Abstract
Attention is thought to enhance perceptual performance at attended locations due to top-down attention signals that modulate activity in visual cortex. Here, we show that activity in early visual cortex is sustained during maintenance of attention in the absence of visual stimulation. We used functional magnetic resonance imaging (fMRI) to measure activity in visual cortex while human subjects performed a visual detection task in which a variable-duration delay period preceded target presentation. Portions of cortical areas V1, V2, and V3 representing the attended part of the visual field exhibited sustained increases in activity throughout the delay period. Portions of these cortical areas representing peripheral, unattended parts of the visual field displayed sustained decreases in activity. The data were well-fit by a model that assumed the sustained neural activity was constant in amplitude over a time period equal to that of the actual delay period for each trial. These results demonstrate that sustained attention responses are present in early visual cortex (including primary visual cortex) in the absence of a visual stimulus, and that these responses correlate with the allocation of visuospatial attention in both the spatial and temporal domains.
Introduction
Following a cue to shift attention to a particular location, attention can be voluntarily maintained at that location, even in the absence of visual stimulation. Previous work in our laboratory demonstrated a large increase in activity in early visual cortical areas (including primary visual cortex, V1) in the absence of a visual stimulus, evoked by the presentation of an auditory cue that signaled the beginning of each trial of a contrast detection task (Ress et al. 2000). However, the temporal proximity of cue and target presentation made it impossible to measure the time course of this activity. Sustained spatial attention signals in the absence of visual stimulation have been reported using fMRI in human visual cortical areas V1, V2, V4, and TEO (Kastner et al. 1999), and similar sustained cue-related attention signals were described in extrastriate cortical areas V2, V3, and V4 (Hopfinger et al. 2000). However, other fMRI studies have found only transient cue-related activity in human visual cortical areas such as MT+, lateral occipital (LO) cortex, and anterior fusiform (Corbetta et al. 2000; 2002; Shulman et al. 1999). Notably, all of the above studies demonstrated sustained cue-related signals in attention control areas in parietal and frontal cortex (see also Colby et al. 1996; Bisley and Goldberg 2003). The studies differ mainly in their descriptions of the time courses of activity in visual cortical areas, which are presumably the recipients of top-down spatial attention signals from parietal and frontal cortex (reviewed in Corbetta and Shulman 2002).
Electrophysiological studies in monkey primary visual cortex have demonstrated attentional modulation of visual responses (McAdams and Maunsell 1999; McAdams and Reid 2005; Mehta et al. 2000; Motter 1993). However, sustained attention responses in the absence of visual stimulation (increases in baseline activity) have been reported only in extrastriate cortex (Haenny et al. 1988; Luck et al. 1997; Reynolds et al. 1999), not in cortical area V1 (Luck et al. 1997; Mehta et al. 2000).
Given the heterogeneous results in the literature regarding the time courses of endogenous attention signals in visual cortex, it is important to replicate and extend the findings of Kastner et al. (1999) to more completely characterize the temporal properties of these signals. Of particular importance is the replication and characterization of sustained activity in human primary visual cortex (V1); there is only one report of sustained V1 activity in the absence of visual stimulation (Kastner et al. 1999), and it was found in only two out of five individual subjects. A detailed description of the time courses of visual cortical spatial attention signals is necessary for understanding the functions of early visual cortical areas and the roles they play in the enhancement of visual perception by endogenous spatial attention (Bashinski and Bacharach, 1980; Posner et al., 1980).
Previous neuroimaging studies of cue-related attention signals have employed a limited range of cue-target intervals. This design made it difficult to unambiguously assess whether attention signals are transient or sustained, because if subjects know in advance when the target will be presented, they can detect or discriminate the target without maintaining attention continuously throughout the cue-target interval. In addition, the sluggishness and intersubject variability of the hemodynamic response (Aguirre et al. 1998) complicated interpretation of the time courses of the attention signals when a small range of cue-target intervals was used.
In the experiments described here, the delay period between cue and target presentation was fully randomized over a large range to determine if early visual cortex is involved in the maintenance of attention for the entire duration of the trial or if it responds only transiently at the beginning of each trial (e.g., in direct response to the attentional cue, to the expectancy of a target when it is known to immediately follow the cue, or to the shifting of attention as directed by the cue). We used fMRI and a visual detection task with a variable-duration delay period to examine the time course of attention-related activity in early visual cortex of humans during sustained attention in the absence of visual stimulation. fMRI responses within the portions of V1, V2, and V3 representing the attended part of the visual field increased when attention was deployed, and these responses were maintained for the duration of the delay period. In contrast, a sustained decrease in fMRI responses was observed in peripheral portions of early visual cortex representing unattended visual field locations. A model in which attention-related activity was maintained at a constant level throughout the delay period described the measured fMRI time courses very well. Thus, our results clearly indicate that sustained activity in early visual cortex is correlated with the maintenance of visual spatial attention.
Materials and Methods
Subjects
Four healthy subjects participated in the study, all of whom had extensive experience as subjects in psychophysical and fMRI experiments. Two of the participants were also authors of the study (subjects MAS and DBR). All subjects provided written consent, and the experiments were carried out in compliance with safety guidelines for MR research. The experimental protocol was approved by the human subjects Institutional Review Board of Stanford University.
Visual detection task
The visual target was a plaid annulus with inner diameter 1.5 and outer diameter 4.5 degrees of visual angle (Figure 1). The spatial frequency of the component sinusoidal gratings was 1 cyc/deg, and the target was presented for 250 ms. The target contrast was ramped on and off smoothly (contrast modulated by one half cycle of a 2-Hz temporal sinusoid). The target contrast varied across subjects and corresponded to individual detection thresholds as determined by extensive behavioral testing (before fMRI scanning commenced). For fMRI experiments, stimuli were presented on a flat-panel LCD monitor (MutliSync 2000; NEC, Itasca, IL) that was encased in a Faraday cage with an electrically-conductive glass front window. The mean luminance of the monitor was 30 cd/m2, and the size of the screen was 40 × 30 cm. The monitor was viewed with an angled mirror positioned above the subjects’ eyes, and the viewing distance was 304 cm. The attended annulus occupied approximately 90% of the monitor screen in the vertical dimension.
Figure 1.
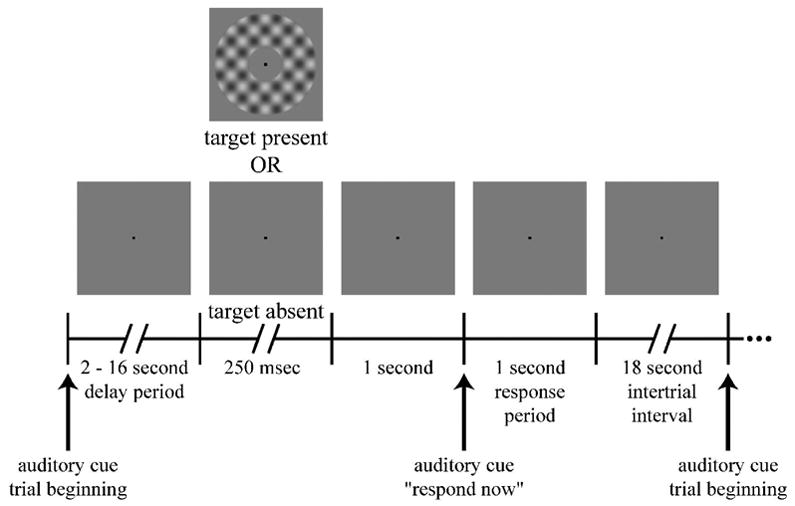
Visual detection task. Each trial began with an auditory cue that was followed by a delay period of variable duration. On approximately half of the trials, a target was presented following the delay period. One second later, a second auditory cue instructed the subjects to respond whether they had perceived a target. A long intertrial interval allowed the hemodynamics to return to baseline before the beginning of the subsequent trial.
Each trial began with a brief auditory stimulus (50 ms in duration; linear frequency sweep from 800 to 1000 Hz). This was followed by a variable (2–16 sec) delay period. The delay-period duration was fully randomized to prevent subjects from using the sounds generated by the MR scanner to predict when the target might be shown. On approximately half of the trials, the target was presented at the end of the delay period, and the screen remained uniform gray for the remaining trials. A second auditory stimulus (60 ms; frequency sweep from 3000 to 1800 Hz) instructed the subject to report whether the target had been shown or not. A one-second interval was inserted between the detection period and response period to allow any visual iconic memory or visual aftereffects of target presentation to dissipate. Thus, subjects were required to maintain attention during the delay period to correctly detect the target, and they could not simply report their current perceptual state when prompted to generate a behavioral response. It is well established that allocation of endogenous spatial attention following a cue reduces behavioral thresholds for detecting low-contrast targets (e.g., Bashinski and Bacharach 1980).
During behavioral training sessions subjects received auditory feedback (distinctive sounds) after each trial that informed them whether the response was correct or incorrect. This feedback was not provided during fMRI experiments. Each trial was followed by a long (18 sec) inter-trial interval to allow the hemodynamics to return to baseline before the initiation of the subsequent trial. Experimental sessions typically included 10 approximately 5-minute runs, corresponding to a total of about 70 trials of the sustained attention task. The stimulus protocol was written in MATLAB (The MathWorks, Inc., Natick, MA) using the Psychophysics Toolbox extensions (Brainard 1997; Pelli 1997).
A 0.125 deg square fixation point was presented continuously to encourage stable eye position, but we did not have the capability to measure eye movements in the MR scanner when these experiments were performed. Although eye position was not monitored in the present study, all of the subjects in this study also participated in another study involving covert attention in which eye position was measured in the scanner (Silver et al. 2005). In that study, subjects were cued to attend to a single peripheral location, and they performed a visual detection task at that location. Subjects showed only a very slight bias in eye position towards the cued location (Silver et al. 2005). The present study, by comparison, cued subjects to attend to an annulus surrounding the fixation point, making it much easier for subjects to maintain fixation. Furthermore, the results of a previously reported control experiment (Ress et al. 2000) demonstrated that the best performance was obtained when the eyes were held steady on the fixation point. In this control experiment, the delay period duration was fixed at 1 sec, the plaid annulus (3 deg inner radius, 6 deg outer radius, spatial frequency of component gratings of 0.5 cyc/deg, 0.75 sec duration, 4 Hz contrast-reversing) was slightly different from that used in the sustained attention experiment (stimulus parameters listed above), and subjects were instructed either to hold central fixation or to move their eyes to the target annulus on each trial.
fMRI data acquisition
fMRI experiments were conducted using a 3-Tesla General Electric Signa LX scanner (Milwaukee, WI). A custom-designed surface coil (NMSC-002-TR-3GE transmit-receive coil, Nova Medical, Wakefield, MA) was employed to improve contrast-to-noise ratio in occipital cortex. A time series of fMRI volumes was acquired using a two shot, spiral-trajectory, gradient-recalled-echo pulse sequence (Glover and Lai 1998; Glover 1999). Other scan parameters were as follows: TE = 30 ms, TR = 0.75 s, FOV = 220 mm. The effective inplane pixel size ranged from 2.2 × 2.2 mm to 3.5 × 3.5 mm, the number of slices was either 12 or 15, and the slice thickness was either 3.5 or 4 mm.
In addition to the functional images, every scanning session included the acquisition of T1-weighted anatomical images, coplanar with the functional images. These were aligned to a high-resolution whole-brain anatomical volume for each subject using custom software (Nestares and Heeger 2000), so that the functional data from a given subject could be combined across multiple scanning sessions. The whole-brain anatomical volumes were T1-weighted to emphasize contrast between gray and white matter and acquired with a birdcage-style head coil on a 1.5-Tesla GE Signa LX scanner using an inversion-recovery prepared 3-D SPGR pulse sequence.
fMRI data preprocessing
Each fMRI run began with a dummy trial in which the delay-period duration was always 2 seconds. Results from these trials were not included in the behavioral analysis, and the fMRI data acquired during these trials (corresponding to 14 frames, or 21 seconds) were discarded to remove artifacts due to incomplete magnetic saturation and to allow the hemodynamics to attain a steady state baseline. Head movements in the remaining frames were corrected using a 3-D image registration algorithm (MCFLIRT; Jenkinson et al. 2002). The time series at each voxel were then high-pass filtered to remove low frequency noise and slow drift (Smith et al. 1999; Zarahn et al. 1997). Finally, each voxel’s time series was divided by its mean intensity to convert the data from arbitrary image intensity units to percentage signal modulation and to compensate for the decrease in mean image intensity as a function of distance from the receive coil.
Definition of regions of interest (ROIs)
The boundaries of early visual areas V1, V2, and V3 were defined using well-established retinotopic mapping methods (DeYoe et al. 1996; Engel et al. 1994, 1997; Sereno et al. 1995). An LCD projector was used to present visual stimuli for the retinotopic mapping experiments. The projector’s field of view was approximately 40 × 40 degrees of visual angle, much larger than the approximately 5 × 7 degree field of view for the LCD monitor in the visual detection task. Each visual area was then restricted based on fMRI responses from a visual localizer experiment, in which subjects passively viewed a visual stimulus in a peripheral annulus around the fixation point (same size and shape as the visual target in the sustained attention experiments). The annulus was a checkerboard (100% contrast, 3 cyc/deg, 4-Hz contrast reversal) presented for blocks of 9 seconds in alternation with 9-second blocks of a uniform gray field. The fMRI data obtained during these localizer experiments were preprocessed as described above. A sinusoid with the same period as the block alternation was fit to the time-series from each voxel, and the coherence and phase of the best-fitting sinusoid were computed (Bandettini et al. 1993; Engel et al. 1997). The ROIs for each visual area were then restricted based on response phase (latency) in the localizer experiment. These phases had a bimodal distribution. One set of voxels was activated by the visual stimulus and represented the portion of the visual field corresponding to the stimulus, and the other set was 180 degrees out-of-phase with the stimulus and represented regions of the visual field more central or more peripheral than the stimulus annulus. The ROIs were, therefore, restricted to include only voxels that responded to the visual stimulus with an increase in BOLD signal.
Finally, a series of coherence threshold values were chosen (ranging from 0.3 to 0.9), and the value that minimized the mean variance of the binned time series in the detection task (e.g., as shown in Figure 2) was selected. The coherence threshold values for each subject and each ROI were independently selected for every fMRI session. Only voxels exceeding this coherence threshold in the localizer experiment were included in the ROIs. By using the variance of the measured responses during the sustained attention experiments as the dependent variable in setting the coherence thresholds, we were able to avoid resorting to arbitrary criteria for the choice of statistical threshold. Note that there is no reason to believe that this procedure for refining the ROIs systematically biased the results to favor sustained time courses. In fact, voxels with transient attention signals are likely to have relatively less variance than those with sustained signals, as the transient responses would be time-locked to the onset of attention. Variance was measured for fMRI time series that were binned (3.5 second bins) and aligned at the beginning of the delay period (as displayed in Figure 2). Therefore, the transient signal will occur at the same time for all time series within a bin. The sustained signal, on the other hand will have relatively more variance, because the average time course in a given bin will combine time series with heterogeneous durations. We examined binned time courses from the detection task over the full range of coherence thresholds (0.3–0.9) and found clear evidence for sustained activity over the entire range of coherence thresholds for subjects MAS, DBR, and RAS. However, reducing the variance by this method of ROI definition facilitated quantitative modeling of the time courses.
Figure 2.
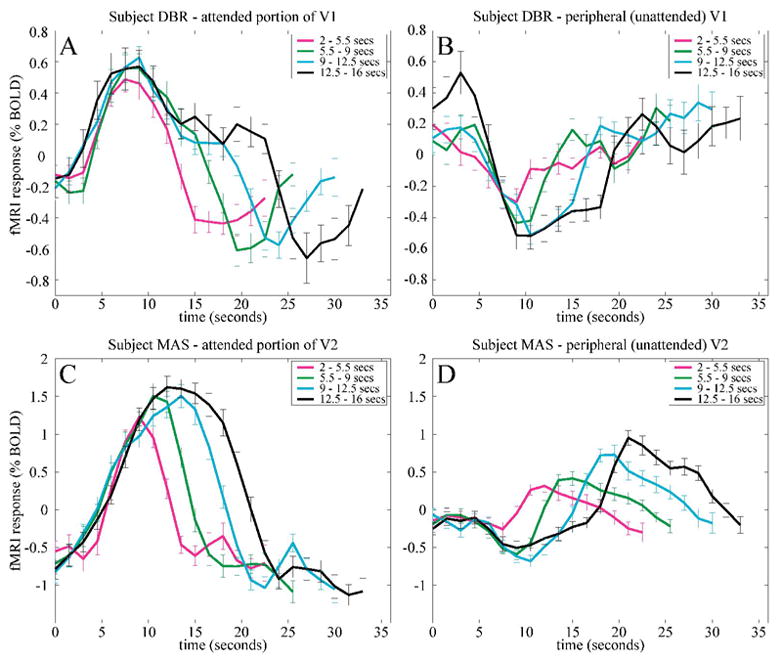
Sustained delay-period activity in early visual cortex (example data from two subjects). fMRI responses were aligned at the beginning of each trial and binned into four groups (magenta, green, cyan, black curves) based on delay-period duration. A, C. fMRI responses in a subregion of early visual cortex corresponding to the attended portion of visual field. Response increases were time-locked to the beginning of the delay period, but they returned to baseline at different times depending on the delay-period duration. B, D. Peripheral, unattended portion of visual field. Activity decreased during the delay period, but the durations of sustained decreases in activity were also a function of delay-period duration.
Regions of interest corresponding to peripheral, unattended portions of V1, V2, and V3 were also defined. These ROIs were the most eccentric portions of each visual area defined with the retinotopic mapping procedure and corresponded to an annular region with diameter centered about an eccentricity of about 20 degrees of visual angle. The annular width of each ROI was chosen to match the volume of the corresponding attended region - specifically, the number of voxels in the attended portion of a given cortical area was equal to the number of voxels in the peripheral unattended portion of that area for each subject. The ROIs corresponding to the attended and unattended visual field representations did not overlap for any of the visual areas.
Finally, we defined ROIs corresponding to the central, foveal regions of early visual cortex. The cortical representation of these central eccentricities is an area on the cortical surface where areas V1, V2d, V2v, V3d, and V3v all converge (the foveal confluence). It is difficult to accurately assign voxels to one of these areas when the cortical representations are so small. In addition, the inner radius of the annulus used in our attention experiments was only 0.75 degrees, leaving only a very small portion of cortex that represented the circular region within the annulus. Consequently, the foveal ROIs were not subdivided by visual area and were smaller than the ROIs corresponding to the target annulus and periphery.
Estimation of sustained neural activity
The data from the main (sustained attention) experiment were analyzed, separately for each subject and each ROI, to estimate the amplitude of the sustained activity for each trial. This was done by adopting a model of the underlying neural activity and a model of the hemodynamics. Neural activity was modeled as a step function that had a value of zero during the intertrial interval and a value of one throughout each trial. Specifically, the onset of activity in the model was coincident with the auditory stimulus at the beginning of each trial, and the activity was assumed to be maintained at a constant level until the end of the behavioral response period (Figure 1). In addition, a positive transient response (200 msec duration) was included in the model at the end of the behavioral response period. This “off-response” has been previously described as a transient response associated with the termination of a sustained state of readiness (Shulman et al. 2002) or with transitions between task components (Jack et al. 2006). In the current task design, a number of components of the trial occurred near the end of the delay period, including target presentation (on 50% of the trials), perceptual judgment, the offset of sustained attention, and the execution of a motor response. Because all of these events occurred within a brief temporal window (Figure 1), they could not be resolved with fMRI. Therefore, although the off-response was clearly a component of the fMRI time courses, the task design employed in the present study was not well suited for investigating its function. As a result, the off-response was included as a parameter in the model but was not studied further.
The model neural activity time course was convolved with a canonical hemodynamic response function, as defined in the SPM99 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm99/). The model time series were also highpass filtered exactly like the measured time series. The amplitudes of the sustained delay-period activity and the off-response were estimated for each trial by minimizing the mean squared difference between the modeled and measured time series.
Results
Subjects performed a visual detection task in which the target was a plaid annulus centered around a fixation point (Figure 1). The contrast of the target corresponded to the detection threshold that had been determined individually for each subject (Table 1). Each trial began with an auditory beep that indicated to the subjects that the target could be presented at some point during the subsequent 2–16 second interval. A second auditory beep was presented which indicated that the delay period had ended and instructed the subjects to report with a button press whether the target had been shown or not. Because the durations of the delay period were fully randomized, subjects had to continuously maintain attention throughout the delay period.
Table 1.
Behavioral performance. Contrast range is the range of stimulus contrasts used during the scanning sessions. A threshold contrast was selected for each subject, before scanning, based on extensive behavioral testing. Occasionally, this contrast was adjusted during a scanning session if performance was too high or too low, resulting in a range of contrasts for some subjects. Response bias is defined as ½ [z(hit rate) + z(false alarm rate)], where z represents the z-transformation, or the inverse of the cumulative normal distribution function (Macmillan and Creelman, 2005). Response bias is greater than zero when subjects are more likely to respond “target absent” than “target present”.
| Subject | contrast range (%) | % correct | d′ | response bias |
|---|---|---|---|---|
| MAS | 1.4–1.5 | 76 | 1.45 | 0.11 |
| DBR | 1.5 | 64 | 0.81 | 0.48 |
| RAS | 1.5 | 74 | 1.29 | 0.16 |
| JM | 1.9–2.1 | 75 | 2.01 | 0.83 |
Subjects practiced the detection task for several hours over several days before participating in fMRI experiments. This allowed them to reach asymptotic levels of behavioral performance which, in turn, allowed a precise estimation of their detection thresholds. In addition, subjects developed an internal representation of the size, shape, and appearance of the target. Therefore, during the delay period, they were able to allocate attention to the annular region of the visual field where the target could appear, even though there was no visual information provided during this time to indicate the target location.
Subregions of cortical areas V1, V2, and V3 that corresponded retinotopically to the attended portion of the visual field exhibited sustained delay-period activity (Figure 2A, C, and Supplemental Figure 1A, C) in three of four subjects. Each fMRI time series was segmented into individual trials. These were sorted into one of four bins based on the duration of the delay period. The initial portions of the fMRI response time courses were similar for all delay periods, with a short (~4 s) hemodynamic latency consistent with a prompt deployment of attention in response to the auditory cue. However, the later portions of the time series indicated sustained cortical activity, and the duration of this sustained activity was positively correlated with delay-period duration. This phenomenon was observed in cortical areas V1, V2, and V3. Because the bins differed only in the duration of a delay period that contained no visual stimulation, it is likely that these sustained responses were associated with the maintenance of attention required to perform the visual detection task.
Portions of visual cortex, by contrast, that corresponded to peripheral unattended visual field locations exhibited sustained decreases in fMRI activity during the delay periods (Figure 2B, D, and Supplemental Figure 1B, D) in all four subjects. This result indicates that attention has two retinotopically-specific effects in early visual cortex: increased activity in cortical regions corresponding to the attended stimulus, and decreased activity in regions representing unattended visual field locations. Additionally, ROIs corresponding to the foveal confluence were defined. These cortical regions represented unattended central visual field locations within the inner boundary of the attended annulus. In contrast to the sustained responses observed in cortical regions representing the location of the stimulus annulus, there was little evidence for sustained activity in the foveal confluence (Supplemental Figure 2). The retinotopic specificity of the attention effects provides evidence against explanations based on eye movements, arousal, or other global, non-spatially-selective processes.
To quantify these results, we estimated the amplitude and duration of the sustained activity from the fMRI measurements by adopting a model of the underlying neural activity and a model of the hemodynamic response. We modeled sustained neural activity in early visual cortex with a step function that started at the beginning of the trial (coincident with the auditory tone that initiated the trials) and persisted with a constant amplitude until the end of the response period. This step function was convolved with a canonical hemodynamic response function (see Materials and Methods) to generate an estimate of the time course of the BOLD signal. The amplitude of the sustained delay-period response that provided the best fit of the observed time courses was computed. The trials were sorted into six bins, and the average measured time course was plotted along with the estimated time course based on the model (Supplemental Figure 3). The fits were very good for three of four subjects, indicating that the model, in which attention was simply switched on and maintained at a constant level until a response was made, effectively described the time course of the measured cortical activity.
The estimated delay-period response amplitudes, in cortical regions corresponding retinotopically to the attended portion of the visual field, were significantly greater than zero in the attended portions of V1, V2, and V3, for three out of four subjects (Figure 3). Although there are only three subjects in this study that exhibited sustained delay-period activity in early visual cortex, similar results have been obtained with a nearly identical experimental protocol in four additional subjects (Offen et al. 2005).
Figure 3.
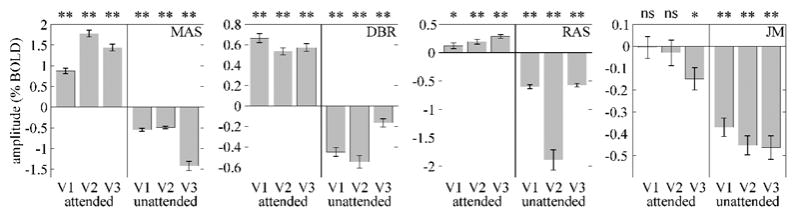
Attention-related activity was retinotopically specific. Bars, estimated amplitudes of sustained delay-period activity. Error bars, standard errors of the mean across trials. Response amplitudes were significantly greater than zero in the portions of each visual area corresponding to the attended visual field for 3 of the 4 subjects. Response amplitudes corresponding to unattended visual field were negative for all 4 subjects. ns = not significant; * = p < 0.05; ** = p < 10−4.
In contrast to the increases in activity in attended portions of early visual cortex in three of four subjects, all subjects displayed significant response decreases in peripheral, unattended portions of V1, V2, and V3 (Figure 3). A reduction of activity in unattended visual cortex has been observed in previous neuroimaging studies of attention (Tootell et al. 1998; Somers et al. 1999; Smith et al. 2000; Slotnick et al. 2003; Müller and Kleinschmidt 2004). Mean estimated amplitudes for the foveal confluence ROIs were -0.27 +/− 0.68 % BOLD for Subject MAS, 0.01 +/− 0.08 for Subject RAS, and 0.36 +/− 0.09 for Subject DBR. These amplitudes were not significantly different from zero for MAS and RAS, but they were for DBR (p < 10−4). However, DBR’s amplitude in the foveal confluence was smaller than in the attended portions of V1, V2, and V3 (0.36 % BOLD versus 0.66, 0.54, and 0.57, respectively).
The amplitude of sustained attention signals was equivalent in areas V1, V2, and V3, and this was true for both attended and peripheral unattended portions. Analysis of variance with subject and cortical area as main factors was performed separately for attended and peripheral unattended portions of early visual cortex. For attended portions, there was a significant effect of subject (p < 0.005) but not for cortical area (p = 0.5). For peripheral unattended portions, neither subject (p = 0.4) nor cortical area (p = 0.6) yielded a significant effect. A similar equivalence of cue-related response amplitudes in areas V1, V2, and V3 was reported by Ress et al. (2000). However, other studies have shown larger amplitude expectancy signals in extrastriate cortex compared to V1 in monkeys (Luck et al. 1997) and humans (O’Connor et al. 2002).
The amount of variance in the fMRI time series that was accounted for by our sustained attention model was computed and compared to an alternative model in which the attention signals were assumed to be transient and time-locked to the auditory cue at the beginning of the delay period. Except for the duration of attention signals, the two models were identical. The model with sustained attention signals consistently fit the observed fMRI time series better than the transient attention model (22 out of 24 ROIs; Table 2). Averaging across both attended and unattended portions of visual cortex and across all three early visual cortical areas, the percent of variance accounted for was 77% (sustained) versus 30% (transient) for subject MAS, 65% versus 15% for DBR, 63% versus 37% for RAS, and 33% versus 3% for JM. We did not determine the statistical significance of the differences between the sustained and transient models because conventional statistical tests require independence of consecutive points in the actual and modeled fMRI time series, an assumption which is not met by these data. However, the sustained attention model clearly fit the observed time courses substantially better than the transient model (Table 2).
Table 2.
Percentage variance accounted for in binned mean fMRI time series by sustained and transient models of attention signals. In the sustained model, the attention signal was assumed to be a step function that began at cue presentation and continued until the end of the response period (as in Figure 3 and Supplementary Figure 2). The transient model assumed an attention signal that was associated with allocation of attention at cue presentation but did not persist through the delay period. The sustained attention model accounted for more variance than the transient attention model for 22 out of 24 subject/cortical area combinations.
| Attended part of visual field
|
Unattended part of visual field
|
|||||
|---|---|---|---|---|---|---|
| Subject | V1 | V2 | V3 | V1 | V2 | V3 |
| MAS - sustained | 76 | 92 | 86 | 74 | 76 | 59 |
| MAS - transient | 16 | 10 | 15 | 49 | 48 | 41 |
| DBR - sustained | 74 | 83 | 85 | 69 | 60 | 21 |
| DBR - transient | 29 | 19 | 20 | 6 | 0.5 | 19 |
| RAS - sustained | 61 | 57 | 74 | 72 | 45 | 67 |
| RAS - transient | 51 | 39 | 38 | 50 | 18 | 25 |
| JM - sustained | 0.1 | 0.3 | 5 | 69 | 68 | 55 |
| JM - transient | 1 | 4 | 0.6 | 6 | 4 | 0.3 |
The durations of sustained neural activity in early visual cortex were also estimated for each trial (see Supplemental Materials and Methods), and the correlations between actual delay-period duration and estimated duration of sustained neural activity were highly statistically significant (Supplemental Figures 4 and 5; Table 3). This relationship was observed for all three visual areas in both attended and unattended visual field representations.
Table 3.
Correlation coefficients relating the estimated duration of neural activity to the actual delay-period duration (interval between beginning of each trial and the end of the behavioral response period). Asterisks indicate a significant positive correlation and evidence for rejecting the null hypothesis of no sustained activity during the delay period
| Attended part of visual field
|
Unattended part of visual field
|
||||||
|---|---|---|---|---|---|---|---|
| Subject | V1 | V2 | V3 | V1 | V2 | V3 | # trials |
| MAS | 0.44*** | 0.55*** | 0.65*** | 0.67*** | 0.57*** | 0.44*** | 145 |
| DBR | 0.27** | 0.48*** | 0.32** | 0.31** | 0.36*** | 0.38*** | 164 |
| RAS | 0.52*** | 0 49*** | 0.43*** | 0 59*** | 0 39*** | 0.48*** | 218 |
| JM | 0.15* | 0.23** | 0.21* | 0.30** | 0.32*** | 0 35*** | 213 |
= p < 0.05;
= p < 0.001;
= p < 1 × 10−5.
Interestingly, the fourth subject in the current study (JM), who did not exhibit significant increases in activity during the delay period, had a pattern of behavioral performance that was unlike the other three subjects. Subject JM had a strong response bias and was much more likely to respond “target absent” than “target present” (Table 1). This subject responded “target absent” for 97% of the trials when no target was shown and for 43% of the trials when a target was actually present. In addition, subject JM’s detection thresholds were approximately 35% higher than the other subjects (Table 1). This combination of conservative response bias and high threshold raises the possibility that subject JM was employing an alternative strategy that did not rely on endogenous, voluntary attention. If visual stimuli are sufficiently salient, they will be detected even if attention is not allocated to the stimulus location at the time of stimulus presentation, a phenomenon known as stimulus-driven, or exogenous attention (reviewed in Yantis 2000). By relying on such exogenous attention mechanisms, subject JM could have correctly detected targets (albeit at a higher contrast than the other subjects) without employing sustained, endogenous attention. This interpretation is also consistent with JM’s conservative response bias, because the false alarm rate would be expected to be extremely low if target detection occurred via exogenous attention mechanisms.
However, this account cannot explain why subject JM exhibited sustained decreases in activity in peripheral early visual cortex. It has been hypothesized that there are two component processes in sustained spatial attention - signal enhancement and noise (distractor) suppression – and that they can be dissociated using psychophysical methods (Lu and Dosher 1998; Carrasco et al 2000; Dosher and Lu 2000; Solomon 2004; Pestilli and Carrasco 2005). One admittedly speculative possibility is that subject JM only engaged processes of noise suppression and not signal enhancement, resulting in sustained decreases in ignored portions of visual cortex but no change in the portions of visual cortex corresponding to the target annulus. Our behavioral data do not permit a dissociation of these possible component processes. Without a more complete description of JM’s behavioral performance in other attention tasks, it is difficult to reconcile this subject’s behavioral and fMRI results with those of the other subjects.
Discussion
We found that early visual cortical areas exhibit increased and sustained fMRI responses during periods of sustained visual spatial attention. These responses corresponded to the allocation of spatial attention required to perform the visual detection task, both spatially and temporally. In the spatial domain, fMRI responses increased for attended and decreased for unattended visual field representations. In the temporal domain, the time courses of activity were well described by a step-function model that assumed that neural activity in early visual cortex paralleled the time course of the attentional demands of the task.
Previous studies of top-down attention signals in early visual cortex
A number of studies have used fMRI to examine the responses to a cue that directs spatial attention to a particular visual field location (Kastner et al. 1999; Corbetta et al. 2000; 2002; 2005; Hopfinger et al. 2000; Ress et al. 2000; Müller and Kleinschmidt 2003; Müller et al. 2003; Müller and Kleinschmidt 2004; Serences et al. 2004). These studies employed either a fixed interval between cue and target presentation or a limited range of cue-target intervals. The use of a small range of cue-target intervals introduces two difficulties in estimating the time course of attention signals. First, the hemodynamic response is sluggish and highly variable across subjects (Aguirre et al. 1998). This makes it difficult to accurately estimate the time course of neural activity of an interval of fixed duration unless the hemodynamic response function is measured precisely for each individual subject. Second, if subjects were aware that there were a small number of cue-target intervals, they may have been able to perform the task without maintaining attention continuously throughout the delay period.
Our current results go beyond previous studies in showing that retinotopically-specific activity is maintained in early visual cortex throughout a period of sustained visuospatial attention. By fully randomizing the delay-period durations over a wide range (2–16 seconds), we obtained a correlation between delay-period duration and estimated duration of sustained neural activity for individual subjects. In addition, the cue that resulted in the allocation of attention was an auditory stimulus, and there was no visual stimulation during the delay period. This allowed the isolation of attention signals during the delay period without contribution from visually-evoked responses related to the cue or target. Finally, the task was designed to require sustained attention throughout the delay period.
Yantis et al. (2002) compared fMRI responses associated with shifting versus maintenance of visual spatial attention during performance of a rapid serial visual presentation (RSVP) task. The superior parietal lobule exhibited bilateral transient increases in cortical activity associated with shifts of attention, while bilateral extrastriate cortex and left IPS displayed retinotopically-specific persistent activity during periods of sustained attention. These results are generally similar to our observations in extrastriate cortex. However, we observed sustained delay-period activity in V1 as well as extrastriate cortex. These delay-period responses were evident even in the complete absence of visual stimulation, unlike the activity reported by Yantis et al. (2002), which was due to attentional modulation of visual responses to a continuously changing stimulus. In addition, we performed gray-matter cortical segmentation and retinotopic mapping to define cortical areas in early visual cortex. This allowed us to determine the time courses of the attention signals separately for each of these areas and to subdivide early visual cortical areas into attended and unattended visual field representations.
A large number of studies have examined the effects of top-down attention on single-unit responses to visual stimuli in monkeys. The electrophysiological measurement most similar to the delay-period activity described in the present study is a baseline shift in activity during the interval between cue and target presentation. An increase in activity during this delay period relative to the spontaneous firing rate has been described in the lateral intraparietal area (LIP) (Colby et al. 1996; Bisley and Goldberg 2003) and in extrastriate cortex (Haenny et al. 1988; Luck et al. 1997; Reynolds et al. 1999). Although attention has been shown to modulate the gain of stimulus-evoked neural responses in V1 (McAdams and Maunsell 1999; McAdams and Reid 2005; Mehta et al. 2000; Motter 1993), the increases in baseline firing rates reported in extrastriate cortex have not been found in cortical area V1 (Luck et al. 1997; Mehta et al. 2000). By contrast, clear evidence of cue-related activity, with little or no additional visual stimulation, has been obtained in humans with fMRI in portions of V1 corresponding to the attended visual field (Kastner et al. 1999; Ress et al. 2000; Müller and Kleinschmidt 2003; Müller et al. 2003; Müller and Kleinschmidt 2004; Serences et al. 2004). A discussion of some of the possible explanations of discrepancies between the monkey electrophysiology and human neuroimaging data regarding attention signals in V1 can be found in Ress et al. (2000).
Spatial specificity of attention signals
In addition to sustained fMRI responses in attended portions of early visual cortex, we also observed sustained decreases in fMRI activity in unattended portions of these same cortical areas. Similar reductions in cortical activity due to withdrawal of attention have been observed for spatial attention using event-related potentials (Luck et al. 1994) and fMRI (Tootell et al. 1998; Somers et al. 1999; Smith et al. 2000; Slotnick et al. 2003; Müller and Kleinschmidt 2004). This decrease in activity is unlikely to be due to central portions of early visual cortex “stealing” blood from the neighboring peripheral regions, as similar decreases have been observed in the hemisphere contralateral to the hemisphere exhibiting the increase in attention-related activity (Tootell et al. 1998; Müller and Kleinschmidt 2004). In addition, decreases in fMRI visual responses (so-called “negative BOLD”) have been associated with decreases in neural firing rates in primary visual cortex (Shmuel et al. 2006).
The sustained decreases in activity parallel behavioral results demonstrating improved visual target detection at cued locations but diminished performance at remote locations (e.g., Posner et al. 1980; Bashinski and Bacharach 1980). Hence, the sustained decreases in fMRI activity in the present study might be causally related to changes in behavioral performance by suppressing irrelevant neural signals corresponding to peripheral visual field locations. A different possibility is that spatial attention may have been distributed over a large portion of the visual field during the intertrial interval, while during the delay period, attention was focused on the part of the visual field corresponding to the target to be detected. Therefore, at the beginning of each trial, attention would have been allocated to the target location and removed from the peripheral regions far from the target, giving rise to sustained increases and decreases in cortical activity relative to the amount of activity during the intertrial interval. It should be noted that Subject JM’s results are inconsistent with this model of redistribution of spatial attention. This subject displayed sustained decreases in peripheral visual cortex but no sustained increases in areas corresponding to the location of the target annulus.
Analysis of attention signals in the foveal confluence, which represents central visual field locations within the inner boundary of the attended annulus, generally did not show sustained positive or negative responses. Presumably, these visual field locations were far enough away from the attended portion of the visual field that they did not display sustained positive signals, but they were not sufficiently far from the attended region to exhibit sustained decreases in activity during the delay period.
Similarities between attention and imagery
A possible alternative interpretation of our results concerns mental imagery. Subjects in our experiment maintained attention during a delay period while anticipating a threshold-contrast target. Because subjects practiced the visual detection task extensively before the collection of any fMRI data, each subject developed a perceptual template that represented the appearance, size, and location of the target. One possible task strategy for target detection would have been to continuously compare the visual input during the delay period to this template. This process of recalling a visual memory in the absence of visual stimulation is a form of visual mental imagery. Some component of the delay-period activity observed in the present study may have been due to visual imagery of the target, at least for the three subjects that exhibited sustained increases in fMRI responses during the delay period. Visual imagery has been shown to increase activity in early visual cortex, including V1 (reviewed in Kosslyn and Thompson 2003). Like the delay-period activity described in the present study, activity in early visual cortex evoked by visual imagery has been reported to be retinotopically-specific: imagery of small objects increased activity in central visual field representations, while imagery of large objects increased activity in more peripheral representations (Kosslyn et al. 1995). Additionally, direct comparisons of retinotopic maps of early visual cortex obtained using visual stimulation, visual imagery, and spatial attention have been reported to be in close correspondence (Klein et al. 2004; Slotnick et al. 2005). Others, however, have failed to find activity in early visual areas during mental imagery (Roland and Gulyás 1994; D’Esposito et al. 1997; Mellet et al. 1998; Ishai et al. 2000). Finally, we observed large individual differences in sustained attention signal amplitudes (Figure 3), and substantial individual differences in behavior and patterns of brain activity across multiple visual imagery tasks have been reported (Ganis et al. 2005). Further experiments will, therefore, need to be performed to determine the possible contribution of mental imagery to our experimental results.
Conclusions
What is the function of sustained delay-period activity in early visual cortex, and what is its impact on performance? Signal detection theory offers a framework for understanding how increases in the relevant neuronal signals can lead to improved performance (e.g., Palmer et al., 2000). Accuracy is improved by boosting (via sustained increases in activity) the relevant neuronal signals (e.g., from neurons with receptive fields that overlap the stimulus aperture) relative to other signals (e.g., from neurons with receptive fields outside the stimulus aperture), which contribute only noise to the detection process. Likewise, accuracy is improved by suppressing (via sustained decreases in activity) irrelevant neuronal signals. Selecting the responses of relevant sensory neurons and/or suppressing the responses of irrelevant sensory neurons, therefore, facilitates the decision process.
Changes in activity due to attention could reflect either of two mechanisms that we will term the neuronal hypothesis and the hemodynamic hypothesis. According to the neuronal hypothesis, the observed fMRI responses would correspond to alterations in spike rates and/or subthreshold synaptic activity. Increases in baseline firing rates have been reported to occur following allocation of spatial attention in extrastriate cortex, but not in V1 (Haenny et al. 1988; Luck et al. 1997; Mehta et al. 2000; Reynolds et al. 1999). However, attention has been reported to increase the gain of sensory-evoked responses in V1 (McAdams and Maunsell 1999; McAdams and Reid 2005; Metha et al. 2000; Motter 1993), suggesting that subthreshold membrane depolarization can occur even in the absence of increases in baseline firing rates. The neuronal hypothesis addresses a puzzling question about attention: Why not deploy attention everywhere all the time? According to this hypothesis, attention improves target detection by boosting the relevant neural signals corresponding to attended locations and possibly by suppressing neural activity corresponding to unattended regions.
The hemodynamic hypothesis posits that during a state of sustained attention, the brain responds by increasing the flow of oxygenated blood selectively to regions where it may be needed in anticipation of future metabolic demand. Hemodynamic responses could increase during delay periods with little concurrent change in spiking or subthreshold synaptic activity. For example, the hemodynamic responses could be mediated by a small subpopulation of neurons. The resulting fMRI responses would then be only indirectly related to the behavioral performance improvements associated with attention. Even so, it is worth considering the possibility that attentional processing (which anticipates future metabolic demand) may be more accurately assessed with fMRI (which reflects changes in metabolic supply) than with electrophysiological measurements.
Supplementary Material
Acknowledgments
The authors thank J. Larsson for gray matter segmentation and cortical flattening software.
Grants
This research was supported by National Eye Institute Grant RO1 EY-11794 and National Research Service Award F32 EY-14520.
References
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Res Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bashinski HS, Bacharach VR. Enhancement of perceptual sensitivity as the result of selectively attending to spatial locations. Percept Psychophys. 1980;28:241–248. doi: 10.3758/bf03204380. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Eckstein M. Spatial covert attention increases contrast sensitivity across the CSF: support for ignal enhancement. Vision Res. 40:1203–1215. doi: 10.1016/s0042-6989(00)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel J-R, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationship to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Tansy AP, Stanley CM, Astaflev SV, Snyder AZ, Shulman GL. A functional MRI study of preparatory signals for spatial location and objects. Neuropsychologia. 2005;43:2041–2056. doi: 10.1016/j.neuropsychologia.2005.03.020. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippet LJ, Farah MJ. A functional MRI study of mental image generation. Neuropsychologia. 1997;35:725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu Z-L. Noise exclusion in spatial attention. Psychol Sci. 11:139–146. doi: 10.1111/1467-9280.00229. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky E-J, Shadlen MN. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM. Understanding the effects of task-specific practice in the brain: Insights from individual-differences analysis. Cogn Affect Behav Neurosci. 2005;5:235–245. doi: 10.3758/cabn.5.2.235. [DOI] [PubMed] [Google Scholar]
- Glover GH. Simple analytic spiral k-space algorithm. Magn Res Med. 1999;42:412–415. doi: 10.1002/(sici)1522-2594(199908)42:2<412::aid-mrm25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Res Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Haenny PE, Maunsell JHR, Schiller PH. State dependent activity in monkey visual cortex. II. Retinal and extraretinal factors in V4. Exp Brain Res. 1988;69:245–259. doi: 10.1007/BF00247570. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28:979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Jack AI, Shulman GL, Snyder AZ, McAvoy M, Corbetta M. Separate modulations of human VI associated with spatial attention and task structure. Neuron. 51:135–147. doi: 10.1016/j.neuron.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Klein I, Dubois J, Mangin J-F, Kherif F, Flandin G, Foline J-B, Denis M, Kosslyn SM, Le Bihan D. Retinotopic organization of visual mental images as revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2004;22:26–31. doi: 10.1016/j.cogbrainres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL. When is early visual cortex activated during visual mental imagery? Psychol Bull. 2003;129:723–746. doi: 10.1037/0033-2909.129.5.723. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Kim IJ, Alpert NM. Topographical representations of mental images in primary visual cortex. Nature. 1995;378:496–498. doi: 10.1038/378496a0. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Dosher BA. External noise distinguishes attention mechanisms. Vision Res. 38:1183–1198. doi: 10.1016/s0042-6989(97)00273-3. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. J Exp Psychol: HPP. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. I. Distribution and timing of effects across visual areas. Cereb Cortex. 2000;10:343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- Mellet E, Petit L, Mazoyer B, Denis M, Tzourio N. Reopening the mental imagery debate: Lessons from functional anatomy. Neuroimage. 1998;8:129–139. doi: 10.1006/nimg.1998.0355. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Müller NG, Bartelt OA, Donner TH, Villringer A, Brandt SA. A physiological correlate of the “zoom lens” of visual attention. J Neurosci. 2003;23:3561–3565. doi: 10.1523/JNEUROSCI.23-09-03561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller NG, Kleinschmidt A. Dynamic interaction of object- and space-based attention in retinotopic visual areas. J Neurosci. 2003;23:9812–9816. doi: 10.1523/JNEUROSCI.23-30-09812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller NG, Kleinschmidt A. The attentional ‘spotlight’s’ penumbra: center-surround modulation in striate cortex. Neuroreport. 2004;15:977–980. doi: 10.1097/00001756-200404290-00009. [DOI] [PubMed] [Google Scholar]
- Nestares O, Heeger DJ. Robust multiresolution alignment of MRI brain volumes. Magn Res Med. 2000;43:705–715. doi: 10.1002/(sici)1522-2594(200005)43:5<705::aid-mrm13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Offen S, Schluppeck D, Heeger DJ. The role of human V1 in working memory and attention. Soc Neurosci Abstr. 2005;286:13. [Google Scholar]
- Palmer J, Verghese P, Pavel M. The psychophysics of visual search. Vision Res. 40:1227–1268. doi: 10.1016/s0042-6989(99)00244-8. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The Video Toolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Pestilli F, Carrasco M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Res. 45:1867–1875. doi: 10.1016/j.visres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. J Exp Psychol: Gen. 1980;109:160–174. [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, Gulyás B. Visual imagery and visual representation. TINS. 1994;17:281–287. doi: 10.1016/0166-2236(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S, Culberson A, Awh E. Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. J Neurophysiol. 2004;92:3538–3545. doi: 10.1152/jn.00435.2004. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RBH. Borders of multiple visual areas in humans revealed by functional MRI. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. J Neurosci. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Tansy AP, Kincade M, Petersen SE, McAvoy MP, Corbetta M. Reactivation of networks involved in preparatory states. Cereb Cortex. 2002;12:590–600. doi: 10.1093/cercor/12.6.590. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. Neuroimage. 2003;19:1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Thompson WL, Kosslyn SM. Visual mental imagery induces retinotopically organized activation of early visual areas. Cereb Cortex. 2005;15:1570–1583. doi: 10.1093/cercor/bhi035. [DOI] [PubMed] [Google Scholar]
- Smith AM, Lewis BK, Ruttimann UE, Ye FQ, Sinnwell TM, Yang Y, Duyn JH, Frank JA. Investigation of low frequency drift in fMRI signal. Neuroimage. 1999;9:526–533. doi: 10.1006/nimg.1999.0435. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW. Attentional suppression of activity in the human visual cortex. Neuroreport. 2000;11:271–277. doi: 10.1097/00001756-200002070-00010. [DOI] [PubMed] [Google Scholar]
- Solomon JA. The effect of spatial cues on visual sensitivity. Vision Res. 44:1209–1216. doi: 10.1016/j.visres.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RBH. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RBH, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Yantis S. Goal-directed and stimulus-driven determinants of attentional control. In: Monsell S, Driver J, editors. Attention and Performance XVIII. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D’Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions. Neuroimage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


