Abstract
The objective of this study was to examine the opposite behavior responses of conditioned fear extinction and renewal and how they are represented by network interactions between brain regions. This work is a continuation of a series of brain mapping studies of various inhibitory phenomena, including conditioned inhibition, blocking and extinction. A tone-footshock fear conditioning paradigm in rats was used, followed by extinction and testing in two different contexts. Fluorodeoxyglucose autoradiography was used to compare mean regional brain activity and interregional correlations resulting from the presentation of the extinguished tone in or out of the extinction context. A confirmatory structural equation model, constructed from a neural network proposed to underlie fear extinction, showed a reversal from negative regional interactions during extinction recall to positive interactions during fear renewal. Additionally, the magnitude of direct effects was different between groups, reflecting a change in the strength of the influences conveyed through those pathways. The results suggest that the extinguished tone encountered outside of the extinction context recruits auditory and limbic areas, which in turn influence the interactions of the infralimbic cortex with the amygdala and ventrolateral periaqueductal gray. Interestingly, the results also suggest that two independent pathways influence conditioned freezing: one from the central amygdaloid nucleus and the other from the infralimbic cortex directly to the ventrolateral periaqueductal gray.
Keywords: Path analysis, Structural equation modeling, Brain mapping, Prefrontal cortex, Hippocampus, Amygdala
In the past few years, there has been increased interest in the neural substrates of fear extinction, partly due to the relevance of fear extinction to the treatment of anxiety disorders (Craske, 1999). Brain lesion, stimulation, recording and metabolic mapping studies have addressed the question of which brain areas are important in fear extinction learning and memory in both animals and humans (Quirk et al., 2000, Davis, 2002, Garcia, 2002, Barrett et al., 2003, Quirk et al., 2003, Phelps et al., 2004, Straube et al., 2006). From these findings, an emerging picture has developed, implicating an interaction between prefrontal and subcortical output regions as contributing to conditioned fear extinction (Quirk et al., 2006). In this study, we tested the hypothesis that fear extinction is functionally associated with inhibitory influences from the medial prefrontal cortex to the central amygdala (CEA) and ventrolateral periaqueductal grey (vlPAG), regions mediating the output freezing behavior.
Animal studies implicate the medial prefrontal cortex-amygdala interaction in the extinction of conditioned fear (Quirk, Russo, Barron, & Lebron, 2000; Herry & Mons, 2004; Milad, Vidal-Gonzalez, & Quirk, 2004; Pare, Quirk, & Ledoux, 2004; Garcia, Chang, & Maren, 2006; Morgan, Schulkin, & Ledoux, 2003). One of the first studies that examined the role of the medial prefrontal cortex (mPFC) in fear extinction was conducted by Morgan et al. (1993), who reported that rats with mPFC lesions had an increased resistance to extinction. Since damage to the prefrontal cortex had long been known to produce emotional disturbances and an increase in certain perseverative responses (Nauta, 1971; Goldman-Rakic, 1987; Sotres-Bayon, Bush, & Ledoux, 2004), Morgan et al. speculated that the resistance to extinction following mPFC lesions corresponded to perseverative tendencies in the emotional realm. Furthermore, the authors proposed that mPFC-amygdala connections normally allowed an animal to adjust its emotional behavior when environmental circumstances changed, and that a loss of prefrontal influence on the amygdala might bring about the reduced capacity of people with anxiety disorders to regulate their emotions. Subsequent research has examined the role of mPFC in extinction in more detail. For example, when brain lesions were limited to only dorsal or ventral mPFC (vmPFC), it was found that vmPFC lesions selectively impaired extinction over days without increasing the expression of fear within trials (Morgan & Ledoux, 1995). Quirk et al. (2000) followed this work with further lesions of yet a smaller subdivision of vmPFC, and found that lesions of the infralimbic cortex (IL) in rats also interfered with between-days extinction. In other words, IL is not necessary for extinction learning, but it does play a role in either consolidation or retrieval of fear extinction. Furthermore, IL stimulation has been found to simulate extinction learning through experiments that paired the tone CS with brief IL stimulation, which resulted in reduced freezing in rats (Milad et al., 2004; Milad & Quirk, 2002). Data from our laboratory also support the hypothesis that mPFC plays a role in the retrieval of fear extinction memory. Barrett et al. (2003) demonstrated that mice with higher prefrontal metabolic activity more successfully inhibited the CR when presented with an extinguished auditory CS.
It has been proposed that basolateral amygdala (or perhaps just the lateral amygdaloid nucleus) plays a role in the acquisition and extinction of conditioned fear (Fanselow & Ledoux, 1999; Akirav, Raizel, & Maroun, 2006; Berlau & McGaugh, 2006), in part because this region receives converging sensory input from auditory, somatosensory and visual areas (Ghashghaei & Barbas, 2002). Basolateral nucleus of the amygdala (BLA) projects to the intercalated amygdaloid cells (Pare et al., 2004), which synapse onto the central amygdaloid nucleus, an area that organizes the conditioned fear response via its influence on ventromedial and lateral hypothalamus (VMH, LH) and ventrolateral periaqueductal grey (vlPAG), among others. This fear response could be inhibited by the top-to-bottom control of the amygdala firing by the medial prefrontal cortex, more specifically the infralimbic cortex (Quirk et al., 2000). According to Pare et al. (2004), IL sends projections to the GABA-ergic intercalated cells of the amygdala, thus inhibiting the activation of the medial central nucleus by the BLA when the CS is encountered in the extinction context. This model of CR inhibition in fear extinction does not account for how the contextual influence modifies extinction responding. In this study we also tested the hypothesis that context-dependent fear renewal is functionally associated with an increased influence of the hippocampus on the medial prefrontal cortex.
One possible way for contextual cues to exert their influence during extinction memory retrieval is through the interaction of the hippocampal formation with the basal nucleus of the amygdala, as proposed by Sotres-Bayon et al. (2004). However, since bilateral lesions of the basal amygdaloid nucleus have no effect on fear extinction (Anglada-Figueroa & Quirk, 2005), another possibility is that hippocampal formation directly influences the vmPFC, thus modifying vmPFC control of the amygdala.
Anatomical, as well as electrophysiological studies of these regions show that there are strong functional connections between the hippocampus and mPFC (Jay & Witter, 1991; Tierney, Degenetais, Thierry, Glowinski, & Gioanni, 2004; Ishikawa & Nakamura, 2003; Conde et al., 1995). These anatomical and electrophysiological findings, together with the lesion studies and behavioral findings (Corcoran, Desmond, Frey, & Maren, 2005; Quirk et al., 2000; Sotres-Bayon, Cain, & Ledoux, 2006), suggest that hippocampal inputs to mPFC cells may subserve contextual constraints on the retrieval of cued fear extinction. Corcoran et al. (2005) have found that inactivation of DHC impairs both ABA and ABC fear renewal in rats. However, other findings are in disagreement with these results (Frohardt, Guarraci, & Bouton, 2000; Wilson, Brooks, & Bouton, 1995). Other than hippocampal formation, no other lesion or inactivation studies have been conducted to investigate involvement of areas such as mPFC, amygdala or sensory areas in conditioned fear renewal. Since Bouton proposed that contextual cues gate the recall of extinction memory (Bouton et al., 1983; Bouton et al., 1998), it was hypothesized that the hippocampal formation might show altered metabolic activity associated with fear renewal. A recent human imaging study has shown that context-dependent fear memory is mediated by a hippocampal and ventromedial prefrontal network (Kalisch et al., 2006).
In addition, other brain areas, such as auditory regions TE1 and MGD (Ledoux, Sakaguchi, & Reis, 1984; Teich et al., 1989) have also been shown to play a role in fear extinction. While the current opinion favors the role of certain brain areas, such as MGD, as simple relay stations to, for example, amygdala, it is not unlikely that these regions also contribute to the processing and storage of the associative properties of the conditioned stimuli. The third hypothesis tested in this study was that fear renewal may be functionally associated with a greater excitatory influence from the medial geniculate nucleus (MGD) to the lateral amygdala (LA). A consistent finding from our laboratory using metabolic mapping techniques has been that the auditory system activity results not only from the tone CS processing, but also from the associative effects of excitatory conditioning (Gonzalez-Lima & Scheich, 1984a; Gonzalez-Lima, Finkenstadt, & Ewert, 1989; Gonzalez-Lima, 1992; Jones & Gonzalez-Lima, 2001a; Jones & Gonzalez-Lima, 2001b; Barrett, Shumake, Jones, & Gonzalez-Lima, 2003).
Rather than focusing on independent brain regions and their role in fear extinction, this study emphasizes how the changing relationships among numerous neural areas lead to the appropriate learned behavior. Fluorodeoxyglucose (FDG), an autoradiographic method that measures the rate of glucose use within the first few minutes following the administration in an intact brain (Gonzalez-Lima, 1992) was used. Learning-related changes in neural plasticity were mapped in rats which underwent fear conditioning and extinction training. A tone conditioned stimulus (CS) was first paired with a mild footshock as the unconditioned stimulus (US), and then the conditioned response (CR) was extinguished through repeated presentations of unreinforced CSs. FDG was administered during a test trial in which the CS was presented either in the context of extinction or in another, neutral context. Usually, when an extinguished CS is encountered outside the extinction context, there is a renewal of the CR. Thus learned behavior associated with an extinguished CS depends, at least in part, on contextual cues present during the CS presentation (Bouton 2004). Therefore, fear extinction can be viewed as an example of an inhibitory learning paradigm, in which the conditioned behavioral response is determined by the differential associative properties of the same physical stimulus in two different contexts.
Structural equation modeling quantifies the impact of links between variables in a causally structured network. Directional links between elements of the network (regions of interest, ROIs) are assigned based on the anatomical pathways, and the correlation matrices of activity between ROIs are used to compute path coefficients of the influences mediated by individual functional pathways. The application of structural equation modeling to neural data assumes that ROI correlations reflect common influences and direct anatomical connections between them (McIntosh and Gonzalez-Lima, 1994, McIntosh and Gonzalez-Lima, 1995). Thus, a neural network model combines known anatomical connections between ROIs and a functional network model in which the interregional correlations of activity are decomposed to assign path coefficients to these connections. Specific functional interactions within the same anatomical model can then be compared for the strength of path coefficients to identify task-specific functional interactions within that network. Hence, structural equation models reveal patterns of interrelations between brain ROIs within an anatomically constrained neural system, but extend beyond interregional pairwise correlations to include direct causal influences.
The first application of structural equation modeling to brain mapping data was from a study of auditory system activity following long-term habituation, which successfully demonstrated that interactions between auditory pathways changed depending on the experience with the acoustic stimulus (McIntosh and Gonzalez-Lima, 1991). Since then, other brain mapping studies utilized structural equation modeling to analyze functional neural networks related to behavior in both animals and humans (McIntosh and Gonzalez-Lima, 1992, McIntosh and Gonzalez-Lima, 1993, Buchel and Friston, 1997, Seminowicz et al., 2004, Grady et al., 2005, Horwitz et al., 2005) validating the combined use of functional brain mapping and anatomically based structural equation modeling as an analytical tool for advancing our understanding of brain-behavior relations at the systems level. In the present study, brain regions of interest were defined based on previous findings (Davis et al., 1982, Ledoux et al., 1984, Maren et al., 1997, Quirk et al., 2000, Maren, 2001, Davis, 2002, Quirk et al., 2003, Maren and Quirk, 2004, Pare et al., 2004, Phelps et al., 2004, Maren, 2005, Quirk et al., 2006, Straube et al., 2006), and, in particular, structural equation modeling nodes (ROIs) were selected based on the cortical and hippocampal circuitry model of fear extinction proposed by Maren (2005).
Maren’s model (Fig. 1) was chosen because it considers the role of environmental context, its inter-regional connections are anatomically sound, and many of the model’s ROIs were identified in this study as having either mean differences in metabolic activity or having task-specific functional interactions. According to this model, a CS undergoing extinction recruits prefrontal cortical (PFC) and hippocampal circuits involved in regulating CR behavioral output through the amygdala. The context specificity of extinction is conferred by the hippocampal formation, which detects CS and context mismatches. A detected CS-context mismatch inhibits the PFC circuit, which reduces the inhibitory influence of the PFC on the amygdala resulting in CR renewal.
Fig. 1.
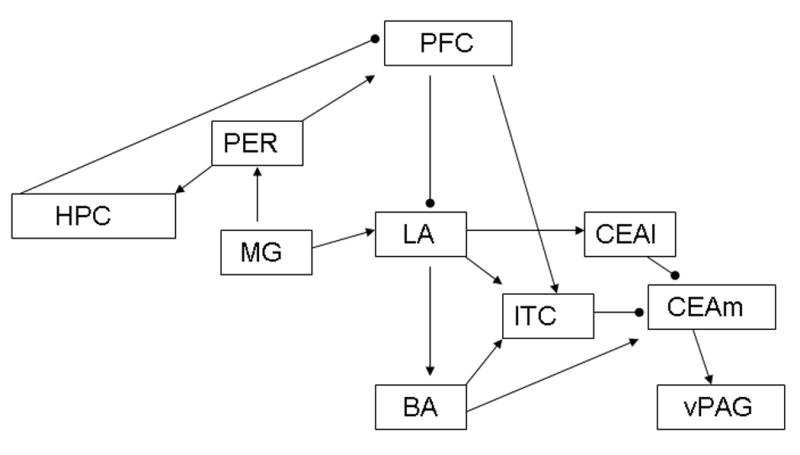
Maren’s model of fear extinction. Excitatory projections are indicated by arrowheads, and inhibitory projections by circles. PFC = prefrontal cortex; PER = perirhinal cortex; HPC = hippocampus; MG = medial geniculate nucleus; LA = lateral amygdala; BA = basal amygdala; CEAl = lateral division of the central amygdaloid nucleus; CEAm = medial division of the central amygdaloid nucleus; ITC = intercalated nuclei of amygdala; vPAG = ventral periaqueductal gray.
EXPERIMENTAL PROCEDURES
The behavioral and autoradiographic methods are similar to those used for a previous report (Barrett et al., 2003) and are outlined below.
Subjects and apparatus
Subjects were 36 one-month old male Long-Evans rats purchased from Harlan, Inc. (Houston, TX). Rats were divided into three groups (extinction, renewal and pseudorandom groups; n = 12 per group). The extinction group was tested for FDG uptake in the same context used for extinction. The renewal group was tested for FDG uptake in a context different from the extinction context. The pseudorandom group did not undergo acquisition and extinction. The three groups were exposed to the same physical stimuli and contexts during training. All subjects were housed under standard laboratory conditions (12 hour light/dark cycle), 3 per cage, with food and water available ad libitum. Upon their arrival to the colony, rats were handled daily for a week to habituate them to the experimenters. At the time of FDG administration subjects weighed 172 g on average (range 157–187 g). The protocol used was approved by the University of Texas Institutional Animal Care and Use Committee and complied with all applicable federal and National Institute of Health guidelines.
The acquisition phase of the experiment took place in MED Associates (St. Albans, VT) sound-attenuated operant chambers (context A) illuminated by a bright red light. The chambers had a speaker mounted at the top, through which Wavetek Sweep/Modulation generators (Wavetek, San Diego, CA) produced 65 dB frequency-modulated tones of 1–2 kHz, and 15 seconds in duration that were used as the conditioned stimulus (CS). The chambers were also equipped with a grid floor through which an electric shock could be delivered as programmed into a MED-PC protocol supplied by MED Associates. Before each session the chambers were cleaned with diluted detergent which also served as an olfactory cue for this context.
The extinction phase of the experiment took place either in context B or context C. Context B was a metal cage with a distinct metal floor, a diluted iodine odor and dim red light illumination. Context C consisted of a clear plastic cage with a speaker mounted on top, dim white light and diluted Bioclean (Nitritex Ltd, Ontario, Canada) odor.
Training procedure
Initial pilot studies conducted in our laboratory determined the parameters best suited to produce a strong renewal effect in the ABC paradigm, as described by Bouton (2004). The present experiment was optimized to produce a robust FDG renewal effect, whereas previous FDG studies were optimized to produce strong extinction effects (Barrett et al., 2003).
Acquisition phase
Animals were habituated to the conditioning chambers for two days, one hour each day. After habituation, the extinction and the renewal group rats received two days of four tone-shock pairings. The US consisted of a footshock of 0.5 mA, 0.75 sec in duration and it co-terminated with a tone CS. Each 15 sec CS was divided into five three second bins and subjects’ behavior was scored for each of the five bins. Pre-CS behavior was recorded for the 15 sec preceding each CS presentation. The same experimenter, a trained observer unaware of group assignment, made all the behavioral observations. The CR that was measured was freezing behavior, defined as lack of any movement except for rapid, shallow breathing, with all feet on the floor. Subjects in the pseudorandom group were presented with the same number of tones and shocks, but these stimuli were paired only once out of eight total stimulus presentations. Pseudorandomly trained subjects were used to control for any possible non-associative effects of stimulus presentation. The average inter-trial interval (ITI) was three minutes. Presentation of stimuli was controlled by computer programs created using MED-PC behavioral programming language (MED Associates). Twenty four hours following the acquisition training, one ten-minute probe trial consisting of three CS presentations was conducted out of the acquisition context to test the CR independently of any possible contextual influence. Freezing behavior was measured in three-second bins, over the entire period of CS presentation (15 seconds), as well as 15 seconds prior to the onset of the CS (pre-CS freezing). Thus, maximum freezing duration was 15 seconds (same duration as the CS). The pre-CS freezing measure was used to account for any non-specific freezing due to generalization from context A to contexts B and C or sensitization effects of the US. Based on the probe trial freezing scores, animals were matched into experimental groups.
Extinction phase
Extinction training took place in context B or C over a period of two days, and it consisted of hour-long session of 18 CS presentations with an average 3 minute ITI. This protocol was based on five pilot studies, and it was chosen as an optimal training procedure for reliable ABC renewal effect. Contexts B and C were counterbalanced between different cohorts of animals. Exposure to two counterbalanced contexts (B and C) was used to ensure that fear renewal was dependent only on the shift to a context different from the context used in acquisition and extinction, as opposed to any non-specific aspect of the physical exposure to contexts B or C.
FDG test
Before administration of FDG the anticipated behavioral CR was verified in all subjects to ensure that all animals included in this study displayed CR extinction (in the extinction group) and CR renewal (in the renewal group). Twenty four hours following the extinction training, another ten-minute probe trial was conducted in the extinction context for the extinction group subjects and in the renewal context for the renewal group subjects in order to verify that the extinction group showed a reduction in CR, and that renewal group displayed strong CR. FDG was administered two hours later, and it was verified at that time that the renewal animals were displaying a strong CR, and that the extinction animals displayed a reduced CR. Thus, the probe trial administered prior to the FDG session did not seem to reduce the strength of the CR renewal displayed during the critical FDG uptake period.
All subjects received an intraperitoneal injection of 18 μCi/100 gm body weight of 14C(U)-FDG (specific activity, 300–360 mCi/mmol; American Radiolabeled Chemicals) in 0.1 ml of sterile saline. FDG uptake makes it possible to examine functional maps from an entire brain in learning paradigms with great spatial resolution. However, this method allows only one measure per animal, corresponding primarily to the cumulative FDG uptake during the first 10 minutes after the injection (Gonzalez-Lima, 1992). The renewal group subjects were tested out of their extinction context, and the final FDG session context was kept identical for all groups to ensure similar conditions during the critical FDG uptake period. During this period, subjects were exposed to an hour-long 5 seconds on, 1 second off CS tone. This repeated frequency of tone presentations has been determined from previous FDG studies to produce optimal CS-evoked buildup of FDG for mapping the regional effects of the tone in the brain (Jones and Gonzalez-Lima, 2001). The change in tone duration did not affect the level of freezing of animals, as verified by the level of freezing observed after the FDG injection.
Because most of the FDG uptake takes place during the first ten minutes following the intraperitoneal administration of the compound (Gonzalez-Lima, 1992), the cumulative amount of freezing to the CS was measured as the CR for the first ten minutes of the FDG session. Although most of FDG uptake happens in the first ten minutes post-injection, it is necessary to wait for all the remaining circulating FDG to be trapped in the brain during one hour before decapitation of the subjects. If subjects are decapitated after the first 10 minutes, the autoradiographs reflect not only brain FDG uptake, but also circulating FDG, which reduces the signal to noise ratio. Following the FDG test, rats were decapitated and their brains were quickly removed and frozen in −40°C isopentane, then stored in plastic bags in a −40°C freezer.
FDG autoradiography
The standard FDG autoradiographic procedure (Gonzalez-Lima, 1992) was performed on 40 μm thick frozen brain sections. Briefly, frozen sections were picked up on slides and immediately dried on a 60°C hot plate. Then, slides and standards of known 14C concentration were apposed to radiographic film for a period of 14 days. Films were developed, dried and stored in protective covers. After the films were developed, subjects were dropped out of the final analysis if they showed poor FDG uptake, which was most likely caused by injection errors. The final number of subjects was n = 9 for extinction and pseudorandom groups, and n = 10 for the renewal group.
Image analysis
FDG uptake was quantified by film densitometry as described previously by Jones and Gonzalez-Lima (2001). Briefly, developed films were placed on a light box and optical density of images was captured by the video camera. Digitized images were corrected for film and light box artifacts through subtraction of the background (film background and optical distortions from camera and light source) by image analysis software JAVA (Jandel Scientific Corp.). 14C standards from Amersham were used to calculate a calibration curve for each film and convert optical density values to 14C incorporation per gram of brain tissue. The film developing parameters were optimized to obtain maximum linearity between isotope concentration and optical density.
Each ROI was measured from the right hemisphere from the three adjacent sections, and the mean optical density value was used for calculating the FDG uptake. Mean activity readings were expressed as nanocuries per gram of tissue. Additionally, for each slide containing the ROIs, the overall FDG uptake was determined for the entire set of sections (white and gray matter) by means of the object threshold function in the JAVA software. The average of all readings served as an index of whole brain isotope incorporation.
Statistical analysis
Behavioral data were analyzed based on the post-acquisition, extinction and post-extinction freezing scores, as well as FDG session freezing scores. Repeated measures ANOVA with tests for simple effects was used for differences within (pre and post extinction) and between groups. After it was determined that there were no between group differences in the whole brain FDG incorporation, regional activity was normalized using whole brain uptake values for each subject to account for individual variability in FDG uptake. In addition, interregional within-group correlations were examined to determine the pattern of relationships among the various brain regions and systems. More specifically, Pearson product-moment correlations were obtained for each region that was included in the final network model. Multiple linear regression analysis with ROIs represented in the model as predictor variables and behavior during the FDG session as dependent variable was also performed to examine how the network metabolic activity of these regions predicts the freezing behavior.
Structural equation modeling
Structural equation modeling algorithms strive to account for an observed pattern of correlations based on the causal structure provided by the directional anatomical connectivity of the system (McIntosh and Gonzalez-Lima, 1995). If a brain regional network is represented by a set of path equations where the correlations between regions are the sum of the compound paths connecting those regions, then path coefficients can be obtained through algebraic substitution (McIntosh & Gonzalez-Lima, 1991). While path equations specify the components of each correlation coefficient, another representation of the causal order of the system can be obtained by a set of structural equations. These equations specify the influences on the variance for each ROI, and their solutions are obtained using matrix operations best described as simultaneous multiple regressions (McIntosh & Gonzalez-Lima, 1995). Structural equation modeling allows for unaccounted influences on an ROI to be incorporated in the model as residuals (PSI), which would include the influence of ROI upon itself, as well as the combined influence of areas outside the model (McIntosh & Gonzalez-Lima, 1995). Although we are using structural equation modeling to calculate direct effects, total effects and residuals in our models, our variables have only one indicator (FDG uptake), so we will refer to this analysis as path analysis.
Path coefficients representing the influences through the anatomical pathways were computed using LISREL (version 8.54, Scientific software). A statistical comparison of the models was done using the multiple group stacked model approach through which functional models whose path coefficients are constrained to be equal between conditions are compared with those where the coefficients are allowed to differ (McIntosh & Gonzalez-Lima, 1995).
Total effects were calculated as the algebraic sum of direct and indirect effects (McIntosh & Gonzalez-Lima 1994). Decomposition of effects into total, direct and indirect can be very informative in that it can give an indication of the total influence of a brain region or pathway and whether the influence is modified at any stage in the system.
RESULTS
Behavioral tests
Behavioral results for the extinction group are reported here for the first time, whereas behavioral results for the pseudorandom and renewal groups have been reported elsewhere (Bruchey and Gonzalez-Lima, 2006), and are briefly summarized below. Extinction group animals displayed strong conditioning to the tone CS, as evidenced by their high (12.9 ± 1.44 seconds) freezing when presented with a conditioned CS out of the acquisition context. This CR was successfully extinguished, as suggested by diminished freezing response (0.3 ± 0.36 seconds) displayed during the last 3 presentations of the CS at the end of the extinction training, as well as the post-extinction renewal probe (probe II, table 2).
Table 2. Behavioral results.
Means and standard errors of CR (seconds of freezing during the first CS presentation minus seconds of freezing during the first pre-CS period) during the post-acquisition (probe I), average CR (seconds of freezing) during the last three tones of extinction training (extinction), and means and standard errors of CR (seconds of freezing during the first CS presentation minus seconds of freezing during the first pre-CS period) during the renewal probe (probe II).
| Group | Probe I | Extinction | Probe II |
|---|---|---|---|
| Extinction | 12.9 ± 1.44 | 0.3 ± 0.36 | 2.4 ± 1.5 |
| Renewal | 13.5 ± 0.6 | 0.12 ± 0.18 | 12.6 ± 1.5* |
| Pseudorandom | 2.4 ± 1.8* | 0.3 ± 0.39 | 0.3 ± 0.6 |
p < 0.01 significant group differences in probe I, II
The probe trials indicated that conditioned fear acquisition and extinction training was successful. Freezing CR was significantly greater (F(1,18) = 20.87; p < 0.01) in the renewal group when that group was tested out of the extinction context, as compared to the extinction group (tested in the context of extinction), although there were no differences in freezing to the CS at the end of extinction training between these two groups (F(1,18) = 0.25; p > 0.05). Pseudorandomly trained animals displayed a low level of conditioned freezing to the tone CS following acquisition, indicating that for this group the CS did not acquire associative properties consistent with a conditioned excitor.
During the FDG session, pseudorandom group rats froze only 3.9 ± 2.5 %, while extinction group rats froze 0.6 ± 0.46 % of the time. In contrast, renewal group froze 66.25 ± 12.9 % of the time. Renewal rats did not show a slope of change in response to the CS during the first 10 minutes. The average number of seconds spent freezing was 76.5 during minutes 1–2, 93 during minutes 3–4, 99.4 during minutes 5–6, 82.2 during minutes 7–8 and 90.4 during minutes 9–10. This confirms that presentation of an extinguished CS out of the extinction context leads to strong and relatively persistent CR renewal.
Correlational analyses
When all the regions represented in the model were used as predictors of freezing behavior in multiple linear regression analysis, it was found that there was a strong (r = 0.9) correlation between the FDG uptake in these regions and freezing across subjects. Bivariate correlations of relative FDG uptake for each group of rats are presented in table 4.
Table 4.
Bivariate correlations of relative FDG uptake for each group of rats.
| MGD | PER | ILA | LA | BA | IC | CEAl | CEAm | vlPAG | CA1 | CG2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Extinction group | |||||||||||
| MGD | 1.00 | ||||||||||
| PER | 0.73 | 1.00 | |||||||||
| ILA | 0.08 | −0.39 | 1.00 | ||||||||
| LA | 0.74 | 0.79 | −0.14 | 1.00 | |||||||
| BA | −0.23 | −0.15 | 0.05 | −0.40 | 1.00 | ||||||
| IC | 0.85 | 0.82 | −0.13 | 0.96 | −0.47 | 1.00 | |||||
| CEAl | 0.19 | 0.13 | −0.05 | −0.14 | 0.54 | −0.11 | 1.00 | ||||
| CEAm | 0.69 | 0.68 | −0.14 | 0.92 | −0.61 | 0.93 | −0.39 | 1.00 | |||
| vlPAG | 0.26 | 0.37 | −0.20 | −0.10 | 0.52 | −0.03 | 0.91 | −0.32 | 1.00 | ||
| CA1 | 0.65 | 0.72 | −0.39 | 0.34 | 0.10 | 0.43 | 0.65 | 0.20 | 0.78 | 1.00 | |
| CG2 | 0.43 | 0.14 | 0.29 | 0.60 | −0.82 | 0.62 | −0.58 | 0.72 | −0.65 | −0.24 | 1.00 |
| B. Renewal group | |||||||||||
| MGD | 1.00 | ||||||||||
| PER | 0.82 | 1.00 | |||||||||
| ILA | 0.31 | −0.11 | 1.00 | ||||||||
| LA | 0.72 | 0.87 | −0.24 | 1.00 | |||||||
| BA | −0.06 | 0.35 | −0.70 | 0.20 | 1.00 | ||||||
| IC | 0.73 | 0.83 | −0.14 | 0.97 | 0.16 | 1.00 | |||||
| CEAl | 0.74 | 0.91 | −0.17 | 0.83 | 0.43 | 0.80 | 1.00 | ||||
| CEAm | 0.79 | 0.92 | −0.16 | 0.95 | 0.29 | 0.96 | 0.84 | 1.00 | |||
| vlPAG | 0.47 | 0.35 | 0.18 | 0.44 | 0.23 | 0.53 | 0.40 | 0.56 | 1.00 | ||
| CA1 | 0.91 | 0.92 | 0.03 | 0.75 | 0.26 | 0.75 | 0.87 | 0.82 | 0.32 | 1.00 | |
| CG2 | 0.55 | 0.66 | 0.13 | 0.32 | 0.26 | 0.25 | 0.73 | 0.36 | −0.01 | 0.68 | 1.00 |
Abbreviations are as in Table 3.
Path analysis
The final model was based on Maren’s model of fear extinction outlined in Fig. 1.
However, since this model was not able to account for some of the variability observed in the extinction and the renewal groups, another region (anterior cingulate cortex, CG2) and another anatomical pathway (infralimbic cortex to ventrolateral periaqueductal gray; ILA to vlPAG) were added to the path analysis in order to better represent brain changes found utilizing the FDG metabolic mapping technique. This one additional region and one more path were chosen by iteration after nine alternative structural equation models including additional brain ROIs (dentate gyrus, CA3, posterior parietal cortex, external cuneate nucleus, septum, nucleus accumbens and caudate putamen) and combinations of connections between these ROIs were tested. The ROIs tested were selected based on the literature implicating their possible role in fear extinction and renewal, anatomical connectivity, and our empirical data showing between-group differences in some of these regions. The final model reported here was found to yield optimal solution out of all the models tested. The rejected models had non-significant chi square values given their degrees of freedom (13.4 to 20.04, p > 0.05) or overall higher residual values (greater than 0.94). The final model consisted of the eleven areas listed in table 3 and their connections presented in Fig 2.
Table 3.
List of abbreviations
| Region of interest | Abbreviation | Bregma level |
|---|---|---|
| Infralimbic cortex, anterior | ILA | 3.2 mm |
| Anterior cingulate cortex | CG2 | 0.7 mm |
| Perirhinal cortex | PER | −2.3 mm |
| Lateral amygdaloid nucleus | LA | −2.3 mm |
| Central amygdaloid nucleus, medial | CEAm | −2.3 mm |
| Central amygdaloid nucleus, lateral | CEAl | −2.3 mm |
| Basal amygdaloid nucleus | BA | −2.3 mm |
| Intercalated amygdaloid cells | ITC | −2.3 mm |
| Hippocampus | CA1 | −3.8 mm |
| Medial geniculate nucleus, dorsal | MGD | −6.04 mm |
| Ventrolateral periaqueductal gray | vlPAG | −8.3 mm |
Fig. 2.
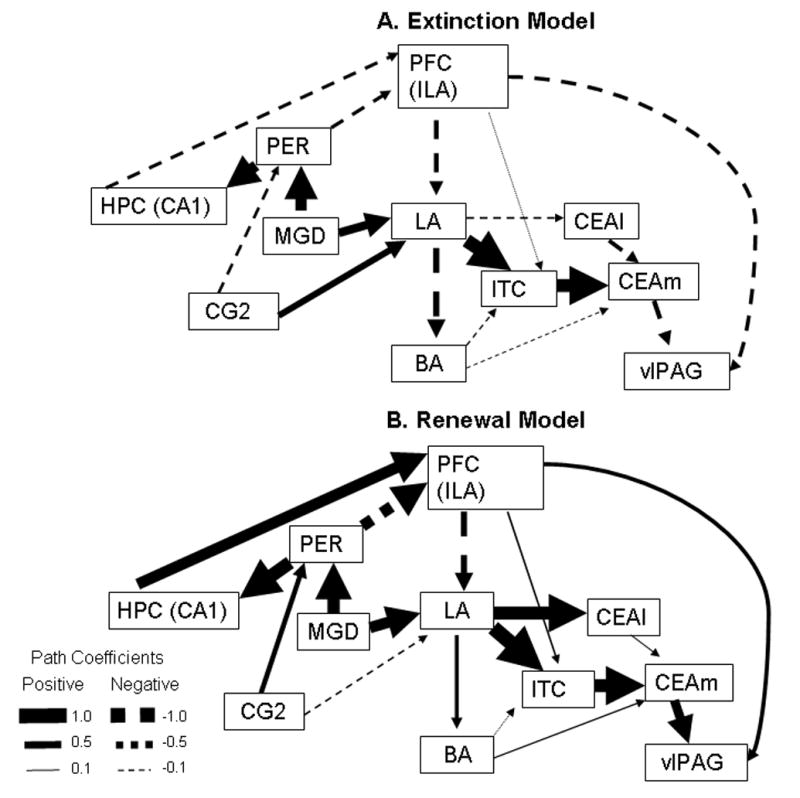
Graphic representation of the direct effects for the a) fear extinction model and b) fear renewal model. Magnitude of the direct effect is proportional to arrow width for each path. Positive path coefficients are shown as solid arrows, whereas negative coefficients are shown as segmented arrows. Values for width gradient are given in the legend at the bottom left of the figure.
In this study, the “all paths free” alternative model reproduced a correlation matrix whose χ2 goodness-of-fit statistic was significantly different (χ2diff(28) = 123; p < 0.05) from the χ2 of the original (null model) correlation matrix, indicating that all path coefficients between the chosen ROIs were statistically different between the renewal and the extinction group.
In both of these graphic representations, the magnitude of the direct effect is proportional to the arrow width for each path (Fig. 2). Positive path coefficients are shown as solid arrows, while negative path coefficients are shown as segmented arrows. As seen, there are several path coefficients that showed changes not only in magnitude, but in sign as well. Perhaps the most clear example of this is the direct effect from CA1 hippocampal field to the anterior infralimbic cortex (ILA). While this path coefficient was strongly positive (0.77) in the fear renewal condition, it was weakly negative (−0.23) in the fear extinction condition. Other path coefficients (direct effects), PSI values (residual influences) and total effects combined (direct and indirect effects) for this model are listed in tables 5 and 6 and described below.
Table 5. Total effects, direct effects and residuals (PSI values) for the functional neural network model of fear extinction.
Total effects are the algebraic sum of the direct and indirect effects. Direct effects represent the influence of one brain region on another with the impact of other regions left constant. Residual influences include the combined influences of areas outside the model and the influence of a brain region upon itself. The columns list origins of effect and the rows list structures being affected. Values given are coefficients for total, direct and residual effects. Effects that can not occur given the direct anatomical connections are left blank.
| ROI | ILA | CG2 | PER | CA1 | MGD | LA | BA | ITC | CEAl | CEAm | vlPAG |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Effects | |||||||||||
| ILA | 0.08 | −0.41 | −0.23 | −0.31 | |||||||
| CG2 | |||||||||||
| PER | −0.2 | 0.76 | |||||||||
| CA1 | −0.15 | 0.74 | 0.57 | 0.05 | −0.32 | ||||||
| MGD | |||||||||||
| LA | −0.32 | 0.42 | 0.13 | 0.07 | 0.68 | ||||||
| BA | 0.13 | −0.17 | −0.05 | −0.03 | −0.27 | −0.4 | |||||
| ITC | 0.96 | 0.4 | 0.13 | 0.07 | 0.66 | 0.96 | −0.1 | ||||
| CEAl | −0.13 | −0.06 | −0.02 | −0.01 | −0.09 | −0.13 | |||||
| CEAm | 0.9 | 0.38 | 0.12 | 0.07 | 0.62 | 0.9 | −0.28 | ||||
| vlPAG | −0.33 | −0.16 | 0.06 | 0.04 | −0.14 | −0.33 | −0.13 | 0.88 | 0.1 | −0.37 | |
| Direct Effects | |||||||||||
| ILA | −0.24 | −0.23 | |||||||||
| CG2 | |||||||||||
| PER | −0.2 | 0.76 | |||||||||
| 0.74 | |||||||||||
| CA1 | |||||||||||
| MGD | |||||||||||
| LA | −0.32 | 0.44 | 0.58 | ||||||||
| BA | −0.4 | ||||||||||
| ITC | 0.01 | 0.93 | −0.1 | ||||||||
| CEAl | −0.13 | ||||||||||
| CEAm | −0.04 | 0.88 | −0.28 | ||||||||
| vlPAG | −0.32 | ||||||||||
|
| |||||||||||
| Residuals | |||||||||||
|
| |||||||||||
| (PSI) | 0.81 | 1 | 0.38 | 0.45 | 1 | 0.27 | 0.84 | 0.05 | 0.98 | 0.04 | 0.9 |
Table 6. Total effects, direct effects and residuals (PSI values) for the functional neural network model of fear renewal.
Total effects are the algebraic sum of the direct and indirect effects. Direct effects represent the influence of one brain region on another with the impact of other regions left constant. Residual influences include the combined influences of areas outside the model and the influence of a brain region upon itself. The columns list origins of effect and the rows list structures being affected. Values given are coefficients for total, direct and residual effects. Effects that can not occur given the direct anatomical connections are left blank.
| ROI | ILA | CG2 | PER | CA1 | MGD | LA | BA | ITC | CEAl | CEAm | vlPAG |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Effects | |||||||||||
| ILA | −0.03 | −0.09 | 0.77 | −0.07 | |||||||
| CG2 | |||||||||||
| PER | 0.34 | 0.75 | |||||||||
| CA1 | −0.08 | 0.91 | 0.68 | ||||||||
| MGD | |||||||||||
| LA | −0.41 | −0.1 | 0.04 | −0.32 | 0.82 | ||||||
| BA | −0.11 | −0.03 | 0.01 | −0.08 | 0.21 | 0.26 | |||||
| ITC | −0.41 | −0.1 | 0.04 | −0.31 | 0.8 | 0.98 | −0.04 | ||||
| CEAl | −0.36 | −0.09 | 0.03 | −0.28 | 0.72 | 0.88 | |||||
| CEAm | −0.4 | −0.09 | 0.04 | −0.31 | 0.79 | 0.96 | 0.06 | 0.88 | 0.08 | ||
| vlPAG | −0.08 | 0.54 | 0.69 | 0.04 | 0.63 | 0.06 | 0.71 | ||||
| Direct Effects | |||||||||||
| ILA | −0.79 | 0.77 | |||||||||
| CG2 | |||||||||||
| PER | 0.34 | 0.75 | |||||||||
| CA1 | 0.91 | ||||||||||
| MGD | |||||||||||
| LA | −0.42 | −0.12 | 0.79 | ||||||||
| BA | 0.26 | ||||||||||
| ITC | 0.12 | 1 | 0.04 | ||||||||
| CEAl | 0.87 | ||||||||||
| CEAm | 0.1 | 0.89 | 0.06 | ||||||||
| vlPAG | 0.62 | ||||||||||
|
| |||||||||||
| Residuals | |||||||||||
|
| |||||||||||
| (PSI) | 0.89 | 1 | 0.32 | 0.18 | 1 | 0.15 | 0.93 | 0.03 | 0.24 | 0.03 | 0.61 |
Direct effects
Overall, this model revealed many more negative direct influences on brain regions in the fear extinction than the fear renewal condition, suggesting a reversal in the functional interactions from negative during extinction recall to positive during fear renewal. Additionally, the magnitude of direct effects was often different between groups, reflecting a change in the strength of the influences conveyed through those pathways. Path weights of regional influences are essentially linear regression coefficients with the sign implying the positive or negative influence of one region on another. The magnitude of the weight gives the strength of the regression. It has been suggested that relative suppression of activity at one region with respect to another produces path weights that are negative, implying that this represents an inhibitory process at the neuronal level (Nyberg et al., 1996). However, interpretation of path coefficient signs as representing excitatory or inhibitory influences in the electrophysiological sense is unwarranted, considering that the relationship between FDG uptake and action potential rates may differ in various brain regions (McIntosh & Gonzalez-Lima, 1994). Rather, weight of a positive path coefficient indicates the proportion of the activity in the target region which will increase given a unit increase in the source region; a negative coefficient indicates the proportion the activity in the target region will decrease given a unit increase in the source region.
Path analysis of the proposed extinction and fear renewal neural circuitry suggests that there are several functional pathways through which neural representations of the CS and context might come to influence behavioral output. While the proposed auditory CS input through the dorsal medial geniculate nucleus (MGD) seemed to exert similar direct effects on perirhinal cortex (PER) and lateral amygdala (LA) in both extinction and renewal groups, possibly reflecting similar learned behavioral significance of the tone in the two conditions, direct effects from the lateral to central amygdala became strongly positive in the renewal condition. Likewise, there was a switch in LA to basal amygdaloid nucleus (BA) and ILA to vlPAG path coefficient signs from negative in extinction to positive in renewal.
Another interesting finding was that the infralimbic cortex may not need to recruit the amygdala in order to influence the CR. The pathway between the ILA and vlPAG displayed a reversal of sign between the extinction and renewal groups, indicating that the ILA may be able to influence freezing directly via its projection to vlPAG. Direct effects from anterior cingulate to PER and LA also displayed a reversal of sign between the two experimental conditions.
Residual influences
From the χ2 difference test the residual influences (PSI) on the lateral division of the central amygdaloid nucleus (CEAl) was found to vary significantly between the groups. The value of PSI for CEAl was significantly smaller (0.24) in the renewal group compared to the extinction (0.98) group; χ2diff(1) = 5.74; p < 0.05), indicating that less of the variance in the CEAl was accounted for by the selected pathways in the extinction group. The results from direct path analysis also suggests that neural influences through the intercalated cells of the amygdala may not be of critical importance for CR expression; rather, neural influences between lateral and central amygdala may play an important role in organization of the final behavioral output.
Total effects
Total effects represent the impact of regions both through direct anatomical links and any influences transmitted through indirect routes. Thus, total effects can be described as the algebraic sum of direct and indirect effects. While in some instances direct and total effects of one brain area on another did not differ to any great extent, in other cases indirect effects considerably contributed to the total effects. For example, total effects computed for the influence of ILA on certain amygdaloid nuclei were −0.4 in renewal, while in the extinction group this influence was found to be greater than or equal to 0.9 (Tables 5 and 6). Therefore, effect decomposition revealed that in the extinction group ILA had a large positive influence on amygdaloid nuclei implicated in the regulation of CR behavior, while in the renewal group this influence was negative (Fig. 3). This finding lends support to the hypothesis that infralimbic cortex can greatly influence amygdaloid nuclei following the extinction of fear conditioning, possibly governing the inhibition of the CR (Maren et al., 1997, Maren and Fanselow, 1997, Maren, 1999, Quirk et al., 2000).
Fig. 3.
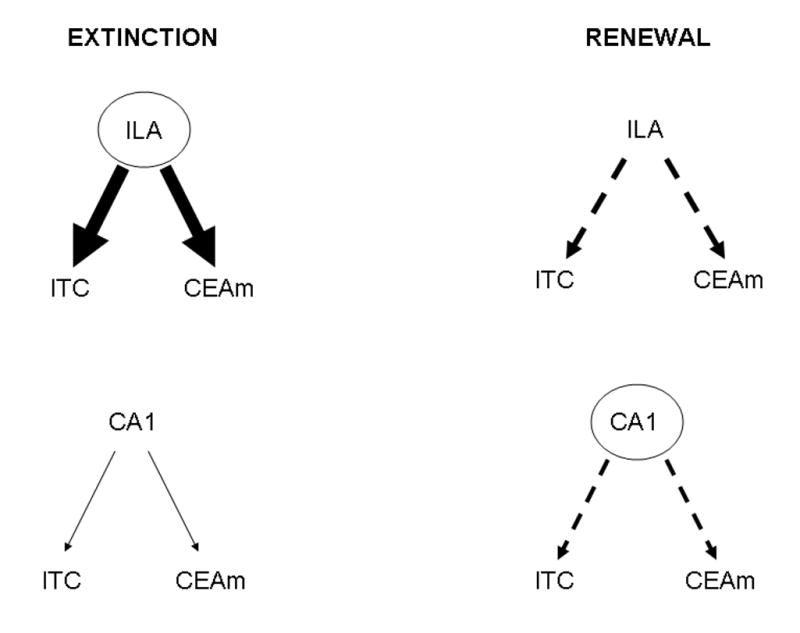
Graphic representation of the total effects on amygdaloid nuclei for the fear extinction and renewal models. The diagrams contrast the large positive total effects of prefrontal (ILA) cortex on amygdaloid nuclei (ITC and CEAm) activity during extinction with the negative total effects of ILA and CA1 on these amygdaloid nuclei during renewal. Positive total effects are shown as solid arrows, whereas negative ones are shown as segmented arrows. Magnitude of the total effect is proportional to arrow width.
Similarly, an opposite pattern of total effects between the two groups was also observed for the influence of hippocampus (CA1) on the intercalated amygdaloid cells (ITC) and medial central amygdaloid nucleus (CEAm). While in extinction these total effects were found to be very close to 0, in the renewal group they were approximately −0.3, suggesting that hippocampal influence on the amygdala through both direct and indirect routes plays a larger role in the renewal group than it does in the extinction group. This is consistent with the concept that the hippocampal formation may participate in the representation and the processing of contextual cues, thought to be important in context-dependent fear renewal (Corcoran and Maren, 2001, Corcoran and Maren, 2004, Corcoran et al., 2005, Ji and Maren, 2005, Bouton et al., 2006).
Finally, total influences of higher brain regions on the vlPAG showed a change of sign between the extinction and the renewal groups as well (Fig. 4). Total effects of MGD, LA and CEAm on vlPAG were found to be negative in the extinction group, and positive in the renewal group. Therefore, the analysis of total effects clearly showed different patterns of brain regional interactions between the fear extinction recall and fear renewal condition.
Fig. 4.
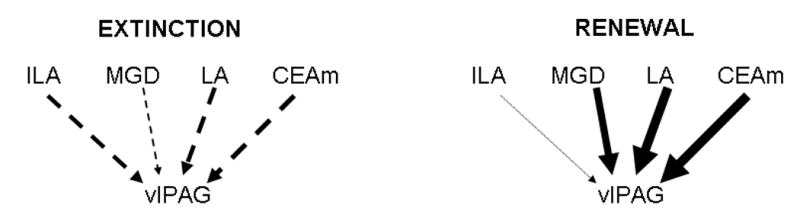
Graphic representation of the total effects on ventrolateral periaqueductal gray (vlPAG) for the fear extinction and renewal models. The diagrams contrast the shift from negative to positive total effects of higher regions on the vlPAG in the extinction and renewal groups. Negative total effects are shown as segmented arrows. Magnitude of the total effect is proportional to arrow width.
While the above described model of neural interactions between multiple regions was solvable for both extinction and renewal groups FDG data, the pseudorandom group data were not solvable using this combination of neural nodes and pathways. The inability of the model to account for the variability in the regional FDG uptake observed in the pseudorandom group may partially be due to high colinearity between some of the brain areas represented in the model, and partially due to the fact that no CS-US association was formed as a result of pseudorandom training in this group.
DISCUSSION
The network model of fear extinction supports the hypothesis that fear extinction is functionally associated with inhibitory influences from the medial prefrontal cortex to the central amygdala (CEA) and ventrolateral periaqueductal grey (vlPAG), regions mediating the output freezing behavior. The initial network brain areas were selected based on an existing model of neural substrates of conditioned fear extinction compiled by Maren (2005). However, it is of interest to note that very similar results were obtained by a data-driven approach, in which the neural systems involved in fear extinction were defined by the data and constructed based on regions showing significant differences between groups in previous statistical examinations (Bruchey & Gonzalez-Lima, unpublished data). When direct effects and residual values for each ROI obtained from our FDG data were applied to Maren’s model of extinction pathways, it was found that this model was unable to account for a large amount of influences on some of the critical regions (such as the prefrontal cortex and the lateral amygdaloid nucleus). Thus, another ROI and inter-regional connection were added to the model in an attempt to better approximate the neural network of fear extinction. The anterior cingulate was included in the model because of recent work suggesting that this region may play an important role in memory for footshock (Malin and McGaugh, 2006), extinction of specific phobias (Straube et al., 2006), and gating among external sensory and internally driven stimuli and limbic structures involved in emotional and behavioral responses to CSs (Hamner et al., 1999).
The anterior cingulate region added is the ventral anterior cingulate cortex in rats [Cg2 of Zilles and Wree (1985)], which corresponds to human supragenual anterior cingulate (area 24), based on the homology analysis made by Uylings & Van Eden (1990). It is the area of the anterior cingulate immediately dorsal to the corpus callosum at Bregma 0.7 mm, labeled as Cg2 in Paxinos & Watson (1997). Metabolic suppression in the ventral anterior cingulate cortex is found in congenitally helpless rats (Shumake and Gonzalez-Lima, 2003), which show persistent fear memories and impaired fear extinction (Shumake et al., 2005).
Although all the pathways indicated in Maren’s extinction model are anatomically correct, they are not complete. For example, many of the ROIs shown to have only one-way connectivity in fact have reciprocal connections, and some of the known inter-regional connections are not represented. Therefore, this model is a simplified representation of the actual neural network that may participate in some aspects of conditioned fear extinction. However, there are limits as to how many regions and connections one can consider in order to have solvable structural equations, so construction of this model represents a compromise between anatomical accuracy and the ability to interpret the model.
Another limitation of Maren’s extinction model is that it does not take into account the neural representation of the US, which may prove important when considering emergent properties of distributed brain activity and network interactions in extinction learning. In addition, several large residual values (PSI) were present, which may have occurred through a combination of influences from brain regions not included in this model and internal processes within these regions. Some residual values, such as those of the lateral central amygdaloid nucleus and ventrolateral periaqueductal gray, were lower in the functional network model of fear renewal, as compared to extinction model. This indicates that some influences on these regions were not accounted for by the presented extinction model.
Nevertheless, the application of anatomically based path analysis to metabolic brain mapping data can broaden our understanding of brain-behavior relations at the systems level. For example, one possible application of this model would be to evaluate how a functional disconnection would be reflected in the functional connectivity maps of the proposed regions of interest, such as in transgenic mice that do not show fear renewal (Waddell et al., 2004), or in phobics who do not extinguish their fear responses (Craske 1999). Furthermore, metabolic brain mapping in conjunction with path analysis could be utilized to relate differences in the functional interactions between brain regions to the learned significance of stimuli and their behavioral outcomes.
For instance, metabolic mapping together with network modeling can be used to test the hypothesis that fear extinction is functionally associated with inhibitory influences from the mPFC to the CEA and vlPAG, regions mediating the output freezing behavior. This hypothesis is difficult to test using the brain lesion approach, given that lesion of CEA and vlPAG disrupt the freezing behavior (Vianna et al., 2001b, Wilensky et al., 2006). There are also conflicting lesions reports regarding the role of mPFC in fear extinction. For example, some studies have found no mPFC lesion effects (Gewirtz et al., 1997, Vouimba et al., 2000), while others have found deficits in extinction (Morgan et al., 2003). Our model of fear extinction may help clarify these conflicting lesion results, because mPFC is part of a larger network of regions that collectively influence the freezing response (Barrett et al., 2003). Dysfunction of more than one of these regions may be necessary for observing extinction deficits, as unaffected regions with parallel influences to CEA and vlPAG may compensate for any single region’s influence.
The results from the combination of FDG brain imaging with path analysis suggest that the functional interactions between multiple neural pathways depend not only on the acquired behavioral significance of the tone CS, but also on the environmental context in which that tone was encountered. Bouton (2004) proposed that an extinguished CS may be particularly sensitive to context shift due to its ambiguity (tone was first encountered as an excitatory CS, then extinguished, so that it may have two competing meanings, which contextual cues help disambiguate).
The structural equation model presented in this study suggests that the hippocampal formation and its associated areas (CA1 and PER) may be involved in this type of “occasion setting” in fear extinction and renewal of conditioned fear. For example, while the CA1-ILA pathway is weakly negative in the extinction group, it is strongly positive in the renewal group, suggesting that the information transmitted from CA1 hippocampal cells to the infralimbic cortex may guide the further interaction between the prefrontal cortical areas with other neural nodes of interest. Additionally, greater hippocampal activation was observed in the renewal as compared to extinction group, lending further support to the importance of hippocampus in fear renewal (Corcoran and Maren, 2001, Corcoran and Maren, 2004, Corcoran et al., 2005, Ji and Maren, 2005). Therefore, Maren’s model is supported in part insofar as greater hippocampal activation was apparent in the renewal condition relative to the extinction condition. The network model of fear renewal supports the hypothesis that context-dependent fear renewal is functionally associated with an increased influence of the hippocampus on the mPFC.
The renewal model suggests that the PER is contributing an important influence to the mPFC. Although there are no lesion studies of PER in renewal, PER lesions disrupt auditory, visual and contextual fear conditioning, as well as fear-potentiated startle (Rosen et al., 1992; Sacchetti, Lorenzini, Baldi, Tassoni, & Bucherelli, 1999; Burwell, Bucci, Sanborn, & Jutras, 2004). Rostral PER lesions following fear conditioning interfere with CRs evoked by auditory stimuli when these stimuli are presented in contexts that differ from the initial conditioning context (Corodimas & Ledoux, 1995). Therefore, PER may participate in novel/familiar context discrimination during ABC renewal. In addition, PER has reciprocal connections to the hippocampus (Naber, Witter, & Lopes da Silva, 1999; Burwell et al., 2004). Hippocampal lesions impair renewal in some studies (Corcoran & Maren, 2004; Ji & Maren, 2005), but not others ( Wilson et al., 1995; Frohardt et al., 2000). Perhaps hippocampal lesions disrupt incoming PER influences, especially novelty/familiarity discriminations (Bussey, Muir, & Aggleton, 1999; Liu & Bilkey, 2001; Murray & Richmond, 2001).
It is possible that the ambiguity of an extinguished tone encountered out of the extinction context is reflected by the incongruent signals from PER and CA1 to the ILA in the renewal group, and ILA is recruited to resolve the inconsistency. The hypothesis that the functional relevance of a brain area depends on the status of other connected areas was proposed by McIntosh (1999), and it can be illustrated in our model as a shift in functional connectivity between CA1 and ILA. The hippocampal formation may serve as a behavioral catalyst, enabling the transition between behavioral states (McIntosh et al., 2003, McIntosh, 2004). Our model suggests that context-dependent fear renewal is functionally associated with an increased influence of the hippocampus on the medial prefrontal cortex, and it lends support to evidence from lesion studies which indicate that lesions of the hippocampus disrupt fear renewal in rats (Corcoran and Maren, 2001, Corcoran and Maren, 2004, Corcoran et al., 2005, Ji and Maren, 2005).
The relationship between the activity in prefrontal cortex and amygdala has been implicated in emotional control in both human and animal studies (Hariri et al., 2000, Quirk et al., 2006). Effects decomposition of the presented models suggested that rats showing renewal behavior had less of the infralimbic cortical influence transmitted to the intercalated cells of amygdala and the medial central amygdaloid nucleus, as compared to the extinction group animals. However, activity of the hippocampal CA1 region had more bearing on these amygdaloid areas in the renewal group, possibly reflecting influence of context on the renewal process (Fig. 3). Similarly, infralimbic cortex’ total effects on vlPAG, another brain area thought to be involved in creating the behavioral freezing response (Vianna et al., 2001a, Farook et al., 2004, De Luca-Vinhas et al., 2006), were found to be negative in the extinction group, and added up to near zero in the renewal group. Total effects from MGD, LA and CEAm on vlPAG showed a reversal of sign, indicating that increase in activity in those three areas in the extinction group may lead to a proportional decrease in activity in the vlPAG, while an increase in those three areas in the renewal group may lead to an increase in the vlPAG’s activity in the renewal group. This is in agreement with the previous findings which suggest that stimulation of vlPAG neurons results in freezing behavior in rats (Vianna et al., 2001; deLuca-Vinhas et al., 2006), and illustrates how cortical, auditory and limbic regions may work together to produce the appropriate behavioral response.
Another network analysis of Pavlovian auditory conditioning completed by our laboratory showed similar trends of path coefficient sign reversal between tone-excitor and tone-inhibitor functional neural network models (McIntosh & Gonzalez-Lima, 1994). In the tone-excitor model, similar to our renewal model, some of the effects were transmitted through positive covariance relationships, and, in the tone-inhibitor model, there was a change in those relationships to negative (McIntosh & Gonzalez-Lima, 1994). In our extinction model, it appears that contextual cues might have acquired some inhibitory stimulus properties, given that direct influence of the hippocampal CA1 field on ILA was found to be negative in the extinction group.
For the structural equation models, the connections between the selected regions were based on the known neuroanatomical pathways. Thus, data were interpreted with regard to the interactions among brain regions, mediated through the underlying neuroanatomy. Notable differences between the tone tested in extinction vs. neutral context were present in both the magnitude and the sign of the path coefficients. In some cases the pattern of differences in the path coefficients was consistent with the differences in mean FDG uptake between the two groups. For example, the path coefficient for the basal amygdaloid nucleus differed between models as did mean FDG activity in this region. There were also coefficients that differed between models, but where FDG uptake did not differ in connected regions, such as CA1 and ILA. These brain regions may thus act as behavioral catalysts and enable the change in dominant interactions from one set of regions to another (McIntosh, 2004). In other words, activity of an isolated region is not likely the critical factor which determines the behavioral expression. Rather, neural context, or the status of the rest of the brain during the time that a brain region is engaged in a mental operation is the most direct link between metabolic activity patterns and behavioral expression (McIntosh, 2004). This emphasizes the usefulness of analyzing not only mean activity levels in the brain mapping studies, but also patterns of functional relationships between anatomically connected brain regions in order to better understand brain function. It is possible that learning-related brain changes start as a change in the covariance between neural nodes, thus leading to later changes in regional brain activity (Ahissar et al., 1992).
The network models support the hypothesis that fear renewal may be functionally associated with a greater excitatory influence from the medial geniculate nucleus (MGD) to the lateral amygdala (LA). Lesions of either the medial geniculate, lateral amygdala or central amygdala disrupt fear conditioning associated with tone CSs (Iwata et al., 1986, Wilensky et al., 2006). This, and our previous studies, support the hypothesis that associative changes in the significance of an auditory stimulus occur in the auditory system, as evidenced by the total positive effects of MGD on the vlPAG during renewal and by increased FDG uptake found in the medial geniculate in the renewal, as compared to the extinction and the pseudorandom groups (Gonzalez-Lima et al., 1989), 1992; McIntosh & Gonzalez-Lima, 1993). This hypothesis is supported by electrophysiological and brain-imaging studies that have given support to the idea that learning is a property distributed over many regions, including sensory regions, rather than the result of one specialized region. Hence, neural pathways involved in a particular kind of learning depend on the requirements for behavioral change (John and Schwartz, 1978, Wolpaw and Lee, 1989, Gonzalez-Lima, 1992).
Others have proposed that any behavioral response depends not only on the brain regional activity, but is closely related to the interregional functional connectivity of the brain as well (McIntosh, 2004). Thus, selected interregional functional connectivity changes, reflected by the changing interregional correlations between the interconnected brain regional pairs, were correlated to the FDG freezing behavior. This demonstrated that more than one brain region may contribute to the freezing behavior, and that behavior can change as a function of a changing relationship between neural nodes. Particularly, it was found using multiple regression analysis that the network regional activity was strongly correlated with the freezing behavior across subjects. Recent evidence shows that inactivation of the medial amygdala decreases periaqueductal gray-related freezing response in rats (Herdade et al., 2006), suggesting the importance of interaction between these two brain regions on freezing behavior. However, just as one should look beyond the “one region, one function” interpretation of brain organization, one should also acknowledge the complexity of an interconnected brain system and recognize that in any neural network model the final behavioral output results from the combined influences of many regions on each other, not just a single pathway.
By examining the changes in the functional interactions between different brain systems, we can begin to better appreciate the mechanisms supporting neural plasticity associated with learning. However, it is important to keep in mind that while structural equation modeling provides a useful tool for the quantification of the functional interactions that take place between interconnected neural structures, a neural model can only represent a system that operates in isolation of other neural structures. Therefore, it is difficult to assess precisely what the influence of brain regions not included in the presented model may be. For example, it has been shown that stimulation of midbrain reticular formation can lead to freezing behavior (Gonzalez-Lima and Scheich, 1984), yet this brain region is not even included in our model. Likewise, some of the residual values for certain brain regions in our model are rather high, indicating that there are strong outside influences on the system which were not accounted for by the presented model. Nevertheless, structural equation modeling applied to FDG neural mapping data can help us understand the combined actions of interconnected brain regions and how their activity is related to behavior, as illustrated by the application of the structural equation modeling to an existing neural model of fear extinction discussed here.
In summary, we applied structural equation modeling to data from an FDG brain mapping study which assessed changes in rat brain metabolic activity associated with conditioned fear extinction and renewal to a neural network model of fear extinction proposed by Maren (2005). Changes in both direct and total functional influences mediated by specific neuroanatomical pathways were found between the extinction and the renewal group. As expected, total effects of the infralimbic cortex, as well as the influence of the hippocampal CA1 region on amygdala were found to differ between these two groups. There was also a reversal of both direct and total influence of higher brain areas on vlPAG, from negative in the extinction group, to positive in the renewal group. Thus, the interactions between the examined brain systems seem to change during the recall of extinction learning in the extinction vs. the neutral context. These changes are reflected in the observed differences in the metabolic brain activity and interactivity, as well as the subject’s final behavioral output.
Table 1A.
Experimental design
| Groups | Phase I | Test I | Phase II | Test II | Fluorodeoxyglucose |
|---|---|---|---|---|---|
| Renewal | T -> S in context A | T in context B or C | T in context B or C | T in context C or B | T in context C or B |
|
| |||||
| Extinction | T -> S in context A | T in context B or C | T in context B or C | T in context B or C | T in context B or C |
T, low-frequency tone; S, footshock. Contexts B and C were counterbalanced.
Table 1B.
Behavioral Conditioning Protocol, days 1–7
| Day 1–2 | 1h Habituation to conditioning context A. |
| Day 3–4 | 15 min session of paired presentations of T and S (4) in context A. |
| Day 5 | 10 min Probe session. 3 T, context B or C. |
| Day 5–6 | 1h Extinction session, 18 T, context B or C. |
| Day 7 | 10 min Probe session. 3 T, context C or B. |
| Day 7 | 1h FDG session. 500 T, context C or B. |
List of abbreviations
- FDG
Fluorodeoxyglucose
- CS
Conditioned stimulus
- US
Unconditioned stimulus
- CR
Conditioned response
- ROI
Region of interest
- ITI
Inter-trial interval
- PFC
Prefrontal cortex
- CG2
Anterior cingulate cortex
- ILA
Infralimbic cortex anterior
- vlPAG
Ventrolateral periaqueductal gray
- MGD
Medial geniculate nucleus
- PER
Perirhinal cortex
- LA
Lateral amygdala
- BA
Basal amygdaloid nucleus
- CEAl
Lateral division of the central amygdaloid nucleus
- ITC
Intercalated amygdaloid cells
- CEAm
Medial central amygdaloid nucleus
- HPC
Hippocampus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahissar M, Ahissar E, Bergman H, Vaadia E. Encoding of sound-source location and movement: activity of single neurons and interactions between adjacent neurons in the monkey auditory cortex. J Neurophysiol. 1992;67:203–215. doi: 10.1152/jn.1992.67.1.203. [DOI] [PubMed] [Google Scholar]
- Barrett D, Shumake J, Jones D, Gonzalez-Lima F. Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J Neurosci. 2003;23:5740–5749. doi: 10.1523/JNEUROSCI.23-13-05740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and Behavioral Processes in Extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and Temporal Modulation of Extinction: Behavioral and Biological Mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Gonzalez-Lima F. Brain activity associated with fear renewal. EurJ Neurosci. 2006 doi: 10.1111/j.1460-9568.2006.05229.x. in press. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb Cortex. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal Inactivation Disrupts the Acquisition and Contextual Encoding of Fear Extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal Inactivation Disrupts Contextual Retrieval of Fear Memory after Extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors Regulating the Effects of Hippocampal Inactivation on Renewal of Conditional Fear After Extinction. Learning Memory. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske M. Anxiety Disorders: Psychological approaches to theory and treament. Westview Press Boulder; CO: 1999. [Google Scholar]
- Davis M. Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: clinical implications for exposure therapy. Eur J Neurosci. 2002;16:395–398. doi: 10.1046/j.1460-9568.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca-Vinhas MCZ, Macedo CE, Brandao ML. Pharmacological assessment of the freezing, antinociception, and exploratory behavior organized in the ventrolateral periaqueductal gray. Pain. 2006;121:94–104. doi: 10.1016/j.pain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Farook JM, Wang Q, Moochhala SM, Zhu ZY, Lee L, Wong PT. Distinct regions of periaqueductal gray (PAG) are involved in freezing behavior in hooded PVG rats on the cat-freezing test apparatus. Neurosci Lett. 2004;354:139–142. doi: 10.1016/j.neulet.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, synaptic plasticity, and psychopathology. Rev Neurosci. 2002;13:195–208. doi: 10.1515/revneuro.2002.13.3.195. [DOI] [PubMed] [Google Scholar]
- Gewirtz J, Falls WA, Davis M. Normal Conditioned Inhibition and Extinction of Freezing and Fear-Potentiated Startle Following Electrolytic Lesions of Medial Prefrontal Cortex in Rats. Behav Neurosci. 1997;11:712–716. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F. Advances in Metabolic Mapping Techniques for Brain Imaging of Behavioral and Learning Functions. Kluwer Academic Publishers; Boston/London: 1992. Brain imaging of auditory learning functions in rats: studies with fluorodeoxyglucose autoradiography and cytochrome oxidase histochemistry; pp. 39–109. [Google Scholar]
- Gonzalez-Lima F, Finkenstadt T, Ewert JP. Learning-related activation in the auditory system of the rat produced by long-term habituation: a 2-deoxyglucose study. Brain Res. 1989;489:67–79. doi: 10.1016/0006-8993(89)90009-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Scheich H. Functional activation in the auditory system of the rat produced by arousing reticular stimulation: a 2-deoxyglucose study. Brain Res. 1984;299:201–214. doi: 10.1016/0006-8993(84)90702-9. [DOI] [PubMed] [Google Scholar]
- Grady C, McIntosh AR, Craik FIM. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Hamner M, Lorberbaum J, George M. Potential role of the anterior cingulate cortex in PTSD: Review and hypothesis. Depress Anxiety. 1999;9:1–14. [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Herdade KCP, de Andrade Strauss CV, Junior HZ, de Barros Viana M. Effects of medial amygdala inactivation on a panic-related behavior. Behav Brain Res. 2006;172:316–323. doi: 10.1016/j.bbr.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Glabus MF, Michael FG. International Review of Neurobiology. Vol. 66. Academic Press; 2005. Neural Modeling and Functional Brain Imaging: The Interplay between the Data-Fitting and Simulation Approaches; pp. 267–290. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383:195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ER, Schwartz EL. The Neurophysiology of Information Processing and Cognition. Annu Rev Psychol. 1978;29:1–29. doi: 10.1146/annurev.ps.29.020178.000245. [DOI] [PubMed] [Google Scholar]
- Jones D, Gonzalez-Lima F. Mapping pavlovian conditioning effects on the brain: Blocking, contiguity, and excitatory effects. J Neurophysiol. 2001;86:809–823. doi: 10.1152/jn.2001.86.2.809. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-Dependent Human Extinction Memory Is Mediated by a Ventromedial Prefrontal and Hippocampal Network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and Burying Fear Memories in the Brain. The Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic Lesions of the Fimbria/Fornix, Dorsal Hippocampus, or Entorhinal Cortex Produce Anterograde Deficits in Contextual Fear Conditioning in Rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7:523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Contexts and catalysts: a resolution of the localization and integration of function in the brain. Neuroinformatics. 2004;2:175–182. doi: 10.1385/NI:2:2:175. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural modeling of functional neural pathways mapped with 2-deoxyglucose: effects of acoustic startle habituation on the auditory system. Brain Res. 1991;547:295–302. doi: 10.1016/0006-8993(91)90974-z. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural modeling of functional visual pathways mapped with 2-deoxyglucose: effects of patterned light and footshock. Brain Res. 1992;578:75–86. doi: 10.1016/0006-8993(92)90232-x. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Network analysis of functional auditory pathways mapped with fluorodeoxyglucose: Associative effects of a tone conditioned as a Pavlovian excitor or inhibitor. Brain Res. 1993;627:129–140. doi: 10.1016/0006-8993(93)90756-d. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Network interactions among limbic cortices, basal forebrain, and cerebellum differentiate a tone conditioned as a Pavlovian excitor or inhibitor: fluorodeoxyglucose mapping and covariance structural modeling. J Neurophysiol. 1994;72:1717–1733. doi: 10.1152/jn.1994.72.4.1717. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Functional network interactions between parallel auditory pathways during Pavlovian conditioned inhibition. Brain Res. 1995;683:228–241. doi: 10.1016/0006-8993(95)00378-4. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Rajah MN, Lobaugh NJ. Functional Connectivity of the Medial Temporal Lobe Relates to Learning and Awareness. J Neurosci. 2003;23:6520–6528. doi: 10.1523/JNEUROSCI.23-16-06520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, Ledoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. BehavBrain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Cabeza R, Nilsson LG, Houle S, Habib R, Tulving E. Network Analysis of Positron Emission Tomography Regional Cerebral Blood Flow Data: Ensemble Inhibition during Episodic Memory Retrieval. J Neurosci. 1996;16:3753–3759. doi: 10.1523/JNEUROSCI.16-11-03753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New Vistas on Amygdala Networks in Conditioned Fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, Ledoux JE. Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal Mechanisms in Extinction of Conditioned Fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The Role of Ventromedial Prefrontal Cortex in the Recovery of Extinguished Fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: Reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav Brain Res. 2005;164:222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain Systems Underlying Susceptibility to Helplessness and Depression. Behav Cogn Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WHR. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Vianna DML, Graeff FG, Brandao ML, Landeira-Fernandez J. Defensive freezing evoked by electrical stimulation of the periaqueductal gray: comparison between dorsolateral and ventrolateral regions. Neuroreport. 2001a;12:4109–4112. doi: 10.1097/00001756-200112210-00049. [DOI] [PubMed] [Google Scholar]
- Vianna DML, Graeff FG, Landeira-Fernandez J, Brandao ML. Lesion of the Ventral Periaqueductal Gray Reduces Conditioned Fear but Does Not Change Freezing Induced by Stimulation of the Dorsal Periaqueductal Gray. Learn Mem. 2001b;8:164–169. doi: 10.1101/lm.36101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba RM, Garcia R, Baudry M, Thompson RF. Potentiation of conditioned freezing following dorsomedial prefrontal cortex lesions does not interfere with fear reduction in mice. Behav Neurosci. 2000;114:720–724. doi: 10.1037//0735-7044.114.4.720. [DOI] [PubMed] [Google Scholar]
- Waddell J, Dunnett C, Falls WA. C57BL/6J and DBA/2J mice differ in extinction and renewal of extinguished conditioned fear. Behav Brain Res. 2004;154:567–576. doi: 10.1016/j.bbr.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the Fear Circuit: The Central Nucleus of the Amygdala Is Required for the Acquisition, Consolidation, and Expression of Pavlovian Fear Conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Lee CL. Memory traces in primate spinal cord produced by operant conditioning of H-reflex. J Neurophysiol. 1989;61:563–572. doi: 10.1152/jn.1989.61.3.563. [DOI] [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: Areal and laminar structure. In: Paxinos G, editor. The rat nervous system. Academic Press; Sydney, Australia: 1985. pp. 374–415. [Google Scholar]


