Abstract
The most reliable and robust risk factor for some neurodegenerative diseases is aging. It has been proposed that processes of aging are associated with the generation of reactive oxygen species and a disturbance of glutathione homeostasis in the brain. Yet, aged animals have rarely been used to model the diseases that are considered to be age-related such as Parkinson's or Alzheimer's disease. This suggests that the results from these studies would be more valuable if aged animals were used. The present study was designed to provide insight into the glutathione redox state in young and aged rat siblings of both genders by studying the enzyme activities related to glutathione synthesis, cycling, and usage. The results suggested a significant age-related reduction of reduced glutathione (GSH) level in all brain regions examined, associated with an increase of GSH oxidation to glutathione disulfide (GSSG) and decrease of the GSH/GSSG ratio. These changes were accompanied by diminished γ-glutamylcysteine synthetase activity in de novo glutathione synthesis and increased lipid peroxidation. In addition, these changes were associated with increased enzyme activities related to the GSH usage (glutathione peroxidase, γ-glutamyl transpeptidase, and glutathione S-transferase). The results indicate that aged animals are likely more vulnerable to oxidative stress and insinuate the roles of aged animals in modeling age-related neurodegeneration diseases.
Keywords: Aging, Glutathione, γ-glutamylcysteine synthetase, Oxidative stress, Rat
1. Introduction
Oxidative stress, an unavoidable consequence in the metabolism of oxygen in aerobic cells, is a major factor in the aging process and in the course of many chronic diseases associated with aging (Ames et al., 1993). There is substantial evidence that aging is one of the most robust and significant risk factors for age-related neurodegenerative diseases, such as Parkinson's disease (Olanow and Tatton, 1999). In study of such diseases, however, young animals, which do not display characteristics found in the central nervous system of older animals, are often used to model the age-related diseases (Vu et al., 2000; Brown et al., 2005; Ossowska et al., 2005).
Aging-related changes in the brain include reduction of trophic supports (Ling et al., 2000; Sortwell et al., 2001), decreased proteasomal enzyme activities (Gray et al., 2003; Zeng et al., 2005), mitochondrial dysfunction (Hampton, 2005), change into more pro-inflammatory environment (Gayle et al., 1999), and, more importantly, aging is associated with an increase in reactive oxygen species (ROS) (Ames et al., 1993).
Glutathione (GSH) is the most abundant non-protein thiol that buffers ROS in the brain tissue (Dringen et al., 2000). It eliminates H2O2 and organic peroxides by glutathione peroxides (GPx) (Meister, 1988). GSH also transports amino acids across the cellular membrane by the γ-glutamyl cycle and detoxifies foreign agents by glutathione S-transferase (GST) (Meister, 1988). Normal levels of glutathione are maintained by de novo glutathione synthesis and the salvage pathway. GSH is comprised of three amino acids, glutamate, cysteine, and glycine. The first enzyme in de novo synthesis is γ-glutamylcysteine synthetase (GCS) that catalyzes glutamate and cysteine to form γ-glutamylcysteine. γ-Glutamylcysteine and glycine form GSH by second enzyme glutathione synthatase (GS). During detoxification, ROS are reduced by GPx at the expense of GSH to form glutathione disulfide (GSSG). GSH is regenerated by redox recycling, in which GSSG is reduced to GSH by glutathione reductase (GR) with a consumption of one NADPH. During transportation of amino acids into mammalian cells, one GSH is lost for each amino acid transported, but it can be restored by the salvage pathways including recovery of cysteine and de novo GSH synthesis. These salvage pathways maintain homeostasis of glutathione system (Griffith et al., 1978; Dickinson and Forman, 2002).
The aims of the present study were to define the levels of glutathione (GSH and GSSG) and GSH-related enzymes activities of aged rats in both genders and compare them with those found in young littermates. The results reveal relatively complete view of glutathione system in conjunction with lipid peroxidation and provide useful information for those who may use aged rats to study neurodegenerative diseases such as Parkinson's disease. Our findings also highlight the role of GCS in maintaining GSH homeostasis in the rat brain.
2. Results
2.1. Age-related changes in GSH and GSSG in rat brain
To gauge glutathione levels in the brain, GSH and GSSG were determined in the frontal cortex, striatum, midbrain, and cerebellum. Overall, aging caused GSH reduction in all brain regions examined (F1,76 = 93.34, P < 0.001). These age-related changes in GSH level equally occurred in both genders (F1,76 = 0.12, P = 0.73). As shown in Fig. 1A, in the midbrain of aged male rats, there was a 25 ± 4% decrease in GSH level when compared with the young rats (P < 0.01). GSH was also reduced in three other regions in both genders when compared with the young rats (Fig. 1A).
Fig. 1.
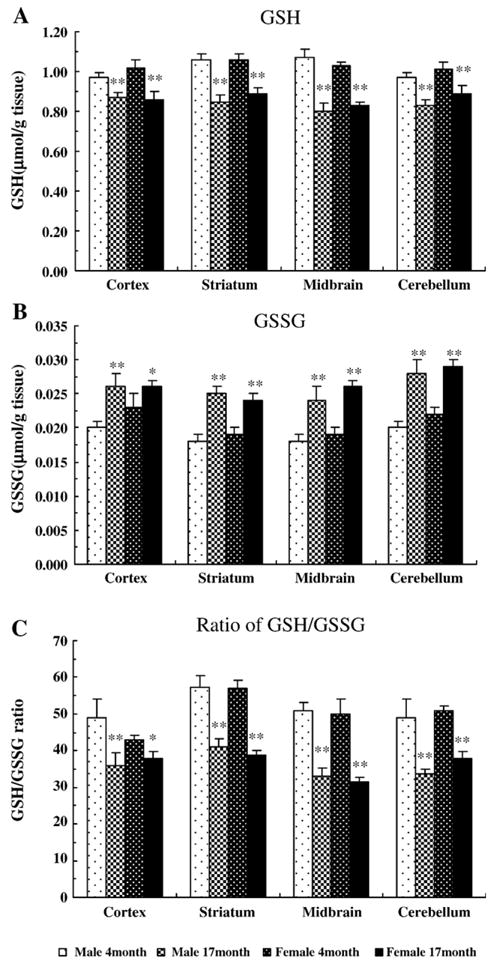
The levels of reduced glutathione (GSH), glutathione disulfide (GSSG), and GSH/GSSG ratio in rat regional brain tissues. GSH and GSSG were measured in cortex, striatum, midbrain, and cerebellum of male and female rats at 4 and 17 months of age. (A) GSH, (B) GSSG, and (C) ratio of GSH over GSSG. Data are the means ± SEM from 5 animals in each group. *P < 0.05, **P < 0.01 compared with the corresponding young group.
When GSH is oxidized by GPx to form GSSG, GSSG level is increased, and the GSH/GSSG ratio is proportionately decreased. As shown in Figs. 1B and C, there was a significant increase in the GSSG level and a decrease in the GSH/GSSG ratio in aged rat brains compared with the young brains in all regions examined (P < 0.05). In particular, there was a 20% to 35% decline in the GSH/GSSG ratio in aged animals compared with the young rats. As was true for GSH, the age effects on GSSG level and GSH/GSSG ratio were equivalent in both genders.
2.2. Age-related changes of lipid peroxide (LPO) in rat brain
To evaluate the possible consequences of oxidative stress in the brain, lipid peroxide was measured in different regions of brain. As shown in Fig. 2, there were significant increases in LPO production in cortex (male 64 ± 9% and female 51 ± 8%), striatum (male 61 ± 8% and female 44 ± 9%), midbrain (male 61 ± 9% and female 44 ± 8%), and cerebellum (male 64 ± 11% and female 51 ± 6%) compared with the respective regions in young rats (P < 0.01). In addition, two-way ANOVA showed that the effect of age on LPO was independent of gender in all regions examined (F1,76 = 0.38, P = 0.59).
Fig. 2.
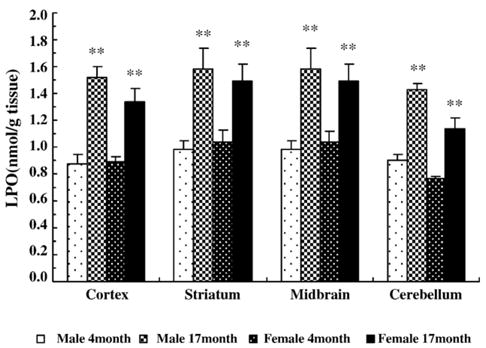
The levels of lipid peroxide (LPO) in rat brain tissues. LPO was measured in cortex, striatum, midbrain, and cerebellum of male and female rats at 4 and 17 months of age. Data are the means ± SEM from 5 animals in each group. **P < 0.01 compared with the corresponding young group.
2.3. Age-related changes in de novo GSH synthesis and GCS protein expression in rat brain
To determine whether GCS or GS was affected by age, GCS and GS activities were measured in the brain tissues. Overall, aging caused an increase in GCS activity in all brain regions examined (F1,76 = 117.55, P < 0.001). However, as summarized in Table 1, a pronounced decrease in GCS activity was observed in the midbrain of rats at 17 months of age (by 30 ± 7% in male and 21 ± 5% in female, respectively.). In the cortex, striatum, and cerebellum, the change was less dramatic but still statistically significant (Table 1). The effect of age on GCS activity was independent of gender in all regions examined (F1,76 = 0.82, P = 0.37). In contrast, there were no age-or gender-associated changes in GS activity in rat brain except in the midbrain of female rats with a significant decrease relative to the midbrain of young females (P < 0.05).
Table 1.
Summary of enzyme activities related to glutathione metabolism in rat brain
| Enzyme | Gender | Age (month) | Cortex | Striatum | Midbrain | Cerebellum |
|---|---|---|---|---|---|---|
| GCS | M | 4 | 0.78 ± 0.05 | 0.78 ± 0.03 | 0.93 ± 0.06 | 0.95 ± 0.04 |
| 17 | 0.63 ± 0.05* | 0.70 ± 0.04* | 0.65 ± 0.03** | 0.79 ± 0.04** | ||
| F | 4 | 0.81 ± 0.04 | 0.87 ± 0.06 | 0.96 ± 0.06 | 0.92 ± 0.04 | |
| 17 | 0.65 ± 0.05** | 0.60 ± 0.02** | 0.73 ± 0.05** | 0.75 ± 0.04** | ||
| GS | M | 4 | 0.62 ± 0.02 | 0.71 ± 0.07 | 0.68 ± 0.04 | 0.63 ± 0.07 |
| 17 | 0.56 ± 0.03 | 0.63 ± 0.05 | 0.61 ± 0.05 | 0.65 ± 0.02 | ||
| F | 4 | 0.68 ± 0.04 | 0.76 ± 0.04 | 0.86 ± 0.05 | 0.70 ± 0.04 | |
| 17 | 0.55 ± 0.08 | 0.68 ± 0.02 | 0.71 ± 0.05* | 0.65 ± 0.03 | ||
| GR | M | 4 | 4.86 ± 0.33 | 4.48 ± 0.55 | 4.14 ± 0.32 | 3.76 ± 0.34 |
| 17 | 5.96 ± 0.23** | 5.92 ± 0.19** | 6.12 ± 0.53**,Δ | 5.20 ± 0.45** | ||
| F | 4 | 4.11 ± 0.44 | 3.91 ± 0.46 | 3.75 ± 0.23 | 3.89 ± 0.52 | |
| 17 | 4.90 ± 0.20* | 5.36 ± 0.30 | 6.36 ± 0.39**,Δ | 5.45 ± 0.22 | ||
| GPx | M | 4 | 7.51 ± 0.62 | 7.70 ± 0.63 | 8.27 ± 0.46 | 7.70 ± 0.57 |
| 17 | 9.41 ± 0.82* | 10.10 ± 0.12** | 10.96 ± 0.33**,Δ | 9.31 ± 0.94** | ||
| F | 4 | 7.81 ± 0.14 | 6.11 ± 0.24 | 8.87 ± 1.00 | 7.95 ± 0.50 | |
| 17 | 8.78 ± 0.71* | 10.20 ± 0.21** | 10.97 ± 0.24**,Δ | 9.71 ± 0.45** | ||
| γ-GT | M | 4 | 0.52 ± 0.06 | 0.75 ± 0.06 | 0.65 ± 0.04 | 0.66 ± 0.10 |
| 17 | 0.86 ± 0.08** | 1.15 ± 0.15** | 1.02 ± 0.09** | 0.97 ± 0.05** | ||
| F | 4 | 0.62 ± 0.04 | 0.71 ± 0.05 | 0.78 ± 0.05 | 0.66 ± 0.06 | |
| 17 | 0.98 ± 0.12** | 1.16 ± 0.09** | 1.15 ± 0.14** | 1.07 ± 0.04** | ||
| GST | M | 4 | 9.38 ± 0.37 | 8.31 ± 0.45 | 8.68 ± 0.23 | 10.50 ± 0.42 |
| 17 | 12.02 ± 0.79** | 10.90 ± 0.52** | 10.57 ± 0.46** | 13.10 ± 0.34** | ||
| F | 4 | 9.00 ± 0.43 | 7.59 ± 0.51 | 7.59 ± 0.51 | 8.70 ± 0.58 | |
| 17 | 10.70 ± 0.81* | 9.96 ± 0.25** | 10.10 ± 0.42** | 10.41 ± 0.75**,†† |
GCS: γ-glutamylcysteine synthetase; GS: glutathione synthetase; GR: glutathione reductase; GPx: glutathione peroxidase; γ-GT: γ-glutamyl transpeptidase; GST: glutathione S-transferase; M: male; F: female. All the enzyme activities are expressed as nmol/min/mg protein. Data are means ± SEM from 5 animals in each group.
P < 0.05, significant changes compared with the corresponding region in the same gender.
P < 0.01, significant changes compared with the corresponding region in the same gender.
P < 0.01, significant change compared with the corresponding region in opposite gender.
P < 0.05, significant changes compared with other brain regions in the same age/same gender.
Structurally, GCS is composed of heavy and light subunits (GCS-HS and GCS-LS), which are dissociated under reducing conditions and can be detected with specific antibodies (Yan and Meister, 1990). Western blot and band density analysis data showed that there were no significant changes of GCS-HS protein amount among young and aged male and female rats in the four regions examined (Figs. 3A, C). However, the amount of GCS-LS was significantly decreased in all regions of the aged animals compared with the young rats (P < 0.05), although the changes were not the same across all regions examined (Figs. 3B, D).
Fig. 3.
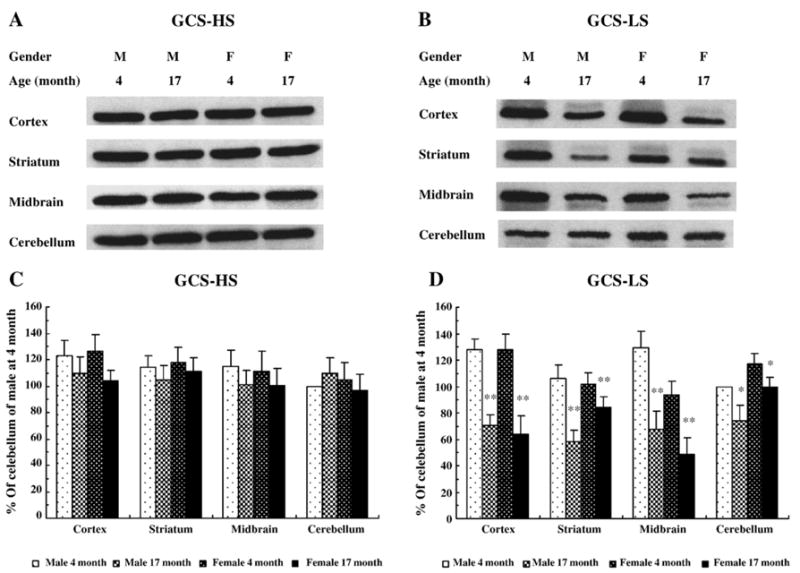
The subunits of γ-glutamylcysteine synthetase (GCS) protein in rat brain tissues. Heavy subunit of GCS (GCS-HS) and light subunit of GCS (GCS-LS) were immunoblotted for cortex, striatum, midbrain, and cerebellum of male and female rats at 4 and 17 months of age. A and B were representative Western blot images for GCS-HS (A) and GCS-LS (B). C (GCS-HS) and D (GCS-LS) represent percent of optical density of immunoblot bands relative to the band density in cerebellum in male rat at 4 months of age. Data are the means ± SEM from 5 animals in each group. *P < 0.05; **P < 0.01 compared with the corresponding young group.
Overall, there was no significant difference between male and female groups when region and gender were used as two factors in the two-way ANOVA on GCS-LS. However, post hoc analysis in one-way ANOVA indicated a male and female GCS-LS difference in midbrain of 4-month-old animals. Similarly, there was a male and female GCS-LS difference in the striatum of 17-month-old animals. These differences were not seen in other regions suggesting regional effects. Pearson 2-tailed test showed significant correlation between GCS activity and GCS-LS protein levels (r2 = 0.595, P < 0.01).
2.4. Age-related changes in GR activity in rat brain
GR participates in glutathione redox cycling by converting GSSG back to GSH. As summarized in Table 1, GR activity was increased in both male or female aged brains compared with the young male or female rat brains, respectively (P < 0.05). The most prominent increase in GR activity was seen in the midbrain of the aged rats, although other regions also displayed increases in GR activities (P < 0.05). In addition, the effect of age on GR activity was independent of gender in all regions examined (F1,76 = 0.96, P = 0.33).
2.5. Age-related changes of GPx, γ-glutamyl transpeptidase (γ-GT), and GST activity in rat brain
GPx, γ-GT, and GST are important GSH-using enzymes and play important roles in maintaining GSH homeostasis and tissue detoxification. As summarized in Table 1, all these enzyme activities were significantly increased in both male or female aged rat brains compared with the young animals in all brain regions examined (P < 0.05). However, GPx activity was significantly higher in the midbrain compared with other regions (P < 0.05). In addition, the effect of age on these enzyme activities did not depend on gender in all regions examined.
3. Discussion
Increasing evidences suggest that the excessive production of free radicals in brain, and the imbalance between oxidative species and antioxidant defenses is related to aging and the pathogenesis of neurodegenerative diseases (Schulz et al., 2000; Kasapoglu and Ozben, 2001). Glutathione redox cycling is an extremely important system in cellular free radical detoxification. Glutathione homeostasis is maintained by de novo glutathione synthesis and redox cycling. In order to realize how aging may alter this system, we measured reduced and oxidized glutathione in multiple regions of brain, we also assessed activities of those enzymes involved in de novo glutathione synthesis, glutathione redox cycling, and glutathione consumption in the same tissues. Thus, results of this study firstly reveal a relatively complete view of glutathione metabolism in young and aged rat brains of both genders, which provides useful information for those who may use aged rats to study neurodegenerative diseases, such as Parkinson's disease.
3.1. Age effects on GSH, GSSG, and GSH/GSSG ratio in rat brain
GSH is present in the brain in millimolar concentrations (Dringen et al., 2000). However, reports on the GSH level among various brain areas are inconsistent (Chen et al., 1989; Abbott et al., 1990; Hussain et al., 1995). In the present study, GSH level was similar in all brain tissues examined (cortex, striatum, midbrain, and cerebellum) regardless of genders. This is in agreement with Liu's (2002) study, in which there were no significant variations of GSH levels in cortex, cerebellum, and hippocampus of 3-month-old Fisher 344 rats. However, Abbott et al. (1990) and Kang et al. (1999) reported that the levels of GSH in 4-month-old mouse brain varied from region to region with cortex > cerebellum > hippocampus > striatum > substantia nigra. This discrepancy could be due to the use of different species (rats versus mice) or differences in animal housing or other environmental conditions. In stressful conditions (Bottje et al., 1998) or in the mild pathological conditions (Maher, 2005), animals may show elevations in GSH levels in tissues or organs that are targets of such insults. Nevertheless, our data suggests that GSH is homogeneous across the midbrain, striatum, cortex, and cerebellum in both male and female Sprague–Dawley rats within the same age group.
An age-associated decline in GSH level has been reported in the brain tissue of various species (Chen et al., 1989; Liu, 2002; Kim et al., 2003), which are consistent overall with the findings of current study. Increases in GSSG were significant in all four brain regions of the aged rats. The decrease in GSH level and increase in GSSG level in aged rats produces a significant decrease in the GSH/GSSG ratio, which is often used to indicate the redox state within the tissue (Dringen et al., 2000; Lin et al., 2002; Samuele et al., 2005). In previous studies, the ratios of GSH/GSSG spread from 7 to 200 (Lin et al., 2002; Lipton et al., 2003; Samuele et al., 2005). The ratios in this study (31 to 57) are clearly within this range. Among the factors that may contribute to this range are strain of rat, age, tissue preparation, and the methodology that was employed to assess GSH and GSSG (Lin et al., 2002; Lipton et al., 2003; Samuele et al., 2005). In the present study, the reduction in GSH/GSSG ratio in aged rats suggested a deficiency in the aged brains to detoxify the ROS, which normally occurs in the brain (Dringen et al., 2000), or resulting from neurotoxin insults.
3.2. Lipid peroxidation in aged rat brain
Quantification of lipid peroxidation is widely used to indicate oxidative injury in diseases (Porter et al., 1995; Halliwell, 1996). We observed a significant increase in LPO production in the brains of aged animals consistent with the recent report by Subathra et al. (2005). Lipid peroxidation is a complex process, and polyunsaturated fatty acids are readily susceptible to autoxidation. The results of this study have led us to believe that deficiency of non-protein thiol is an important, but not an exclusive factor, in aging-related brain injury as indicated by increases in LPO and GSSG productions.
3.3. Age effects on glutathione-related enzymes activities in rat brain
The mechanisms underlying the age-related decline in the GSH level are not well understood. It may result from the reduction in de novo glutathione synthesis. We assessed two de novo synthetic enzyme activities, GCS and GS. Significant reductions in GCS but not in GS activities were observed. Therefore, GCS is more likely responsible for the reduction in GSH level. Recently, Liu and Choi (2000) reported that an age-associated decline in GCS gene expression was accompanied by a decline in total GSH level in aged rats. This suggests that decrease in de novo GSH synthesis may be responsible for the age-associated decrease in GSH content.
GCS catalyzes the first reaction in de novo GSH synthesis. Mammalian GCS is a heterodimer consisting of a catalytic heavy subunit (GCS-HS,73 kDa) and a regulatory light subunit (GCS-LS, 31 kDa), which are encoded by different genes and are dissociated under reducing conditions (Yan and Meister, 1990; Huang et al., 1993). GCS-HS exhibits all of the catalytic activity of the isolated enzyme (Seelig et al., 1984). GCS-LS is enzymatically inactive but plays an important regulatory function by lowering the Km of GCS for glutamate and raising the Ki for GSH (Huang et al., 1993). Regulation of GCS-LS gene expression appears to be critical for GSH homeostasis. Kang et al. (1999) found that GCS activity is highly correlated with GCS-LS mRNA levels in different brain regions. In addition, the activity of GCS is also under post-translational regulation by protein kinases. In 1996, Sun et al. (1996) first reported that GCS was phosphorylated by protein kinase A (PKA), protein kinase C (PKC), and Ca2+/calmodulin-dependent kinase (CMK). This suggests that phosphorylation or dephosphorylation may represent an important regulatory mechanism of GCS activity. In the present study, protein analysis for the subunit of GCS showed that there were no changes in the GCS-HS protein in aged rats compared with young rats. In contrast, a reduction of GCS-LS protein in old rats was seen in all regions observed, which is consistent with another report (Liu, 2002). The fact that these two proteins are translated from two distinct genes and regulated differently in various tissues under physiological conditions (Gipp et al., 1995; Liu et al., 1998) may explain why we observed a difference in translational levels between GCS-HS (no change in aged animals, Figs. 3A, C) and GCS-LS (decreased in aged animals, Figs. 3B, D).
GR was found increased in midbrain particularly in the midbrain of older animals. This is likely resulted from the fact that midbrain contains high level of dopamine which is not sequestered in synaptic vesicles, promoting a higher turnover of dopamine which produces hydroxyl radicals, and hydrogen peroxide. During aging, this turnover becomes more prominent (Ling et al., 2000) which depletes more GSH to cause a greater increase in GSSG, thus stimulating an increase in GR to remove excess GSSG. However, whether increase in GR activity reciprocally down-regulates GCS-LS activity in de novo GSH synthesis is not clear. GSH is a product of both de novo synthesis and glutathione redox cycling. GSH is a nonallosteric feedback inhibitor of the GCS enzyme (Richman and Meister, 1975). In the presence of thiols like GSH, the disulfide bridge connecting the heavy and light subunits of GCS heterodimer is reduced, leading to a conformational change within the substrate-binding site of GCS-HS. The relaxed substrate-binding site can accommodate tripeptides like GSH with greater affinity, thereby inhibiting substrate binding leading to decreased synthesis of GSH (Richman and Meister, 1975). However, in the aged group, this did not seem the case since GSH, as an inhibitor for GCS activity, was reduced significantly regardless of an increase in GR activity. This seems to support a view that GCS activity is independently affected by aging process.
GSH is transported from the intracellular space to extra-cellular space by glutathione transporter. Peroxides and other oxidative species are disposed from cells through this transporter. Such reactions result in a loss of GSH involving the conversion of GSH to GSSG by GPx. GPx activity appears to be increased in aged brain (Carrillo et al., 1992). In the present study, GPx was significantly increased in all four brain regions of aged rats compared to the young rats regardless of genders, demonstrating an adaptive enzymatic alteration in the aged rats secondary to the increase of oxidative stress, as evidenced by the increase in LPO.
GST is another detoxification enzyme which catalyzes the glutathione moiety to a great variety of acceptor molecules including toxins, organic hydroperoxides, and lipid peroxides to form conjugates. These conjugates are degraded in the γ-glutamyl cycle. In one study, GST activity was 1.7-fold increased in the brains of 9-month-old rats compared with those of 5-week-old rats (Kim et al., 2003). In our current study, GST was increased significantly in all brain regions examined in aged male or female rats. Like the elevation of GPx activity, GST is assumed to respond to aging as an adaptive mechanism secondary to increases of oxidative stress.
γ-GT initiates the degradation of extracellular GSH by catalyzing the transfer of the γ-glutamyl moiety from GSH onto an acceptor molecule, usually an amino acid, to form γ-glutamyl-amino acid and cysteinlyglycine, which then is broken down by dipeptidase to produce free cysteine for reuse. Because of its degradative function, increases in γ-GT activity would theoretically lead to a decrease in GSH level. In fact, Sian et al. (1994) have reported that the decrease in GSH in the substantia nigra in Parkinson's disease was due to an increase in γ-GT activity. In the present study, the increase of γ-GT activity was 30 to 40% higher in aged animals relative to the young animals regardless of brain region or gender, suggesting an excessive degradative metabolism of GSH in the aged brains.
3.4. Gender effect on glutathione metabolism in rat brain
The present study showed that the aged-associated changes in GSH, GSSG, and GSH/GSSG ratio, as well as GSH-related enzyme activities were gender-independent with an exception of GST in female cerebellum (Table 1). These findings differed from a report by Wang et al. (2003), which showed that male mice had a more dramatic age-associated change in GSH level than did female mice. The discrepancy may be associated with the animal species (mouse vs. rat) and the subject selection. In the present study, male and female pups were siblings and only one male or female pup was allowed to be assigned into an age group to avoid litter effects. In addition, dividing siblings into young and aged groups also allowed better comparisons cross two time points. This setting might explain why there were no gender-associated variation in GSH and GSH-related enzyme activities in the present study.
Another exception was the gender effect on GCS-LS protein expression. Difference was seen only in midbrain (lower in aged female group) and striatum (lower in aged male group). These differences were not seen in other regions of both young and aged groups suggesting regional effects. The mechanism underlying such differences was not clear and warrants further investigation. However, the differential post-translational regulation among regions may play a role (Sun et al., 1996). The positive correlation between GCS-LS protein levels (Fig. 3D) and GCS activity levels (Table 1) was in agreement with a report by Kang et al. (1999) who found that GCS activity is highly correlated with GCS-LS mRNA levels in different brain regions.
In conclusion, data from this study indicated that the aging-associated GSH decline was accompanied by an increase in oxidative load in the aged rat brains. Data also demonstrated that the age-associated GSH decline was due to both the reduction of GCS-mediated de novo GSH synthesis and the increase of consumption in aged brain. This study provides invaluable data for modeling diseases with neurodegenerative nature, especially age-related diseases such as PD.
4. Experimental procedures
4.1. Chemicals and reagents
2,3-Naphthalenedicarboxyaldehyde (NDA), GSH, γ-glutamylcysteine, ATP, 5-sulfosalicylic acid (SSA), L-glutamic acid, L-cysteine, L-serine, glycylglycine, protease inhibitor cocktail, and other analytical grade reagents were purchased from Sigma Chemical Co. (St. Louis, MO). γ-Glutamyl-7-amino-4-methyl-coumarin (γ-glutamyl-AMC) was from Bachem Bioscience (Torrance, CA). AMC was from Anaspec (San Jose, CA). Assay kits for GSH, GPx, GR, GST, and LPO were from Cayman Chemical (Ann Arbor, MI). Rabbit anti-GCS-HS was from Abcam Inc (Cambridge, MA), and rabbit GCS-LS was a generous gift from Dr. Terrance J. Kavanagh (University of Washington and Bristol-Myers Squibb Company, Seattle, Washington).
4.2. Animals
All animal protocols in the present study were approved by the Institutional Animal Care and Utilization Committee (IACUC) of Rush University and met the guidelines of the Council on Accreditation of the Association for Assessment and Accreditation of Laboratory Animals Care (AAALAC).
Timed-gravid female Sprague–Dawley rats (Zivic-Miller, Allison Park, PA) were purchased at embryonic day 9 (E9) and maintained in an environmentally regulated animal facility. Females were housed individually, and all pups were born normally at E22. The offspring were weaned at postnatal day 21, and the dams were sacrificed. Only one male or female from each of five litters was assigned to the 4- and 17-month groups to avoid litter effect. After 4 and 17 months, the pups were anesthetized using sodium pentobarbital (Sigma, 60 mg/kg, i.p.) and perfused transcardially with ice-cold saline. The brains were quickly removed and frozen in 2-methylbutane and stored at −80 °C for less than 2 months for further analyses.
4.3. Preparation of brain homogenates
The brains were dissected while still frozen as previously described (Ling et al., 2004). The striatum, midbrain, frontal cortex and cerebellum were isolated and weighted. The tissues were homogenized with an ultrasonic dismembrator (Biologics Inc, Gainesville, Virginia) for 12 rapid pulses in ice-cold 0.1 M phosphate-buffered saline (PBS, pH 7.4) containing 0.1 mM EDTA. The homogenates were centrifuged at 14,000 × g for 30 min at 4 °C, and the supernatants were collected for different analyses. Protein concentrations were determined using the Bio-Rad (Hercules, CA) DC protein assay kit using bovine serum albumin as a standard.
4.4. Measurement of GSH and GSSG
Reduced glutathione (GSH) and GSSG concentrations were measured by a commercial kit supplied by Cayman Chemical. This kit utilizes an optimized enzymatic GR recycling method for quantification of GSH (Baker et al., 1990). Briefly, 100 μl of brain homogenate was added to an equal volume of the metaphosphoric acid and then centrifuged at 2000 × g for 2 min to remove protein. Then, 50 μl of 4 M triethanolamine was added for each milliliter of homogenate to increase the pH. For total GSH assay, 50 μl of sample was added to 150 μl of a reaction mixture containing 0.4 M 2-(N-morpholino) ethane-sulfonic acid, 0.1 M phosphate (pH 6.0), 2 mM EDTA, 0.24 mM NADPH, 0.1 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), and 0.1 unit GR. The reaction was carried out at 37 °C for 25 min, and then total glutathione was determined by absorbance at 412 nm using GSSG as standard. For the measurement of GSSG, GSH was removed from the reaction by adding 10 μl of 1 M 2-vinylpyridine solution for each milliliter of homogenate. Then, the remaining GSSG in the reaction was quantified as total GSH assay. The amount of reduced GSH was obtained by subtracting GSSG from total glutathione. Each sample was assessed in duplicates, and the levels of GSH and GSSG were expressed as μmol/g tissue. The ratio of GSH over GSSG was used to indicate redox status that inferences the detoxification capacity.
4.5. LPO measurement
Lipid peroxidation results in a formation of LPO that can therefore be used to indicate the levels of lipid peroxidation in tissue. LPO was quantified with the Cayman's LPO assay kit. This kit measures hydroperoxides directly utilizing the redox reactions with ferrous ions (Mihaljevic et al., 1996). Briefly, 100 μl of brain homogenate was added to an equal volume of saturated methanol and 1 ml of cold chloroform. The mixture was mixed thoroughly and then centrifuged at 2000 × g for 5 min to extract LPO into chloroform layer. Then, 500 μl of chloroform extract was mixed with 450 μl of chloroform–methanol solvent and 50 μl of freshly prepared chromogen (containing 4.5 mM ferrous sulfate in 0.2 M hydrochloric acid) in glass tube. Absorbance was measured at 500 nm after 5 min incubation. The LPO levels in sample homogenates were calculated with standard curve of LPO. Each homogenate was assessed in duplicates, and LPO was expressed as nmol/g tissue.
4.6. Enzyme activity assays
4.6.1. GCS and GS activity
Activity of GCS and GS were determined by a fluorescence-based microtiter plate assay originally developed by White et al. (2003) with little modification. Briefly, 50 μl of homogenate was centrifuged in micro-tubes for 20 min at 4 °C at 12,000 × g to remove endogenous GSH and other inhibitors (Liu et al., 1998). Then, 0.3 ml of 0.1 M PBS was added, and then the samples were centrifuged for another 20 min to wash and concentrate the protein. Final concentrates were tested for their protein content with Bio-Rad DC protein assay kit. For GCS activity assay, 50 μl of sample was added to 50 μl of GCS reaction cocktail containing 5 mM L-cysteine, 100 mM Tris, 10 mM ATP, 20 mM L-glutamic acid, 2 mM EDTA, 20 mM sodium borate, 2 mM serine, and 40 mM MgCl2 then was incubated at 37 °C. The GCS reaction was initiated by addition of 50 μl of 2 mM cysteine. For GS activity assay, L-glutamic acid was substituted with 30 mM glycine, and L-cysteine was substituted with 3 mM γ-glutamylcysteine (γ-GC). After incubation for 20 min, the GCS and GS reactions were stopped by addition of 50 μl of 200 mM SSA. Following deproteination, the supernatants were incubated with 180 μl of 10 mM NDA to form NDA-γ-GC and NDA-GSH. Then, NDA-γ-GC or NDA-GSH fluorescence intensity was measured (472 excitation/528 emission) on a fluorescence plate reader (Molecular Devices, Menlo Park, CA). The production of γ-GC and GSH was calculated with standard curves for NDA-γ-GC and NDA-GSH. Each assay was performed in duplicate, and GCS and GS activity were reported as nmol/min/mg protein.
4.6.2. GPx activity
GPx activity was determined using a commercial kit supplied by Cayman Chemical. In this assay system, the oxidation of glutathione (GSH) was coupled to NADPH oxidation by GR (Paglia and Valentine, 1967). Briefly, the reaction mixture (200 μl) contained 20 μl of brain homogenate, 5.0 mM GSH, 0.1 mM NADPH, 50 mM Tris–HCl (pH 7.6), 5 mM EDTA, and 0.1 unit of GR. The reaction was initiated by addition of 20 μl of 0.2 mM cumene hydroperoxide at room temperature. The decrease in absorbance at 340 nm was recorded at 60 s intervals for 6 min. The rate of decrease in the absorbance is directly proportional to the GPx activity in the sample. Each assay was performed in duplicate, and enzyme units were recorded as nmol NADPH oxidized/min/mg protein.
4.6.3. GR activity
GR activity was assayed spectrophotometrically by monitoring the oxidation of NADPH to NADP+ by GR at 340 nm with the Cayman's GR assay kit at room temperature (Carlberg and Mannervik, 1985). Briefly, the reaction mixture (200 μl) contained 50 mM potassium phosphate (pH 7.5), 1 mM EDTA, 1 mM GSSG, and 0.1 mM NADPH. The reaction was initiated by addition of 20 μl of brain homogenate. The decrease in absorbance at 340 nm was recorded at 60 s intervals for 6 min. Each assay was performed in duplicate, and enzyme units were recorded as nmol NADPH oxidized/min/mg protein.
4.6.4. GST activity
GSTs have been grouped into species-independent classes of isozymes, including both cytosolic and microsomal enzymes (Morgenstern et al., 1979; Mannervik, 1985). Three families of cytosolic soluble GSTs, α (GSTA), μ (GSTM), and π (GSTP), were known in the brain (Theodore et al., 1985; Board et al., 1990). Cayman's GST assay kit measured total GST activity (cytosolic and microsomal) using 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate. Briefly, the reaction mixture (200 μl) contained 20μl of brain homogenate, 100 mM potassium phosphate (pH 6.5), 0.1% (v/v) Triton X-100, 5.0 mM reduced GSH. The reaction was initiated by addition of 10 μl of 20 mM CDNB at room temperature. The increase in absorbance at 340 nm was recorded at 60 s intervals for 7 min. The rate of increase in the absorbance is directly proportional to the GST activity in the sample. Each assay was performed in duplicate, and enzyme units were recorded as nmol/min/mg protein.
4.6.5. γ-GT activity
γ-GT activity was measured following the procedure of Forman et al. (1995). Briefly, a 0.1 ml of brain homogenates was added to 0.25 ml of a reaction mixture containing 50 mM Tris–HCl (pH 7.6), pH 8.6, 20 mM glycylglycine, 20 μM γ-glutamyl-7-amino-4-methyl-coumarin (γ-glutamyl-AMC), and 0.1% (v/v) Triton X-100. The reaction was carried out at 37 °C for 30 min and terminated by adding 1.5 ml cold 50 mM glycine. The fluorescence was measured at 440 nm with excitation wavelength of 370 nm. The enzyme activity, represented by product formation, was calculated and compared with a standard curve of AMC. Each assay was performed in duplicate, and enzyme units were recorded as nmol/min/mg protein.
4.7. Western blot for analysis of GCS protein
The GCS heavy subunit (GCS-HS) and light subunit (GCS-LS) proteins were determined by Western blot as described by Liu et al. (1998). Briefly, the homogenates were mixed with a protease inhibitor cocktail, and then the samples were prepared as described for GCS activity measurement. For each sample, 30 μg of total protein was electrophoresed in 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and blotted onto a nitrocellulose membrane. After blocking with milk, the membrane was incubated with a primary antibody to GCS-HS or GCS-LS. Anti-GCS-HS polyclonal antibody was diluted at 1:1000. Anti-GCS-LS polyclonal antibody was used at 1:2000. Second antibodies to anti-GCS-HS or GCS LS were conjugated with peroxidase. Films were exposed using enhanced chemiluminescence. The X-ray film was scanned, and the density of band was quantified using Scion Image software (Scion Corporation, Frederick, MD). All band densities were compared with that of cerebellum of male rat at 4 months old.
4.8. Statistical analyses
Data are expressed as mean ± SEM. Differences among groups and regions were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's method. Two-way ANOVA was used to analyze interaction of gender and age. Pearson 2-tailed test was used to detect correlation between GCS activities and GCS protein levels. A probability value of less than 0.05 was considered statistically significant.
Acknowledgments
This study was supported by NINDS NS045316, NIEHS 012307, USARMRA W81XWH-04-01-0365, and grant from the Michael J. Fox Foundation. The authors also thank Dr. Terrance J. Kavanagh at University of Washington for his generosity of providing GCS-LS anti sera.
Abbreviations
- γ-GC
γ-glutamylcysteine
- GCS
γ-glutamylcysteine synthetase
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GS
glutathione synthetase
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- γ-GT
γ-glutamyl transpeptidase
- LPO
lipid peroxide
- NDA
2,3-naphthalenedicarboxyaldehyde
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- SSA
5-sulfosalicylic acid
References
- Abbott LC, Nejad HH, Bottje WG, Hassan AS. Glutathione levels in specific brain regions of genetically epileptic (tg/tg) mice. Brain Res Bull. 1990;25:629–631. doi: 10.1016/0361-9230(90)90124-i. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- Board P, Coggan M, Johnston P, Ross V, Suzuki T, Webb G. Genetic heterogeneity of the human glutathione transferases: a complex of gene families. Pharmacol Ther. 1990;48:357–369. doi: 10.1016/0163-7258(90)90054-6. [DOI] [PubMed] [Google Scholar]
- Bottje WG, Wang S, Beers KW, Cawthon D. Lung lining fluid antioxidants in male broilers: age-related changes under thermoneutral and cold temperature conditions. Poult Sci. 1998;77:1905–1912. doi: 10.1093/ps/77.12.1905. [DOI] [PubMed] [Google Scholar]
- Brown AM, Deutch AY, Colbran RJ. Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur J Neurosci. 2005;22:247–256. doi: 10.1111/j.1460-9568.2005.04190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Kanai S, Sato Y, Kitani K. Age-related changes in antioxidant enzyme activities are region and organ, as well as sex, selective in the rat. Mech Ageing Dev. 1992;65:187–198. doi: 10.1016/0047-6374(92)90035-c. [DOI] [PubMed] [Google Scholar]
- Chen TS, Richie JP, Jr, Lang CA. The effect of aging on glutathione and cysteine levels in different regions of the mouse brain. Proc Soc Exp Biol Med. 1989;190:399–402. doi: 10.3181/00379727-190-42879. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Shi MM, Iwamoto T, Liu RM, Robison TW. Measurement of gamma-glutamyl transpeptidase and gamma-glutamylcysteine synthetase activities in cells. Methods Enzymol. 1995;252:66–71. doi: 10.1016/0076-6879(95)52009-0. [DOI] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Romanovitch AE, Peloso E, Satinoff E, Plata-Salaman CR. Basal and IL-1beta-stimulated cytokine and neuropeptide mRNA expression in brain regions of young and old Long–Evans rats. Brain Res Mol Brain Res. 1999;70:92–100. doi: 10.1016/s0169-328x(99)00134-5. [DOI] [PubMed] [Google Scholar]
- Gipp JJ, Bailey HH, Mulcahy RT. Cloning and sequencing of the cDNA for the light subunit of human liver gamma-glutamylcysteine synthetase and relative mRNA levels for heavy and light subunits in human normal tissues. Biochem Biophys Res Commun. 1995;206:584–589. doi: 10.1006/bbrc.1995.1083. [DOI] [PubMed] [Google Scholar]
- Gray DA, Tsirigotis M, Woulfe J. Ubiquitin, proteasomes, and the aging brain. Sci Aging Knowledge Environ. 2003:RE6. doi: 10.1126/sageke.2003.34.re6. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Bridges RJ, Meister A. Evidence that the gamma-glutamyl cycle functions in vivo using intracellular glutathione: effects of amino acids and selective inhibition of enzymes. Proc Natl Acad Sci U S A. 1978;75:5405–5408. doi: 10.1073/pnas.75.11.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radical Res. 1996;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Hampton T. Study reveals mitochondrial role in aging. JAMA. 2005;294:672. doi: 10.1001/jama.294.6.672. [DOI] [PubMed] [Google Scholar]
- Huang CS, Anderson ME, Meister A. Amino acid sequence and function of the light subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268:20578–20583. [PubMed] [Google Scholar]
- Hussain S, Slikker W, Jr, Ali SF. Age-related changes in antioxidant enzymes, superoxide dismutase, catalase, glutathione peroxidase and glutathione in different regions of mouse brain. Int J Dev Neurosci. 1995;13:811–817. doi: 10.1016/0736-5748(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Kang Y, Viswanath V, Jha N, Qiao X, Mo JQ, Andersen JK. Brain gamma-glutamyl cysteine synthetase (GCS) mRNA expression patterns correlate with regional-specific enzyme activities and glutathione levels. J Neurosci Res. 1999;58:436–441. [PubMed] [Google Scholar]
- Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001;36:209–220. doi: 10.1016/s0531-5565(00)00198-4. [DOI] [PubMed] [Google Scholar]
- Kim HG, Hong SM, Kim SJ, Park HJ, Jung HI, Lee YY, Moon JS, Lim HW, Park EH, Lim CJ. Age-related changes in the activity of antioxidant and redox enzymes in rats. Mol Cells. 2003;16:278–284. [PubMed] [Google Scholar]
- Lin AM, Chen CF, Ho LT. Neuroprotective effect of intermittent hypoxia on iron-induced oxidative injury in rat brain. Exp Neurol. 2002;176:328–335. doi: 10.1006/exnr.2002.7938. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Collier TJ, Sortwell CE, Lipton JW, Vu TQ, Robie HC, Carvey PM. Striatal trophic activity is reduced in the aged rat brain. Brain Res. 2000;856:301–309. doi: 10.1016/s0006-8993(00)01945-4. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Chang Q, Lipton JW, Tong CW, Landers TM, Carvey PM. Combined toxicity of prenatal bacterial endotoxin exposure and postnatal 6-hydroxydopamine in the adult rat midbrain. Neuroscience. 2004;124:619–628. doi: 10.1016/j.neuroscience.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Lipton JW, Gyawali S, Borys ED, Koprich JB, Ptaszny M, McGuire SO. Prenatal cocaine administration increases glutathione and alpha-tocopherol oxidation in fetal rat brain. Brain Res Dev Brain Res. 2003;147:77–84. doi: 10.1016/j.devbrainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Liu RM. Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J Neurosci Res. 2002;68:344–351. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- Liu R, Choi J. Age-associated decline in gamma-glutamylcysteine synthetase gene expression in rats. Free Radical Biol Med. 2000;28:566–574. doi: 10.1016/s0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- Liu RM, Gao L, Choi J, Forman HJ. Gamma-glutamylcysteine synthetase: mRNA stabilization and independent subunit transcription by 4-hydroxy-2-nonenal. Am J Physiol. 1998;275:L861–L869. doi: 10.1152/ajplung.1998.275.5.L861. [DOI] [PubMed] [Google Scholar]
- Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- Mihaljevic B, Katusin-Razem B, Razem D. The reevaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanistic aspects of the response. Free Radical Biol Med. 1996;21:53–63. doi: 10.1016/0891-5849(95)02224-4. [DOI] [PubMed] [Google Scholar]
- Morgenstern R, DePierre JW, Ernster L. Activation of microsomal glutathione S-transferase activity by sulfhydryl reagents. Biochem Biophys Res Commun. 1979;87:657–663. doi: 10.1016/0006-291x(79)92009-6. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Wardas J, Smialowska M, Kuter K, Lenda T, Wieronska JM, Zieba B, Nowak P, Dabrowska J, Bortel A, Kwiecinski A, Wolfarth S. A slowly developing dysfunction of dopaminergic nigrostriatal neurons induced by long-term paraquat administration in rats: an animal model of preclinical stages of Parkinson's disease? Eur J Neurosci. 2005;22:1294–1304. doi: 10.1111/j.1460-9568.2005.04301.x. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]
- Samuele A, Mangiagalli A, Armentero MT, Fancellu R, Bazzini E, Vairetti M, Ferrigno A, Richelmi P, Nappi G, Blandini F. Oxidative stress and pro-apoptotic conditions in a rodent model of Wilson's disease. Biochim Biophys Acta. 2005;1741:325–330. doi: 10.1016/j.bbadis.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- Seelig GF, Simondsen RP, Meister A. Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem. 1984;259:9345–9347. [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Sortwell CE, Camargo MD, Pitzer MR, Gyawali S, Collier TJ. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp Neurol. 2001;169:23–29. doi: 10.1006/exnr.2001.7644. [DOI] [PubMed] [Google Scholar]
- Subathra M, Shila S, Devi MA, Panneerselvam C. Emerging role of Centella asiatica in improving age-related neurological antioxidant status. Exp Gerontol. 2005;40:707–715. doi: 10.1016/j.exger.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Sun WM, Huang ZZ, Lu SC. Regulation of gamma-glutamylcysteine synthetase by protein phosphorylation. Biochem J. 1996;320 (Pt 1):321–328. doi: 10.1042/bj3200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore C, Singh SV, Hong TD, Awasthi YC. Glutathione S-transferases of human brain. Evidence for two immunologically distinct types of 26500-Mr subunits. Biochem J. 1985;225:375–382. doi: 10.1042/bj2250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TQ, Ling ZD, Ma SY, Robie HC, Tong CW, Chen EY, Lipton JW, Carvey PM. Pramipexole attenuates the dopaminergic cell loss induced by intraventricular 6-hydroxydopamine. J Neural Transm. 2000;107:159–176. doi: 10.1007/s007020050014. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu H, Liu RM. Gender difference in glutathione metabolism during aging in mice. Exp Gerontol. 2003;38:507–517. doi: 10.1016/s0531-5565(03)00036-6. [DOI] [PubMed] [Google Scholar]
- White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ. Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem. 2003;318:175–180. doi: 10.1016/s0003-2697(03)00143-x. [DOI] [PubMed] [Google Scholar]
- Yan N, Meister A. Amino acid sequence of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1990;265:1588–1593. [PubMed] [Google Scholar]
- Zeng BY, Medhurst AD, Jackson M, Rose S, Jenner P. Proteasomal activity in brain differs between species and brain regions and changes with age. Mech Ageing Dev. 2005;126:760–766. doi: 10.1016/j.mad.2005.01.008. [DOI] [PubMed] [Google Scholar]


