Abstract
Disturbances in glutamate neurotransmission are thought to be one of the major contributing factors to the pathophysiology of schizophrenia. In the dorsolateral prefrontal cortex (DLPFC), glutamate neurotransmission is largely mediated by AMPA receptors. Data regarding alterations of subunit expression in the brains of patients with schizophrenia remain equivocal. This may be due to differences in technique sensitivity, endogenous control selection for normalization of data, or effect of antipsychotic drug treatment in different cohorts of schizophrenia. This study attempted to address these issues by examining the expression of AMPA receptor subunits and splice variants in the DLPFC of two schizophrenia cohorts using quantitative PCR (qPCR) with normalization to the geometric mean of multiple endogenous controls. In addition, a non-human primate model of chronic antipsychotic drug administration was used to determine the extent to which the transcript expression may be altered by antipsychotic drug treatment in the primate DLPFC. AMPA receptor subunits and flip and/or flop splice variants were not significantly different in the DLPFC of schizophrenia subjects versus controls in either of the two cohorts. However, in rhesus monkeys chronically treated with antipsychotic drugs, clozapine treatment significantly decreased GRIA1 and increased GRIA3 mRNA expression, while both clozapine and haloperidol increased the expression of GRIA2 subunit mRNA. Expression of AMPA receptor splice variants was not significantly altered by antipsychotic drug administration. This is the first study to show that AMPA receptor subunit mRNAs in the primate DLPFC are altered by antipsychotic drug administration. Antipsychotic drug induced alterations may help explain differences in human post-mortem studies regarding AMPA receptor subunit expression and provide some insight into the mechanism of action of antipsychotic drugs.
Keywords: AMPA, schizophrenia, antipsychotic, rhesus monkey, dorsolateral prefrontal cortex, flip flop splice variants
1. Introduction
Altered glutamatergic neurotransmission is associated with schizophrenia (Coyle et al. 2002; Goff and Coyle 2001; Olney and Farber 1995; Tsai and Coyle 2002), particularly in the dorsolateral prefrontal cortex (DLPFC) (Bunney and Bunney 2000; Dracheva et al. 2001; Mueller and Meador-Woodruff 2004; Vawter et al. 2002), a region implicated in the pathophysiology of schizophrenia (Bunney and Bunney 2000; Goff and Coyle 2001; Weinberger et al. 1986). Most research has focused on the role of NMDA receptors due to the ability of NMDA receptor antagonists to induce schizophrenia-like symptoms in normal individuals (Domino et al. 1965; Javitt and Zukin 1991; Krystal et al. 1999; Krystal et al. 1994; Luby et al. 1959; Snyder 1980) and exacerbate symptoms in patients (Lahti et al. 1995; Lahti et al. 2001). It remains unclear whether NMDA receptor hypofunction is attributable to a primary deficit in NMDA receptor function or to a modulator of NMDA receptor function. AMPA receptor activation plays a large role in cellular depolarization and regulation of NMDA receptor signaling. Therefore, deficiencies in AMPA receptor signaling could underlie this apparent NMDA receptor hypofunction. Further, some studies with NMDA antagonists suggest that schizophrenia involves an increase in glutamate release in the prefrontal cortex and increased activation of non-NMDA post-synaptic receptors, such as the AMPA receptors (Anand et al. 2000; Moghaddam et al. 1997; Olney and Farber 1995). Given the dynamic nature of AMPA receptors and their important role in synaptic plasticity and fast glutamatergic signaling (Carroll et al. 2001; Malenka 2003), it is therefore important to understand how these receptors may be altered schizophrenia.
AMPA receptors are tetrameric structures assembled from four subunits GRIA1–4, each of which exists in splice variant forms (Palmer et al. 2005). Alternative splicing in the extracellular N-terminal region of the fourth transmembrane domain yields variants termed “flip” and “flop” for each subunit (Sommer et al. 1990). Stoichiometry of the functional ionophore is controlled by the expression levels of individual subunits which affect several properties of the mature receptor including receptor trafficking and cellular physiology (Koike et al. 2000; Mosbacher et al. 1994; Palmer et al. 2005). Therefore altered expression of AMPA receptor subunit or splice variants might underlie disturbances in glutamatergic neurotransmission in schizophrenia patients.
Several studies have examined AMPA receptor subunit mRNA and protein expression in the brains of patients with schizophrenia (Dracheva et al. 2005; Healy et al. 1998; Hemby et al. 2002; Meador-Woodruff and Healy 2000; Vawter et al. 2002). While alterations in AMPA receptors in temporal lobe regions are observed consistently (Hemby et al. 2002; Meador-Woodruff and Healy 2000), findings in the DLPFC have been equivocal. An early in situ hybridization study reported no change in AMPA receptor subunit expression(Healy et al. 1998), while later studies using microarray analysis and quantitative real time PCR, respectively, reported either a decrease in GRIA2 subunit mRNA expression(Vawter et al. 2002) or increases in GRIA 1 and GRIA4 subunit mRNA(Dracheva et al. 2001). Discrepancies between these studies may be due in part to the use of different cohorts, differences in technique sensitivity, or the possible influence of antipsychotic drug (APD) treatment history. Resolution of this issue will be important for understanding the pathophysiology of schizophrenia and to identify targets for medication development.
Expression of flip and flop variants have been largely overlooked in the schizophrenia literature. Flop containing AMPA receptors desensitize more quickly than flip containing receptors (Sommer et al. 1990) and expression of flip and flop variants is developmentally regulated (Stine et al. 2001). Altered flip/flop expression has even been reported in hippocampal regions in schizophrenia patients (Eastwood et al. 1997). However, to date expression of flip and flop variants in the DLPFC has not been examined even though GRIA3 flop expression was decreased in the prefrontal cortex in the ventral hippocampal lesion rodent model of schizophrenia (Stine et al. 2001).
Human post-mortem schizophrenia brain tissue available for study is typically from patients administered APDs for many years. Studies in rodents have shown that APD treatment alters mRNA and protein levels of AMPA receptor subunits in the PFC (Fitzgerald et al. 1995; Healy and Meador-Woodruff 1997), while reports of APD induced effects on flip and flop expression for GluR1 and GluR2 subunits are conflicting (Eastwood et al. 1996; McCoy et al. 1998). Of course, significant differences exist between rodent and primate PFC (Preuss 1995), making a non-human primate model of APD administration ideal for investigating drug effects on gene expression in the uniquely primate DLPFC.
The present study was undertaken to use new analytic methods to examine AMPA receptor subunit and AMPA subunit flip and flop mRNA levels in the DLPFC of two postmortem cohorts of schizophrenia subjects. In addition, the effects of chronic antipsychotic drug administration on AMPA subunit mRNA expression in the DLPFC of rhesus monkeys administered oral clozapine or haloperidol for 180 days was compared with controls.
2. Materials and Methods
2.1 Subjects
2.1.1. Human
Two cohorts of human frozen brain samples from DLPFC (Brodmann’s area 46) were used for this study. The first cohort included16 chronically hospitalized patients with schizophrenia and 11 age-matched controls from the established brain collection at the University of Pennsylvania (Table I). The schizophrenia subjects were elderly, "poor-outcome" patients who were participants in a clinicopathological studies program at the University of Pennsylvania, School of Medicine in collaboration with eight state hospitals in eastern and central Pennsylvania. All patients were prospectively accrued, had clinical interviews and assessments, and were diagnosed according to DSM-IV (DSM-IV 1994) criteria by research psychiatrists (Arnold et al. 1995). In general, clinical features included prominent negative symptoms, relatively mild positive symptoms, moderate to severe cognitive dysfunction, and impairments in basic self-care activities that warranted their chronic hospitalization. Antipsychotic treatment 1 month prior to death was reported. The control subjects were obtained via the Center for Neurodegenerative Disease Research at the University of Pennsylvania and were without history of neurological or major psychiatric illness, as determined in extensive, retrospective chart review. Gross and microscopic diagnostic neuropathologic examinations, which included examination of multiple cortical and subcortical regions, were performed in all cases and no neuropathological abnormalities relevant to mental status were found. The second cohort included 60 subjects [schizophrenia (n=15), bipolar disorder (n=15), major depressive disorder (n=15), and non-diseased controls (n=15)] obtained from the Stanley Foundation Neuropathology Consortium. Subject selection and tissue collection/preparation have been described previously (Torrey et al. 2000). For this study, 14-μm frozen sections through the frontal region were used. General demographic information for each group is shown in Table 1.
Table 1.
Demographic Information for Human Subjects
| N | Sex (M:F) | Race (W:B) | PMI (hr ± StErr) | Age at death (years ± StErr) | Brain Weight (± StErr) | Brain pH | Duration of Illness (years ± StErr) | |
|---|---|---|---|---|---|---|---|---|
| Cohort 1 | ||||||||
| Normal Controls | 11 | 5:6 | 8:3 | 9.4 ± 1.7 | 80.4 ± 2.7 | 1159.9 ± 42.4 | 6.4 ± 0.06 | n/a |
| Schizophrenia | 16 | 6:10 | 16:0 | 10.7 ± .8 | 80 ± 1.7 | 1222.6 ± 42.1 | 6.4 ± 0.04 | 54.9 ± 2.1 |
| Cohort 2 | ||||||||
| Normal Controls | 15 | 9:6 | 23.7 ± 2.7 | 48.1 ± 2.1 | 1501 ± 43.9 | 6.3 ± 0.1 | n/a | |
| Schizophrenia | 15 | 9:6 | 33.7 ± 3.9 | 44.5 ± 3.5 | 1471.7 ± 28.9 | 6.2 ± 0.1 | 21.3 ± 3.1 | |
| Bipolar Disorder | 15 | 9:6 | 32.5 ± 4.3 | 42.3 ± 3.1 | 1441.2 ± 45.8 | 6.2 ± 0.1 | 20.1 ± 2.6 | |
| Major Depressive Disorder | 15 | 9:6 | 27.5 ± 2.9 | 46.5 ± 2.5 | 1462 ± 38 | 6.2 ± 0.1 | 12.7 ± 3.0 | |
2.1.2. Monkey
Fifteen rhesus macaques were randomly assigned to one of three treatment groups: haloperidol, clozapine, or control (n=5/group; 3 males and 2 females/group; age range: 3.7–7.3 years). There was no significant difference in the age of subjects between groups (F(2,14)=0.0221, p=0.978). Using previously established protocols, haloperidol (0.14 mg/kg daily dose) (Castner et al. 2000; O'Connor et al. 2006) or clozapine (5.2 mg/kg daily dose) (Lidow et al. 1997; Lidow and Goldman-Rakic 1994; Lidow and Goldman-Rakic 1997b) were administered mixed with powdered sugar and given orally in peanut butter or fruit treats. Doses approximate the range used clinically in psychiatric patients (PDR 1996; PDR 2006) and correspond to doses used by research groups in the field (Castner et al. 2000; Lidow et al. 1997; Lidow and Goldman-Rakic 1994; Lidow and Goldman-Rakic 1997a; O'Connor et al. 2006). After six months of treatment, monkeys were anesthetized with pentobarbital and transcardially perfused with ice cold saline. Brains were removed and cut into 4 mm slabs in the coronal plane using a brain matrix (EMS, Fort Washington, PA). The ventral bank of the principal sulcus was dissected as a sample of DLPFC (Area 46). The post-mortem interval (time from overdose to freezing of last piece of tissue) from the animals ranged from 38 to 87 minutes and the mean time for the three treatment groups did not differ significantly (F(2,14)=0.517, p=0.61; HAL = 52 +/− 6.9, CLOZ = 53.6 +/− 8.5, control = 44.8 +/− 2.8). A previous study indicates that glutamate receptor subunits are stable up to 18 hr PMI (Wang et al. 2000). The care of the animals and euthanasia procedures in this study were performed according to the National Institutes for Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Emory University.
2.2. RNA Isolation
Dissected tissue samples of DLPFC from Cohort 1 and monkeys were pulverized under liquid nitrogen. An aliquot of pulverized tissue was then used for RNA isolation. Approximately 50–100 mg of tissue specimen from each subject was placed in Trizol® (Invitrogen Corporation, Carlsbad, CA) and disrupted using a PowerGen tissue homogenizer. Samples were incubated at 25ºC for 5 min to allow complete dissociation of nucleoprotein complexes. Chloroform was added and samples were centrifuged for 15 min for phase separation. The aqueous phase was retained and total RNA was precipitated with isopropyl alcohol and linear acrylamide (5 μg) and washed with 75% ethanol. The RNA pellet was re-suspended in RNase-free water and stored at −80°C. RNA was quantified using spectrophotometric analysis and the integrity of total RNA for each sample was assessed using an Agilent 2100 Bioanalyzer and RNA 6000 Nano Lab Chip according to the manufacturer’s protocol.
For Cohort 2, frozen tissue from Area 46 was scraped from a total of three slides per subject and total RNA was isolated with the mirVana miRNA Isolation Kit (Ambion #1560) using the total RNA isolation protocol. Dissected tissue was placed in 400 μL lysis/binding buffer and sonicated for 2–10 seconds at 4ºC. 40 μl homogenate additive was added to samples and samples were vortexed and incubated at 4ºC for 10 min. RNA was extracted using 400 μl acid-phenol chloroform added to the mixture, vortexed, and centrifuged for phase separation. 1.25 volumes room temperature 100% ethanol was added to the aqueous phase, the solution was mixed thoroughly, and the lysate/ethanol mixture was passed through a filter cartridge. Purified total RNA was eluted with nuclease free water and subjected to spectrophotometric analysis and Agilent 2100 Bioanalyzer with RNA 6000 Nano Lab Chip for quantification and examination of quality.
2.3. Synthesis of cDNA
One μg (cohort 1) or 500 ng (cohort 2) of total RNA from each human DLPFC sample and a pooled sample of total RNA from each cohort, was reverse transcribed using random hexamers and SuperScript III (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. For the rhesus monkey samples, 2 ug of total RNA from each sample and from a pool of total RNA combined from all monkey samples was reverse transcribed using random hexamers and superscript III. Resulting cDNA product was diluted 1: 100 with RNAse-free water for samples, and cDNA from the pooled samples was serially diluted in 2 fold dilutions from 1:20–1:1280 for use as standards.
2.4. AMPA receptor splice variant Taqman assay design
Custom Taqman assays were designed for the flip and flop splice variants of the four AMPA receptors (GRIA1–4) in collaboration with Applied Biosystems (Foster City, CA). Sequence information for these assays and the accession numbers for which the assays were designed are listed in Table 2. All assays use FAM reporter dye and NFQ quencher. Assays were designed such that the Taqman probe spanned the exon-exon junction (or included exon) that is specific to the splice variant being measured. Standard Taqman assays were purchased for measurement of specific subunit levels and endogenous controls (Applied Biosystems; GRIA1, Hs00181348_m1; GRIA2, Hs00181331_m1; GRIA3, Hs00241485_m1; GRIA4, Hs00168163_m1; PGK1(phosphoglycerate kinase 1), Hs99999906_m1; PPIA (peptidylprolyl isomerase A (cyclophilin A)), Hs99999904_m1; GAPDH (glyceraldehyde-3-phosphate dehydrogenase), Hs99999905_m1; and TBP (TATA box binding protein), Hs99999910_m1.
Table 2.
Sequence Information for Custom Designed Taqman Assays
| Gene | Probe Sequence | Primer Sequerices | Size |
|---|---|---|---|
| GRIA1 flip | CACTGAGTTTCAAAACCG | F-CTCCTGGAGTCCA.CC AT GAATG
R-CGGAGTCCTTGCTTCCACATT |
224 |
| GRIA1 flop | CCCCTGCTCGTTTAGTTT | F-TGGAGTCCACCATGAATGAGTACA
R-ACCTCCCCCGCTGC |
229 |
| GRIA2 flip | ACCCCAGTAAATCTTG | F-TGGATTCCAAAGGCTATGGCATC
R-CCTTGGCTCCACATTCACCTT |
150 |
| GRIA2 flop | CCTCGCAGTACTAAAAC | F-AACCTGGATTCCAAAGGCTATGG
R-CCGCTGCCGCACTCT |
152 |
| GRIA3 flip | CAGTGAACAAGGCATCTTA | F-AGGCTATGGTGTGGCAACC
R-GGAGTCCTTGGCTCCACATTC |
145 |
| GRIA3 flop | AACTGAATGAGCAAGGCCT | F-TGGATTCCAAAGGCTATGGTGTG
R-CCGCTGCCGCACTC |
148 |
| GRIA4 flip | CAGTGAGGCAGGCGTC | F-AGCAGCGAAAGCCATGTGA
R-AGAGTCTTTGGGTCCACATTCAC |
197 |
| GRIA4 flop | AAGGCCTCTTGGACAAAT | F- GAGCAGCGAMGCCATGTG
R- AGTCACCTCCCCCGCT |
202 |
2.5. Real-time quantitative PCR
Using a 384 well format with the ABI Prism 7900HTS real-time detector, 0.5 μl aliquots of Taqman Expression Assay (20X), 5.5μl 2X Absolute QPCR ROX PCR Mastermix (Abgene), and 4.5μl diluted cDNA (either sample or pooled standard) were mixed together and pipetted into single wells of the PCR plate. For no template controls (NTC) for each gene tested, water was added in lieu of cDNA. Each sample, including NTC was run in triplicate. Thermocycling conditions: 1) one cycle 2 min at 50°C, 2) one cycle 15 min at 95°C, and 3) 40 cycles 15 sec at 95°C and 1 min at 60°C. Fluorescence was measured during the 60ºC step for each cycle. Reactions were quantified by the standard curve method using SDS2.1 software (as described in User Bulletin #2, Applied Biosystems) generating a mean quantity value (Qty mean) for each sample from the triplicates of that sample for each gene of interest. Endogenous controls were selected for each experiment from a set of eleven candidate reference transcripts: β-2 microglobulin (B2M), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal protein, large (RPLPO), β-actin (ACTB), ribosomal protein 18S (18S), TATA box binding protein (TABP), hypoxanthine phosphoribosyltransferase 1 (HPRT1), transferrin receptor (TFRC), peptidylprolyl isomerase A (cyclophilin A) (PPIA), β-glucuronidase (GUSB), and phosphoglycerate kinase 1 (PGK1) using geNorm software. geNorm is a collection of VBA macros for Microsoft Excel which allows the determination of the most stable reference genes from a given test panel of genes. By computing the average pairwise variation V for each control gene paired with all other tested control genes, geNorm calculates the gene expression stability measure M (Vandesompele et al. 2002). This allows for the selection of the most stably expressed control genes in a given sample set, minimizing any bias in the data as a result of normalization. The gene expression normalization factor is then calculated based on the geometric mean of a user-defined number of reference genes(Vandesompele et al. 2002). Three candidate genes (PGK1, PPIA, GAPDH) were selected from this list to serve as endogenous controls in each of the human studies based on the criteria set by Vandesompele and colleagues. Likewise, two candidate genes (TBP and PGK1) were selected from this list to serve as controls in the non-human primate studies. Thus, data for each gene of interest was expressed as Qty mean for the gene of interest/ geometric mean of Qty mean values for the selected endogenous control genes. qPCR experiments for each human cohort and for antipsychotic drug treated monkey samples were run independently. Normalized values were then expressed as percent control.
2.6. Data Analysis
Experiments determining relative gene expression for each candidate gene were run independently. Likewise, experiments in the distinct cohorts were run independently. In Cohort 1, we used two-tailed t-tests to compare group means from the non-schizophrenic control group and schizophrenic subjects for each of the candidate genes. Because there were three disease groups and a control, we used a one way analysis of variance (ANOVA) to compare group means for the non-schizophrenic control groups, schizophrenic group, bipolar group, and major depressive disorder group for each of the candidate genes in Cohort 2. For both types of analyses, the null hypothesis was rejected if p < 0.05. In the antipsychotic drug treated monkey studies, one way ANOVAs were used to determine if there was a significant effect of haloperidol or clozapine treatment upon each subunit or splice variant expression. The Holm-Sidak method was used for post hoc comparisons, and the null hypothesis was rejected if p< 0.05. All statistical analyses were performed using SigmaStat 3.1 (Systat Software) and Statistica (StatSoft, Inc.) software packages.
3. Results
3.1 Demographic Data
In Cohort 1, the schizophrenia and control groups were matched for age (schizophrenia group age= 80 ± 1.7; control age= 80 ± 2.7) with equivalent male: female ratios per group (schizophrenia group M:F = 6:10; control group M:F = 5:6). There were no significant differences between the groups in age (p= 1), PMI (p= 0.468), or brain weight (p= .314). In Cohort 2, there were no significant differences between the diagnosis groups in age (F= 0.727, p=0.539), pH (F=0.602, p=.616), brain weight (F= 0.419, p=.739), or PMI (F=1.857, p=0.147). There was a significant difference between the groups in number of days the tissue had been stored in the freezer (F= 5.35, p=0.003). However, there was not a significant correlation between number of days in the freezer and expression of any of the dependent variables: GRIA1 (R2=0.24, p=0.06), GRIA2 (R2=0.19, p=0.14), GRIA3 (R2=0.06, p=0.67), GRIA4 (R2=0.11, p=0.40), GRIA2flip (R2=0.12, p=0.36), GRIA2flop (R2=0.03, p=0.80), GRIA3flip (R2=−0.01, p=0.95), GRIA3flop(R2=−0.06, p=0.66).
All schizophrenia subjects in this study were exposed to antipsychotic drug treatment in their lifetime. In Cohort 1, data regarding antipsychotic drug exposure one month prior to death was available (see Table 3): four subjects were neuroleptic free, five subjects received typical antipsychotic drugs, and three subjects received atypical antipsychotic drugs; however, information for four subjects was unavailable. Cohort 2 schizophrenic subjects were all receiving antipsychotic drug treatment at the time of death and the life-time fluphenazine equivalent dose received is reported in Table 3.
Table 3.
Human Subjects Antipsychotic Drug Treatment
| Cohort 1 | ||
|---|---|---|
| Schizophrenia Group | ||
| Subject ID# | Antipsychotic Drug Treatment (1 month prior to death) | Dose(mg/day) |
| 01-103 | clozapine | 250 |
| 02-217 | not available, probably none | |
| 90-265 | 0 | 0 |
| 92-217 | chlorpromazine | 1350 |
| 93-208 | not available | |
| 94-041 | 0 | 0 |
| 94-098 | haloperidol | 1.5 mg |
| 94-188 | not available | |
| 94-202 | thioridzine | 300 |
| 95-104 | thiothixene | 30 |
| 96-039 | risperidone | 4 |
| 96-044 | 0 | 0 |
| 96-137 | 0 | 0 |
| 97-032 | haloperidol | 2 |
| 97-087 | risperidone | 1 |
| 97-236 | not available | |
| Cohort 2 | ||
| Schizophrenia Group | ||
| Subject ID# | Lifetime Qty fluphenazine or equivalent (mg) | |
| 93 | 34955 | |
| 120 | 35116 | |
| 64 | 34836 | |
| 100 | 34996 | |
| 30 | 34673 | |
| 43 | 34712 | |
| 13 | 34581 | |
| 116 | 35096 | |
| 18 | 34503 | |
| 173 | 35451 | |
| 81 | 34898 | |
| 118 | 35103 | |
| 41 | 34711 | |
| 66 | 34843 | |
| 82 | 34904 | |
| Bipolar Group | ||
| Subject ID# | Lifetime Qty fluphenazine or equivalent (mg) | |
| 34 | 32000 | |
| 89 | 0 | |
| 72 | 60000 | |
| 83 | 200 | |
| 128 | 60000 | |
| 91 | 12000 | |
| 88 | 30000 | |
| 33 | 7500 | |
| 103 | 40000 | |
| 147 | 0 | |
| 68 | 0 | |
| 47 | 1200 | |
| 75 | 7000 | |
| 48 | 2500 | |
| 60 | 60000 | |
3.2. Expression of GRIA1–4 subunit mRNA in the DLPFC of two separate human cohorts
In the DLPFC of both human subject cohorts, GRIA1, GRIA2, GRIA3, and GRIA4 mRNA expression levels were assessed relative to the geometric mean of the endogenous controls PGK, PPIA, and GAPDH. In Cohort 1, no significant differences were observed between schizophrenia (n=16) and control (n=11) groups for any of these transcripts: GRIA1 (p= 0.67), GRIA2 (p= 0.37), GRIA3 (p= 0.62), and GRIA4 (p= 0.13) (Figure 1a). In a second cohort of human subjects representing normal controls (n=15), schizophrenia (n=15), bipolar disorder (n=15), and major depressive disorder (n=15), there was no significant effect of diagnosis on AMPA receptor subunit mRNA expression levels: GRIA1 (F= 1.489, p= 0.228), GRIA2 (F=1.855, p= 0.148), GRIA3 (F= 1.055, p= 0.376), GRIA4 (F= 0.273, p= 0.845) (Figure1b). For GRIA1, GRIA2, and GRIA4 subunits, gene expression values were greater than two standard deviations below the mean for one subject from the bipolar group, so this subject was omitted from analysis, resulting in a sample size of 14 for the bipolar group in Cohort 2 for in these studies.
Figure 1.
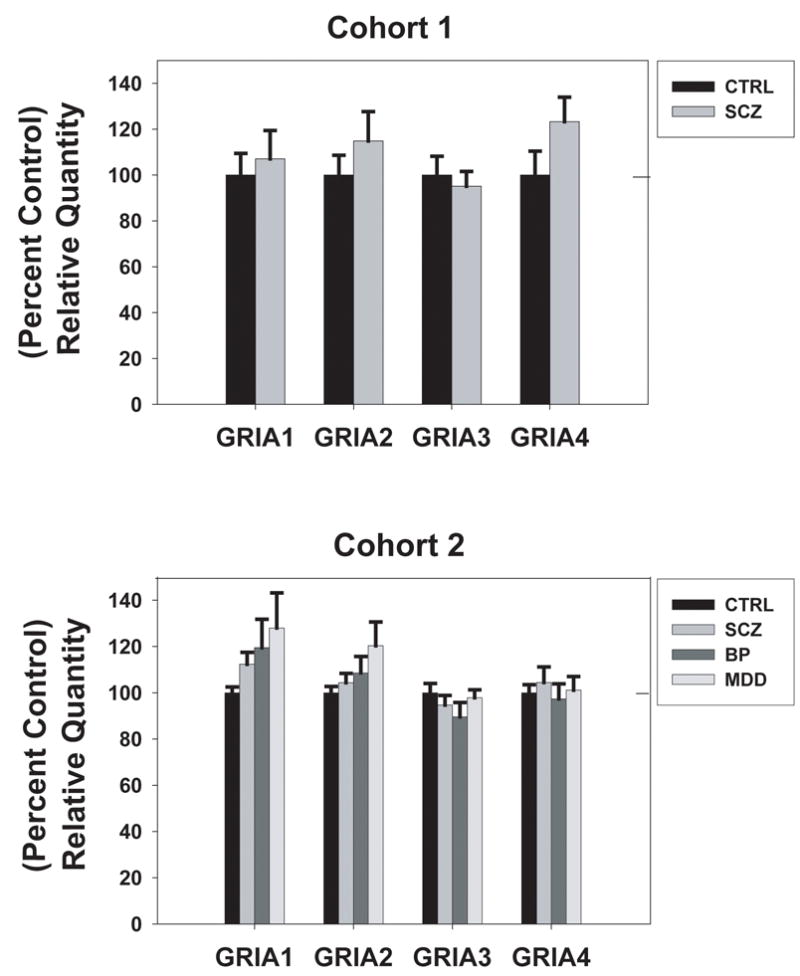
AMPA receptor subunit mRNA levels in the dorsolateral prefrontal cortex (DLPFC) of an elderly cohort of schizophrenia patients (SCZ) and controls (CTRL) (top) and a younger cohort of schizophrenia patients (SCZ), bipolar disorder patients (BP), major depressive disorder patients (MDD), and controls (bottom). Data are expressed as mean (± S.E.M.) of the percent control values for normalized relative quantities. There were no significant differences compared to control subjects (p< 0.05).
3.3 Expression of GRIA1–4 flip and flop variants in the DLPFC of schizophrenia patients and controls
Relative expression levels of the flip and flop variant mRNAs of the GRIA1, GRIA2, GRIA3, and GRIA4 genes were assessed using quantitative qPCR in the DLPFC of two human cohorts. In both cohorts, signals for GRIA1 flop and GRIA4 flip were undetectable, likely because of the low abundance of GRIA1 and GRIA 4 transcripts in the DLPFC (Dracheva et al. 2005). Similarly, the signal for GRIA4 flop was unreliable, with standard deviations among triplicates >1 cycle for most sets of triplicates. Therefore, this data was not included. In cohort 1, no significant differences were observed between the schizophrenia and control groups for any of the remaining splice variants: GRIA1 flip (p=0.28), GRIA2 flip (p=0.2), GRIA2 flop (p=0.23), GRIA3 flip (p=0.07), and GRIA3 flop (p=0.5) (Figure 2a). In the second cohort, there was no significant effect of diagnosis on splice variant expression except for GRIA2 flip (F= 2.867, p= 0.045); however, post hoc analysis failed to confirm a significant difference between controls and any of the disease groups for GRIA2 flip (Figure 2b). Again, any expression values that were greater than two standard deviations from the mean were removed as outliers. No outliers were identified in Cohort 1, but for Cohort 2 one subject from the bipolar group consistently measured below two standard deviations from the mean and was therefore omitted from analysis for GRIA 2 flip and flop as well as GRIA 3 flip and flop. In addition, one subject was removed from the schizophrenia group and one subject was removed from the major depressive disorder group for GRIA 2 flip studies.
Figure 2.
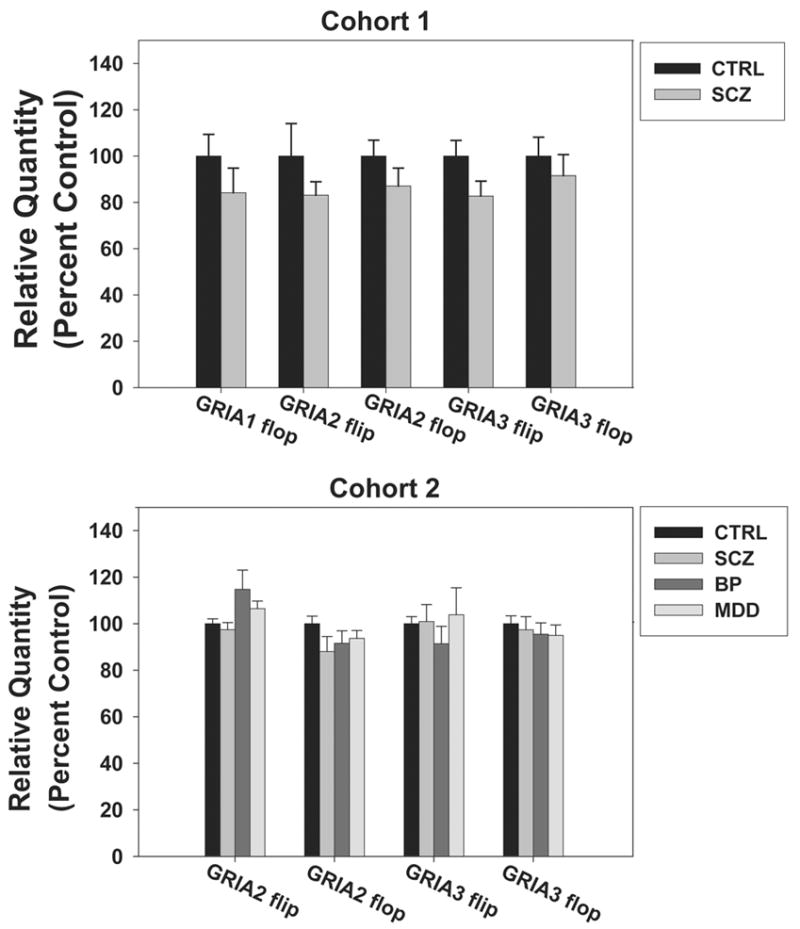
AMPA receptor flip and flop splice variant mRNA levels in the dorsolateral prefrontal cortex (DLPFC) of an elderly cohort of schizophrenia patients (SCZ) and controls (CTRL) (top) and a younger cohort of schizophrenia patients (SCZ), bipolar disorder patients (BP), major depressive disorder patients (MDD), and controls (bottom). Data are expressed as mean (± S.E.M.) of the percent control values for normalized relative quantities. There were no significant differences compared to control subjects (p< 0.05).
3.4. Effect of APD treatment on the expression of GRIA 1–4 and flip/flop splice variants in the DLPFC
In Cohort 1, the schizophrenia group could be divided into those patients receiving APDs within one month of death (n=8) and those subjects who were medication free for the last month of their life (n=4). There was no significant group effect (drug treated n=8, drug free n=4, or control n=11) for any of the subunits or splice variants measured: GRIA 1 [F= 0.27, p= 0.76], GRIA 2 [F= 2.09, p= 0.15], GRIA 3 [F= 0.04, p= 0.95], GRIA4 [F= 1.27, p= 0.31], GRIA2 flip [F= 0.97, p= 0.41], GRIA2 flop [F= 0.71, p= 0.55], GRIA3 flip [F= 1.09, p= 0.37], GRIA3 flop [F= 0.29, p= 0.83]. For Cohort 2, the Stanley Foundation reports what the equivalent dose of fluphenazine would be for their lifetime APD exposure. Correlating gene expression with APD exposure is one method to determine a relationship between gene expression and APD exposure. AMPA receptor subunit and splice variant expression was not significantly correlated with the lifetime fluphenazine equivalent dose: GRIA 1 [R2=−.16, p= 0.39], GRIA2 [R2= −0.22, p= 0.24], GRIA3 [R2= −0.13, p= 0.49], GRIA4 [R2= 0.1, p= 0.94], GRIA 2 flip [R2= −0.21, p= 0.29], GRIA 2 flop [R2= −0.22, p= 0.25], GRIA 3 flip [R2=−0.07, p= 0.71], GRIA3 flop [R2= −0.04, p= 0.82].
3.5. Expression of GRIA1–4 and flip/flop splice variants in the DLPFC of APD treated monkeys
To further investigate whether chronic APD treatment may alter GRIA1–4 subunit or splice variant expression, we examined the expression of these subunits in the DLPFC of rhesus monkeys that received six months of treatment with clozapine, haloperidol, or no drug. There was an APD treatment effect on GRIA1 subunit mRNA [F=10.28, p=0.003], GRIA2 subunit mRNA [F=8.83, p=0.004], and GRIA3 subunit mRNA [F=11.33, p=0.002], with no significant treatment effect on GRIA4 subunit mRNA [F=2.29, p=0.144]. Post hoc analysis confirmed that clozapine treatment significantly reduced GRIA1subunit mRNA expression (p=0.017) and increased GRIA3 subunit mRNA expression (p=0.017) while GRIA2 subunit mRNA expression was increased by both clozapine and haloperidol (p=0.017 and p=0.025 respectively), see Figure 3. GRIA1 flip, GRIA1 flop, and GRIA4 flip were not reliably detected in these samples. For the remaining splice variants, there was no significant effect of APD treatment: GRIA2 flip [F=0.57, p=0.581], GRIA2 flop [F=2.29, p=0.144], GRIA3 flip [F=1.65, p=0.233], GRIA3 flop [F=3.66, p=0.057], GRIA4 flop [F=2.291, p=0.144] (Figure 4).
Figure 3.
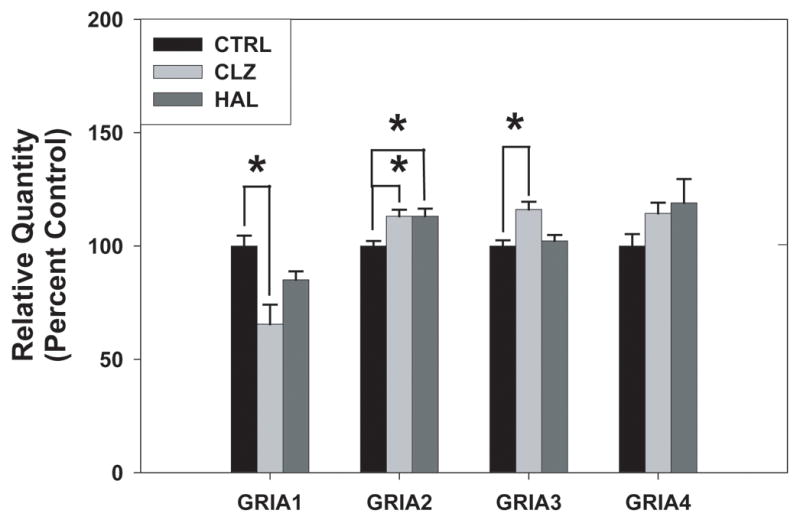
AMPA receptor subunit mRNA levels in the dorsolateral prefrontal cortex (DLPFC) of rhesus monkeys following six month administration of haloperidol, clozapine, or no drug. Data are expressed as mean (± S.E.M.) of the percent control values for normalized relative quantities. Asterisks indicate a significant difference compared to controls (p< 0.05). There was a significant decrease in GRIA 1 with clozapine treatment, a significant increase in GRIA 2 with clozapine and haloperidol treatment, and a significant increase in GRIA3 with clozapine treatment.
Figure 4.
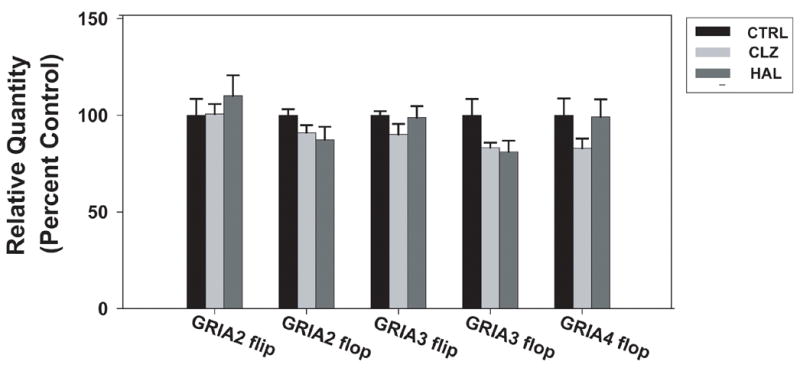
AMPA receptor flip and flop splice variant mRNA levels in the dorsolateral prefrontal cortex (DLPFC) of rhesus monkeys following six month administration of haloperidol, clozapine, or no drug. Data are expressed as mean (± S.E.M.) of the percent control values for normalized relative quantities. There were no significant differences compared to control subjects (p< 0.05).
4. Discussion
Previous studies assessing AMPA receptor subunit mRNA expression in the DLPFC of schizophrenic subjects have been equivocal with reports of no change (Healy et al. 1998), decreased GRIA2 (Vawter et al. 2002), and increased GRIA1 and GRIA4 mRNA levels (Dracheva et al. 2005). In agreement with the initial report of no change in AMPA receptor subunit mRNA between schizophrenia and controls (Healy et al. 1998), our study found no difference between schizophrenia and controls in the expression of any AMPA receptor subunits or splice variant mRNAs in either of the two schizophrenia cohorts investigated. Possible explanations for the differences between the results of this study and previous work include technical differences, as well as differences in the schizophrenia cohorts studied. In situ hybridization and microarray methods have advantages in cellular resolution and ability to study large numbers of transcripts, respectively; however, qPCR, used by Dracheva et al. and in this study has significant advantages in sensitivity and reliability for quantification (Bonnet et al. 1999; Bustin 2000; Bustin and Nolan 2004; Tyagi and Kramer 1996). A caveat of qPCR studies is the selection of endogenous controls for normalization. It is possible that previous reports of increased GRIA1 and GRIA4 mRNA in the schizophrenia DLPFC (Dracheva et al. 2005) reflect normalization to a single endogenous control which can cause significant errors in quantification (Ohl et al. 2005; Vandesompele et al. 2002). Alternatively, it is possible that increases in GRIA1 and GRIA4 mRNA expression were unique to the elderly cohort examined in the Dracheva et al study. Cohort 1 of our study shared a very similar clinical profile to that previous cohort, and an increase in GRIA 4 was observed in our Cohort, although it did not reach significance. However, this explanation seems unlikely for GRIA1 given the absence of even a trend toward increase in Cohort1 of this study. While, the negative results of this study must be interpreted cautiously given that the power of these findings was below the desired power of β = 0.8, a sample size calculation based on the observed difference between the group means and observed standard deviation in each of the Cohort1 studies determined that a sample size of 253, 64, 615, or 37 for each group would be necessary to determine a significant difference (α ≤ 0.05) with the desired power (β= 0.8) for GRIA1, 2, 3 and 4 subunit mRNA, respectively. Aside from the practical infeasibility of such large sample sizes, this need suggests that altered AMPA receptor subunit expression is not a prominent feature of schizophrenic pathology.
All schizophrenia subjects studied here were previously treated with APDs, and data from the rodent literature suggests that APD administration alters AMPA receptor subunit expression in the brain (Brene et al. 1998; Healy and Meador-Woodruff 1997; Meador-Woodruff et al. 1996; Spurney et al. 1999). In an attempt to determine the effect of APD exposure on AMPA receptor subunit mRNA in schizophrenia subjects, the schizophrenia group was separated into those receiving APDs at the time of death and those that were medication free one month prior to death (Cohort 1) for further analysis, and AMPA receptor subunit expression was correlated with lifetime exposure of APD (Cohort 2). These strategies are typically employed when investigating APD effect relative to gene expression in human schizophrenia post-mortem tissue; however, there are several limitations to this approach. First, limited sample size makes it difficult to determine significant drug effect when comparing treated versus untreated subjects or correlating APD treatment with gene expression. In addition, chronic APD treatment is thought to induce long term cellular and neuroadaptive changes that would influence gene expression (Hyman and Nestler 1996; MacDonald et al. 2005). Precedence for persisting drug effects exists in the dopamine system literature, e.g. the sensitization to motor activation effects of psychostimulants (Hummel and Unterwald 2002; Kalivas and Stewart 1991; Robinson and Berridge 2000; Vanderschuren and Kalivas 2000) or long lasting improvement in cognition due to repeated, intermittent stimulation of prefrontal D1 receptors (Castner and Goldman-Rakic 2004; Castner et al. 2000). It is therefore possible that chronic effects of APD treatment persist beyond the cessation of treatment, and subdividing schizophrenia subjects into drug treated and drug free at the time of death is only accounting for acute effects of the APDs on gene expression. As a result of these limitations, researchers have suggested a multi-tiered approach to investigating APD treatment effects in human schizophrenia studies that includes an assessment of chronic APD treatment effects in non-human primates in addition to the aforementioned strategies (Dorph-Petersen et al. 2005; Lewis 2002).
In this study, a non-human primate model of chronic APD administration similar to those used previously (Dorph-Petersen et al. 2005; Lidow et al. 1997; Lidow and Goldman-Rakic 1997a; Mirnics et al. 2000; Mirnics et al. 2001), demonstrated that chronic administration of clozapine decreased the expression of GRIA1 mRNA and increased the expression of GRIA2 and GRIA3 mRNA in the primate DLPFC. Haloperidol administration also increased the expression of GRIA2 mRNA in the primate DLPFC, but had no effect on expression of the other subunits. While these findings are consistent with rodent studies demonstrating that APDs alter AMPA receptor subunit expression (Brene et al. 1998; Healy and Meador-Woodruff 1997; Meador-Woodruff et al. 1996; Spurney et al. 1999), there were differences in the specific changes observed. There is no simple way to resolve the differences observed in this study and previous studies in rats, but differences in metabolism, route of administration, and length of exposure may all contribute to differences between rodents and primates. Furthermore, direct comparisons of drug -induced alterations in prefrontal cortical regions between rodents and primates is difficult due to the marked expansion and differentiation of the primate cerebral cortex compared to the rat (Lewis and Akil 1997; Preuss 1995; Preuss 2001) and in the complexity of primate thalamocortical projections (Haber and Gdowski 2004) and mesocortical dopamine projections in primates (Francois et al. 1999; Lynd-Balta and Haber 1994; Williams and Goldman-Rakic 1998). Also, use of primates enables a closer approximation of antipsychotic doses used in psychiatric patients (Castner et al. 2000; Lidow et al. 1997; Lidow and Goldman-Rakic 1994; Lidow and Goldman-Rakic 1997a, 2006), and offers similar metabolism of clozapine and haloperidol in humans and monkeys (Bun et al. 1999; Stafford et al. 1981).
Given the lack of observed changes in AMPA receptor subunit mRNA in the two cohorts of human schizophrenia subjects studied here, it may be that observations in drug treated patients represent normalization of pre-existing, disease-related alterations (e.g. schizophrenia may be associated with a reduction in GRIA2 expression which is normalized by the effects of antipsychotics). It is prudent to recall the limitations of dividing schizophrenia subjects into drug-treated and drug-free at the time of death to determine APD effect: limited power and no control for chronic drug effects. Therefore, the potential normalization of pre-existing, disease related alterations in schizophrenia is a viable explanation for the lack of observed alterations in AMPA receptor subunit mRNA in the schizophrenic DLPFC. Alternatively, the finding of a drug effect in non-human primates, while no effect was observed in drug treated patients may be due to differences between a non-diseased brain (monkey) and a disease brain (human SCZ). Resolution of this issue will require future studies in neuroleptic naïve schizophrenia patients, or in animal models of psychosis.
AMPA receptor subunit expression controls aspects of synaptic plasticity and calcium signaling, deficits of which are thought to be involved in schizophrenia. While APDs are known to act partially through their interactions with the dopamine system, the glutamate system has also been implicated in APD mechanism of action. This is the first study to demonstrate that chronic APD administration alters AMPA receptor subunit expression in the primate DLPFC and suggests that APD mechanism of action may involve altered AMPA receptor subunit expression and may alleviate deficiencies in synaptic plasticity or calcium signaling in schizophrenia. For example, the GluR1 subunit (encoded by GRIA1) is required for NMDAR-dependent synaptic delivery of AMPA receptors to the membrane (Hayashi et al. 2000; Shi et al. 2001; Shi et al. 1999). Interestingly, chronic APD administration has been shown to decrease expression of dopamine D1 receptor mRNA in the primate DLPFC (Lidow et al. 1997; Lidow and Goldman-Rakic 1994). Also, repeated intermittent stimulation of D1 receptors is known to increase the presence of GluR1 containing AMPA receptors in the synapse (Smith et al. 2005). Therefore, it is possible that APD administration decreases the availability of D1 receptors for activation which results in secondary decreases in GluR1. Our study showed that both clozapine and haloperidol decreased expression of GRIA1; however, only decreases with clozapine were significant. Given that decreases in D1 receptor mRNA were observed with slightly higher daily doses of haloperidol, it is possible that decreases in GRIA1 mRNA expression might also be observed at a slightly higher dose. Experiments determining the dose effect curves in these APD treated monkeys are underway in our laboratory, but are beyond the scope of this study. Interestingly, clozapine binds with high affinity to D1 receptors in the primate prefrontal cortex (Chou et al. 2006), and it is therefore possible that this higher affinity for D1 receptors is responsible for the drug selective effects on GRIA1 subunit mRNA expression observed here. Therefore our data suggests that one potential mechanism by which clozapine could alter AMPA-mediated glutamate neurotransmission in the DLPFC is by decreasing the expression of GRIA1, potentially reducing the number of GluR1 containing AMPA receptors trafficked to the membrane. In addition, the GluR2/3 subunits (encoded for by GRIA2 and/or GRIA3) are important for maintenance of stable basal synaptic response (Passafaro et al. 2001; Shi et al. 2001). Clozapine and haloperidol both increased GRIA2 mRNA levels in the primate DLPFC. All APDs, including haloperidol and clozapine exert their affects, at least in part, through the antagonism of D2 receptors (Kapur and Mamo 2003). Previous studies have shown a direct link between stimulation of D2 receptors and GluR2 subunit expression in the plasma membrane (Zou et al. 2005). Given the importance of GluR2 in synaptic stability, this increase in GRIA2 mRNA may reflect a potential mechanism of action for APDs in the DLPFC. Similarly, clozapine administration resulted in an increase in GRIA3 mRNA. Similar to GluR2, GluR3 expression is also important to maintaining synaptic strength (Meng et al. 2003). An increase in GRIA3 may reflect an additional mechanism by which clozapine strengthens synaptic plasticity in the DLPFC of schizophrenia. Finally, altered calcium signaling has been proposed as a unifying hypothesis of schizophrenic pathology (Lidow 2003), and the calcium permeability of AMPA receptors is determined by the presence or absence of GluR2 (Dingledine et al. 1999; Jayakar and Dikshit 2004). This elevation in GRIA2 subunit mRNA is also important because GluR2 subunit expression in AMPA receptors renders AMPA receptor impermeable to calcium (Dingledine et al. 1999; Palmer et al. 2005). Therefore, increasing GRIA2 expression and decreasing calcium permeability of AMPA receptors expressed in the DLPFC is also a potential mechanism of action for APDs. Further studies into the functional consequences of these transcriptional alterations in AMPA-receptor mediated glutamate neurotransmission are warranted, but are beyond the scope of this study.
To summarize, this study used a superior quantification method and normalization strategy to assess differences in AMPA receptor subunit and splice variant mRNA in the DLPFC of two distinct cohorts of schizophrenia subjects. Consistent with an earlier study, no differences in AMPA receptor subunit or splice variant mRNA were observed between schizophrenia and controls in either cohort. All schizophrenia subjects in this study had a history of chronic APD treatment. This is the first study to show that chronic APD treatment induces alterations in AMPA receptor subunit mRNA in the primate DLPFC. These data from the APD treated monkeys not only suggest that alterations in AMPA receptor subunit mRNA expression might be difficult to detect in human subjects with a history of chronic APD treatment, but also provide some preliminary insight into potential mechanisms of action of clozapine and haloperidol.
Acknowledgments
The research was funded in part by NIH grants MH074313 (SEH), MH01194 (ECM), by the Stanley Medical Research Foundation (ECM & SEH), and by RR00165.
Abbreviations
- APD
antipsychotic drug
- DLPFC
dorsolateral prefrontal cortex
- qPCR
quantitative polymerase chain reaction
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-D-aspartic acid
- GRIA
glutamate receptor ionotropic AMPA
- B2M
β-2 microglobulin
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- RPLPO
ribosomal protein, large
- ACTB
β-actin
- 18S
ribosomal protein 18S
- TABP
TATA box binding protein
- HPRT1
hypoxanthine phosphoribosyltransferase 1
- TFRC
transferrin receptor
- PPIA
peptidylprolyl isomerase A (cyclophilin A)
- GUSB
β-glucuronidase
- PKG1
phosphoglycerate kinase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J.A. O’Connor, Email: joconnor@wfubmc.edu.
E.C. Muly, Email: ecmuly@rmy.emory.edu.
S.E. Arnold, Email: sarnold@mail.med.upenn.edu.
S.E. Hemby, Email: shemby@wfubmc.edu.
References
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270–6. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Gur RE, Shapiro RM, Fisher KR, Moberg PJ, Gibney MR, Gur RC, Blackwell P, Trojanowski JQ. Prospective clinicopathologic studies of schizophrenia: accrual and assessment of patients. Am J Psychiatry. 1995;152(5):731–7. doi: 10.1176/ajp.152.5.731. [DOI] [PubMed] [Google Scholar]
- Bonnet G, Tyagi S, Libchaber A, Kramer FR. Thermodynamic basis of the enhanced specificity of structured DNA probes. Proc Natl Acad Sci U S A. 1999;96(11):6171–6. doi: 10.1073/pnas.96.11.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brene S, Messer C, Nestler EJ. Expression of messenger RNAs encoding ionotropic glutamate receptors in rat brain: regulation by haloperidol. Neuroscience. 1998;84(3):813–23. doi: 10.1016/s0306-4522(97)00490-9. [DOI] [PubMed] [Google Scholar]
- Bun H, Disdier B, Aubert C, Catalin J. Interspecies variability and drug interactions of clozapine metabolism by microsomes. Fundam Clin Pharmacol. 1999;13(5):577–81. doi: 10.1111/j.1472-8206.1999.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev. 2000;31(2–3):138–46. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25(2):169–93. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15(3):155–66. [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2(5):315–24. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Enhancement of working memory in aged monkeys by a sensitizing regimen of dopamine D1 receptor stimulation. J Neurosci. 2004;24(6):1446–50. doi: 10.1523/JNEUROSCI.3987-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020–2. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- Chou YH, Halldin C, Farde L. Clozapine binds preferentially to cortical D1-like dopamine receptors in the primate brain: a PET study. Psychopharmacology (Berl) 2006;185(1):29–35. doi: 10.1007/s00213-005-0219-9. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff DC. Ionotropic glutamate receptors as therapeutic targets in schizophrenia. Curr Drug Target CNS Neurol Disord. 2002;1(2):183–9. doi: 10.2174/1568007024606212. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Domino EF, Chodoff P, Corssen G. Pharmacologic Effects of Ci-581, a New Dissociative Anesthetic, in Man. Clin Pharmacol Ther. 1965;6:279–91. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–61. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;158(9):1400–10. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Dracheva S, McGurk SR, Haroutunian V. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J Neurosci Res. 2005;79(6):868–78. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Eastwood SL, Burnet PW, Harrison PJ. GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT-PCR) study. Brain Res Mol Brain Res. 1997;44(1):92–8. doi: 10.1016/s0169-328x(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Porter RH, Harrison PJ. The effect of chronic haloperidol treatment on glutamate receptor subunit (GluR1, GluR2, KA1, KA2, NR1) mRNAs and glutamate binding protein mRNA in rat forebrain. Neurosci Lett. 1996;212(3):163–6. doi: 10.1016/0304-3940(96)12801-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Deutch AY, Gasic G, Heinemann SF, Nestler EJ. Regulation of cortical and subcortical glutamate receptor subunit expression by antipsychotic drugs. J Neurosci. 1995;15(3 Pt 2):2453–61. doi: 10.1523/JNEUROSCI.15-03-02453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois C, Yelnik J, Tande D, Agid Y, Hirsch EC. Dopaminergic cell group A8 in the monkey: anatomical organization and projections to the striatum. J Comp Neurol. 1999;414(3):334–47. [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367–77. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Haber SN, Gdowski MJ. The Basal Ganglia. In: Paxinos G, Mai JK, editors. The Human Nervous System. San Diego: Elsevier; 2004. pp. 676–738. [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287(5461):2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Healy DJ, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ, Meador-Woodruff JH. AMPA receptor binding and subunit mRNA expression in prefrontal cortex and striatum of elderly schizophrenics. Neuropsychopharmacology. 1998;19(4):278–86. doi: 10.1016/S0893-133X(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Healy DJ, Meador-Woodruff JH. Clozapine and haloperidol differentially affect AMPA and kainate receptor subunit mRNA levels in rat cortex and striatum. Brain Res Mol Brain Res. 1997;47(1–2):331–8. doi: 10.1016/s0169-328x(97)00064-8. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Ginsberg SD, Brunk B, Arnold SE, Trojanowski JQ, Eberwine JH. Gene expression profile for schizophrenia: discrete neuron transcription patterns in the entorhinal cortex. Arch Gen Psychiatry. 2002;59(7):631–40. doi: 10.1001/archpsyc.59.7.631. [DOI] [PubMed] [Google Scholar]
- Hummel M, Unterwald EM. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J Cell Physiol. 2002;191(1):17–27. doi: 10.1002/jcp.10078. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153(2):151–62. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jayakar SS, Dikshit M. AMPA receptor regulation mechanisms: future target for safer neuroprotective drugs. Int J Neurosci. 2004;114(6):695–734. doi: 10.1080/00207450490430453. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16(3):223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1081–90. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Koike M, Tsukada S, Tsuzuki K, Kijima H, Ozawa S. Regulation of kinetic properties of GluR2 AMPA receptor channels by alternative splicing. J Neurosci. 2000;20(6):2166–74. doi: 10.1523/JNEUROSCI.20-06-02166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB, Jr, Vegso S, Heninger GR, Charney DS. Interactive effects of subanesthetic ketamine haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145(2):193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist ketamine in humans. Psychotomimetic perceptual cognitive and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–67. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA. The human brain revisited: opportunities and challenges in postmortem studies of psychiatric disorders. Neuropsychopharmacology. 2002;26(2):143–54. doi: 10.1016/S0893-133X(01)00393-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Akil M. Cortical dopamine in schizophrenia: strategies for postmortem studies. J Psychiatr Res. 1997;31(2):175–95. doi: 10.1016/s0022-3956(96)00057-x. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev. 2003;43(1):70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Elsworth JD, Goldman-Rakic PS. Down-regulation of the D1 and D5 dopamine receptors in the primate prefrontal cortex by chronic treatment with antipsychotic drugs. J Pharmacol Exp Ther. 1997;281(1):597–603. [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. A common action of clozapine, haloperidol, and remoxipride on D1- and D2-dopaminergic receptors in the primate cerebral cortex. Proc Natl Acad Sci U S A. 1994;91(10):4353–6. doi: 10.1073/pnas.91.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. Differential regulation of D2 and D4 dopamine receptor mRNAs in the primate cerebral cortex vs. neostriatum: effects of chronic treatment with typical and atypical antipsychotic drugs. Journal of Pharmacology & Experimental Therapeutics. 1997a;283(2):939–46. [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. Differential regulation of D2 and D4 dopamine receptor mRNAs in the primate cerebral cortex vs. neostriatum: effects of chronic treatment with typical and atypical antipsychotic drugs. J Pharmacol Exp Ther. 1997b;283(2):939–46. [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81(3):363–9. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994;59(3):609–23. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- MacDonald ML, Eaton ME, Dudman JT, Konradi C. Antipsychotic drugs elevate mRNA levels of presynaptic proteins in the frontal cortex of the rat. Biol Psychiatry. 2005;57(9):1041–51. doi: 10.1016/j.biopsych.2005.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- McCoy L, Cox C, Richfield EK. Antipsychotic drug regulation of AMPA receptor affinity states and GluR1, GluR2 splice variant expression. Synapse. 1998;28(3):195–207. doi: 10.1002/(SICI)1098-2396(199803)28:3<195::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Healy DJ. Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev. 2000;31(2–3):288–94. doi: 10.1016/s0165-0173(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, King RE, Damask SP, Bovenkerk KA. Differential regulation of hippocampal AMPA and kainate receptor subunit expression by haloperidol and clozapine. Mol Psychiatry. 1996;1(1):41–53. [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39(1):163–76. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28(1):53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Molecular Psychiatry. 2001;6(3):293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266(5187):1059–62. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr Res. 2004;71(2–3):361–70. doi: 10.1016/j.schres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- O'Connor JA, Hasenkamp W, Horman BM, Muly EC, Hemby SE. Region Specific Regulation of NR1 in Rhesus Monkeys Following Chronic Antipsychotic Drug Administration. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Ohl F, Jung M, Xu C, Stephan C, Rabien A, Burkhardt M, Nitsche A, Kristiansen G, Loening SA, Radonic A, Jung K. Gene expression studies in prostate cancer tissue: which reference gene should be selected for normalization? J Mol Med. 2005;83(12):1014–24. doi: 10.1007/s00109-005-0703-z. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev. 2005;57(2):253–77. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4(9):917–26. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga A. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1999. [Google Scholar]
- PDR. Physician's Desk Reference. Oradell, NJ: Medical Economics; 1996. [Google Scholar]
- PDR. Physicians' Desk Reference. Montvale, NJ: Thomson PDR; 2006. [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The Rose- Woolsey-Akert Program reconsidered. Journal of Cognitive Neuroscience. 1995;7(1):1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Preuss TM. The discovery of cerebral diversity: An unwelcome scientific revolution. In: Falk D, Gibson K, editors. Evolutionary Anatomy of the Primate Cerebral Cortex. Cambridge: Cambridge University Press; 2001. pp. 138–164. [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 (Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105(3):331–43. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284(5421):1811–6. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45(5):765–79. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Phencyclidine. Nature. 1980;285(5764):355–6. doi: 10.1038/285355a0. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249(4976):1580–5. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Spurney CF, Baca SM, Murray AM, Jaskiw GE, Kleinman JE, Hyde TM. Differential effects of haloperidol and clozapine on ionotropic glutamate receptors in rats. Synapse. 1999;34(4):266–76. doi: 10.1002/(SICI)1098-2396(19991215)34:4<266::AID-SYN3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Stafford JE, Jackson LS, Forrest TJ, Barrow A, Palmer RF. Haloperidol pharmacokinetics: a preliminary study in rhesus monkeys using a new radioimmunoassay procedure. J Pharmacol Methods. 1981;6(4):261–79. doi: 10.1016/0160-5402(81)90067-x. [DOI] [PubMed] [Google Scholar]
- Stine CD, Lu W, Wolf ME. Expression of AMPA receptor flip and flop mRNAs in the nucleus accumbens and prefrontal cortex after neonatal ventral hippocampal lesions. Neuropsychopharmacology. 2001;24(3):253–66. doi: 10.1016/S0893-133X(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The stanley foundation brain collection and neuropathology consortium. Schizophr Res. 2000;44(2):151–5. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–79. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14(3):303–8. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151(2–3):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res. 2002;58(1):11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, TesFaye E, Yasuda RP, Mash DC, Armstrong DM, Wolfe BB. Effects of post-mortem delay on subunits of ionotropic glutamate receptors in human brain. Brain Res Mol Brain Res. 2000;80(2):123–31. doi: 10.1016/s0169-328x(00)00111-x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2):114–24. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex. 1998;8(4):321–45. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Zou S, Li L, Pei L, Vukusic B, Van Tol HH, Lee FJ, Wan Q, Liu F. Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity. J Neurosci. 2005;25(17):4385–95. doi: 10.1523/JNEUROSCI.5099-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


