Abstract
This study tested the hypotheses that older adults make less advantageous decisions than younger adults on the Iowa gambling task (IGT). Less advantageous decisions, as measured by the IGT, are characterized by choices that favor larger versus smaller immediate rewards, even though such choices may result in long-term negative consequences. The IGT, and measures of neuropsychological function, personality, and psychopathology were administered to 164 healthy adults 18–85 years of age. Older adults performed less advantageously on the IGT compared with younger adults. Additionally, a greater number of older adult’s IGT performances were classified as ‘impaired’ when compared to younger adults. Less advantageous decisions were associated with obsessive symptoms in older adults and with antisocial symptoms in younger adults. Performance on the IGT was positively associated with auditory working memory and psychomotor function in young adults, and in immediate memory in older adults.
Keywords: decision-making, aging, cognition, gambling task, frontal lobe function, executive function
Introduction
Older adults are faced with many situations that require making important decisions about financial management, medical care, retirement, housing, and transportation. Unfortunately, it is not uncommon to read of accounts in the local newspaper of elderly individuals being victimized by a range of scams involving home-repair, fraudulent lottery or sweepstake winnings, identity theft, and fraudulent charitable organizations. It is a fact that older adults are preferentially targeted by fraudulent and misleading advertising (American Association of Retired Persons, 1996), presumably because they are more likely to make ill-informed decisions that make them easier prey for scam artists.
The poor decisions observed in some older adults are thought to be due to either age-related declines in general cognitive function (Band et al., 2002) or to age-related declines in specific executive cognitive abilities associated with changes in the frontal lobe (Raz et al., 1998; West, 1996). For instance, older adults deliberate less when solving problems and have lower working memory capacity, short-term memory capacity, and speed of information processing (Johnson, 1990; Kim et al., 2002; MacPherson et al., 2002; Salthouse, 1996; Schaie & Willis, 1999). Lower working memory capacity or reduced deliberation / reflection time when making decisions are associated with impulsive decisions (Finn et al., 2002). Studies show that lower working memory capacity in younger populations is associated with less advantageous, impulsive decision-making (Bechara & Martin, 2004; Finn et al., 2002; Hinson et al., 2003). Furthermore, MRI studies have shown that aging is highly correlated with shrinkage in prefrontal regions such as the lateral prefrontal cortex, the orbitofrontal cortex, and in prefrontal grey and white matter volumes (Raz et al., 2004, 1997).
The last decade has seen a substantial amount of research on decision-making in populations, such as alcoholics, drug abusers, and those with antisocial traits, who typically display patterns of impulsive decisions characterized by choices that favor larger versus smaller immediate rewards even though such choices may result in long-term negative consequences. This pattern of decision-making, labeled as a disadvantageous ‘myopia for the future’, is usually assessed using the Iowa Gambling Task (IGT) developed by Bechara and colleagues (1994) to study the decision-making impairments of patients with lesions of the ventromedial prefrontal cortex who develop what is called acquired sociopathy. Decision-making impairments on this task are also associated with substance abuse problems, antisocial behavior, and personality traits reflecting low levels of socialization and higher levels of impulsivity (Bechara et al., 2001; Fein et al., 2004; Mazas et al., 2000).
Studies conducted in recent years that examine decision-making on similar tasks in older adults have yielded mixed results. Recent studies using the IGT suggest that decision-making in older adults is associated with different cognitive processes than in younger adults (Wood et al., 2005), and there may be some evidence that at least some older adults make less advantageous decisions than younger adults (Deakin et al., 2004; Denburg et al., 2005). Denburg and colleagues (2005) found that older adults (56 – 85 years) made generally less advantageous decisions compared with their sample of younger adults (26 – 55 years). Denburg et al’s (2005) analyses revealed that nearly one half of their older adults were clearly impaired in their decisions, and never learned to shift their decision-making strategies in order to make more advantageous decisions. However, the other half of the older adults were not impaired at all and showed the same pattern of advantageous decisions as the younger adults in their sample. Wood and colleagues (2005) also used the IGT to assess decision-making in younger (18–34 year old) and older (65–88 year old) adults. While Wood et al (2005) did not report any significant age-related differences in advantageous decisions, the mean performance for older adults was non-significantly lower than that of younger adults and older adults used different strategies than younger adults when learning the task. Finally, MacPherson and colleagues (2002) reported no significant differences in advantageous decision-making between younger and older adults; however, as pointed out by Denburg et al. (2005), older adults had generally flatter learning curves compared with younger adults, which seems to suggest some impairment in the ability to learn to make more advantageous decisions over time.
The current study follows up on these studies by assessing decision-making differences between older and younger adults using the IGT and by assessing cognitive and other psychological correlates of decision-making in these two age groups. The motivation for our study was two-fold. First, in anticipation of examining IGT performance in elderly samples of long-term abstinent alcoholics (and comparing those data to that we have previously published on young-to-middle-aged long-term abstinent alcoholics), we wanted to compare the elderly control group to the younger control subjects used in our earlier studies. Secondly, we wanted to replicate the findings of the Denburg paper (2005). Our primary hypotheses is that older adults would show less advantageous decisions compared with younger adults. Furthermore, consistent with studies linking less advantageous decisions in young adults with more antisocial behavior, we proposed that antisocial behavior (and psychological measures of deviance proneness) would be associated with less advantageous decisions in young adults, but not in older adults. The basic idea is that different mechanisms will contribute less to advantageous decisions in younger versus older adults.
Methods
Participants
A total of 164 men and women ranging in age from 18 to 85 were recruited from the San Francisco Bay Area via an Internet posting, newspaper ads, café postings, and subject referrals. All participants were chosen as healthy comparison subjects for one of two different studies on the effects of alcoholism. One study examined abstinent alcoholics ranging from 35 to 85 years of age, and the other examined active, treatment-naïve alcoholics that were 18 to 55 years of age. Following Denburg et al.(2005), participants were divided into younger and older groups at a breakpoint of 55 years of age. The younger group consisted of 49 men, and 63 women from 18 through 55 years old (M = 37.8, SD = 10.7). The older group consisted of 18 men, and 34 women ranging from 56 to 85 years old (M = 73.7, SD = 7.4). Table 2 presents subject demographics as well as a summary of the gambling task (IGT) and neuropsychological results.
Table 2.
Demographics, Gambling Game, and Neuropsychological Measures
| Younger Group Ages 18 – 55
|
Older Group Ages 56–85
|
Effect Size (%)
|
|||||
|---|---|---|---|---|---|---|---|
| Variable | Male (n=49) | Female (n=63) | Male (n=18) | Female (n=34) | Group | Gender | Group x Gender |
| Demographics | |||||||
| Age (years) | 35.4 ± 10.1 | 39.7 ± 10.8 | 71.5 ± 7.6 | 74.8 ± 7.2 | N/A | N/A | N/A |
| Years Education | 16.3 ± 2.0 | 16.4 ± 1.6 | 16.5 ± 2.5 | 15.9 ± 1.9 | 0.1 | 0.4 | 0.8 |
|
| |||||||
| Gambling Game | |||||||
| Sum of the ‘Good’ - ‘Bad’ Decksa | 36.6 ± 31.2 | 34.3 ± 29.3 | 21.7 ± 31.8 | 8.2 ± 35.1 | 8.1*** | 1.3 | 0.7 |
|
| |||||||
| Neuropsychological Domains | |||||||
| Average Z Score | 0.28 ± 0.46 | 0.25 ± 0.45 | 0.33 ± 0.40 | 0.26 ± 0.52 | 0.1 | 0.2 | 0.1 |
| Attention | −0.07 ± 0.64 | 0.06 ± 0.49 | −0.15 ± 0.78 | 0.24 ± 0.51 | 0.1 | 4.0** | 1.1 |
| Auditory Working Memory | 0.15 ± 0.85 | −0.17 ± 1.04 | −0.06 ± 0.58 | −0.50 ± 1.09 | 1.6 | 3.0* | 0.1 |
| Verbal | 0.98 ± 0.62 | 1.01 ± 0.63 | 0.88 ± 0.76 | 0.93 ± 0.48 | 0.5 | 0.1 | 0.0 |
| Abstraction/Cognitive Flexibility | 0.17 ± 0.59 | 0.18 ± 0.55 | 0.42 ± 0.58 | 0.21 ± 0.53 | 1.2 | 0.7 | 0.8 |
| Psychomotor | −0.07 ± 0.93 | −0.07 ± 0.88 | −0.21 ± 0.67 | −0.34 ± 0.64 | 1.2 | 0.1 | 0.1 |
| Immediate Memory | 0.64 ± 0.57 | 0.62 ± 0.57 | 0.52 ± 0.44 | 0.39 ± 0.79 | 1.6 | 0.3 | 0.2 |
| Delayed Memory | 0.24 ± 0.82 | 0.41 ± 0.84 | 0.62 ± 0.70 | 0.71 ± 1.09 | 3.0* | 0.4 | 0.0 |
| Reaction Time | 0.06 ± 0.61 | 0.07 ± 0.61 | 0.45 ± 0.50 | 0.30 ± 0.59 | 5.4** | 0.3 | 0.4 |
| Spatial Processing | 0.37 ± 0.56 | 0.14 ± 0.54 | 0.45 ± 0.63 | 0.35 ± 0.68 | 1.3 | 1.6 | 0.3 |
Measures are reported mean ± standard deviation. Effect is significant:
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
N/A = Not applicable
The total sum of the cards drawn from ‘Good’ decks minus the sum of the cards drawn from the ‘Bad’ Decks.
Participants were excluded for the following reasons: (1) lifetime diagnosis of schizophrenia or schizophreniform disorder; (2) lifetime history of alcohol or drug (other than nicotine or caffeine) dependence or abuse; (3) significant history of head trauma (head injury with loss of consciousness) or cranial surgery; (4) history of diabetes, stroke, or other significant neurological disease that required medical intervention; and (5) clinical evidence of Wernicke-Korsakoff syndrome. These exclusion criteria were developed to support our studies and assessments of active alcoholics and abstinent alcoholics, including a focus on the prevalence and manifestation of co-morbid mood, anxiety and externalizing disorders in the study samples.
Assessment
Subjects participated in a total of four sessions that lasted between 1.5 and 3 hours. The sessions included clinical, neuropsychological, electrophysiological, and neuroimaging assessments. All participants were asked to abstain from drinking alcohol for 24 hours prior to each lab visit, and a Breathalyzer was administered before each session. No participants in the current study had positive Breathalyzer results (>.000) on any of their study sessions. Furthermore, participants were asked to get a blood draw to test hepatic function (all participants in the current study had normal hepatic function). Participants were informed of the study’s procedures and aims and signed a consent form prior to participation. Those who completed the sessions were given monetary compensation for their time and travel expenses, plus an additional bonus once they had completed the study. All of the procedures, measures, and compensation were reviewed and approved by an IRB.
Psychodiagnostic and Personality Assessment
Lifetime and current (prior year) psychiatric diagnoses and symptom counts were determined using the computerized version of the Diagnostic Interview Schedule (CDIS) (Robins et al., 1998). Table 1 presents the number of positive diagnoses by group and gender. Personality traits reflecting antisocial tendencies (Finn & Hall, 2004) were assessed using the Psychopathic Deviance scale of the MMPI-2 (MMPI_Pd) (Hathaway & McKinley, 1989), the Socialization scale of the California Psychological Inventory (CPI_So) (Gough, 1969), and the sum of Conduct Disorder and Antisocial Personality Disorder symptom counts from the CDIS (CD_ASPD). Research shows that these different measures of personality and antisocial symptoms are significantly associated with disadvantageous decisions on the IGT in young adult samples (Mazas et al., 2000; Stout et al., 2005).
Table 1.
CDIS Current & Lifetime Diagnoses
| Younger Group Ages 18 – 55 | Older Group Ages 56–85 | |||
|---|---|---|---|---|
| Variable | Male (n=49) | Female (n=63) | Male (n=18) | Female (n=34) |
| Antisocial Personality | 1, 6 | 0, 0 | 0, 0 | 0, 0 |
| Bipolar Disorder | 0, 2 | 0, 0 | 0, 0 | 0, 0 |
| Conduct Disorder (CD) | 0, 1 | 0, 0 | 0, 0 | 0, 0 |
| Compulsive Disorder | 0, 0 | 0, 1 | 0, 0 | 0, 0 |
| Major Depressive Disorder | 3, 10 | 7, 32 | 0, 4 | 1, 8 |
| Obsessive Disorder | 0, 0 | 1, 3 | 0, 0 | 0, 0 |
| Panic Disorder | 0, 0 | 1, 4 | 0, 0 | 1, 1 |
| Post-Traumatic Stress Disorder | 0, 1 | 0, 5 | 0, 0 | 0, 1 |
| Social Phobia | 0, 0 | 1, 4 | 0, 1 | 0, 1 |
Results are reported as the number of people with: Current Diagnosis, Lifetime Diagnosis
No participant had current or lifetime diagnoses for ADHD (Attention Deficit Hyperactivity Disorder), Agoraphobia, Dysthymic Disorder, Hypomanic Episode, or Manic Episode and thus these disorders were not included in the table.
Neuropsychological Assessment
A neuropsychological assessment was administered on the second day of testing. This assessment began with the administration of the following individual tests: Rey-Osterrieth Complex Figure (copy, immediate, and delay) (Osterrieth, 1944), Trail Making Test A and B (Reitan & Wolfson, 1985), Symbol Digit Modalities Test (written administration only) (Smith, 1968), American version of the Nelson Adult Reading Test (AMNART) (Grober & Sliwinski, 1991), Short Category Test (booklet format) (Wetzel, 1982), Controlled Oral Word Association Test (COWAT) (Benton & Hamsher, 1983), Paced Auditory Serial Addition Test (PASAT) (Gronwall, 1977), Block Design (WAIS) (Wechsler, 1981), Stroop Color and Word Test (Golden, 1975), Fregly Ataxia Battery (Fregly et al., 1973), and the Iowa Gambling Task (IGT described in detail below) (Bechara et al., 1994). After a 15 minute break the subject completed the MicroCog (MC) Assessment of Cognitive Functioning (standard version) (Powell et al., 1993), a computerized battery that includes 18 subtests, and takes approximately 1 hour to complete.
Normative scores derived from a nationally representative sample of adults are available for each test, either from the creators or distributors of the tests. Z-scores for the neuropsychological domains and measures were computed based on standardized norms adjusted for age [Stroop (Golden, 1978), Short Categories (Wetzel & Boll, 1987), PASAT (Stuss et al., 1988), Block Design (Wechsler, 1997), and Rey (Denman, 1987)], years of education [AMNART (Grober & Sliwinski, 1991)], age and years of education [Symbol Digit Modalities (Smith, 1982), MicroCog (Powell et al., 1993)], and age, gender, and years of education [(Trails A and B (Heaton et al., 1991), COWAT (Ruff et al., 1996)]. The Stroop, Symbol Digit Modalities, and the MicroCog test norms are not specific to gender, since gender did not significantly affect scores in the normative samples (Golden, 1978; Powell et al., 1993; Smith, 1982). The AMNART is used as a measure of pre-morbid intelligence (Grober & Sliwinski, 1991). The AMNART did not have age norms because the test was designed to be resistant to the effects of normal aging and most neurodegenerative diseases. Additionally, Grober and Sliwinski (1991) have reported that gender does not influence AMNART scores.
The final NP battery consisted of the following 9 domains, and their component tests: (1) Attention (Stroop Color, MC Numbers Forward, MC Numbers Reversed, MC alphabet, MC Word List 1) (2) Verbal Ability (COWAT, AMNART), (3) Abstraction/Cognitive Flexibility (Short Categories, Stroop interference score, Trail Making Test B, MC Analogies, MC Object Match A and B), (4) Psychomotor (Trails A, Symbol Digit), (5) Immediate Memory (MC Story immediate 1 and 2, Rey immediate recall, MC Word List 2), (6) Delayed Memory(MC Story Delay 1 and 2, MC Address delay, Rey delayed recall), (7) Reaction Time (MC Timers 1 and 2), (8) Spatial Processing (MC Tic Tac 1 and 2, MC Clocks, Block Design), and (9) Auditory Working Memory (PASAT at delays of 2.4, 2.0, 1.6, and 1.2 seconds).
Decision-Making
The Iowa gambling task (IGT), used to assess decision making, was administered during the second day. Participants were seated in front of a computer, and the rules were both explained on the computer screen and reviewed by the experimenter. Participants started out with $2000 of fake money. The object of the task was to end up with as much money as possible. Participants select one card at a time from any of four different decks. This card would indicate an amount either ‘won’ or ‘lost’, and would add or subtract that amount from the participant’s running total. Participants were told that they could switch from one deck to another at any time and as often as they wished. Each deck had a total of 60 cards, and the game ended after a total of 100 cards were selected. Participants were told that they would not know when the game would end, that some decks are better than others, and that the computer does not change the order of the cards after the game starts. Within the gambling game, the ‘good’ decks (decks C & D) have smaller immediate rewards and lower long-term punishment, and the ‘bad’ decks (decks A& B) have higher immediate rewards, but also higher long-term punishment. The participant’s performance on the task is reflected by a measure of advantageous decision bias quantified as the number of cards chosen from the good decks minus the number of cards chosen from the bad decks (Fein et al., 2004; Mazas et al., 2000).
Statistical Analysis
The data were analyzed with SPSS (SPSS Inc., 2004). Comparisons between the two groups were done with the general linear models procedure. The different psychological and neuropsychological measures were examined as covariates in an analysis of variance and/or Pearson’s correlations in order to determine their association with IGT performance. Multiple regression analyses of the psychological and neuropsychological predictors of decision-making were also performed.
Results
Gambling Task Performance
Analysis of variance on IGT performance revealed significant group differences (F1, 160 = 14.08, p < 0.001), with the older group performing less advantageously than the younger group, but no significant gender or group by gender interactions. Participants were divided into two groups with the cutoff at age 55 based upon Denburg’s paper (Denburg et al., 2005). We performed polynomial regression of IGT performance as a function of age to examine how age affected IGT performance over the entire study age range. A linear fit was significant (F1,162 = 19.1, p < 0.0001, adjusted rsquared = .100), but the best fit was a quadratic function of age (F2, 161 = 13.20, p < 0.0001, IGT = 12.045 + 1.38 * age − 0.019 * age2, adjusted rsquared = .130). The quadratic term accounted for additional IGT variance with no improvement to the prediction of IGT performance by adding a cubic age term (p > 0.83). This best fit quadratic is displayed in Figure 1, where it shows that the negative association of advantageous decision bias on the IGT with age does begin in the mid fifties. An advantageous decision bias was not associated with age in the under 55 group (r = −0.03, p = 0.79), but was negatively associated with age in the over 55 group (r = −0.34, p < 0.05). IGT performance was also associated with years of education (r = 0.18. p < 0.03) (Figure 2), but not, however, with indices of pre-morbid intelligence as assessed by the AMNART (r = 0.11, p = 0.15) (Figure 3).
Figure 1.
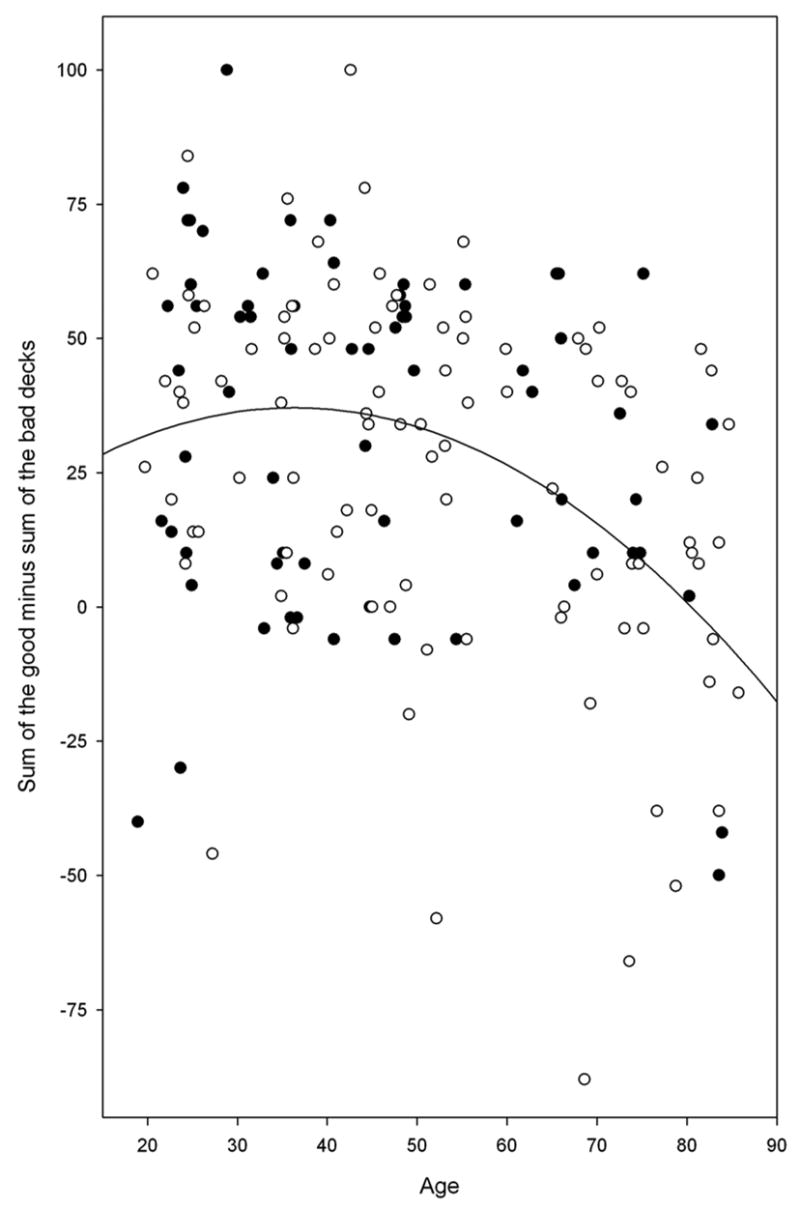
Figure 1 depicts the best-fit quadratic equation (IGT = 12.045 + 1.38 * age − 0.019 * age2), which was a result of polynomial regression of IGT performance as a function of age. It shows that the negative association of advantageous decision bias on the IGT with age does begin in the mid fifties.
●Black circle = Men; ○White circle = Women
Figure 2.
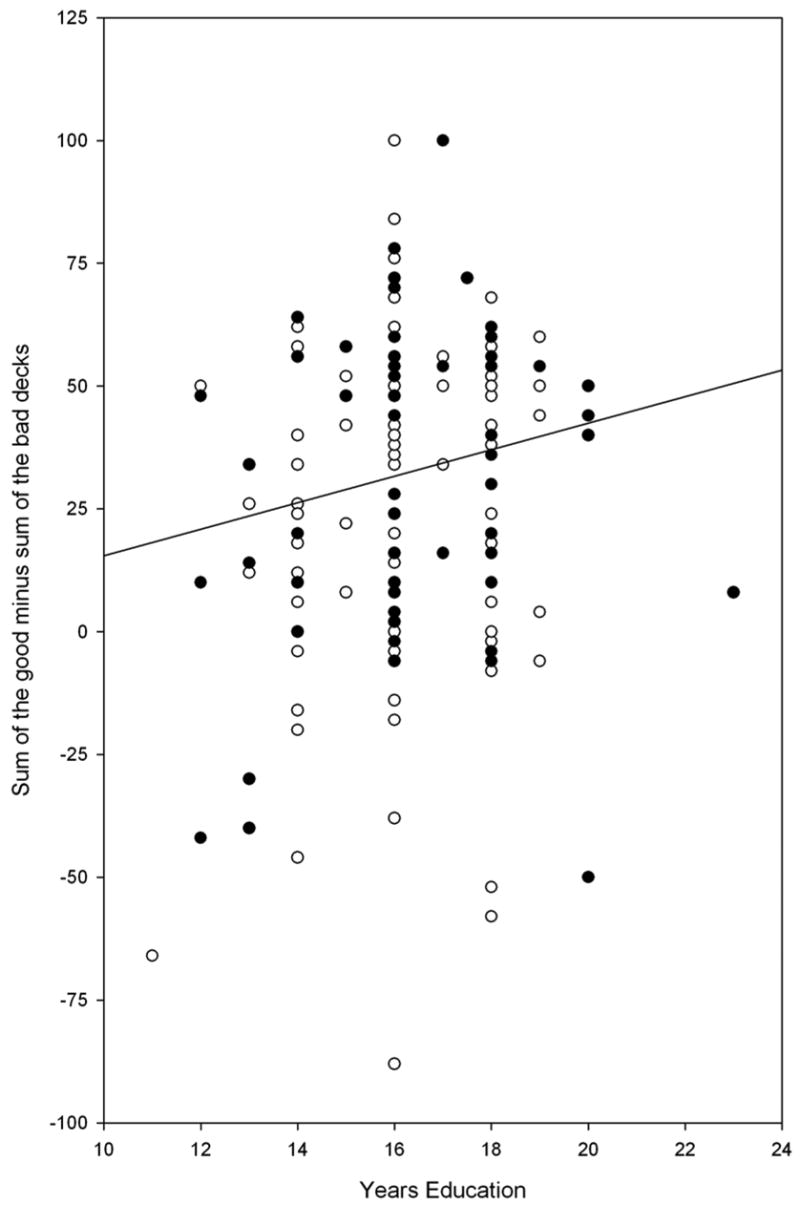
Figure 2 plots the association between IGT performance (total number of cards drawn from the ‘good’ decks minus the total number of cards drawn from the ‘bad’ decks) and years of education.
●Black circle = Men; ○White circle = Women
Figure 3.
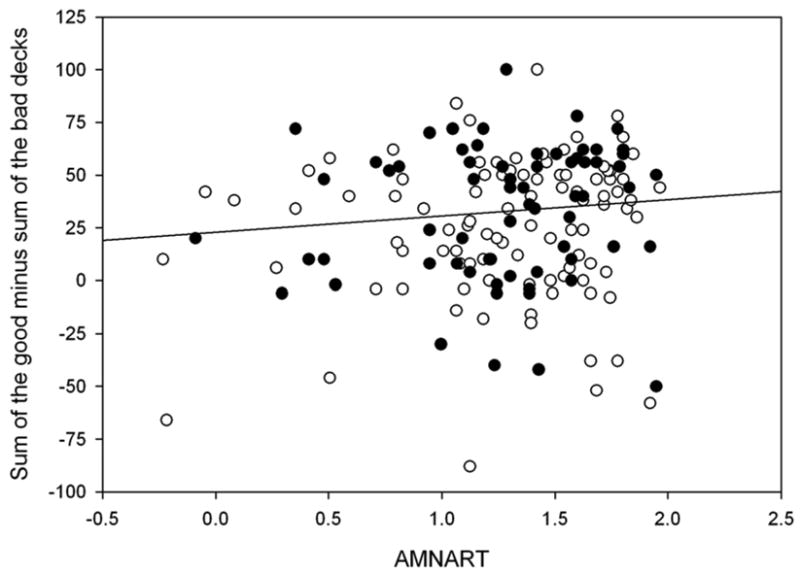
Figure 3 plots the association between IGT performance (total number of cards drawn from the ‘good’ decks minus the total number of cards drawn from the ‘bad’ decks) and the AMNART (measure of pre-morbid intelligence).
●Black circle = Men; ○White circle = Women
We also examined decision bias on the IGT broken down into five blocks of twenty cards each. Unfortunately, card sequence was not originally saved during the testing, so these analyses were performed only on subjects who had card sequence data (18 younger women, 16 younger men, 24 older women, and 11 older men). The older subjects performed more poorly across blocks(F 4, 62 = 3.13, p < 0.02), but there were no significant block by group, block by gender, or block by gender by group interactions (all F2, 62‘s < 1.46, p’s > 0.23). Figure 4 shows the performance of the younger and older groups across blocks, with the performance curves for the older and younger groups roughly parallel across blocks.
Figure 4.
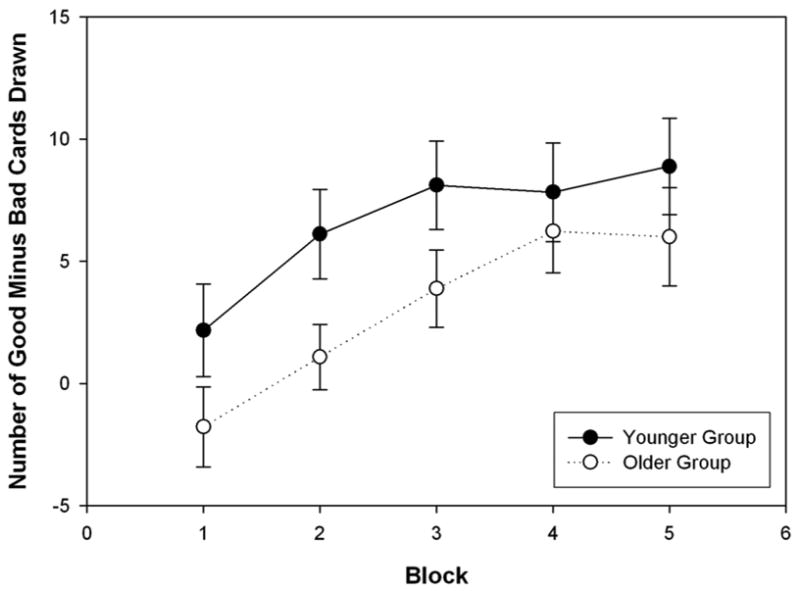
Figure 4 plots the average IGT performance with standard error bars across blocks for the younger and the older groups, where blocks are sequences of 20 cards.
Furthermore, in following with Denburg et al (2005) we used binomial probability to determine whether each participant’s IGT performance was significantly different from random performance. Denburg categorized individuals who performed significantly better than chance as ‘unimpaired’ and individuals performing significantly worse than chance as ‘impaired’. We found a higher percentage of ‘impaired’ individuals in the older compared to the younger group (8 of 52, ~15%; vs. 5 of 112, ~4%) (Fisher’s exact test p = .03), and a lower percentage of individuals who were ‘unimpaired’ in the older group compared to the younger group (24 of 52, ~46%; vs. 79 of 112, ~71%) (χ2 = 9.03, p < 0.01). IGT block information was available on 3 out of the 8 older ‘impaired’ participants, none of whom demonstrated learning as the task progressed.
Neuropsychological variables and their association with Advantageous Decision Biases
Analysis of variance revealed significant group differences on various age-adjusted z-scores for neuropsychological domains (see Table 2, Table 3 contains results from analyses on the raw scores). There was no difference in the average z-score across domains (F1,160 = 0.15, p > 0.70). On specific domains, the younger group performed more poorly than the older group in delayed memory (F1, 160 = 4.88, p < 0.03) and reaction time (F1, 159 = 9.09, p < 0.01). The average z-score across domains was associated with IGT performance across all subjects (r = 0.29, p < .001). Within the younger group advantageous decision-making was associated with auditory working memory (r = 0.32, p = 0.001), psychomotor ability (r = 0.29, p = 0.002), and the average z-score across domains (r = 0.28, p = 0.003). Within the older group decision-making was associated with abstraction/cognitive flexibility (r = 0.40, p = 0.003), immediate memory (r = 0.44, p = 0.001), spatial processing (r = 0.45, p = 0.001), and the average z-score across domains (r = 0.39, p = 0.004). Multiple regression analysis for the young and old groups was analyzed separately, first entering all neuropsychological domains, followed by backward deletion. For the young group, this resulted in the combination of auditory working memory and psychomotor ability together accounting for 12.0 % of the IGT performance measure (adjusted R-square). For the old group, there was a single predictor, immediate memory, accounting for 19.1% of the IGT performance measure.
Table 3.
Neuropsychological Domains and Individual Tests (Raw Scores)
| Younger Adults | Older Adults | Effect Size (%) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Male (n=49) | Female (n=63) | Male (n=18) | Female (n=34) | Group | Gender | Group by Gender |
| Neuropsychological Domains (Raw scores) | |||||||
| Abstraction/Cognitive Flexibility | |||||||
| MicroCog Analogies | 11.65 ± 3.47 | 12.13 ± 4.27 | 14.44 ± 2.53 | 12.79 ± 3.63 | 4.2** | 0.5 | 1.6 |
| MicroCog Object Match A | 9.77 ± 2.99 | 10.41 ± 1.98 | 11.56 ± 2.21 | 11.46 ± 2.38 | 6.6*** | 0.3 | 0.5 |
| Short Categories (Errors) | 25.08 ± 14.70 | 26.73 ± 13.86 | 36.61 ± 16.75 | 40.73 ± 13.78 | 13.9*** | 0.8 | 0.2 |
| Stroop-Interference | 44.73 ± 9.08 | 46.03 ± 9.30 | 32.33 ± 10.89 | 33.06 ± 8.32 | 26.8*** | 0.2 | 0.0 |
| Trails B | 53.57 ± 16.51 | 58.68 ± 21.22 | 87.89 ± 34.44 | 89.09 ± 30.69 | 27.2*** | 0.4 | 0.1 |
| Attention | |||||||
| MicroCog Alphabet | 10.37 ± 1.68 | 10.63 ± 1.45 | 11.22 ± 2.44 | 11.32 ± 2.45 | 3.4* | 0.2 | 0.0 |
| MicroCog Numbers Forward | 9.86 ± 3.51 | 9.97 ± 2.90 | 9.67 ± 4.28 | 10.74 ± 3.49 | 0.2 | 0.6 | 0.4 |
| MicroCog Numbers Reversed | 9.51 ± 3.93 | 9.94 ± 2.81 | 8.39 ± 3.79 | 10.68 ± 3.38 | 0.1 | 3.2* | 1.5 |
| MicroCog Wordlist 1 | 10.29 ± 2.81 | 10.84 ± 2.13 | 10.33 ± 3.12 | 11.62 ± 2.74 | 0.5 | 2.5* | 0.4 |
| Stroop-Color | 74.02 ± 10.91 | 76.43 ± 12.27 | 58.20 ± 15.23 | 65.28 ± 11.19 | 19.6*** | 2.9* | 0.7 |
| Auditory Working Memory | |||||||
| PASAT 2.4 (seconds delay) | 47.20 ± 9.02 | 42.62 ± 12.30 | 38.63 ± 11.16 | 32.70 ± 14.15 | 11.0*** | 3.8* | 0.1 |
| PASAT 2.0 (seconds delay) | 41.20 ± 10.23 | 37.68 ± 12.86 | 35.00 ± 8.73 | 29.61 ± 13.47 | 6.6*** | 2.7* | 0.1 |
| PASAT 1.6 (seconds delay) | 37.33 ± 11.33 | 33.16 ± 12.59 | 30.40 ± 8.53 | 26.69 ± 13.91 | 5.5** | 2.0 | 0.0 |
| PASAT 1.2 (seconds delay) | 27.81 ± 9.97 | 22.86 ± 9.98 | 21.36 ± 7.67 | 19.13 ± 10.94 | 4.6** | 2.4 | 0.3 |
| Immediate Memory | |||||||
| MicroCog Story Recall | 9.76 ± 1.16 | 9.65 ± 1.22 | 9.17 ± 1.15 | 8.44 ± 2.18 | 7.3*** | 1.7 | 0.9 |
| MicroCog Wordlist 2 | 14.55 ± 3.25 | 14.79 ± 3.01 | 14.06 ± 2.07 | 13.53 ± 3.48 | 1.6 | 0.0 | 0.3 |
| Rey-Immediate Recall | 49.43 ± 11.49 | 47.41 ± 13.51 | 36.44 ± 9.39 | 35.56 ± 15.25 | 15.9*** | 0.3 | 0.0 |
| Delayed Memory | |||||||
| MicroCog Story-Delayed Recall | 9.94 ± 3.41 | 11.21 ± 3.26 | 11.89 ± 2.74 | 12.38 ± 4.03 | 4.1** | 1.4 | 0.3 |
| Rey-Delayed Recall | 49.16 ± 11.95 | 46.98 ± 13.08 | 36.00 ± 9.14 | 35.09 ± 14.35 | 16.8*** | 0.3 | 0.1 |
| Psychomotor | |||||||
| Symbol Digit Modalities | 58.04 ± 9.99 | 57.94 ± 9.39 | 42.00 ± 8.38 | 40.15 ± 9.13 | 40.0*** | 0.2 | 0.2 |
| Trails A | 27.00 ± 10.59 | 29.00 ± 9.66 | 39.50 ± 12.70 | 44.38 ± 15.95 | 22.3*** | 1.7 | 0.3 |
| Reaction Time | |||||||
| MicroCog Cued Timers | 10.27 ± 1.97 | 10.11 ± 2.15 | 11.56 ± 1.80 | 11.24 ± 2.12 | 6.7*** | 0.3 | 0.0 |
| MicroCog Simple Timers | 10.09 ± 2.01 | 10.33 ± 1.92 | 11.17 ± 1.55 | 10.59 ± 1.88 | 2.5* | 0.2 | 0.9 |
| Spatial Processing | |||||||
| Block Design | 52.82 ± 10.07 | 45.81 ± 10.13 | 38.00 ± 8.49 | 32.59 ± 11.34 | 27.5*** | 6.9*** | 0.1 |
| MicroCog Clocks | 10.65 ± 1.92 | 11.13 ± 1.66 | 12.56 ± 2.18 | 12.24 ± 2.92 | 9.5*** | 0.0 | 0.7 |
| MicroCog Tic Tac | 8.92 ± 2.30 | 8.11 ± 2.38 | 8.94 ± 2.53 | 9.38 ± 2.16 | 1.6 | 0.1 | 1.5 |
| Verbal | |||||||
| AMNART | 33.73 ± 6.52 | 34.70 ± 7.44 | 35.61 ± 7.78 | 34.26 ± 8.16 | 0.2 | 0.0 | 0.5 |
| COWAT | 45.02 ± 12.29 | 45.33 ± 12.05 | 38.17 ± 11.80 | 39.88 ± 9.03 | 5.5** | 0.2 | 0.1 |
Measures are reported mean ± standard deviation.
Effect is significant:
= p ≤ 0.05,
= p ≤ 0.01,
= p ≤ 0.001
Psychological variables and their association with Advantageous Decision Biases
Analysis of variance revealed significant group differences on a number of CDIS symptom counts. Compared to the older group, the younger group reported more manic symptoms (F1,160 = 5.76, p < 0.02), PTSD symptoms (F1,160 = 3.89, p = 0.05), psychotic symptoms (F1,160 = 5.53, p = 0.02), and conduct disorder symptoms (F1,160 = 5.89, p < 0.02). The older group did not have higher symptom counts on any measures.
We examined the effects of each symptom count variable on IGT performance by using that symptom count variable as a covariate in an analysis of variance. Group by covariate interactions were present only for obsessive symptoms (F1, 158 = 3.14, p < 0.05), hyperactivity symptoms (F1, 157 = 3.27, p < 0.05), and antisocial personality symptoms (F1, 157 = 3.10, p < 0.05). Obsessive symptoms and hyperactivity symptoms were more negatively associated with advantageous decision bias in the older compared to the younger group (r = −0.32, p < 0.02 vs. r = −0.07, p = 0.46, and r = −0.20, p = 0.15 vs. r = 0.15, p = 0.12), while antisocial personality symptoms were more negatively associated with advantageous decisions in the younger compared to the older group (r = −0.18, p =0.06 vs. r = 0.12, p = 0.39). Multiple regression analysis of the symptom count variables was performed in the same manner as for the neuropsychological variables. For the young group, antisocial personality disorder symptoms were a single predictor, accounting for 2.2 % of the IGT performance measure. For the old group, there was also a single predictor, with obsessive symptoms accounting for 8.7 % of the IGT performance measure.
Group analysis of variance for the MMPI_Pd and CPI_So scale revealed higher MMPI_Pd scores in the younger group (F1,160 = 7.37, p < 0.01), but no difference on the CPI_So scale or CD_ASPD symptom counts. There were no associations between IGT performance and any of these variables, nor were there differences in the associations between groups.
Discussion
The current study replicates the major finding of Denburg et al. (2005) that individuals over age 55 exhibit less advantageous decisions on the IGT compared to individuals below age 55. The analysis of IGT performance versus age data using polynomial regression found that a quadratic curve best fit the data. As shown in Figure 1, IGT performance as a function of age revealed that age 55 was a reasonable cutoff for demarking the beginning of an age-related impairment. Using Denburg’s definition of ‘impaired’ and ‘unimpaired,’ IGT performance as being significantly worse than chance and significantly better than chance, respectively, we replicate their finding of more impaired performance in the over 55 compared to more unimpaired performance in the under 55 group. Our percentage of older individuals with unimpaired performance was lower than that in our younger sample and was comparable to Denburg’s (46% vs. 38%); however, our percentage of older individuals with impaired performance, while higher than that in our younger sample, was lower than that reported by Denburg (15% vs. 35%). In addition, Denburg did not find any associations between the IGT and performance on cognitive tests, while we reported an association with the average z-score across all nine cognitive domains assessed. Furthermore, Wood et al (Wood et al., 2005) found that older and younger adults employ different cognitive strategies in regards to decision-making as assessed by the IGT. Analyses of our participants also revealed that different cognitive processes are associated with decision-making in older versus younger adults. Auditory working memory and psychomotor abilities were associated with IGT within the younger group, and immediate memory was associated with IGT performance in the older group.
Regarding neuropsychological performance, it is important to note that all participants performed within the normal range compared to age and education adjusted norms (see Table 2). The groups did not differ on age and education adjusted scores, except for the older group performing better on reaction time and delayed memory. These differences were subtle impairments, and were not clinically significant. Furthermore, looking at the raw scores (Table 3), older adults performed worse than younger adults on these two domains. The better performance in terms of the scaled scores suggests that older adults in the Bay Area (from which our sample was drawn) perform better than the older sample from which the normative data were derived. For all subjects the average z-score across domains was positively associated with advantageous decisions on the IGT, and there was no significant difference in this association between groups.
Within the younger groups, especially among the young women, there were a relatively high proportion of individuals who met lifetime criteria for depression. Analysis of covariance did not, however, reveal any association between depressive symptoms and performance on the IGT. This higher rate of depression reported in the younger groups may reflect a combination of factors. Depressed older individuals are subject to survivor effects since depressed individuals die at an earlier age than non-depressed individuals (Schulz et al., 2002). In addition, it is possible that our elderly participants were a select sample (with a relatively low rate of depression) because of their propensity to volunteer for a four-day research study. They reported that their participation was mostly for altruistic reasons and to learn more about themselves, as compared to the younger subjects who more often reported monetary considerations as a motivating factor. Finally, possible cohort differences in the acknowledgement of psychological distress may also have played a part.
The results also indicate different psychological correlates of impaired decision-making in older versus younger adults. For younger adults, even though these subjects were healthy normal individuals, antisocial behavior was significantly associated with less advantageous decisions. This result is consistent with studies that show that young adults with diagnosable disinhibitory syndromes, such as conduct disorder, antisocial personality disorder, and substance abuse, demonstrate less advantageous decisions (Mazas et al., 2000). However, for older adults, symptoms of obsessiveness were associated with less advantageous decisions. More studies are needed to replicate these findings and to determine what aspects of obsessiveness might contribute to impaired decisions in older adults. We are very aware of the difference between diagnosable psychiatric illness and sub-diagnostic signs and symptoms associated with such illness. We have a recent manuscript in which we examined both phenomena in long-term abstinent alcoholics compared to age and gender comparable controls (Fein et al., In press). We found that the major difference between the groups in psychiatric illness was carried by sub-diagnostic psychopathology. We believe that, in comparison to the limited view provided by using only symptomatology that meets meet criteria for a diagnosis, the use of continuous measures of psychiatric symptomatology and psychological abnormality yields a much more accurate picture of psychiatric illness; however, we are very aware that the measures we are discussing here are sub-diagnostic.
Despite our findings that older adults demonstrate less advantageous decision-making than younger adults, the IGT remains a laboratory task in a fixed environment. Although the gambling task models real-life decisions that involve weighing short-term rewards and long-term consequences, it remains an artificial task and it may not be sensitive to more subtle deficits in decision-making, or to the influence of context on decision-making. Given these limitations, one cannot conclude that older adults would demonstrate impairment regarding decisions in other aspects of their lives.
Finally, the results reported here have implications for the prognosis of the alcohol or drug-dependent individual as they age. Aging may independently affect a brain system (evaluation of rewards and punishments) that is already compromised in the alcoholic or drug abuser. This would result in even greater impairments in decision-making ability in elderly individuals with a history of alcohol or drug abuse, and may make them particularly vulnerable to making disadvantageous decisions. Studies examining decision-making ability in elderly individuals with a history of alcohol or drug abuse are warranted.
Acknowledgments
This work was supported by Grants AA11311 (GF) and AA13659 (GF), both from the National Institute of Alcoholism and Alcohol Abuse. We also express our appreciation to the NRI recruitment and assessment staff, and to each of our volunteer research participants.
References
- American Association of Retired Persons. Telemarketing Fraud and Older Americans: An AARP Survey. Washington, DC: 1996. [Google Scholar]
- Band GP, Ridderinkhof KR, Segalowitz S. Explaining neurocognitive aging: is one factor enough? Brain Cogn. 2002;49(3):259–267. doi: 10.1006/brcg.2001.1499. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18(1):152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination. Iowa City: AJA Associates; 1983. [Google Scholar]
- Deakin J, Aitken M, Robbins T, Sahakian BJ. Risk taking during decision-making in normal volunteers changes with age. J Int Neuropsychol Soc. 2004;10(4):590–598. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43(7):1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Denman SB. Denman Neuropsychology Memory Scale. Charleston, SC: SB Denman; 1987. [Google Scholar]
- Fein G, Di Sclafani V, Finn P, Scheiner DL. Sub-diagnostic psychiatric comorbidity in alcoholics. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2006.08.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a Simulated Gambling Task in Long-Term Abstinent Alcoholics. Alcohol Clin Exp Res. 2004;28(10):1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Hall J. Cognitive ability and risk for alcoholism: short-term memory capacity and intelligence moderate personality risk for alcohol problems. J Abnorm Psychol. 2004;113(4):569–581. doi: 10.1037/0021-843X.113.4.569. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcohol Clin Exp Res. 2002;26(2):186–206. [PubMed] [Google Scholar]
- Fregly AR, Smith MJ, Graybiel A. Revised normative standards of performance of men on a quantitative ataxia test battery. Acta Otolaryngol. 1973;75(1):10–16. doi: 10.3109/00016487309139631. [DOI] [PubMed] [Google Scholar]
- Golden CJ. A group form of the Stroop color and word test. Journal of Personality Assessment. 1975;39:386–388. doi: 10.1207/s15327752jpa3904_10. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Los Angeles, CA: Western Psychological Services; 1978. [Google Scholar]
- Gough HGPD. Manual for the California Psychological Inventory (So Scale) Palo Alto, CA: Consulting Psychological Press; 1969. [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intellegence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991a;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991b;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gronwall D. Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hathaway S, McKinley J. MMPI-2: Minnesota Multiphasic Personality Inventory. Minneapolis: The University of Minnesota Press; 1989. [Google Scholar]
- Heaton RK, Grant I, Maththews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, FL: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ. Automatic and attentional processing: an event-related brain potential analysis of semantic priming. Brain and Language. 1988;35:66–85. doi: 10.1016/0093-934x(88)90101-0. [DOI] [PubMed] [Google Scholar]
- Johnson MM. Age differences in decision making: a process methodology for examining strategic information processing. J Gerontol. 1990;45(2):75–78. doi: 10.1093/geronj/45.2.p75. [DOI] [PubMed] [Google Scholar]
- Kim SY, Karlawish JH, Caine ED. Current state of research on decision-making competence of cognitively impaired elderly persons. Am J Geriatr Psychiatry. 2002;10(2):151–165. [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging. Psychol Aging. 2002;17(4):598–609. [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24(7):1036–1040. [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complex. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog Assessment of Cognitive Functioning. San Antonio, TX: The Psychological Corporation; 1993a. [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog Assessment of Cognitive Functioning. San Antonio, TX: The Psychological Corporation; 1993b. [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25(3):377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- Robins LN, Cottler L, Buckholz K, Compton W. The Diagnostic Interview Schedule for DSM-IV. St. Louis, MO: Washington University School of Medicine; 1998. [Google Scholar]
- Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schaie K, Willis S. Theories and everyday competence and aging. In: Bengston V, Schaie K, editors. Handbook on Theories on Aging. New York: Springer; 1999. pp. 174–195. [Google Scholar]
- Schulz R, Drayer RA, Rollman BL. Depression as a risk factor for non-suicide mortality in the elderly. Biol Psychiatry. 2002;52(3):205–225. doi: 10.1016/s0006-3223(02)01423-3. [DOI] [PubMed] [Google Scholar]
- Smith A. The symbol digit modalities test: A neuropsychological test of learning and other cerebral disorders. In: Helmuth J, editor. Learning Disorders. Seattle, WA: Special Child Publications; 1968. [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- SPSS Inc. SPSS 13.0 for Windows (Version 13.0) Chicago IL: SPSS Inc; 2004. [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, Finn PR. Psychological processes underlying risky decisions in drug abusers. Psychol Addict Behav. 2005;19(2):148–157. doi: 10.1037/0893-164X.19.2.148. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Pelchat G. Three tests of attention and rapid information processing: an extension. The Clinical Neuropsychologist. 1988;2:246–250. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale -- Third Edition: Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wetzel L. Development of a short, booklet form of the category test: Correlational and validity data. Unpublished Doctoral Dissertation, University of Health Sciences / The Chicago Medical School; Chicago: 1982. [Google Scholar]
- Wetzel L, Boll T. Short Category Test, Booklet Format. Los Angeles, CA: Western Psychological Services; 1987. [Google Scholar]
- Wood S, Busemeyer J, Koling A, Cox CR, Davis H. Older adults as adaptive decision makers: evidence from the Iowa Gambling Task. Psychol Aging. 2005;20(2):220–225. doi: 10.1037/0882-7974.20.2.220. [DOI] [PubMed] [Google Scholar]


