Abstract
Selenium, a nutrient, and methylmercury, a developmental neurotoxicant, are both found in fish. There are reports that selenium sometimes ameliorates methylmercury’s neurotoxicity, but little is known about the durability of this protection after low-level gestational exposure. Developmental methylmercury exposure disrupts behavioral plasticity, and these effects extend well into adulthood and aging. The present experiment was designed to examine interactions between developmental low-level methylmercury and nutritionally relevant dietary selenium on discrimination reversals in adulthood. Female rats were exposed, in utero, to 0, 0.5, or 5 ppm mercury as methylmercury via drinking water, approximating mercury exposures of 0, 40, and 400 μg/kg/day. They also received both prenatal and postnatal exposure to a diet containing selenium from casein only (0.06 ppm) or 0.6 ppm selenium, creating a 2 (chronic Se) x 3 (gestational MeHg) full factorial design, with 6 – 8 rats per cell. Behavior was evaluated with a spatial discrimination procedure using two levers and sucrose reinforcers. All groups acquired the original discrimination similarly. Rats exposed to low selenium (0.06 ppm), regardless of MeHg exposure, required more sessions to complete the first reversal and made more omissions during this reversal than high selenium (0.6 ppm) animals, but the two diet groups did not differ on subsequent reversals. Rats exposed to MeHg, regardless of selenium exposure, made more errors than controls on the first and third reversals, which was away from the original discrimination. MeHg-exposed animals also had shorter choice latencies than controls during the first session of a reversal. Low selenium increased the number of omissions during a reversal, whereas high MeHg exposure produced perseverative responding (errors) on the lever that was reinforced during the original discrimination. However, there was no interaction between selenium and MeHg exposure.
Keywords: Methylmercury, Selenium, Development, Operant behavior, Prenatal exposure, perseveration, discrimination reversal, rats
INTRODUCTION
Fish consumption is the primary source of human exposure to methylmercury, a known developmental neurotoxicant (Clarkson, 2002; Schober, 2003), but fish are also a source of important nutrients such as selenium (Chapman and Chan, 2000). When administered concurrently with methylmercury, selenium confers some protection against certain neurological effects of chronic, high-level MeHg exposure (Magos, 1991; Moller-Madsen and Danscher, 1991) even as it elevates mercury concentration in many regions of the nervous system (Imura and Naganuma, 1991; Moller-Madsen, 1994; Moller-Madsen and Danscher, 1991; Prohaska and Ganther, 1977; Thomas and Smith, 1984), as well as in blood, liver, and testes (Whanger, 1992).
Less is known about whether selenium plays a protective role against the long-term effects of developmental methylmercury exposure. Selenium deficiency has been reported to exacerbate methylmercury’s neurodevelopmental effects as expressed in early development (Fredriksson, et al., 1993; Satoh, et al., 1985; Watanabe, 2001; Watanabe, et al., 1999). With the exception of methylmercury-induced hypoactivity tested at two months of age (Fredriksson, et al., 1993) this protection disappears before adulthood.
Effects of developmental methylmercury exposure that extend well into adulthood include slowed transitions during a choice-in-transition procedure (Newland, et al., 2004; Newland, et al., 1994). In this procedure, an animal faces two levers. When the relative reinforcement available from one lever changed, i.e., one lever becomes richer than the other, the behavior of all animals shifted so that most responding occurred on the richer lever in accordance with the “matching law” (Baum, 1979; Davison and McCarthy, 1988), but MeHg-exposed animals accomplished this transition more slowly than unexposed controls (Newland, et al., 2004; Newland, et al., 1994).
Another effect seen in adult animals exposed developmentally to MeHg is the more rapid acquisition of lever-pressing under large fixed-ratio schedules of reinforcement (Paletz, et al., 2006), a paradoxical-sounding effect that has also been reported after developmental exposure to cadmium (Newland, et al., 1986). In the methylmercury study, a fixed ratio (FR) schedule changed rapidly from FR1 to FR5, FR25 and FR75 (1, 5, 25, and 75 responses were required for a reinforcer, respectively) where each schedule was presented for only three days. Unexposed animals showed ragged, low-rate responding, called “ratio strain,” during initial sessions of the FR 75 schedule of reinforcement. Mercury and cadmium exposed animals, however, showed robust responding at the FR 75 schedule, almost as though the response requirement had not changed. This behavior pattern could be viewed as a methylmercury-induced alteration in the sensitivity of behavior to a change in the reinforcement contingency. Such insensitivity would result in a perseverative behavior pattern that, in turn, would facilitate acquisition of these large FR schedules, insofar as perseveration is manifested as persistent responding even after reinforcers are no longer delivered.
The present experiment was designed to examine the effects of developmental MeHg exposure on behavioral plasticity using a spatial discrimination reversal procedure, and the potential protective role of selenium against these effects. The experiments were conducted using a 2 (chronic Se) x 3 (gestational MeHg) full factorial design, which allows for the direct examination of interactions between MeHg and selenium, as well as the main effects of either element. Female rats were exposed gestationally to 0, 0.5, or 5 ppm of mercury as MeHg, producing levels relevant to human exposure (Burbacher, et al., 1990; Newland and Reile, 1999b). They were also exposed, pre- and postnatally, to a diet that was either marginal (0.06 ppm) or rich (0.6 ppm) in selenium. The lower concentration is the lowest possible with a casein-based diet; the selenium content comes only from casein and can vary somewhat. This is a low but still nutritionally adequate level of selenium for rodents (National Research Council, 1995; Reeves, et al., 1993). The higher concentration represents an excess over the AIN-93 formulation, which contains 0.15 ppm of selenium (Reeves, 1997; Reeves, et al., 1993), but is below that thought to be toxic (Abdo, 1994). Upon reaching adulthood, female offspring were trained to lever-press under a spatial discrimination procedure. Initially, only left-lever presses were followed by reinforcement (original discrimination, or OD-left). Once subjects reached a criterion of 85% or more of the left-lever trials ending in reinforcement, only right-lever presses were reinforced (first reversal, or R1-right). After reaching the same performance criterion on the right lever, the contingency reversed back to the left lever (second reversal, or R2-left), and so on, resulting in a total of 4 discrimination conditions (OD – R3).
METHODS
Subjects
The subjects were 44 female Long-Evans rats (F1 generation) housed in environmentally-controlled colony rooms with a 12:12 light-dark cycle (lights on at 7:00 a.m.). Subjects were bred in the laboratory, and each was randomly selected from a separate litter, so the litter served as the statistical unit in all analyses. These rats were exposed in utero to methylmercury via maternal consumption of drinking water containing 0, 0.5, or 5 ppm of mercury as methylmercuric chloride (Alfa Aesar, Ward Hill, MA) and a diet containing approximately 0.06 or 0.6 ppm selenium throughout life (detailed below) forming a 2 (chronic diet) x 3 (developmental MeHg) factorial design. There were six to eight rats per experimental group.
After weaning on postnatal day (PND) 21, the female offspring were injected subcutaneously with an electronic identification chip (Biomedic Data Systems, Seaford, DE). They were housed two per cage and separated by a transparent divider diagonally placed in the cage so that feeding could be tailored to each individual rat’s requirement while maintaining adequate space requirements for each rat. During adulthood, after PND 90, their food was rationed to approximately 10 gm/day so as to maintain their body weight at 250 grams. Rats that shared a home cage also received the same diet (see Exposures) so that diets were never mixed. To prevent excessive tooth growth, a cleaned, nylon chew “bone” was freely available in the home cage. They were 12 ± 1 months of age at the beginning of the present experiment.
Selenium Exposure
At 18 weeks (125 days) of age, female F0 breeders were placed on one of two diets, each based on the AIN-93 formula for laboratory rodents but customized for selenium concentration (Time line in Figure 1). The “low selenium” diet contained selenium from casein only, and the concentration was 0.06 ppm. The “high selenium” diet was supplemented with sodium selenite to produce 0.6 ppm. Selenium content of the diets was analyzed using inductively coupled plasma mass spectrometry (ICP-MS). Analyses revealed actual concentrations between 0.05 and, in one shipment used for adult consumption, 0.1 ppm in the low-Se and 0.6 and 0.9 ppm in the high-Se diets. Between mating and lactation, the base diet was an AIN 93 growth diet containing 7% fat from soybean oil. A maintenance diet of an AIN 93 diet with 4% fat was used at all other times. Both diets were obtained from Research Diets Inc (New Brunswick, NJ.). Dietary mercury was below the detectable level of 50 ppb. Male breeders were maintained on the chow diet, except when briefly exposed to the female’s diet during breeding (see Breeding). All F1 offspring received the same diet as their maternal dams throughout life.
Figure 1.
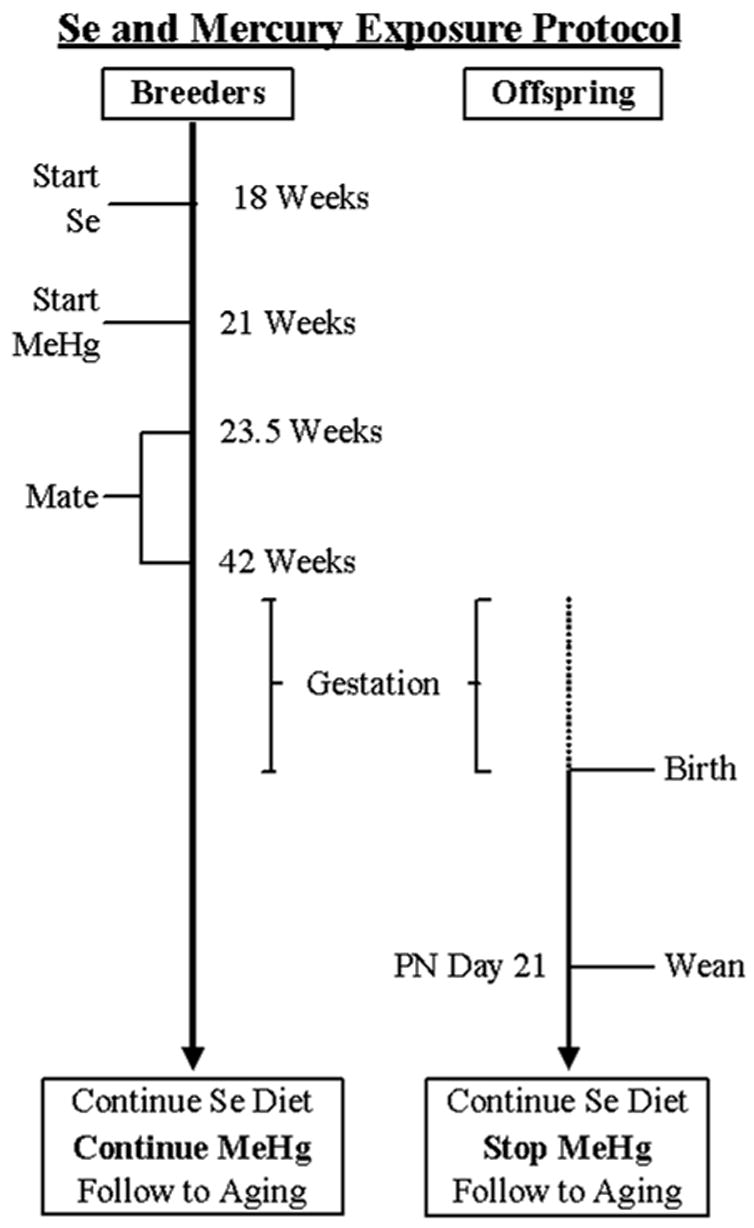
Timeline for breeding and exposure for F0 breeders and F1 offspring. Note exposure to methylmercury ended for offspring at weaning, including those that were used as subjects in the present experiment. Breeders were not included in the present experiment. See text for details.
Methylmercury Exposure
At approximately 21 weeks (145 days of age), after three weeks (20 days) on the custom selenium diets, each selenium group of F0 breeders was further divided into three methylmercury exposure groups to create 6 experimental groups. Methylmercury was added to drinking water of F0 breeders in concentrations of 0, 0.5, or 5 ppm of mercury as methylmercuric chloride, (Alfa Aesar, Ward Hill, MA). These concentrations produce exposures of about 0, 40 and 400 μg/kg/day respectively, based on average daily consumption, with some elevation during gestation due to increased fluid consumption (Newland and Reile, 1999b). Sodium carbonate (< 5 nanomolar), which can buffer the MeHg (Stern, et al., 2001), was added to all three water mixtures. Maternal exposure to the MeHg-containing water was discontinued on post-natal day 16 when the F1 pups were capable of reaching the water spout. Throughout the remainder of life, all F1 rats received plain tap water to drink. Male breeders received exposure to plain tap water only.
Breeding
Beginning at approximately 23.5 weeks of age, after two and half weeks of MeHg exposure, and continuing to 42 weeks of age, 58 male and 114 female Long-Evans rats (F0 generation; Harlan, Indianapolis, IN) were bred. Breeding cages contained the female’s diet and tap water, so males were never exposed to MeHg. Each male was paired with a single female during every other dark cycle. Most males were paired with a second female during alternating dark cycles. A male was paired with the same female(s) throughout breeding. When a male was bred with two females, the females were always members of different exposure groups. Breeding of females continued until systematic increases in daily body weight were observed, suggesting gravidity. Births before 5:00 pm were assigned to PND 0 for that day. All births after 5:00 pm were assigned to PND 0 for the subsequent day. Large litters were culled and small litters combined to produce 8 F1 pups including at least three females when possible, but only one female per litter was included in the present study. Behavior of the F0 rats will not be described here.
All procedures were approved by the Auburn University Institutional Animal Care and Use Committee. The colony was housed in an AAALAC-accredited facility that also met PHS guidelines for animal care. All rats were monitored daily by the research staff and personnel from the Department of Laboratory Animal Health at Auburn University.
Testing Apparatus
The experiments were conducted in 16 commercially purchased operant chambers (Med-Associates, Model ENV-008) containing one rear-mounted lever and two front, retractable levers (each calibrated so that 0.20 N registered a press), a pellet dispenser situated between the two front levers and filled with 20 mg sucrose pellets (Research Diets, Inc., New Brunswick, NJ), Sonalert tones™ (2900 and 4500 Hz, nominally; adjusted to an amplitude of 70 dbC), a house light (28 V 100 ma), and a light emitting diode (LED) above each lever. Drinking water was freely available through a custom mounted bottle with a spout to the left of the rear lever. Each chamber was surrounded by a sound-attenuating cabinet with built-in ventilating fan that circulated air into the experimental environment and provided masking white noise. Programs for experimental procedures and data collection were written using MED-PC IV (Med-Associates, Georgia, VT). Session events were recorded with 0.01” resolution.
Behavioral Methods
At the beginning of the study and throughout experimental testing, body weights did not differ among any of the exposure groups. Morning sessions for each of three squads of subjects were conducted daily at different, but consecutive, times; assignment of subjects to squads and chambers was distributed across exposure groups. Fans, lights, tones, levers, and pellet dispensers were tested before and after sessions for each squad of rats to ensure that equipment was functioning properly. Electronic identification chips were used to track subjects, and rats were scanned prior to each session to insure they were placed in the appropriate chamber and home cage.
Autoshaping of lever-pressing
Left lever-pressing was autoshaped first in an overnight session, as follows. The lever was extended and a light over the lever was lit for 30 seconds or until the lever was pressed. Either event resulted in the retraction of the lever, darkening of the light, pellet delivery, and the beginning of a five minute intertrial interval. After the left lever was pressed 10 times, the autoshaping procedure ended, a fixed ratio (FR) 1 schedule was in effect, and the session continued until 100 left lever presses occurred. In the next overnight session, the right lever was extended, the right lever-light was lit continuously, and the left lever remained retracted; this session continued until 100 right lever presses occurred. In a third overnight session, both left and right levers remained retracted, a light over the rear lever was lit continuously, and the session continued until 100 responses occurred on the rear lever.
Two-lever training
The goal of this training phase was to establish a two-lever response sequence in which a rear-lever press extended a front lever that, if pressed, resulted in the delivery of a sucrose pellet. Each trial began with an alternating 2900 Hz tone (0.3-seconds on, 0.6-seconds off). When the rear lever was pressed, the tone stopped and either the left or right front levers extended into the chamber, determined pseudo-randomly, and the LED above the extended lever was lit until that lever was pressed. After being pressed, the lever retracted, the light turned off, a pellet was delivered, and the trial ended. To limit position bias, the lever was extended into the same side of the chamber until it was pressed (correction procedure); only after this response was the side for the subsequent trial redetermined. If the rear lever was not pressed within 5 minutes after the onset of the tone, the trial ended without a sucrose pellet. If the front lever was not pressed within 5 minutes after it was extended, the trial ended without a sucrose pellet. There was a 10-second interval between trials (intertrial interval, or ITI). Initially, rear-lever presses during the ITI had no programmed consequences, but after six trials ending with sucrose reinforcement, ITI responses reset the incrementing timer to 0 seconds. The two-lever response sequence was used to prevent rats from standing in one place throughout the course of the session (i.e., in front of either retractable lever). Sessions lasted 100 trials or until 12 cumulative trials ended in reinforcement, with the latter also being the criterion for ending training.
Original Discrimination and Spatial Discrimination Reversal
The procedure for the original discrimination (OD) and spatial discrimination reversal task (SDR) was similar to that of training with some exceptions. First, both front levers extended (and LEDs above them turned on) when the rear lever was pressed. Second, if the rear lever was not pressed within 15 seconds after the onset of the alternating tone, or a front lever was not pressed within 15 seconds after it was extended, the levers retracted and the trial ended without reinforcement. Third, responding on only one of the front levers was reinforced; responses on the other lever ended the trial without reinforcement. Fourth, there was no correction procedure during testing.
Initially, only left-lever presses were followed by reinforcement (original discrimination, or OD-left). The requirement that a trial begin with a rear lever-press remained in place throughout the experiment. After subjects completed three consecutive sessions with 85% (51 out of 60 trials) or more of the left-lever trials ending in reinforcement, only right-lever presses were reinforced (first reversal, or R1-right). After reaching the same performance criterion, only left lever-presses were reinforced (second reversal, or R2-left). Following criterion performance on the left lever, only right lever-presses were reinforced (third reversal, or R3-right), resulting in a total of 4 discrimination conditions (OD – R3). Each session lasted 60 trials.
Individual graphs for each rat were made and assessed on a daily basis. Three rats did not reach criterion because of a failure to initiate the response chain when a trial began. This failure to reach criterion was not because of the occurrence of errors, and original acquisition of the task was similar to that of other rats. The animals failed to initiate the response sequence, suggesting that the sucrose pellet was not sufficiently reinforcing for these three animals. To increase the reinforcing efficacy of the sucrose pellet, these animals’ bodyweights were reduced to 220 grams. All three animals were on the low-selenium diet but differed in methylmercury exposure. Two rats exposed to 0 ppm Hg received this manipulation during the first reversal (R1-right), and one 0.5 ppm Hg-exposed rat received it during the original discrimination phase (OD-left). This manipulation was used after 15 to 30 sessions passed with fewer than four errors, stable and accurate performance, but many omissions (trials without a response). Responding was reinstated in these animals at the lower body weight. The criterion of 51 correct responses for three consecutive sessions was met within three to five days of this intervention. All sessions were included in the data analysis. The lower bodyweight was maintained throughout the remainder of the experiment for these rats.
A second intervention was imposed for two low-selenium (one exposed to 0 and the other to 5.0 ppm Hg) animals that did not reach criterion because of a failure to change levers after 17 or 21 sessions into the first reversal (R1-right). Sessions consisted of many omissions and errors, but no correct responses. The second intervention consisted of trials in which only the correct lever was inserted following a rear lever press. These animals are described in more detail below. These intervention sessions were not included in data analyses.
Data and Statistical Analyses
All statistical analyses were performed using SYSTAT® 11 (SYSTAT Software Inc. Richmond, CA, USA). The Type I error rate (α) was set at 0.05 for all tests. In order to provide a full characterization of behavior, six dependent variables were analyzed, and reversal was treated as a within-subject variable for the original discrimination and each reversal:
Sessions – the total number of sessions required to reach criterion for each reversal.
Reinforced trials– the total number of reinforced trials during each reversal.
Omissions- the total number of trials without a rear-lever or front-lever press for each reversal.
Errors – the total number of unreinforced trials on a front lever for each reversal.
Rear-lever latency - average time per trial between the onset of the trial (alternating tone) and the rear-lever press for the first session of each reversal. Trials without a rear lever-press were scored as 15′′.
Choice latency – average time per trial between a rear-lever press and a front-lever press for the first session of each reversal. Trials without a choice response were scored as 15′′.
For the repeated-measures analysis of variance (RMANOVA), MeHg (0, 0.5, 5 ppm) and Se (0.06 ppm, 0.6 ppm) served as the two between-subjects factors, with 6 – 8 rats per cell. Reversal (OD-R3) served as the within-subject factor. Each omnibus RMANOVA permitted the detection of four within-subject effects (Reversal, MeHg*Reversal, Se*Reversal, and MeHg*Se*Reversal) and three between-subjects effects (MeHg, Se, and MeHg*Se). The Huynh-Feldt correction was always used to adjust degrees of freedom for the tests of within-subjects-effect.
When the RMANOVA identified a significant effect, one-way analyses of variance (ANOVA) were conducted for each reversal in order to identify those that contributed to between or within subject effects. Significant ANOVAs were followed by pairwise, post hoc comparisons among the three MeHg dose groups to determine which differed from each other; post hoc comparisons were unnecessary for Se, as it involved only a single comparison. Log transformations were performed on some dependent measures so as to equate variability across groups. F- ratios, degrees of freedom, and p-values are reported for all RMANOVAs and one-way ANOVAs, and p-values are reported for post-hoc contrasts and comparisons. Only significant effects are reported for each variable. If not reported, the p-value was greater than 0.1.
RESULTS
Training
There were no main effects of either MeHg or Se, and no interaction between them, on acquisition of lever-pressing or of the two-lever response chain.
Spatial Discrimination Reversal Phase
There was a within-subject effect of reversal on all dependent measures (Ps<.01). Sessions to criterion, errors and omissions all increased on reversal 1 and declined between reversals 1 and 3. Choice latency during the first [F(3,114)=30.775,P<.001] and last [F(3,114) = 4.87, P = 0.008] session of each reversal declined across reversals. In addition, choice latency during the first session of a reversal was always longer than the latency seen on subsequent sessions. On no analysis conducted was an interaction between selenium and MeHg detected.
MeHg Effects
Figure 2 illustrates three types of performances observed. The top panel represents behavioral measures for most low-selenium, 0 Hg rats and all high-selenium, 0 Hg rats throughout the reversals. There was a large increase in errors during the first session of each reversal, but these declined quickly, and most lever-presses occurred on the correct lever (black circles) after the first session of a reversal. Some omissions (trials with no response, unfilled circles) occurred, but these were infrequent. Choice latency increased during the first session of a reversal but shortened in subsequent sessions. Latency to press the rear lever after trial onset did not change systematically. Criterion was typically reached within four to five sessions.
Figure 2.
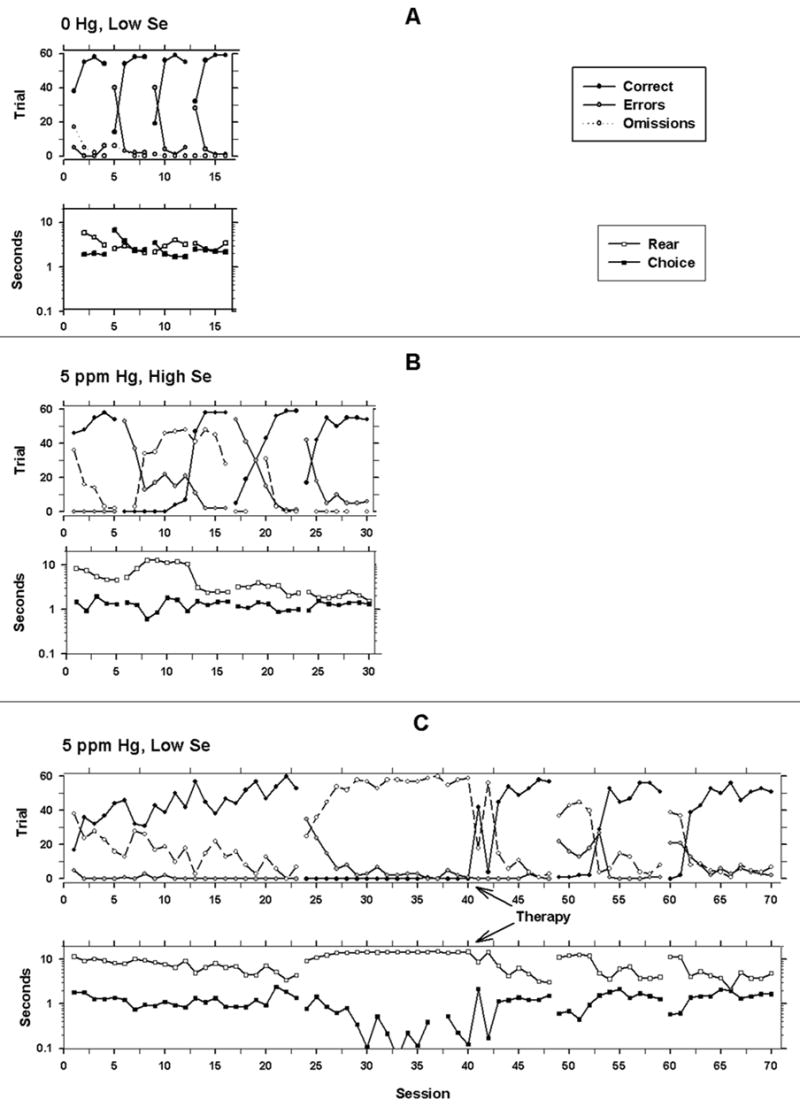
Representative graphs of the dependent measures for each of the conditions for a 0 ppm, low selenium rat (A), 5 ppm Hg, high selenium rat (B), and a 5 ppm Hg, low selenium rat (C). The abscissas show sessions. The ordinate is trials (top graph in each panel) or seconds (bottom graph in each panel with logarithmic scaling). The gaps in data represent reversals. The filled circles show correct trials (reinforced trials); the gray circles show errors, and the unfilled circles show omission trials for the top graph in each panel. The open squares show latency (in seconds) to make a rear lever press to initiate the trial. The filled squares show the latency to make a choice between the front two levers.
The methylmercury-exposed rats (Figure 2, middle panel) exhibited response patterns similar to control animals during the original discrimination, but made more errors during reversals to the right lever, or the 1st and 3rd reversals. This MeHg-exposed animal, like many others, did not exhibit increases in choice latencies for the first session of each reversal. The bottom panel of Figure 2 illustrates the response pattern seen in the two low-selenium animals that required the second therapeutic intervention. No responses occurred on the correct (right) lever, so no reinforcers were delivered and responding gradually declined to very low rates. When only the right lever was inserted during therapy, responding occurred on that lever and continued after both levers were inserted. Subsequent transitions occurred without requiring such an intervention. Behavior measures were collected and analyzed for all sessions except the sessions during which only one lever was available.
RMANOVA analyses revealed a between-subjects effect of methylmercury on choice latency in the first session [F(2,38)=3.47 P=.041] of each reversal. There was a marginal between-subjects effect of mercury on errors [F(2,38)=3.004, P=.061].
One-way ANOVAs confirmed that the between-subjects effect appeared on more than one reversal. Methylmercury exposure increased the number of errors during R1-right [F(2,38)=3.348, P=.046, log-transform] and R3-right [F(2,38)=3.948, P=.028] as illustrated in the top panel of Figure 3. Post-hoc tests revealed that the 5 ppm Hg group made more errors than the controls (P=.017) in R1-right and more errors than both the controls (P=.023) and 0.5 ppm Hg group (P=.02) during R3-right. The 0.5 ppm group was indistinguishable from the 5 ppm group (P= .605) during R1-right, and it was indistinguishable from the 0 ppm group (P= .103) on the number of errors during R3-right. Inspection of the figures suggests that this low-dose group nearly overlapped the high-dose group on the first transition but approached the 0 ppm Hg groups more rapidly. The elevated variability in this low-dose group may have precluded the ability to distinguish it from controls statistically during R1-right. An increase in errors was not detectable with one-way ANOVAs during OD-left or R2-left (Ps > 0.5).
Figure 3.
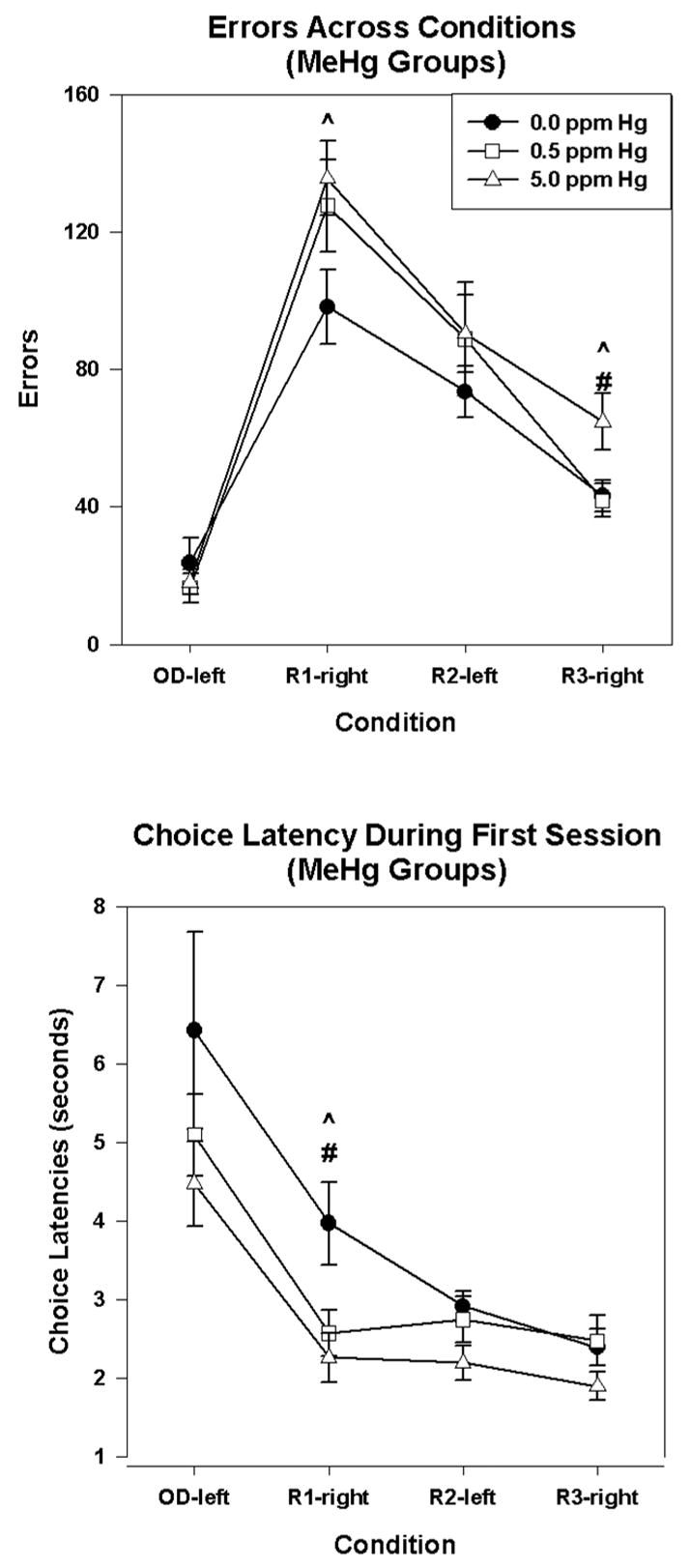
Condition by MeHg interaction showing the total number of errors (top) and choice latency during the first session of each condition (bottom) for the 0.0 ppm Hg group (filled circles), 0.5 ppm Hg group (open squares), and 5 ppm Hg group (open triangles), combined across selenium exposure, for each condition.(^) p<0.05 for the 5 ppm group compared to controls. (#) p<0.05 for the 5 ppm group compared to the 0.5 ppm Hg group. Error bars represent ± 1 SEM.
The bottom of Figure 3 confirms that the between-subjects effect of mercury on choice latency occurred across multiple reversals. MeHg exposure decreased choice latencies during the first session of R1-right [F(2,39)=12.44, P=.01] and, marginally, for R2-left [F(2,39)=2.22, P=.081]. Post-hoc tests revealed that both the 0.05 and 5 ppm group had shorter choice latencies than controls (P=0.018 and 0.003, respectively) for R1-right. Mercury-exposed animals did not differ on choice latencies during OD-left or R3-right (Ps>0.1).
Selenium Effects
There was a between-subjects effect of selenium on sessions to criterion [F(1,38)=4.206, P=.047] and omissions [F(1,38)=4.769, P=.035]. One-way ANOVAs showed that low selenium animals required more sessions to complete R1-right [F(1,38)=4.240, P=.046; Figure 4, top], made more omissions [ F(1,38)=6.283, P=.017, log transformed data; Figure 4, bottom] and had longer rear-lever latencies [F(1,38)=3.808, P=.058] during this reversal than high selenium animals. The selenium groups did not differ on any of these variables during OD-left or the last two reversals (i.e. R2-left and R3-right) (Ps>.1) except that choice latency was marginally longer for the low-Se group during OD left [F (1,39) = 3.75, P = .06].
Figure 4.

Condition by selenium interactions showing the number of sessions to reach criterion (top) and the total number of omissions (bottom) for low selenium (open circles) and high selenium (filled circles) rats combined across MeHg groups. Graphs on the left show the mean number of sessions (top) and mean number of omissions (bottom) to reach criterion during each reversal. The graphs on the right represent individual rats from the low selenium (middle) and high selenium (right) groups. (*) p<0.05 for the low selenium rats compared to high selenium rats. Error bars represent ± 1 SEM.
The large error bars for the low selenium groups seen in the left-most panels of Figure 4 suggest the presence of two populations of animals within this group. Plots indicating the performance of individual animals confirm this observation (Figure 4, right panels). Both sessions and omissions to criterion were tightly clustered for the high and low selenium animals on R2-left and R3-right, but on the first reversal and, to a lesser degree, the original discrimination, these indicators were highly variable and appeared to form two clusters, one above and one below 20 sessions (or about 450 omissions) for the low selenium animals. This low selenium group comprised three control and two MeHg-exposed rats, so selenium, and not MeHg, was responsible for this effect.
Relationships among errors, omissions, and sessions to criterion
The analyses above suggested that the number of sessions to criterion was related to response omissions but not errors. To confirm this, the relationship between sessions to criterion was examined using least-squares linear regression with either omissions or errors as the predictor. When omissions were the predictor, separate analyses were carried out for (a) all subjects and (b) the two selenium groups independently. When errors were the predictor, analyses were conducted for (a) all subjects and (b) the three MeHg groups separately. The separation of groups was determined by the location of statistically significant main effects. The individual regressions (Se or MeHg exposure) did not differ significantly from one using all animals, so only the latter is shown (Figure 5). Omissions were a strong predictor of sessions to criterion, but errors were only a weak predictor.
Figure 5.

The relationship between sessions to criterion and response omissions (top) and errors (bottom). Groups are separated as shown in the key. The regression determined from all animals did not differ significantly from ones conducted separately in each group, so only the former is shown.
DISCUSSION
Prenatal exposure to MeHg and lifetime exposure to a diet that was either marginal or rich in selenium were manipulated in a factorial design to enable the examination of potential interactions between MeHg and selenium on a spatial discrimination procedure (SDR). In addition, main effects of a diet rich in selenium and developmental exposure to MeHg could also be examined. The ability to examine selenium effects is a particular advantage since little is known about the behavioral effects of this trace element.
The SDR procedure was selected because it taps behavioral plasticity or rigidity in the face of changing reinforcement contingencies. With this procedure, animals acquire a simple discrimination based on the spatial location, i.e., left lever-pressing is reinforced. Then, pressing the right lever is reinforced, and the left is not. This is called the first reversal and is the one that is especially sensitive to neurotoxicant exposure (Gilbert and Rice, 1987; Rice, 1985; Schantz and Bowman, 1989; Widholm, et al., 2001). Subsequent reversals are usually acquired with fewer errors and are less sensitive to exposure.
MeHg Effects
During the first reversal (R1-right), the rats exposed gestationally to 0.5 or 5 ppm of Hg (as MeHg), regardless of selenium exposure, perseverated on the left lever, the lever on which the original discrimination had been acquired. A smaller number of errors occurred on the next reversal (R2-left) back to the original lever. The groups were not statistically distinguishable from one another, although both exposure groups displayed more errors than controls on R2-left. Interestingly, on the next reversal, which, like the first reversal, was away from the original lever, an exposure-related effect reappeared, but this time only in the 5 ppm rats. Despite the elevated number of errors, there was no methylmercury-related increase in the number of sessions required to complete the transition, nor was there a change in the number of reinforced trials required to complete the reversal. Insofar as methylmercury exposure is concerned, once responding began on the correct lever, the transition to that lever proceeded the same for all exposure groups.
Choice latency showed a characteristically declining pattern both within and across reversals. As an animal acquired a discrimination, its performance became faster and more accurate. The within-reversal pattern by which latency changed is illustrated in the representative animal in Fig 2 (top panel). In addition, as an animal acquired experience with reversals, its terminal (i.e. the last session of each reversal) choice performance became faster and more accurate; this conclusion is supported by statistical analysis but was not illustrated graphically.
The first session of a reversal was used to examine relationships among choice latency, accuracy, and exposure. Specifically, the question was whether an increase in errors during this crucial session was related to a shorter, perhaps impulsive, choice latency in exposed rats. Indeed, the elevated error rate in methylmercury-exposed rats was accompanied by shortened choice latencies during the first session of the reversals. Since these animals did not differ on rear-lever latency, the time required to press the back lever when a trial began, the result cannot be attributed to sensory or motor deficits. The animals exposed to the lower, 0.5 ppm dose, fell between the 0 ppm and 5 ppm Hg animals and displayed more variability on this measure.
An elevation in perseverative errors is consistent with the pattern of neuropathology seen after developmental exposure to low levels of methylmercury and indicates that these effects have behavioral consequences. In the cerebral cortex, altered morphology of cortical neurons, reduced widths of cortical lamina (Barone, et al., 1998; Berlin, et al., 1975), and morphological aberrations in mitochondria in cortical neuron fibers (O’Kusky, 1983) have been described after developmental methylmercury exposure. In these reports, the cerebral cortex was among the most sensitive regions to gestational exposure to methylmercury. Perseveration in discrimination reversal tasks appears after excitotoxic damage to the orbital prefrontal cortex in rats (Chudasama and Robbins, 2003) or following lesions to the frontal lobes in marmosets (Ridley, et al., 1993). Rodents rendered microencencephalic during early development have been reported to perseverate on a temporal differentiation task as adults (Ferguson, et al., 1994). In addition, age-related decreases in cortical size in humans have been linked to perseveration on such tasks as the Wisconsin Card Sorting Task (Head, et al., 2002; Raz, et al., 1998).
One possible explanation for MeHg-induced increases in errors, and corresponding decreases in choice latency, is a diminished sensitivity to changes in the reinforcement contingency. Such diminished sensitivity is implied by experimental reports that gestational methylmercury exposure (Paletz, et al., 2006) and lesions to the orbital prefrontal cortex that result in increased responding on large ratio schedules of reinforcement (Kheramin, et al., 2005). The last was described as occurring in a pattern consistent with diminished sensitivity to changes in reinforcement rates, but a related experiment also suggests a link to impulsivity (Kheramin, et al., 2002). This reduced sensitivity would produce a pattern of persistent responding on a previously reinforced response alternative, which would appear as a shortened choice latency. On tasks examining response patterns following reductions in reinforcement rate under fixed and progressive ratio schedules, gestationally exposed MeHg rats exhibited increased and persistent responding relative to controls, a pattern that is consistent with the notion of perseveration (Paletz, et al., 2006). Likewise, the concurrent schedule findings (Newland, et al., 2004; Newland, et al., 1994) are in accordance with the idea of perseveration. MeHg animals continue to respond despite relative decreases in the reinforcement rate. It should be noted that these findings can also be explained in terms of an increase in the efficacy of the reinforcer for MeHg animals, an account that is difficult to disentangle from perseveration.
The suggestion of cortical involvement in the effects reported here is also consistent with the shorter choice latencies that accompanied perseverative errors in MeHg-exposed rats immediately after a reversal. Impulsive individuals, such as those with damage to the orbitofrontal cortex, show a negative correlation between response latency and errors on a variety of tasks (Berlin, et al., 2004; Dougherty, et al., 2000). Under this explanation, the erroneous responding and shorter, perhaps impulsive, choice latencies were both related to impaired cortical development due to gestational MeHg exposure.
The discrimination reversal bears some resemblance to the choice-in-transition procedure (Newland and Reile, 1999a; Newland, et al., 2004; Newland, et al., 1994), but whether they tap the same behavioral or neural mechanisms cannot be ascertained at present. It can be noted that the choice-in-transition procedure is sensitive to developmental methylmercury exposure, and there is evidence that cortical regions also mediate choice-related tasks (Schultz, et al., 2000; Tremblay and Schultz, 1999). Therefore, these effects are consistent with methylmercury’s developmental neurotoxicity (Newland, et al., 2006). In the choice-in-transition procedure, a subject faces a panel containing two levers, just as in the discrimination reversal, and pressing the left and right levers is reinforced under separate schedules of reinforcement. In the discrimination reversal procedure, one lever is associated with a fixed-ratio 1 schedule of reinforcement (one response is required) and the other with extinction (reinforcers are not delivered). However, with the choice-in-transition arrangement, each lever is associated with some non-zero rate of reinforcement, so the distinction between the two levers is characterized by the reinforcer ratio between them. For example, pressing one lever may be reinforced four times/min and the other only once/min, thereby producing a reinforcer ratio between the two levers of 4:1. In the reversal procedure, the reinforcement density changes much more starkly. During R1-right, for example, the reinforcement densities change from 100% to 0% for the left lever and from 0% to 100% for the right
An emphasis on reinforcement mechanisms over discrimination-based mechanisms to understand MeHg’s developmental neurotoxicity, as in the above analysis, was suggested in an earlier review (Newland and Paletz, 2000). For example, developmental methylmercury exposure does not affect behavior in a delayed alternation task. In this procedure, the subject presses one lever on one trial and presses the other lever on the next trial, with a delay separating trials. Thus, a discrimination based on the location of the previous response is crucial to good performance, and the effects of reinforced responding on a particular lever do not accumulate due to the requirement of alternating responses on each trial. Other reports have also failed to find effects of developmental methylmercury on discrimination or memory tasks (Buelke-Sam, et al., 1985; Schreiner, et al., 1986).
Se Effects
A main effect of selenium suggested that dietary levels of this trace element can have behavioral consequences. A diet marginal in selenium content, like mercury excess, impaired behavior during the discrimination reversal, and the first reversal was the most sensitive to dietary selenium. The pattern of disruption, however, differed. Many rats fed a diet marginal in selenium, regardless of MeHg exposure, required more sessions to complete R1-right and made more omissions during these sessions, two effects that were highly correlated, but the errors committed did not differ between the two groups. This differed from the error-increasing effects of MeHg, interpreted above as response perseveration. Where rats on the low-selenium diet sometimes committed many errors but eventually shifted to the newly correct lever, some methylmercury-exposed rats did not make the switch.
The pattern of effects also differed in the distribution of effects across rats. Under conditions of selenium deficiency, some rats displayed an inordinate number of response omissions, directly leading to the selenium effect observed here. Examination of individual rats revealed clear evidence of the presence of responders and nonresponders. These patterns suggest that a diet rich in selenium buffers rats against other, unspecified, challenges that result in behavioral alterations, or that a diet low in selenium makes them more sensitive to such challenges. It is interesting, and unexpected, that one of those challenges does not appear to be methylmercury.
Selenium confers protection against neurological impairments associated with chronic, adult-onset exposure to methylmercury, possibly through very high-affinity binding to form insoluble mercury selenides or by increasing glutathione peroxidase activity in adults (Magos, 1991; Moller-Madsen, 1990; Moller-Madsen and Danscher, 1991; Suzuki, 1997; Whanger, 1992) or in the neonatal brain (El-Demerdash, 2001). There have been reports that selenium reduces some of the developmental neurobehavioral effects of MeHg in early development (Watanabe, 2001; Watanabe, et al., 1999) and after much higher MeHg doses (Satoh, et al., 1985). In one study, a maternal diet rich in selenium resulted in reduced MeHg-induced hypoactivity in offspring at two months of age (Fredriksson, et al., 1993). In that study, MeHg was administered by gavage using a ten-fold higher daily dose than our high dose of 5 ppm, on gestational days 6–9. Taken together, these other reports suggest selenium might ameliorate certain behavioral effects of MeHg, but the protection may be limited to early development, to dosing regimens involving higher doses delivered as a bolus, or to certain behavioral measures.
The extant literature does not support specific predictions regarding the conditions under which selenium may protect against methylmercury’s neurotoxity, especially with regard to developmental exposure, but it can support a hypothesis regarding these interactions. It might be that a molar excess of mercury over selenium elucidates the effects of MeHg (Newland, et al., (in press); Raymond and Ralston, 2004; Watanabe, 2001). An interaction between selenium and methylmercury might then be seen when one condition represents a molar excess of selenium and the other a molar excess of mercury. So long as selenium levels are higher than those of MeHg (on a molar basis), there is sufficient free selenium to create the necessary selenoenzymes. With regard to the present study, the Hg:Se molar ratio at birth was about 20:1 for the 5 ppm group and was close to 1.0 in the 0.5 ppm group (Newland, et al., (in press)). The large molar excess of mercury for the 5 ppm group could, therefore, be related to the effects seen in that group, while a near 1:1 ratio in the 0.5 ppm group could be related to the variability in the effects seen in this group. It has been suggested that if MeHg increases beyond the levels of free selenium, then selenium deficiencies may occur, and this could partly account for the neurobehavioral toxicity of MeHg (Watanabe, et al., 1999). The present study cannot support that hypothesis because selenium’s effects were expressed in a different manner than those of methylmercury exposure.
Therapeutic Interventions
Two animals exposed to low selenium, one a 0 ppm and the other a 5.0 ppm Hg rat, committed so few correct responses and so many errors and response omissions that during the first reversal they nearly stopped responding altogether. After numerous sessions, it was decided to try to recapture behavior with a behavioral intervention: only the “correct” lever was inserted during all trials for a single session. Data from this session were not used in describing the number of errors and omissions for that animal. Thus, offering this intervention probably resulted in an underestimate of the degree of impairment, insofar as it is indicated by the number of sessions required to complete that transition.
This intervention was informative. The case illustrated in the bottom panel of Figure 2 showed that errors declined at a rate similar to that seen in other rats, but the number of correct responses, and therefore the number of reinforcers delivered, remained close to or at zero, and the number of response omissions was large. This pattern appeared both during initial discrimination training and after the first reversal. Many sessions were composed exclusively of trials containing either an incorrect response (error) or no response at all. When only the correct lever was available, however, behavior quickly shifted to that lever and remained there even after both levers were re-presented. Subsequent reversals proceeded much more quickly. This intervention revealed that the animal could respond on the correct lever, and that once responding was reinforced, correct responding continued even after both levers were presented. After experience with one reversal, subsequent reversals could be successfully negotiated.
Summary
Rats were exposed during gestation to methylmercury via maternal drinking water and chronically (gestationally and throughout life) to a diet containing low or high, but nutritionally adequate, concentrations of selenium. As adults, the animals exposed gestationally to methylmercury, regardless of selenium exposure, showed perseverative responding on the previously reinforced response alternative on a discrimination reversal task. The selenium diet, regardless of methylmercury exposure, also affected discrimination reversal, but low selenium acted differently than methylmercury. Some animals on the low-selenium diet failed to respond on a greater number of trials during the early phases, but accuracy was unaffected on the trials in which a response occurred.
The methylmercury concentrations used with the drinking water exposures result in consumption levels of approximately 40 and 400 μg/kg/day for the 0.5 and 5 ppm exposure groups, respectively, although during early gestation the exposure may increase slightly (Newland and Reile, 1999b). Rat blood is especially rich in sulfur-containing hemoglobin (Magos, 1987) and therefore binds much more mercury than the blood of other laboratory species, including other rodents, or humans. Accordingly, the dosing regimen for rats must be nearly ten times higher than that of other species to achieve targeted tissue levels. If a 10-fold factor is used to account for this increased hemoglobin binding, then the effects that appeared in the 0.5 ppm group appeared at an effective daily dosing rate approximately 1.5 times the current RfD of 0.1 μg/kg/day (Keating, et al., 1997). The brain levels in neonates achieved by this dosing regimen are about 0.2 to 0.4 ppm (1 to 2 μM) at birth (Newland, et al., (in press)).
Acknowledgments
Supported by ES10865 from NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdo KM. NTP technical report on toxicity studies of sodium selenate and sodium selenite. Research Triangle Park, NC: U.S. Department of Health and Human Services, NIH; 1994. (No. NIH 94-3387) [Google Scholar]
- Barone S, Jr, Haykal-Coates N, Parran DK, Tilson HA. Gestational exposure to methylmercury alters the developmental pattern of trk-like immunoreactivity in the rat brain and results in cortical dysmorphology. Developmental Brain Research. 1998;109:13–31. doi: 10.1016/s0165-3806(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Baum WM. Matching, undermatching, and overmatching in studies of choice. Journal of the Experimental Analysis of Behavior. 1979;32:269–81. doi: 10.1901/jeab.1979.32-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–26. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Berlin M, Grant CA, Hellberg J, Hellstrom J, Schultz A. Neurotoxicity of methylmercury in squirrel monkeys. Cerebral cortical pathology, interference with scotopic vision, and changes in operant behavior. Archives of Environmental Health. 1975;30:340–8. doi: 10.1080/00039896.1975.10666717. [DOI] [PubMed] [Google Scholar]
- Buelke-Sam J, Kimmel CA, Adams J, Nelson CJ, Vorhees CV, Wright DC, et al. Collaborative Behavioral Teratology Study: results. Neurobehavioral Toxicology & Teratology. 1985;7:591–624. [PubMed] [Google Scholar]
- Burbacher TM, Rodier PM, Weiss B. Methylmercury developmental neurotoxicity: A comparison of effects in humans and animals. Neurotoxicology and Teratology. 1990;12:191–202. doi: 10.1016/0892-0362(90)90091-p. [DOI] [PubMed] [Google Scholar]
- Chapman L, Chan HM. The influence of nutrition on methylmercury intoxication. Environmental Health Perspectives. 2000;108(Supplement 1):29–56. doi: 10.1289/ehp.00108s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–80. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW. The three modern faces of mercury. Environmental Health Perspectives. 2002;110(Suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, McCarthy D. The Matching Law: A Research Review. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Dougherty DM, Bjork JM, Marsh DM, Moeller GF. A comparison between adults with conduct disorder and normal controls on a continuous performance test: Differences in impulsive response characteristics. Psychological Record. 2000;50:203–220. [Google Scholar]
- El-Demerdash F. Effects of selenium and mercury on the enzymatic activities and lipid peroxidation in brain, liver, and blood of rats. Journal of Environmental Science and Health. 2001;36:489–99. doi: 10.1081/PFC-100104191. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Holson RR, Paule MG. Effects of methylazoxymethanol-induced micrencephaly on temporal response differentiation and progressive ratio responding in rats. Behavioral & Neural Biology. 1994;62:77–81. doi: 10.1016/s0163-1047(05)80062-7. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Gardlund A, Bergman K, Oskarsson A, Ohlin B, Danielsson B, et al. Effects of maternal dietary supplementation with selenite on the postnatal development of rat offspring exposed to methyl mercury in utero. Pharmacology & Toxicology. 1993;72:377–82. doi: 10.1111/j.1600-0773.1993.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Rice DC. Low-level lifetime lead exposure produces behavioral toxicity (spatial discrimination reversal) in adult monkeys. Toxicol Appl Pharmacol. 1987;91:484–490. doi: 10.1016/0041-008x(87)90070-6. [DOI] [PubMed] [Google Scholar]
- Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: the role of regional cortical shrinkage and cognitive resources. Psychology and Aging. 2002:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- Imura N, Naganuma A. Possible mechanisms of detoxifying effect of selenium on the toxicity of mercury compounds. In: Suzuki T, Imura N, Clarkson T, editors. Advances in mercury toxicity. New York: Plenum Press; 1991. p. 490. [Google Scholar]
- Keating MH, Mahaffey KR, Schoeny R, Rice GE, Bullock OR. Executive Summary (NT No. EPA/452/R-97/003) Vol. 1. Research Triangle Park, N.C: U. S. EPA; 1997. Mercury Study Report to Congress. [Google Scholar]
- Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, et al. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behavioural Brain Research. 2005;156:145–52. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, et al. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- Magos L. The absorption, distribution, and excretion of methyl mercury. In: Eccles CU, Annau Z, editors. The toxicity of methyl mercury. Baltimore: Johns Hopkins; 1987. pp. 24–44. [Google Scholar]
- Magos L. Overview on the protection given by selenium against mercurials. In: Suzuki T, Nobumasa I, Clarkson TW, editors. Advances in Mercury Toxicology. New York: Plenum; 1991. pp. 289–298. [Google Scholar]
- Moller-Madsen B. Localization of mercury in CNS of the rat. II. Intraperitoneal injection of methylmercuric chloride (CH3HgCl) and mercuric chloride (HgCl2) Toxicol Appl Pharmacol. 1990;103:303–23. doi: 10.1016/0041-008x(90)90232-j. [DOI] [PubMed] [Google Scholar]
- Moller-Madsen B. Localization of mercury in the CNS of the rat. Archives of Toxicology. 1994;66:79–89. doi: 10.1007/BF02342499. [DOI] [PubMed] [Google Scholar]
- Moller-Madsen B, Danscher G. Localization of mercury in CNS of the rat. IV. The effect of selenium on orally administered organic and inorganic mercury. Toxicology & Applied Pharmacology. 1991;108:457–73. doi: 10.1016/0041-008x(91)90092-s. [DOI] [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Laboratory Animals. Washington, D.C: National Academy Press; 1995. [Google Scholar]
- Newland MC, Reile PA. Learning and behavior change as neurotoxic endpoints. In: Tilson HA, Harry J, editors. Target Organ Series: Neurotoxicology. New York: Raven Press; 1999a. pp. 311–338. [Google Scholar]
- Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicological Sciences. 1999b;50:106–16. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- Newland MC, Paletz EM. Animal studies of methylmercury and PCBs: what do they tell us about expected effects in humans? Neurotoxicology. 2000;21:1003–27. [PubMed] [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicology & Teratology. 2004;26:179–94. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposure to lead or methyl mercury: reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicology & Applied Pharmacology. 1994;126:6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- Newland MC, Donlin WD, Paletz EM, Banna KM. Developmental behavioral toxicity of methylmercury: Consequences, conditioning, and cortex. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. CRC Press; 2006. pp. 101–146. [PubMed] [Google Scholar]
- Newland MC, Reed MN, LeBlanc A, Donlin WD. Brain and Blood Mercury and Selenium after Chronic and Developmental Exposure to Methylmercury. NeuroToxicology. doi: 10.1016/j.neuro.2006.05.007. in press. [DOI] [PubMed] [Google Scholar]
- Newland MC, Ng WW, Baggs RB, Gentry GD, Weiss B, Millar RK. Operant behavior in transition reflects neonatal exposure to cadmium. Teratology. 1986;34:231–241. doi: 10.1002/tera.1420340302. [DOI] [PubMed] [Google Scholar]
- O’Kusky J. Methylmercury poisoning of the developing nervous system: morphological changes in neuronal mitochondria. Acta Neuropathologica. 1983;61:116–22. doi: 10.1007/BF00697390. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Craig-Schmidt MC, Newland MC. Gestational exposure to methylmercury and n-3 fatty acids: Effects on high- and low-rate operant behavior in adulthood. Neurotoxicology and Teratology. 2006;28:59–73. doi: 10.1016/j.ntt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Prohaska J, Ganther H. Interactions between selenium and methylmercury in rat brain. Chemico-Biological Interactions. 1977;16:155–67. doi: 10.1016/0009-2797(77)90125-9. [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC. Mercury:selenium interactions and health implications. Seychelles Medical and Dental Journal (SMDJ) 2004;7:72–77. doi: 10.1016/j.neuro.2020.09.020. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. Journal of Nutrition. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. Journal of Nutrition. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rice DC. Chronic low-lead exposure from birth produces deficits in discrimination reversal in monkeys. Toxicol Appl Pharmacol. 1985;77:201–210. doi: 10.1016/0041-008x(85)90319-9. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Clark BA, Durnford LJ, Baker HF. Stimulus-bound perseveration after frontal ablations in marmosets. Neuroscience. 1993:595–604. doi: 10.1016/0306-4522(93)90409-9. [DOI] [PubMed] [Google Scholar]
- Satoh M, Yasuda N, Shimai S. Development of reflexes in neonatal mice prenatally exposed to methylmercury and selenite. Toxicology Letters. 1985;25:199–203. doi: 10.1016/0378-4274(85)90082-7. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Bowman RE. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicology & Teratology. 1989;11:13–9. doi: 10.1016/0892-0362(89)90080-9. [DOI] [PubMed] [Google Scholar]
- Schober S, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, Garrett ES, Canady RA, Dillon CF, Sun Y, Joseph CB, Mahaffey KR. Blood mercury levels in US children and women of childbearing age, 1999–2000. JAMA. 2003;289:1667–74. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- Schreiner G, Ulbrich B, Bass R. Testing strategies in behavioral teratology: II. Discrimination learning Neurobehavioral. Toxicology & Teratology. 1986;8:567–72. [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Stern S, Cox C, Cernichiari E, Balys M, Weiss B. Perinatal and lifetime exposure to methylmercury in the mouse: blood and brain concentrations of mercury to 26 months of age. Neurotoxicology. 2001;22:467–77. doi: 10.1016/s0161-813x(01)00047-x. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Equimolar Hg-Se complex binds to Selenoprotein P. Biochemical and Biophysical Research Communications. 1997;231:7–11. doi: 10.1006/bbrc.1996.6036. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Smith JC. Effects of coadministered sodium selenite on short-term distribution of methyl mercury in the rat. Environmental Research. 1984;34:287–94. doi: 10.1016/0013-9351(84)90097-5. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex [see comment] Nature. 1999;398:704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Watanabe C. Selenium deficiency and brain functions: the significance for methylmercury toxicity. Nippon Eiseigaku Zasshi - Japanese Journal of Hygiene. 2001;55:581–9. doi: 10.1265/jjh.55.581. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicology & Teratology. 1999;21:83–8. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Whanger P. Selenium in the treatment of heavy metal poisoning and chemical carcinogenesis. [Review] Journal of Trace Elements & Electrolytes in Health & Disease. 1992;6:209–21. [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: sex-specific deficits in associative ability and inhibitory control. Toxicology & Applied Pharmacology. 2001;174:188–98. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]


