditions (including personality disorders), and contraindications to MRI. All subjects also underwent the Structured Clinical Interview for the Diagnostic and Statistical Manual 4th edition (DSM-IV) (American Psychiatric Association, 1994) to confirm the absence of DSM-IV Axis I diagnoses (First et al., 1995), including the absence of dementia, and were required to be free of psychoactive medications. All subjects performed normally (m=29.4, sd=0.9, range 27–30) on the 30-point Mini-Mental State Examination (Folstein et al., 1975), which was used as an additional screen for cognitive disorders.
All subjects completed the NEO Five Factor Inventory (Costa and McCrae, 1992) (Table 1). Extraversion and neuroticism T-scores were calculated from gender specific normative data to control for effects of gender on the T-score. These T scores were used as the main variable of interest. As in our previous study we also calculated T-scores for openness, agreeableness and conscientiousness and used these in some analyses as described below. All analyses were confirmed using raw scores unless otherwise stated.
TABLE 1.
Personality Self-Report Group Characteristics
| T-score | Extraversion | Neuroticism | Openness | Agreeableness | Conscientiousness |
|---|---|---|---|---|---|
| Mean | 53.0 | 40.9 | 54.9 | 55.5 | 49.7 |
| S.D. | 10.9 | 7.3 | 9.5 | 7.8 | 10.2 |
| Range | 31–74 | 25–63 | 34–72 | 36–70 | 27–71 |
Imaging
All subjects underwent structural brain imaging using a Sonata 1.5 Tesla whole body imaging device (Siemens Medical Systems, Iselin NJ) with a 3-axis gradient head coil. Head movement was restricted using expandable foam cushions. After an automated scout image was acquired, two high-resolution 3D MPRAGE sequences (TR/TE/flip angle=7.25ms/3ms/7°) with an in-plane resolution of 1.3 mm, and 1 mm slice thickness were collected. The acquisition parameters were empirically optimized to increase gray/white and gray/cerebrospinal fluid contrast.
Cortical Thickness Measurement
These methods have been previously described in detail (Fischl and Dale, 2000; Rauch et al., 2005; Wright et al., 2006b), including in elderly subjects (Salat et al., 2004). Briefly, the two high-resolution structural scans for each participant were motion corrected and averaged to create a single average volume with a high signal-to-noise ratio. The resulting volume was used to segment cerebral white matter (Dale et al., 1999) and to estimate the gray/white interface. Topological defects in the gray/white estimate were corrected (Fischl et al., 2001), and this gray/white estimate was used as the starting point for a deformable surface algorithm designed to find the pial surface with sub-millimeter precision (Fischl and Dale, 2000). The entire cortical surface in each individual subject was then visually inspected and any inaccuracies in segmentation were manually corrected.
Cortical thickness measurements were obtained by reconstructing representations of the gray/white matter boundary and the pial surface, and then calculating the distance between those surfaces at each of approximately 320,000 points across the entire cortical mantle ((Dale et al., 1999; Fischl and Dale, 2000); software and complete documentation is available at http://www.nmr.mgh.harvard.edu/freesurfer). The accuracy of the thickness measures derived from this technique has been previously validated by direct comparisons with manual measures on post-mortem brains (Rosas et al., 2002), on MRI data (Kuperberg et al., 2003), and in elderly subjects (Salat et al., 2004). The surface representing the gray–white border was “inflated” (Fischl et al., 1999a), differences among individuals in the depth of gyri–sulci were normalized, and each subject’s reconstructed brain was then morphed and registered to an average spherical surface representation that optimally aligned sulcal and gyral features across subjects (Fischl et al., 1999a; Fischl et al., 1999b). This spherical morphing procedure was used to construct the cortical thickness correlation brain maps. Thickness measures were then mapped on the ‘inflated’ surface of each participant’s reconstructed brain (Fischl et al., 1999a). The data were smoothed on the surface tessellation using an iterative nearest-neighbor averaging procedure. Fifty iterations were applied, which is approximately equivalent to applying a two-dimensional Gaussian smoothing kernel along the cortical surface with a full-width/half-maximum of 13 mm. Data were then resampled for participants into a common spherical coordinate system (Fischl et al., 1999b). Data were also linearly transformed so that approximate Talairach coordinates (Talairach and Tournoux, 1988) could be derived at each point on the surface (Fischl et al., 2002) to allow comparisons with other studies.
Statistical analysis
Statistical surface maps were generated by computing two separate general linear models (GLMs) for the effects of neuroticism or extraversion T-scores on cortical thickness at each point. Similar maps were also generated for openness, agreeableness and conscientiousness T-scores. All analyses included the covariates of gender and age, which were entered in the model as external regressors to control for their effects. Parallel analyses were performed for raw scores. Based on our previous study in young adults (Wright et al., 2006b), our cortical a priori regions of interest included parts of the lateral and inferior prefrontal cortex. Based on the earlier findings, the degree of smoothing applied in the current study, and the combined surface areas of these regions of interest, we selected a statistical significance threshold of p < .001 (two-tailed), as a correction for multiple comparisons within a priori search territory. For all areas outside of the this territory cortex a threshold of p < .0001 (two-tailed).
Once sites with significant effects were identified as described above, a region of interest (ROI) based approach was used. For these analyses, thickness measures for each subject were obtained at the location corresponding to the coordinates of the peak p-value on the group maps (see Figs. 1–3). These thickness measures were then plotted against individual subject T-scores on a scattergram and fitted with a regression line. Partial correlation coefficients (r values) controlling for age and sex, and the associated p-values were then calculated. This ROI based approach was used primarily to display individual subject data points and obtain correlation coefficients. Because of concerns about collinearity influencing the partial correlation results, we also analyzed the data in a multiple linear regression model with age and sex entered as nuisance regressors. A separate model was utilized for each of the two main behavioral variables (extraversion and neuroticism), asking the question, which of the regions identified were the best predictors of these behavioral measures? To test for significant differences in the laterality of our findings, a similar ROI based approach was used whereby matching points in the left and right hemisphere were compared by calculating the r values at each site, using a Fisher r-to-z transformation and then examining for significant hemispheric differences using the test of differences (Cohen and Cohen, 1983).
Figure 1. Significant Correlations of Cortical Thickness and Extraversion in the Lateral Prefrontal Cortex.
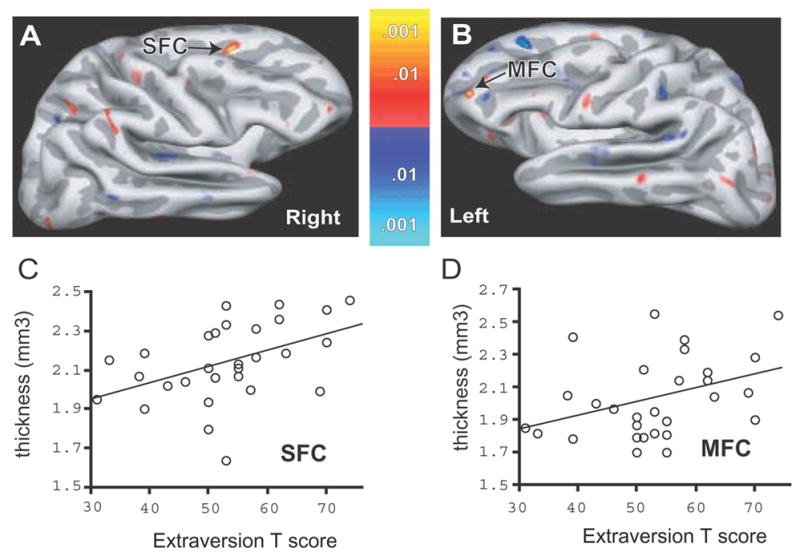
(A) Colorized statistical map superimposed upon a partially inflated group average cortical surface. The lateral aspect the right hemisphere is shown. A significant positive correlation of cortical thickness with extraversion was found in the superior frontal cortex (SFC). (B) Colorized statistical map superimposed upon a partially inflated group average cortical surface. The lateral aspect the left hemisphere is shown with a significant positive correlation between cortical thickness and extraversion in the anterior parts of the middle frontal cortex (MFC). Dark gray regions are sulci, light gray are gyri. Colorized scale bars show the p-value for positive (red-yellow) and negative (blue) correlations. (C) Scatter plot and regression line demonstrating a significant positive correlation between right SFC thickness and extraversion. These values were extracted from the peak surface point of the SFC shown in (A). (D) Scatter plot and regression line from the same MFG site in (B).
Figure 3. Significant Correlations of Cortical Thickness and Openness in the Parietal Cortex.

(A) Colorized statistical map superimposed upon a partially inflated group average cortical surface. The lateral aspect the left hemisphere is shown. Significant inverse correlations of cortical thickness with openness were found in the inferior parietal cortex (IPC). Dark gray regions are sulci, light gray are gyri. Colorized scale bars show the p-value for positive (red-yellow) and negative (blue) correlations. (B) Scatter plot and regression line demonstrating a significant inverse correlation between IPC thickness and neuroticism. These values were extracted from the peak surface point of the IPC shown in (A).
Cortical Nomenclature
For consistency in comparison to our earlier study we use descriptive anatomic terminology to indicate the location of our significant effects. For example, in the lateral prefrontal cortex, we refer to the superior frontal cortex (e.g. the superior frontal gyrus and adjacent sulci), the middle frontal cortex (e.g. the middle frontal gryus and adjacent sulci) and the inferior frontal cortex (e.g. the inferior frontal gyrus and adjacent sulci). This terminology also allows clearer designation of effects in gryal and sulcal portions of cortex. Approximate Brodmann areas and Talairach coordinates are also included for each site of peak effect to allow for comparisons with other studies.
Amygdala Volumetric Analysis and Correlations
The amygdala was manually traced on each individual subjects’ motion corrected, averaged high-resolution 3D image (e.g. see Fig 4A,B). A technician who was blind to the results of the individual personality measures performed tracings. As amygdala volumes are known to vary with head size, we performed our analyses using an amygdala volume corrected for estimated intracranial volume. An automated atlas-based method that has been validated against manual intracranial volume measures (Buckner et al., 2004) was used to calculate estimated total intracranial volume (eTIV). For correction of the effects of head size, amygdala volumes were divided by eTIV then multiplied by 100%. The relationship of these corrected volumes to extraversion and neuroticism was then assessed using partial correlations with age and gender as covariates. Detailed descriptions about the tracing and eTIV procedures, and their reliability, have been presented in earlier studies (Wedig et al., 2005; Wright et al., 2006a; Wright et al., 2006b). For the amygdala-personality factor correlations, a statistical threshold of p < 0.025 was used to correct for examining the right and left amygdala.
Analyses of the other personality factors
Though not the primary purpose of this study we also examined the 3 other factors of the FFM (openness, agreeableness and conscientiousness) using both cortical surface based and amygdala volumetric analyses. We use the same methodology for these analyses as for neuroticisms and extraversion as described above. For cortical surface analyses we applied the non a priori threshold of p<.0001. For the amygdala, we used a threshold of p < .01 as an approximate correction for the number of comparisons that the analyses of these three factors entailed.
Results
Correlations with Extraversion and Cerebral Cortex Thickness
In the right hemisphere, surface-based analyses revealed a significant positive correlation between cortical thickness and extraversion T-scores in the superior frontal cortex (SFC, Fig 1, Table 2). Scatter plots based on ROI analysis at this peak confirmed the significant positive relationship between right superior frontal cortex thickness and extraversion (Fig. 1). Neuroticism scores were not significantly correlated with cortical thickness at this site (Table 2). In the left hemisphere, a significant positive correlation was found between extraversion and the thickness of the middle frontal cortex (MFC, Fig. 1, Table 2). Scatter plots confirmed the relationship between the MFC thickness and extraversion (Fig. 1). Neuroticism scores were not significantly correlated with cortical thickness at this site (Table 2).
TABLE 2.
Brain regions showing significant correlations with cortical thickness, extraversion, and neuroticism are listed and correspond to the sites shown in Figs. 1 and 2. The approximate Brodmann area designations are based on the Talairach coordinates of the peak point on the cortical surface maps. The Talairach X-coordinate indicates left-right, the Y-coordinate anterior-posterior, and the Z-coordinate superior-inferior. R values are for partial correlation coefficients controlling for age and sex.
| Brain Region (Approx. Brodmann Area) | Hemisphere | Talairach Coordinates | Extraversion | Neuroticism | ||
|---|---|---|---|---|---|---|
| X | Y | Z | R value | R value | ||
| Prefrontal Cortex | ||||||
| Extraversion | ||||||
| Superior Frontal Cortex s (A6) | Right | 20 | 12 | 41 | .643* | −.294 |
| Middle Frontal Cortex (A10) | Left | −38 | 56 | 12 | .638* | −.388 |
| Neuroticism | ||||||
| Superior Frontal Cortex g (A6) | Right | 17 | 15 | 44 | .363 | −.605* |
| Inferior Frontal Cortex (A44) | Right | 37 | 21 | 24 | .190 | −.607* |
| Other Regions: | ||||||
| Neuroticism | ||||||
| Anterior Temporal Cortex (A38) | Right | 48 | 15 | −6 | −.373 | .678** |
Statistical significance is as follows:
p < .001,
p < .0001. Note that for the sites where there were significant correlations with extraversion, there were weaker non-significant correlations in the opposite direction with neuroticism. For sites where neuroticism was significantly correlated with cortical thickness there were weaker non-significant correlations in the opposite direction with extraversion. The right superior frontal cortex finding in extraversion was located in the sulcus (s), but located in the gyrus (g) for neuroticism.
No region outside of the prefrontal cortex displayed statistically significant relationships between thickness and extraversion.
We compared the results from the right SFC with those from the corresponding point in the left SFC there was a statistically significant hemispheric difference between the correlations (right vs. left: r=.643 vs. r=.007, p=.007). Comparison of the results from the left MFC with those from the corresponding point in right MFC also indicated a statistically significant difference between the correlations (left vs. right: r=.638 vs. r=.079, p=.0033).
Correlations with Neuroticism and Cerebral Cortex Thickness
Surface bases analyses of the right hemisphere indicated significant inverse correlations between cortical thickness and neuroticism T-scores in the right superior and inferior frontal cortices (SFC, IFC; Fig. 2; Table 2). Scatter plots confirmed the significant negative correlation between neuroticism and cortical thickness in these regions (Fig. 2), but demonstrated no significant relationship with extraversion (Table 2). Of note, the right SFC site that was inversely correlated with neuroticism was located in gyral cortex and exhibited a modest but non-significant positive correlation with extraversion (Table 2). This site was in very close approximation to the right sulcal SFC region that was significantly positively associated with extraversion. When the correlation with neuroticism at identical points in the right and left SFC and IFC were compared, a statistically significant difference between hemispheres was present (right vs. left SFC: r=−.605 vs. r=−.061, p=.021; right vs. left IFC: r=−.607 vs. r=.051, p=.0014).
Figure 2. Significant Correlations of Cortical Thickness and Neuroticism in the Right Hemisphere.
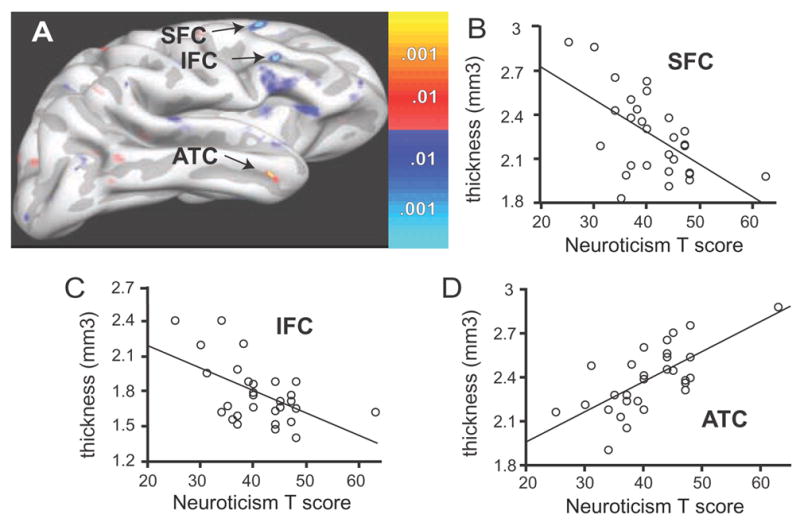
(A) Colorized statistical map superimposed upon a partially inflated group average cortical surface. The lateral aspect the right hemisphere is shown. Significant inverse correlations of cortical thickness with neuroticism were found in the superior and inferior frontal cortex (SFC, IFC). A significant positive correlation between neuroticism and cortical thickness was found in the anterior temporal cortex (ATC). Dark gray regions are sulci, light gray are gyri. Colorized scale bars show the p-value for positive (red-yellow) and negative (blue) correlations. (B) Scatter plot and regression line demonstrating a significant inverse correlation between SFC thickness and neuroticism. These values were extracted from the peak surface point of the SFC locus shown in (A). (C) Scatter plot and regression line from the peak of the IFC locus in (A). (D) Scatter plot and regression line from peak of the ATC locus in (A).
One area in the right hemisphere outside of the PFC met the significance threshold for non a priori regions. This was in the mid-portion of the anterior temporal cortex (ATC), where thickness was positively correlated with neuroticism, but not extraversion, scores (Fig. 2; Table 2). When the correlations at identical points in the right and left were compared, a statistically significant difference was present (right vs. left ATC: r=−.678 vs. r=.062, p=.0014).
No regions of the left hemisphere demonstrated significant correlations between cortical thickness and neuroticism.
Multiple Linear Regression Models of Cortical Thickness, Extraversion and Neuroticism
For the first model, extraversion was entered as the dependent variable, with right SFC and left MFC thickness as the independent variables. The analysis was performed in a stepwise forward multiple linear regression model (p to enter model <.05). Age and sex were forced into the model a nuisance regressors (covariates). Result of this model indicated that both regions were significant predictors (overall model, F=11.6 p<.0005). For the second model, neuroticism was entered as the dependent variable, and analyses were performed similar fashion as for extraversion except that the independent variables were right SFC (gyral portion), right IFC, and right ATC. In this case only two of the regions entered in the model were significant predictors of neuroticism: right ATC and IFC (overall model, F=9.13 p<.0005). The right inferior region did not enter the model because it was strongly correlated with the right SFC (r=.611, p <.0005).
Absence of Correlations with Extraversion, Neuroticism and Amygdala Volume
No significant basic correlations between right or left amygdala volume and extraversion or neuroticism (T or raw scores) were found (all |r|<.2, p>.4). Similar results were obtained when partial correlations adjusting for the effects of gender and age were utilized (|r|<.3, p >.2) and when males and females were analyzed separately.
Anatomic Relationships of the Other NEO Factors: Openness, Agreeableness, and Conscientiousness
A significant negative correlation between the thickness of the right inferior parietal cortex (angular portion) and openness was found (r=−.706; p=.000038; Talairach x=43, y=−74, z=12) (Fig 3). This effect was selective for the right hemisphere (R vs L, r=−.706 vs. −.122; p=.0064), and none of the other personality factors were correlated with cortical thickness at this site (all |r| < .3; p>.1). No significant associations were observed between agreeableness or conscientiousness and cortical thickness. In the left amygdala, there was a trend positive association between agreeableness raw scores and left amygdala volume (r=.48, p=.012; partial correlation corrected for gender and age). Otherwise no significant correlations were found between openness, agreeableness, or conscientiousness (T or raw scores) and cortical thickness or amygdala volumes.
Discussion
The current results demonstrate that specific subregions of the right and left lateral PFC are selectively associated with extraversion and neuroticism in elderly individuals. Specifically, increased thickness of the right SFC and left MFC was selectively associated with greater levels of extraversion in the elderly, while decreased thickness in the right SFC and right IFC was selectively associated with greater levels of neuroticism. In one area outside of the PFC - the right ATC – neuroticism, but not extraversion, was significantly positively associated with thickness. Multiple linear regression modeling confirmed that thickness of the above noted regions were predictive of extraversion and neuroticism even when accounting for the effects of age and gender. Of note, although the regions were selected on the basis of extraversion or neuroticism associations with cortical thickness, there were often modest correlations in the opposite direction for the factor not utilized in the initial region identification process. This effect was particularly of note in the right SFC since in the sulcal region there was a significant positive correlation with extraversion with non-significant effects for neuroticism in the opposite direction. In the closely adjacent gyral portion of the right SFC the neuroticism effects demonstrated statistical significance while weaker effects in the opposite direction were present for extraversion. Although these two factors are considered independent according to the five-factor model of personality, they are often inversely associated. These results suggest that the cortical regions identified represent certain aspects of neuroticism and extraversion that are divergent but related. For example, the tendency to experience positive emotion found with extraversion may be paired with the tendency to experience low levels of negative emotion as found with emotional stability (the inverse of neuroticism).
Structural Correlates of Extraversion Across the Age Range
Two core aspects of extraversion are positive affect and a tendency to seek out and participate in social situations (Lucas and Diener, 2001; Lucas and Baird, 2004). Both these aspects of extraversion are related to hemispheric asymmetries in the PFC, as lesser right vs. left-sided anterior electrophysiological activity is associated with positive affect and approach behaviors (Tomarken et al., 1990; Tomarken et al., 1992; Davidson, 2004b, 2004a). A metabolic study in young adults supports this notion, demonstrating that greater extraversion is associated with lower resting activity in the right PFC (Johnson et al., 1999). Likewise, structural studies in young adults indicate that specific prefrontal cortical regions in extraverts are smaller in the right vs. the left hemisphere (Omura et al., 2005; Wright et al., 2006b). In contrast with those findings in young adults, the present study demonstrates that extraversion in elderly adults is associated with greater cortical thickness in specific regions of both the right and left PFC. Of note, the specific PFC subregions related to extraversion in the elderly were located more superiorly and anteriorly compared to those in the young. Interestingly, inferior and posterior regions of the bilateral PFC demonstrate significant thinning with increasing age in adults (Salat et al., 2004). Taken together, these findings suggest that the neural correlates of extraversion in the prefrontal cortex are altered with aging, perhaps related to atrophy in PFC regions that subserve extraversion in youth. We speculate that, as in young adults, the extraversion-associated PFC regions in the elderly may also be important for regulation of social contacts (i.e. disinhibiting social contacts extraverts, but inhibiting them in intraverts) (Bone and Montgomery, 1970; Paisey and Mangan, 1980; Izard et al., 1993; Wright et al., 2006b), but that age-related anatomic changes, life-time experiences or both alter the direction and laterality of the exact structure-function relationships.
Neuroticism, Cortical Thickness and Aging
An inverse relationship between neuroticism and left medial orbitofrontal cortex thickness was previously reported in healthy young subjects (Rauch et al., 2005; Wright et al., 2006b). Existing evidence indicates that there is extensive individual variation in OFC, particularly in more anterior areas where complex, abstract, or secondary reinforcers are represented (Kringelback & Rolls, 2004). Furthermore, the structural integrity of the OFC is important for the reappraisal of the affective or emotional significance of stimuli. Damage to the OFC in human and other primates impairs learning and reversal of stimulus-reinforcement associations, as well as impairing extinction to conditioned stimuli (Rolls et al., 1994; Rolls, 2004). However, our initial finding in young adults was not reproduced in the current cohort of elderly subjects. Instead we found that there were inverse relationships present between neuroticism and the thickness of specific regions of the right lateral PFC. Of note, the thickness of the medial OFC may increase with age (Salat et al., 2001; Salat et al., 2004; Grieve et al., 2005). Furthermore, research suggests that aging leads to decreased cognitive flexibility due to impaired reversal learning in the OFC (Schoenbaum et al., 2006). This is particularly interesting given that excessive sensitivity to negative cues in the environment is a core feature of neuroticism (Bolger and Schilling, 1991; Mroczek and Almeida, 2004). Taken together, these studies suggest that, as in the case for the lateral PFC and extraversion, age-related OFC structural changes may have led to a shift or change in the cortical thickness-personality relationships we observed. This could be due to age-related (as opposed to personality-related) functional and structural changes in these cortical regions interfering with the ability to detect these relationships. Alternatively, in the setting of the general preservation of personality traits that occurs with aging (Costa and McCrae, 1977, 1986, 1997; McCrae et al., 1999; Costa et al., 2000), age-related dysfunction in the OFC may lead to the compensatory involvement of other cortical areas. Another possibility is that the small decreases in neuroticism that may occur with aging (Costa et al., 1986; McCrae et al., 1999; Costa et al., 2000) result in a change in the underlying structural mechanisms supporting this specific personality factor, thus leading to a weaker relationship between neuroticism scores and OFC thickness. Conversely, the structural changes that occur with aging could lead to decreases in neuroticism levels.
Although OFC thickness was not significantly correlated with neuroticism, there were significant negative correlations with right SFC and IFC thickness, as well as significant positive correlations with right ATC thickness. There is very limited literature on relationships between neuroticism and the structure and function of the right lateral PFC or right ATC with aging. Work in young adults suggests that facets of neuroticism are associated with low levels of serotonin receptor binding in the dorsolateral PFC (Tauscher et al., 2001), and regions IFC have been implicated in self-regulatory control (Marsh et al., 2006) and decoding emotional intonation (Wildgruber et al., 2005). Thus, both neurochemical and functional alterations potentially relevant for neuroticism have been described in the lateral PFC, but additional work is needed to understand how these findings relate to the structural effects reported in the current study. With respect to the right ATC, a recent study suggests that damage to this region leads to decreased memory for unpleasant life events (Buchanan et al., 2006). On this basis we speculate that the positive correlations we observed between neuroticism and right ATC thickness could relate to an increased tendency to recall negative life events (under the assumption that increased thickness underlies functional enhancement). This notion, which also requires additional investigation, is consistent with the observation that those with high levels of neuroticism have a greater sensitivity for recalling events as negative (Schneider, 2004).
Openness, Self Transcendence and the Aging Brain
We are aware of only one earlier study reporting that brain structural differences in the elderly are associated with measures of personality. That study demonstrated grey matter voxel density changes in bilateral perisylvian temporal, parietal, and frontal cortices relate to measures of self transcendence (Kaasinen et al., 2005) using the Temperament and Character Inventory (TCI). However, the present study demonstrates selective structural correlates (i.e. cortical thickness) of personality using the Five Factor Model.
Self-transcendence is one of the three character traits of the TCI. It is characterized by individual openness to unusual and divergent feelings, thoughts, and behaviors, and as such may be most closely related to the openness measure of the FFM. The finding in the present study that openness is related to the thickness of the angular gyrus in the right inferior lateral parietal cortex potentially overlaps anatomically with the region identified in the self-transcendence study. However, although the anatomic localization of these findings was similar, the direction of the effects appears discordant at first glance. We observed an inverse relationship between openness and cortical thickness, but a positive relationship between self-transcendence and cortical gray matter density was described in the other study. It is uncertain how these two structural measures relate to each other, and these apparently discrepant findings may be complementary. For example, decreased cortical thickness could lead to local morphological or structural MRI signal intensity changes that increase the likelihood that voxel-based morphometry (VBM) will assign these voxels as gray matter. In this case, the openness results of this study and the self-transcendence results (Kaasinen et al., 2005) may indicate related underlying structural effects.
Amygdala Structural Metrics and Personality
The structure and function of the amygdala have been associated with measures of extraversion and neutroticism (Johnson et al., 1999; Canli et al., 2002; Omura et al., 2005) as well as with uninhibited and inhibited temperaments (Schwartz et al., 2003). However, we did not find any significant relationship between amygdala volume and these two personality factors in the current study of elderly individuals, or in an earlier study on young subjects (Wright et al., 2006b). There are several potential reasons for the apparent lack of a link between extraversion, neuroticism, and amygdala volumes in the current study. For example, whole amygdala volumes measurements may obscure variations at the level of individual subnuclei. Future studies examining the volume of specific amygdala subregions with very high resolution MRI will be of interest and should help to understand discrepancies between techniques utilizing MRI gray matter voxel density versus amygdala volume.
Potential Limitations, Future Directions, and Clinical Implications
Although the participants performed well on the MMSE and were well characterized from the psychiatric perspective using a structured clinical interview, more extensive cognitive evaluations or confirmatory informant interviews were not performed. This leaves open the possibility that some subjects may have had mild cognitive impairment and that this could have contributed to the findings. The main focus of this initial study was to determine if structural correlates of neuroticism and extraversion exist in the elderly, given the anatomic variability that accompanies aging. The current results support the existence of brain structure-personality correlates in the elderly, and suggest differences in these correlates in the young and elderly. However, the current elderly cohort was not prospectively matched for the personality measures of interest with the young subjects from our earlier study (Wright et al., 2006b), and as such we could not correctly perform direct between groups comparisons. Thus, our results may relate to cohort-specific differences in the distribution of personality measures between the two different cohorts. Future works in young, middle aged, and elderly adults with matching personality characteristics should examine structural (and functional) measures to directly explore the effects of aging on the neural correlates of personality.
Even matching personality measures between groups does not exclude the possibility of cohort effects in studies using cross-sectional design. As with most neuroimaging studies, this current study is cross-sectional in nature. Therefore, the results cannot be used to determine if the cortex structure-personality associations in the elderly reflect changes during a particular individual’s lifetime. Longitudinal studies are required to conclusively investigate aging effects on these structure-personality relations. Furthermore, ultra-high resolution structural MRI, postmortem studies, or both are needed to unravel the possible mechanisms of the anatomic effects we describe. These techniques may address important issues such as whether personality factor-brain structure associations relate to changes in specific cortical layers; in the cellular or subcellular composition of the cortex; or in the subnulcear or subregional structures of subcortical areas (e.g. amygdala).
One implication of having different cellular sources as the underlying cause of an anatomic size difference (e.g. in thickness or volume) is that the consequences for a size difference are not invariant. For example, specific subregions of the frontal lobe may be related to social approach or avoidance. In such a case enhancement or decrement of frontal lobe functions could lead to greater approach behaviors (via diminished inhibitory systems) in extraverts relative to introverts. As our approach at this point is purely anatomic, we cannot determine whether thinning (or thickening) might related to enhance or diminished cortical functioning. Furthermore, thinning due to loss of inhibitory interneurons, might enhance cortical function and activity (via disinhibition); alternatively, thinning due to decreased local cortical connectivity or neuron loss might lead to decreased cortical functioning. On this basis specific predications about the consequences of the direction of the thickness-personality measure relationship must be interpreted with caution. Additional studies assessing structure-function relationships and examining the microstructural contributors to cortical thickness variation are necessary to further address these functional meaning of the personality-structural metric associations we describe.
Finally, extraversion and neuroticism may play an important role in the development and expression of late-life psychiatric disorders in the elderly (Burvill et al., 1991; Henderson and Kay, 1997; Lyness et al., 1998; Seidlitz et al., 2001), and regions of the PFC, parietal and temporal cortices exhibit structural and functional changes in geriatric depression and psychosis (Kumar et al., 1997; Awata et al., 1998; Pantel et al., 1998; Ashtari et al., 1999; Kim et al., 1999; Thomas et al., 2003; Ballmaier et al., 2004; Aizenstein et al., 2005). Going forward it will be of clinical interest to investigate how the anatomical changes associated with specific personality factors in aging relate to the propensity for late-life neuropsychiatric syndromes. However, conceivably, it is only the abnormal representation of traits that might predispose to specific psychiatric disorders. In this regard it may be particularly important to focus on the extremes of personality for such studies.
Acknowledgments
The authors wish to thank Bruce Fischl, Mary Foley, Katherine McMullin, Brian Quinn, and Larry White for technical assistance. This work was supported in part by NIMH grant K23MH64806 and NIA grant R01AG030311 (Dr. Wright), as well as resource grants to the Martinos Center for Biomedical Imaging from the NCRR (P41-RR14075), and the Mental Illness and Neuroscience Discovery (MIND) Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF, 3rd, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58:290–296. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S. Hippocampal/amygdala volumes in geriatric depression. Psychol Med. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- Awata S, Ito H, Konno M, Ono S, Kawashima R, Fukuda H, Sato M. Regional cerebral blood flow abnormalities in late-life depression: Relation to refractoriness and chronification. Psychiatry Clin Neurosci. 1998;52:97–105. doi: 10.1111/j.1440-1819.1998.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: An MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Pietromonaco PR. Accuracy of the five factor model in predicting perceptions of daily social interactions. Personality & Social Psychology Bulletin. 1997;23:1173–1187. [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, Kagan J, Faraone SV. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Bolger N, Schilling EA. Personality and the problems of everyday life: The role of neuroticism in exposure and reactivity to daily stressors. J Pers. 1991;59:355–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Bolger N, Zuckerman A. A framework for studying personality in the stress process. J Pers Soc Psychol. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Bone RN, Montgomery DD. Extraversion, neuroticism, and sensation seeking. Psychol Rep. 1970;26:974. doi: 10.2466/pr0.1970.26.3.974. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Memories for emotional autobiographical events following unilateral damage to medial temporal lobe. Brain. 2006;129:115–127. doi: 10.1093/brain/awh672. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Burvill PW, Hall WD, Stampfer HG, Emmerson JP. The prognosis of depression in old age. Br J Psychiatry. 1991;158:64–71. doi: 10.1192/bjp.158.1.64. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JD. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Canli T, Amin Z, Haas B, Omura K, Constable RT. A double dissociation between mood states and personality traits in the anterior cingulate. Behav Neurosci. 2004;118:897–904. doi: 10.1037/0735-7044.118.5.897. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav Neurosci. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. J Pers Soc Psychol. 2000;79:644–655. [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, N.J.: Erlbaum; 1983. [Google Scholar]
- Costa PT, McCrae RR. Mood and personality in adulthood. In: Magai C, McFadden SH, editors. Handbook of emotional, adult development, and aging. San Diego: Academic Press; 1996. pp. 369–383. [Google Scholar]
- Costa PT, Jr, McCrae RR. Age differences in personality structure revisited: Studies in validity, stability, and change. Int J Aging Hum Dev. 1977;8:261–275. doi: 10.2190/awyd-qt2l-wvnm-4f98. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Cross-sectional studies of personality in a national sample: 1. Development and validation of survey measures. Psychol Aging. 1986;1:140–143. doi: 10.1037//0882-7974.1.2.140. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. The revised neo personality inverntory (neo pi-r) professional manual. Odessa, FL; Psychological Assessment Resources: 1992. [Google Scholar]
- Costa PT, Jr, McCrae RR. Stability and change in personality assessment: The revised neo personality inventory in the year 2000. J Pers Assess. 1997;68:86–94. doi: 10.1207/s15327752jpa6801_7. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Overview: Innovations in assessment using the revised neo personality inventory. Assessment. 2000;7:325–327. doi: 10.1177/107319110000700402. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, Herbst JH, McCrae RR, Siegler IC. Personality at midlife: Stability, intrinsic maturation, and response to life events. Assessment. 2000;7:365–378. doi: 10.1177/107319110000700405. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR, Zonderman AB, Barbano HE, Lebowitz B, Larson DM. Cross-sectional studies of personality in a national sample: 2. Stability in neuroticism, extraversion, and openness. Psychol Aging. 1986;1:144–149. [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Well-being and affective style: Neural substrates and biobehavioural correlates. Philos Trans R Soc Lond B Biol Sci. 2004a;359:1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biol Psychol. 2004b;67:219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Sorensen S, Lyness JM, King DA, Conwell Y, Seidlitz L, Caine ED. Personality is associated with perceived health and functional status in older primary care patients. Psychol Aging. 2003;18:25–37. doi: 10.1037/0882-7974.18.1.25. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV for axis I disorders, patient edition (SCIP-P) New York: Biometrics Research Department; 1995. [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Age-differential patterns of brain activation during perception of angry faces. Neurosci Lett. 2005;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state.” a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS. Affective personality differences in neural processing efficiency confirmed using fMRI. Cogn Affect Behav Neurosci. 2005;5:182–190. doi: 10.3758/cabn.5.2.182. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiol Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Ollinger JM, Shulman GL, Cloninger CR, Price JL, Van Essen DC, Raichle ME. Persistence and brain circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3479–3484. doi: 10.1073/pnas.0538050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AS, Kay DW. The epidemiology of functional psychoses of late onset. Eur Arch Psychiatry Clin Neurosci. 1997;247:176–189. doi: 10.1007/BF02900214. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Izard CE, Libero DZ, Putnam P, Haynes OM. Stability of emotion experiences and their relations to traits of personality. J Pers Soc Psychol. 1993;64:847–860. doi: 10.1037//0022-3514.64.5.847. [DOI] [PubMed] [Google Scholar]
- John OP. The “big five” factor taxonomy: Dimensions of personality in the natural language and in questionanaires. In: Pervin L, editor. Handbook of personality theory and research. New York: Guilford Press; 1990. pp. 66–100. [Google Scholar]
- Johnson DL, Wiebe JS, Gold SM, Andreasen NC, Hichwa RD, Watkins GL, Boles Ponto LL. Cerebral blood flow and personality: A positron emission tomography study. Am J Psychiatry. 1999;156:252–257. doi: 10.1176/ajp.156.2.252. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Maguire RP, Kurki T, Bruck A, Rinne JO. Mapping brain structure and personality in late adulthood. Neuroimage. 2005;24:315–322. doi: 10.1016/j.neuroimage.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. Br J Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kim BL, Sohn SE, Lim SW, Na DG, Paik CH, Krishnan KR, Carroll BJ. Candidate neuroanatomic substrates of psychosis in old-aged depression. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:793–807. doi: 10.1016/s0278-5846(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Schweizer E, Jin Z, Miller D, Bilker W, Swan LL, Gottlieb G. Neuroanatomical substrates of late-life minor depression. A quantitative magnetic resonance imaging study. Arch Neurol. 1997;54:613–617. doi: 10.1001/archneur.1997.00550170085018. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Ketelaar T. Personality and susceptibility to positive and negative emotional states. J Pers Soc Psychol. 1991;61:132–140. doi: 10.1037//0022-3514.61.1.132. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Diener E. Understanding extraverts’ enjoyment of social situations: The importance of pleasantness. J Pers Soc Psychol. 2001;81:343–356. doi: 10.1037//0022-3514.81.2.343. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Baird BM. Extraversion and emotional reactivity. J Pers Soc Psychol. 2004;86:473–485. doi: 10.1037/0022-3514.86.3.473. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Diener E, Grob A, Suh EM, Shao L. Cross-cultural evidence for the fundamental features of extraversion. J Pers Soc Psychol. 2000;79:452–468. doi: 10.1037//0022-3514.79.3.452. [DOI] [PubMed] [Google Scholar]
- Lyness JM, Duberstein PR, King DA, Cox C, Caine ED. Medical illness burden, trait neuroticism, and depression in older primary care patients. Am J Psychiatry. 1998;155:969–971. doi: 10.1176/ajp.155.7.969. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol Sci. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Personality in adulthood. New York: Guilford; 1990. [Google Scholar]
- McCrae RR, Costa PT., Jr Adding liebe und arbeit: The full five-factor model and well-being. Personality & Social Psychology Bulletin. 1991;17 [Google Scholar]
- McCrae RR, John OP. An introduction to the five-factor model and its applications. J Pers. 1992;60:175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT, Jr, Pedroso de Lima M, Simoes A, Ostendorf F, Angleitner A, Marusic I, Bratko D, Caprara GV, Barbaranelli C, Chae JH, Piedmont RL. Age differences in personality across the adult life span: Parallels in five cultures. Dev Psychol. 1999;35:466–477. doi: 10.1037//0012-1649.35.2.466. [DOI] [PubMed] [Google Scholar]
- Mischel W. Toward an integrative science of the person. Annu Rev Psychol. 2004;55:1–22. doi: 10.1146/annurev.psych.55.042902.130709. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. J Pers. 2004;72:355–378. doi: 10.1111/j.0022-3506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Omura K, Todd Constable R, Canli T. Amygdala gray matter concentration is associated with extraversion and neuroticism. Neuroreport. 2005;16:1905–1908. doi: 10.1097/01.wnr.0000186596.64458.76. [DOI] [PubMed] [Google Scholar]
- Paisey TJ, Mangan GL. The relationship of extraversion neuroticism, and sensation-seeking to questionnaire-derived measures of nervous system properties. Pavlov J Biol Sci. 1980;15:123–130. doi: 10.1007/BF03003693. [DOI] [PubMed] [Google Scholar]
- Pantel J, Schroder J, Essig M, Schad LR, Popp D, Eysenbach K, Jauss M, Knopp MV. [Volumetric brain findings in late depression. A study with quantified magnetic resonance tomography] Nervenarzt. 1998;69:968–974. doi: 10.1007/s001150050371. [DOI] [PubMed] [Google Scholar]
- Pickering A, Gray JA. The neuroscience of personality. In: Pervin L, John O, editors. Handbook of personality: Theory and research. 2. New York: Gilford Press; 2001. pp. 227–299. [Google Scholar]
- Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Raz N, Briggs SD, Marks W, Acker JD. Age-related deficits in generation and manipulation of mental images: II. The role of dorsolateral prefrontal cortex. Psychol Aging. 1999;14:436–444. doi: 10.1037/0882-7974.14.3.436. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Hirshfeld DR, Bolduc EA, Faraone SV, Kagan J, Snidman N, Reznick JS. Further evidence of an association between behavioral inhibition and anxiety disorders: Results from a family study of children from a non-clinical sample. J Psychiatr Res. 1991;25:49–65. doi: 10.1016/0022-3956(91)90015-3. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Arch Neurol. 2001;58:1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schneider TR. The role of neuroticism on psychological and physiological stress responses. Journal of Experimental Social Psychology. 2004;40:795–804. [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J Neurophysiol. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Seidlitz L, Conwell Y, Duberstein P, Cox C, Denning D. Emotion traits in older suicide attempters and non-attempters. J Affect Disord. 2001;66:123–131. doi: 10.1016/s0165-0327(00)00300-1. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 3-d proportional system: An approach to cerebral imaging. New York: Thieme Publishers; 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT(1a) receptor binding and anxiety: A [(11)c]way-100635 pet investigation in healthy volunteers. Am J Psychiatry. 2001;158:1326–1328. doi: 10.1176/appi.ajp.158.8.1326. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, O’Brien JT. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry. 2003;18:7–13. doi: 10.1002/gps.720. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. J Pers Soc Psychol. 1990;59:791–801. doi: 10.1037//0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. J Pers Soc Psychol. 1992;62:676–687. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Wallace JF, Newman JP. Neuroticism and the attentional mediation of dysregulatory psychopathology. Cognitive Therapy & Research. 1997;21:135–156. [Google Scholar]
- Wallace JF, Newman JP. Neuroticism and the facilitation of the automatic orienting of attention. Personality & Individual Differences. 1998;24:253–266. [Google Scholar]
- Wallace JF, Newman JP, Bachorowski J. Failures of response modulation: Impulsive behavior in anxious and impulsive individuals. Journal of Research in Personality. 1991;25:23–44. [Google Scholar]
- Watson D, Clark LA. On traits and temperament: General and specific factors of emotional experience and their relation to the five-factor model. J Pers. 1992;60:441–476. doi: 10.1111/j.1467-6494.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Wedig MM, Rauch SL, Albert MS, Wright CI. Differential amygdala habituation to neutral faces in young and elderly adults. Neurosci Lett. 2005;385:114–119. doi: 10.1016/j.neulet.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Seidlitz L. Personality, psychopathology and aging. Journal of Research in Personality. 2002;36:335–362. [Google Scholar]
- Wildgruber D, Riecker A, Hertrich I, Erb M, Grodd W, Ethofer T, Ackermann H. Identification of emotional intonation evaluated by fMRI. Neuroimage. 2005;24:1233–1241. doi: 10.1016/j.neuroimage.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol Aging. 2006a;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, Wedig MM. Neuroanatomical correlates of extraversion and neuroticism. Cereb Cortex. 2006b;16:1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]


