Abstract
Following combustion of fuel containing the additive methylcyclopentadienyl-manganese-tricarbonyl (MMT), manganese phosphate (MnPO4) and manganese sulfate (MnSO4) are emitted in the atmosphere. Manganese chloride (MnCl2), another Mn2+ species, is widely used experimentally. Using rat striatal slices, we found that MnPO4 decreased tissue and media dopamine (DA) and media Dopac (a DA metabolite) levels substantially more than either MnCl2 or MnSO4; antioxidants were partially protective. Also, both MnCl2 and MnPO4 (more potently) oxidized DA and Dopac even in the absence of tissue in the media, suggesting a direct interaction between Mn and DA/Dopac. Because aminochrome is a major oxidation product of DA, we next determined whether MnPO4 will be more potent in forming aminochrome than MnCl2 or MnSO4 which, indeed, was the case. Thus, a potential additional mechanism for the neurotoxic effects of environmentally-relevant forms of Mn, MnPO4 in particular, is the generation of reactive DA intermediates.
Keywords: Manganese, MMT, Dopamine, Aminochrome
1. Introduction
Manganese (Mn) is one of the most common elements in the earth’s crust and an essential metal present in several dietary sources including nuts, grains, and tea (Jankovic, 2005). The recommended dietary intake for Mn is 2.3 and 1.8 mg/day for men and women, respectively. Critical enzymes, such as manganese superoxide dismutase (Mn-SOD) and glutamine synthetase, contain Mn in their structure which is essential for their functions (Wedler et al., 1982).
Although Mn intake is necessary to maintain life, exposure to excessive amounts of this transition metal has been associated with various adverse outcomes. The chief sources of airborne Mn are industrial emissions associated with ferroalloy production, iron and steel foundries, coke ovens and power plant combustion emissions (Lioy, 1983). Occupational exposure to Mn-containing dust is associated with adverse respiratory, reproductive, and, importantly, neurological effects (Iregren, 1999).
Excessive Mn exposure in the brain results in a condition known as manganism, which bears many similarities to Parkinson’s disease. Symptoms of manganism include gait imbalance, rigidity, tremors, dystonia, and bradykinesia (Barbeau et al., 1976). One of the earliest documented cases of excessive Mn exposure causing neurological deficits involved a group of Chilean miners. The symptoms that these miners developed were termed locally as “locura manganica” or manganic madness (Mena et al., 1967). Following exposure, the main areas of the mammalian brain targeted by excessive Mn neurotoxicity are the basal ganglia (caudate, putamen, globus pallidus, substantia nigra, and subthalamic nucleus; Calne et al., 1994; Eriksson et al., 1992; Nagatomo et al., 1999; Brenneman et al., 1999). Of these regions, the globus pallidus appears to be affected the most, a feature of Mn neurotoxicity that is distinct from Parkinsonism (Verity, 1999). Nevertheless, studies show that dopamine (DA), the principal neurotransmitter that is severely reduced in the striatum of Parkinson’s disease patients, is also reduced by Mn both in vivo (Parenti et al., 1986) and in vitro (Vescovi et al., 1991) exposure paradigms. This reduction of DA may be due, at least in part, to direct oxidation of this neurotransmitter by Mn (Donaldson et al., 1982).
Levels of Mn in the brain that are associated with basal ganglia toxicity vary from around 10 to around 350 μM (Yase 1972, Bird et al. 1984, Pomier-Layrargues et al. 1995, Suzuki et al. 1975). For example, non-human primates exposed to Mn (as manganese dioxide) for 3 months had brain Mn concentrations between 35 and 350 μM (Suzuki et al. 1975), whereas a two-year inhalation exposure or rhesus monkeys to occupationally-relevant concentrations of Mn, caused elevated concentrations of Mn in the putamen and the globus pallidus that reached 60 μM (Bird et al. 1984). Moreover, elevated levels of Mn in the basal ganglia are not associated only with occupational exposure, but have been also observed in the brains of PD patients (Yase 1972), as well as in autopsied brains of patients with cirrhosis (Pomier-Layrargues et al. 1995).
Most studies performed with manganese, including ones studying Mn neurotoxicity, have primarily used MnCl2 and, to a lesser extent, MnSO4 salts (Brouillet et al., 1993; Zheng et al., 1998; Brenneman et al., 1999; Erikson et al., 2005). Both of these manganese species are in the Mn2+ state, but Mn neurotoxicity has also been observed with Mn3+ species of Mn, another oxidation state at which this metal can exist (HaMai et al., 2001; Archibald and Tyree, 1987; Ali et al., 1995). Moreover, earlier studies have indicated that Mn3+ is more toxic to the nervous system, including the basal ganglia (Ali et al., 1995). However, the cellular compartment where most Mn accumulates following excessive exposure are the mitochondria with recent findings indicating that Mn present in the brain mitochondria is primarily in the Mn2+ state (Gunter et al., 2004).
Industrial emissions are a major source of airborne Mn, yet, another potential source of Mn in the atmosphere is the use of methylcyclopentadienyl-manganese-tricarbonyl (MMT) as a fuel additive (Loranger and Zayed, 1997). MMT functions as an anti-knock agent by raising the octane of gasoline (Hollrah and Roos, 1997) and is emitted into the atmosphere via automobile exhaust (Vitarella et al., 2000). When DA-producing PC12 cells were exposed MMT, a concentration-dependent decrease in cell viability was observed (Kitazawa et al., 2002). However, when used as a fuel additive, MMT’s main combustion products include manganese phosphate, manganese sulfate, and lesser amounts of manganese tetroxide, with the first two Mn species existing in the Mn2+ oxidation state and Mn3O4 being in the Mn3+ state (Lynam et al., 1999; Vitarella et al., 2000).
To study the MMT-relevant forms of Mn, the pharmacokinetics of inhaled manganese phosphate (as the hureaulite, (Mn5(PO)4)2[(PO3)(OH)]2·4H2O; Mn2+ valence state) have been studied in rats (Vitarella et al., 2000). Besides its commercial availability, hureaulite was chosen for study due to the fact that the MnPO4 emitted during MMT combustion exists in a hydrated form that is similar to the hureaulite (Ressler et al., 1999). Following inhalation exposure to MnPO4, elevated levels of Mn were found in the brain, lungs, and skeletal muscle. However, the levels of Mn in the brain, while greater in the striatum, were not confined to specific brain regions based on the fact that the cerebellum, which is not a known target, also had elevated Mn levels (Vitarella et al., 2000). On the other hand, when rats were exposed to inhaled MnSO4, there was an accumulation of Mn in the olfactory bulb and striatum, while the Mn concentration in the cerebellum remained unchanged (Dobson et al., 2003). These findings suggest that the major MMT combustion product, MnPO4 gains entrance to the brain less-selectively and less efficiently than the other main MMT combustion product, MnSO4.
Because of the fact that one of the targets of excessive Mn exposure appears to be perturbation of striatal DA homeostasis, and due to the lack of data with the MMT combustion products MnPO4 and MnSO4, the objective of the present study was to compare the effects of MnPO4 and MnSO4 on striatal DA metabolism. MnCl2 was also included in the comparisons since it is the Mn2+ species most commonly used in research. Furthermore, by using various pharmacological manipulations, potential mechanism(s) of the effects of Mn on DA neurochemistry were studied.
2. Materials and methods
2.1 Chemicals
Manganese (Mn2+) phosphate, in the mineral form hureaulite (Mn5(PO)4)2[(PO3)(OH)]2 ·4H2O) was acquired from Alfa Aesar (Ward Hill, MA). The material, which is a fine crystalline reddish-white powder, is 37.7% elemental Mn by weight. Manganese (Mn2+) sulfate (MnSO4·H2O) and ethylene diamine tetraacetic acid (EDTA) were obtained from Fisher Scientific (Houston, TX). Zinc Phosphate (Zn3[PO4]2·4H2O), was acquired from ChemService Inc. (West Chester, PA). All other chemicals, including MnCl2 (Mn2+), were of the highest quality available and were purchased from Sigma-Aldrich Company (Saint Louis, MO) unless stated otherwise in the text.
2.2 Preparation of working exposure solutions
Stock 100 mM solutions of manganese chloride (MnCl2) and manganese sulfate (MnSO4) were prepared in ddH2O. Stock 250 mM solutions of manganese phosphate (MnPO4 hureaulite), zinc phosphate (ZnPO4), and cobalt phosphate (CoPO4) were prepared in concentrated HCl (12 M). These stocks were diluted to the actual working concentrations in HEPES buffered Hank’s saline solution (HBHS), pH 7.4, supplemented with 1% v/v horse serum (1% HBHS). In the case of MnPO4, ZnPO4, and CoPO4, the highest working concentration solution was adjusted to pH 7.4 with 2N NaOH. Vehicle controls contained the appropriate amount of either ddH2O or NaOH-buffered HCl. In preliminary experiments, we determined that 200 μM working solutions of MnPO4 precipitate after pH adjustment and a 4 h exposure duration. Therefore, 100μM was the highest working concentration of MnPO4, ZnPO4, and CoPO4 used in the actual experiments.
2.3 Preparation of pharmacological reagents
Stock solutions of (1) reduced L-glutathione (GSH), a major cellular antioxidant known to be depleted in the presence of Mn (Stredrick et al., 2004); (2) N-acetyl-L-cysteine (NAC), a GSH precursor and antioxidant reported to prevent apoptosis in neuronal cells (Ferrari et al., 1995); (3) catalase (CAT) and (4) superoxide dismutase (SOD), two major antioxidant enzymes which protect cells against oxidative damage (Rossman and Goncharova, 1998), were prepared in saline or HBHS. Final exposure working solutions were diluted in 1% HBHS. Working concentrations were: GSH, 1mM; NAC, 1mM; and SOD + CAT, 500 U/ml + 1000 U/ml, respectively. In addition, the metal chelators EDTA and ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), (2mM each) and desferioxamine mesylate (DES, up to 100 μM), were also prepared. Furthermore, 20 μM working concentration of 3-hydroxybenzylhydrazine dihydrochloride (NSD-1015), which is an inhibitor of aromatic amino acid decarboxylase (AAAD, the enzyme responsible for converting L-DOPA to DA) was prepared in 1% HBHS.
2.4 Striatal tissue experiments
Animals
Charles River Sprague Dawley derived Crl-CD(SD)BR rats, 2.5–4 months old, were housed (3–4/cage) on a 12 hr light/dark cycle with food and water available ad libitum. Four or six rats/day were euthanized with CO2 and then rapidly decapitated in accordance with a procedure approved by the Mississippi State University Institutional Animal Care and Use Committee.
Striatal tissue collection and exposure
The whole brain was immediately extracted and washed with ice-cold HBHS. A cross-sectional cut at the level of the hypothalamus was made and the rostral portion was placed in ice-cold HBHS. Several 350-μM thick slices containing the striatum were collected, processed, and prepared for Mn exposure according to Chishti et al. (1996) and Bemis and Seegal (1999). In all cases, striatal slices were incubated in 24-well plates containing 500 μl of 1% HBHS/well and the indicated working concentrations of Mn. Slices were incubated under an atmosphere of 95% O2: 5% CO2 in a Dubnoff metabolic water bath at 37 ºC for a duration of 4 h as described previously (Filipov et al., 2005). The experiments described herein involve working concentrations up to 1000 μM for MnCl2 and MnSO4, and up to 100 μM for MnPO4. The concentrations used in these studies are in line with many other experiments, i.e., Roth et al., (2000) and are representative of levels found in brains of non-human primates following exposure to manganese for 3 months (ranging from 35 to 350 μM; Suzuki et al., 1975).
In cases where a pharmacological agent was used in conjunction with Mn exposure, the agent(s) was added 30 min prior to the addition of Mn to the slices. Following exposure, slices and media were analyzed by high-performance liquid chromatography (HPLC) for DA and its metabolite 3,4-dihydroxyphenylacetic acid (Dopac) as described below.
2.5 Experiments involving direct exposure of Dopac/DA to Mn
Dopac/DA depletion by Mn
The general experimental set up was similar to the one involving striatal tissue. However, in lieu of the tissue, a mixture of Dopac/DA (10 ng each in the case of the experiments involving EDTA and EGTA; 2 ng each in the experiments with desferioxamine) was added to 500 μl of 1% HBHS in each well. Exposure duration was either 4 h (EDTA/EGTA experiments) or 1 h (desferioxamine experiments). Pharmacological agents were added 30 min prior to the addition of Mn. Because all tissue experiments were carried out under an atmosphere of 95% O2/5% CO2, some of the experiments involving only media were, for comparative reasons, performed under the same conditions or under ambient air. At the lower doses of MnPO4, we obtained virtually identical results, regardless of the amount of O2 present during the incubation. HPLC analysis for DA and Dopac was performed as described below.
Dopamine oxidation to aminochrome
Known concentrations of DA (25 μM, 250 μM, and 2500 μM) were incubated with a dose range of MnPO4 (1 μM, 10 μM, 100 μM), or with fixed concentrations of MnPO4, MnCl2, or MnSO4 (100 μM each) in 1% HBHS at 37 ºC for up to 60 min. Aminochrome formation was monitored using a SLM Aminco DW2000 dual-beam spectrophotometer (SLM Instruments, Inc., Urbana, IL, USA) set to 480 nm according to (Donaldson, 1987).
2.6 HPLC analysis
Striatal slices were collected in 1.5-ml tubes to which 100 μl of 0.2N perchloric acid (PA) was added. Samples were sonicated and subsequently centrifuged. Media samples were collected by adding 100 μl of media to 100 μl of 0.4N PA and centrifuged. Twenty μl of striatal tissue supernatant and 50 μl of media supernatant were analyzed using an HPLC equipped with an electrochemical detector (Waters Corp., Milford, MA, USA). The system consists of Waters 2695 Separations Module and a Waters 2465 Electrochemical Detector (set at a range of 20 nA) interfaced with Empower® software. The system was fitted with a Supelcosil ™ LC-18-DB HPLC column maintained at 35 °C. The chromatographic conditions were similar to those already reported (Seegal et al., 1986). An isocratic flow rate of 1 ml/min and a mobile phase consisting of 84 mM of NaH2PO4H2O, 1.15 mM of octyl sodium sulfate, 0.09 mM of EDTA disodium salt, 0.25 mM of triethylamine, and 17.5 % v/v methanol which was adjusted to a pH of 3.65 with 5M H3PO4. Both tissue and media samples were analyzed for Dopac and DA. In certain experiments, media levels of the other DA metabolite, homovanillic acid (HVA), were also determined. In experiments involving the direct addition of Dopac/DA to the exposure medium, the samples for HPLC analysis were treated in the same manner as media samples collected from the striatal slice experiments.
2.7 Protein analysis
Protein content in the tissues was determined by the Bradford method using bovine serum albumin (BSA) as a standard. For the protein analysis, samples were digested with NaOH as described in detail previously (Filipov et al., 2005).
2.8 Tissue viability
In order to ensure that the striatal tissue was viable during our experiments and to determine the effect of Mn exposure on tissue viability, release of lactate dehydrogenase (LDH) into the medium was measured as described previously (Filipov et al., 2005). Data were expressed as LDH activity units/liter (U/L).
2.9 Statistical analysis
Data were analyzed by analysis of variance (ANOVA). Each experiment was performed at least twice and each exposure condition was replicated within an experiment at least three times. Data from identical independent experiments were pooled because initial ANOVA analyses revealed that the data obtained in each experiment were not significantly different (p > 0.5). When ANOVA p-values were ≤ 0.05, means were separated with the Student-Newman-Keul’s (SNK) multiple comparison post hoc test. For graphical presentation, all data were expressed as the percent of their appropriate control values.
3. Results
3.1 Effects of MnCl2, MnSO4, and MnPO4 on tissue viability
As a first step, we sought to determine whether exposure to three different Mn compounds (MnCl2, MnSO4, and MnPO4) would compromise the viability of the striatal tissue. Striatal slices from male Sprague Dawley rats were collected and incubated for 4 h in either control medium or in medium containing MnCl2 (10, 100, and 1000 μM), MnSO4 (10, 100, and 1000 μM), and MnPO4 (1, 10, 100 μM). In order to assess tissue viability, media LDH levels were determined at the end of the incubation period. LDH levels remained stable across the various treatment groups with the exception of a small but significant (P ≤ 0.05; 23%) increase at the 100μM MnPO4 level (Fig. 1).
Figure 1.

Effects of exposure to different manganese compounds on LDH release from rat striatal slices. Striatal slices from male Sprague Dawley rats were collected and incubated for 4 h either in control medium or in medium containing MnCl2 (10, 100, 1000 μM), MnSO4 (10, 100, 1000 μM), or MnPO4 (1, 10, 100 μM). LDH levels in the exposure medium were measured using a UV spectrophotometric assay. Data are expressed in U/L. *Presence of an asterisk indicates significant difference (P ≤ 0.05).
3.2 Effects of MnCl2, MnSO4, and MnPO4 on tissue and media DA and Dopac levels
While Mn exposure did not affect tissue viability, the levels of DA and its metabolite Dopac were significantly decreased by the end of the exposure period. As shown in Figure 2, 4-h incubations with MnCl2 (10, 100, and 1000 μM), MnSO4 (10, 100, and 1000 μM), and MnPO4 (1, 10, 100 μM) caused a dose-dependent decrease (P ≤ 0.05) in tissue levels of DA (Fig. 2a), media levels of DA (Fig. 2b), and media levels of Dopac, which was affected the most (Fig. 2c). At the highest (1 mM) concentrations, the effects of MnCl2 and MnSO4 were comparable, but at the 100 μM MnCl2 was moderately more potent than MnSO4. MnPO4, on the other hand, was markedly more potent than either MnCl2 or MnSO4. For example, media DA levels fell to 33% and 14% of control values at 1 and 100 μM MnPO4, respectively. Similarly, media Dopac levels were reduced to 60% of control by 1 μM MnPO4 and nearly totally depleted at 100 μM MnPO4. We also determined effects of Mn exposure on the other DA metabolite, HVA. Contrary to the significant effects of Mn on DA and Dopac, HVA levels in the medium were not affected by Mn (P > 0.75). For example, media HVA levels in slices exposed to 100 μM MnPO4 and 1 mM MnCl2 were 96.8% and 98.1% of their respective controls.
Figure 2.

Effects of exposure to different manganese compounds on striatal DA and Dopac levels. Striatal slices from male Sprague Dawley rats were collected and incubated for 4 h either in control medium or in medium containing MnCl2 (10, 100, 1000 μM), MnSO4 (10, 100, and 1000 μM), or MnPO4 (1, 10, 100 μM). Tissue DA (Fig. 2a), media DA (Fig. 2b), and media Dopac (Fig. 2c) levels were analyzed by HPLC-ECD and the data was subsequently normalized on a per mg of protein basis and expressed as a percent of vehicle control. *, **, *** Presence and number of asterisks indicate significant differences (P ≤ 0.05).
Figure 4.
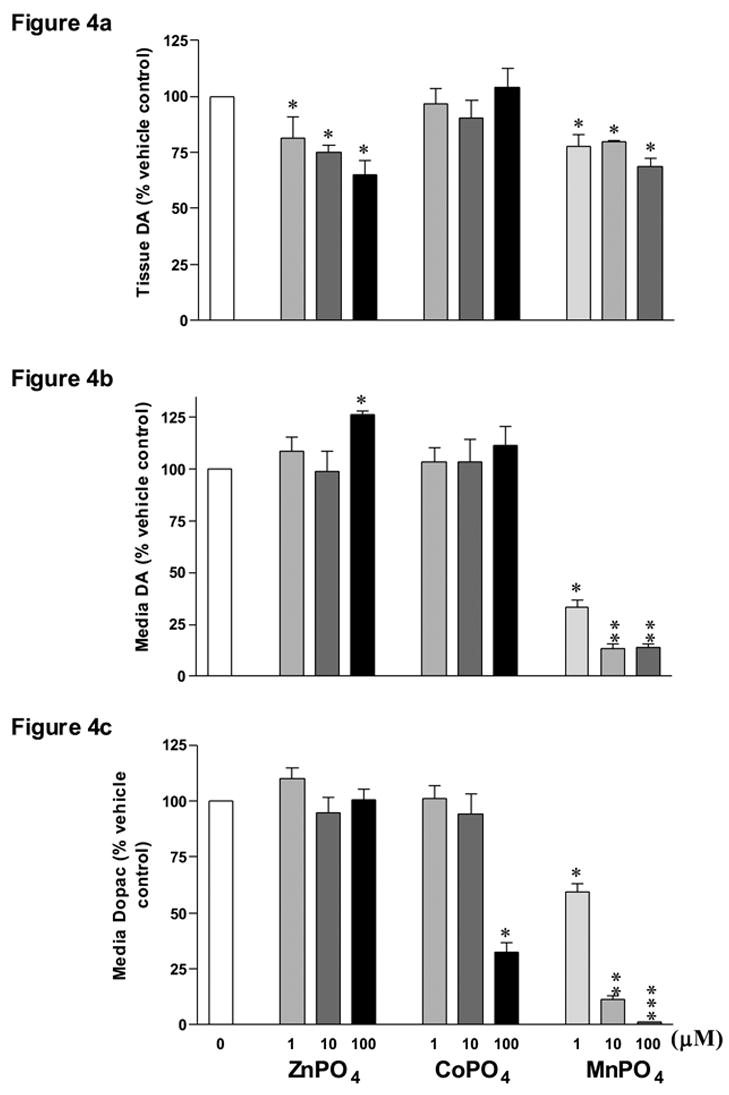
Comparison of the effects of MnPO4 on striatal DA and Dopac levels with the effects of cobalt and zinc phosphate. Striatal slices from male Sprague Dawley rats were collected and incubated for 4 h either in control medium or in medium containing MnPO4, ZnPO4, or CoPO4 at 1, 10, or 100 μM concentrations each. Tissue levels of DA (Fig. 4a), media levels of DA (Fig. 4b) and media levels of Dopac (Fig. 4c) were analyzed by HPLC-ECD and the data was subsequently normalized on a per mg of protein basis and expressed as a percent of vehicle control. *, **, *** Presence and number of asterisks indicate significant differences (P ≤ 0.05).
3.3 Effects of MnCl2, MnSO4, and MnPO4 on L-DOPA
In order to determine the effects of Mn on L-DOPA, we next exposed striatal slices to the three Mn compounds in the presence of NSD-1015, an AAAD inhibitor that prevents conversion of L-DOPA to DA. Total levels of L-DOPA were markedly and dose-dependently (P ≤ 0.05) decreased by all three Mn compounds with MnPO4 again being the most potent (Fig. 3).
Figure 3.

Effects of exposure to different manganese compounds on striatal L-DOPA levels. Striatal slices from male Sprague Dawley rats were collected and incubated for 4 h either in control medium or in medium containing MnCl2 (10, 100, 1000 μM), MnSO4 (10, 100, 1000 μM), or MnPO4 (1, 10, 100 μM). A 20 μM of the AAAD inhibitor NSD-1015 was added 30 min prior to te addition of Mn. Total L-DOPA levels were analyzed by HPLC-ECD and the data was subsequently normalized on a per mg of protein basis and expressed as a percent of vehicle control. *, **, *** Presence and number of asterisks indicate significant differences (P ≤ 0.05).
3.4 Comparison of the effects of MnPO4 on tissue and media DA and Dopac with the effects of ZnPO4 and CoPO4
To determine if the greater potency of MnPO4 was due to the presence of the PO4 moiety, in the next set of experiments we incubated striatal tissue punches with MnPO4, ZnPO4, or CoPO4 (Zn and Co have ionic radii similar to Mn) at 1, 10, and 100 μM concentrations. While MnPO4 decreased (P ≤ 0.05) tissue DA (Fig. 4a), media DA (Fig. 4b), and media Dopac (Fig. 4c), the only significant effect of CoPO4 was a decrease of media Dopac levels at the 100μM dose (P ≤ 0.05). ZnPO4, however, did not affect media Dopac, but it did decrease tissue DA at all dose levels (P ≤ 0.05) and elevated media DA levels at the 100μM dose (P ≤ 0.05). The latter (100μM ZnPO4) was also associated with a 33% increase in media LDH levels, indicating a cytotoxic effect (data not shown).
3.5 Effect of antioxidant supplementation on the MnPO4-caused decreases in media DA and Dopac levels
Although we have shown that MnPO4 was at least an order of magnitude more potent than either MnCl2 or MnSO4 in depleting media DA and Dopac, the mechanism(s) by which it elicits such effects had yet to be examined in our study. To investigate the role of oxidative stress, we supplemented our striatal tissues with various antioxidants. We exposed striatal slices to MnPO4 in the presence of a CAT/SOD mixture, GSH, or its precursor NAC (Fig. 5). The enzyme combination of CAT/SOD had little protective effect, whereas 1mM GSH or NAC significantly (P ≤ 0.05) prevented the MnPO4-mediated degradation of media DA and Dopac (Fig. 5a, b).
Figure 5.
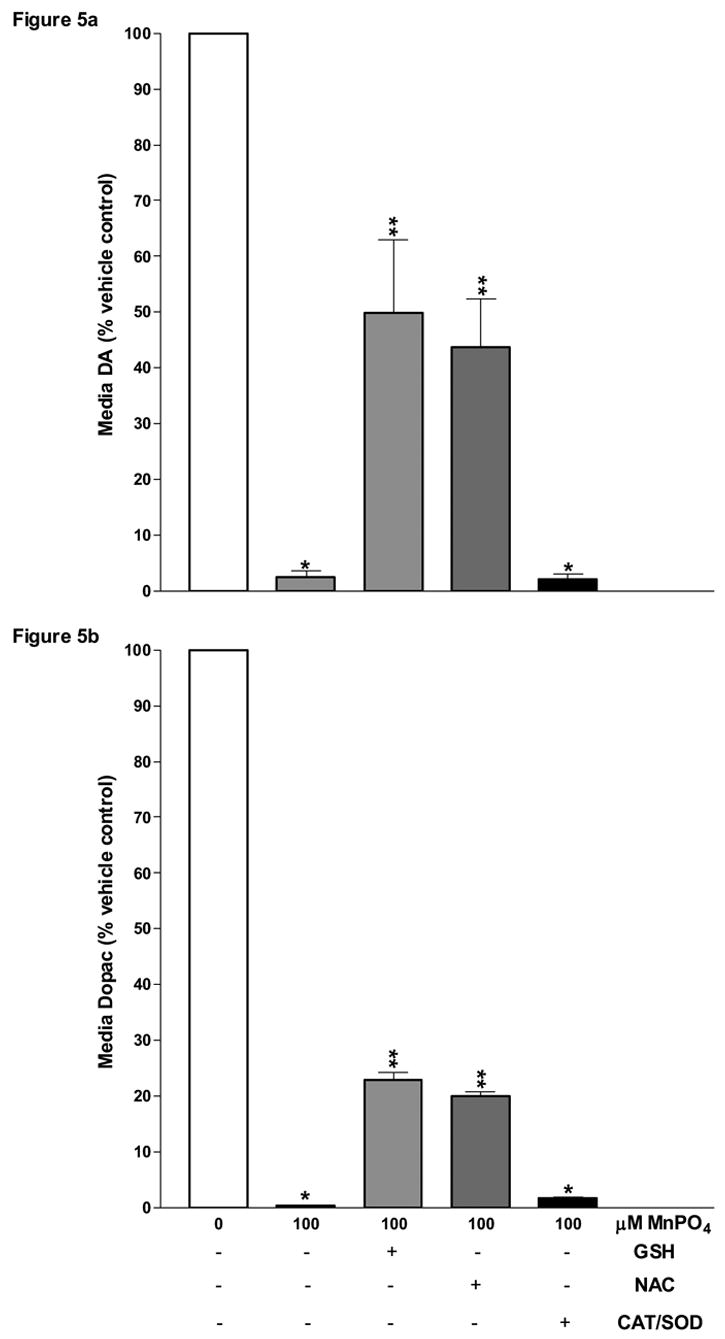
Effects of antioxidant supplementation on MnPO4-caused depletion of DA and Dopac levels released in the culture medium from rat striatal slices. Striatal slices from male Sprague Dawley rats were collected and incubated for 4 h either in control medium or in medium containing 100 μM of MnPO4 or MnPO4 plus GSH(1mM), NAC(1mM), or CAT+ SOD(500+1000 units/ml). Media levels of DA (Fig. 5a) and Dopac (Fig 5b) were analyzed by HPLC-ECD and the data was subsequently normalized on a per mg of protein basis and expressed as a percent of control. *, **, *** Presence and number of asterisks indicate significant differences (P ≤ 0.05).
3.6 Effect of EDTA and EGTA supplementation on the MnPO4-caused DA and Dopac decreases
In all three Mn compounds which were used in our experiments, Mn was likely in the divalent state. However, the possibility exists that in the presence of DA and Dopac some Mn becomes trivalent and initiates redox cycling (HaMai and Bondy, 2004). Our next goal was to determine whether or not Mn3+ was playing a role in the Mn-mediated depletion of DA and Dopac. To this end, we first incubated known amounts of DA and Dopac in medium containing increasing concentrations of MnPO4 supplemented with EDTA or EGTA, which are effective chelators of divalent metals. EDTA and EGTA both attenuated (P ≤ 0.05) the negative effects of MnPO4 on DA and Dopac levels (Fig. 6). EGTA was significantly (P ≤ 0.05) more effective than EDTA in preventing degradation of DA. Both metal chelators also had effects in the absence of Mn. Namely, both significantly increased media DA, while EGTA also decreased media Dopac (P ≤ 0.05), suggesting that other divalent ions present in the media may oxidize DA over time.
Figure 6.

Effects of EDTA/EGDA supplementation on Mn PO4-caused depletion of media DA and Dopac.Ten ng each of DA and Dopac were incubated for 4 h in control medium or in medium containing various concentrations of MnPO4 or Mn PO4 plus 2mM EDTA or EGTA under ambient air conditions (Fig. 6a, c) or under high O2 conditions (Fig. 6b, d). Media levels of DA/Dopac were analyzed by HPLC-ECD and the data was expressed as a percent of vehicle control. *, **, *** Presence and number of asterisks indicate significant effect of Mn (P ≤ 0.05). “a”/”b” indicate significant effects of EDTA and EGTA within a Mn dose (P ≤ 0.05).
3.7 Effect of desferioxamine supplementation on the MnPO4-caused DA and Dopac decreases
We next used desferioxamine (DES) since it is a relatively selective chelator of trivalent metals at low molar ratios. Known amounts of DA and Dopac were added to control medium and incubated for 1 h with MnPO4 (1, 10, 100μM) or MnCl2 (10, 100, 1000μM) in the presence of 1 μM or 10 μM DES. DES prevented the reduction of DA and Dopac levels caused by MnCl2 (Fig. 7a, c). In the case of DA, protection by DES was dose-dependent (P ≤ 0.05) (Fig. 7a). DES pre-treatment also dose-dependently ameliorated the decrease in media DA and Dopac caused by 1μM MnPO4 (P ≤ 0.05). However, 10μM DES was only marginally effective against 10μM MnPO4 and was completely ineffective when 100μM MnPO4 was present in the exposure medium (Figs. 7 b, d).
Figure 7.

Effect of desferioxamine supplementation on manganese-caused depletion of media DA and Dopac.Two ng each of DA and Dopac were incubated for 1 h in control medium, or in a medium containing MnCl2 (10, 100, 1000 μM) (Fig. 7a, c), or MnPO4 (1, 10, 100 μM) (Fig. 7b, d) with and without 1μM or 10μM desferioxamine (DES). Media levels of Dopac/DA were analyzed by HPLC-ECD. *, **, *** Presence and number of asterisks indicate Mn effect (P ≤ 0.05). “a”/”b” indicate DES effect within a Mn dose (P ≤ 0.05).
3.8 Effects of MnCl2, MnSO4, and MnPO4 on DA oxidation to aminochrome
To determine whether the depletion of media DA and Dopac (DA in particular) is due to a direct catecholamine oxidation and a subsequent formation of aminochrome, we monitored aminochrome formation spectrophotometrically. Known concentrations of DA (25, 250, 2500 μM) were incubated with a range of MnPO4 concentrations (1, 10, 100 μM; Fig. 8a), or with fixed concentrations (100 μM each) of MnPO4, MnCl2, and MnSO4 at 37 ºC for up to 60 min (Fig. 8b). First, in the absence of Mn we observed low levels of time-dependent aminochrome formation due to DA autooxidation (Figs. 8a, b). We further observed that MnPO4, regardless of the amount of DA present, was substantially (P ≤ 0.001) more efficient at oxidizing DA and forming aminochrome than the other Mn species (Figs. 8b, c). MnCl2 and MnSO4 catalyzed the oxidation of DA at relatively comparable rates, with MnSO4 being moderately, but significantly (P ≤ 0.05) less efficient than MnCl2 (Figs. 8b, c).
Figure 8.


Manganese-caused oxidation of dopamine to aminochrome. Known concentration of DA (2500 μM) was incubated with a dose range of MnPO4 (1 μM, 10 μM, 100 μM. Fig. 8a), or with MnPO4, MnCl2, and MnSO4 (100 μM each) at 37 ºC for up to 60 min (Fig. 8b). In additional experiments, DA (25 μM, 250 μM, and 2500 μM) was incubated with 100 μM each of MnPO4, MnCl2, and MnSO4 for 60 min at 37 ºC (Fig. 8c). Aminochrome formation was monitored spectrophotometrically at 480 nm. Each bar (Figs. 8b and 8c) represents a minimum of three experiments. *, **, *** Presence and number of asterisks indicate significant differences within a time point (Fig. 8b) or within a DA concentration (Fig. 8c; P ≤ 0.05).
4. Discussion
MnCl2 is a readily available salt and is highly soluble in water. Its properties have led to its extensive use in studies assessing the negative effects of Mn on the basal ganglia (Bird et al., 1984; Sloot et al., 1994; Cano et al., 1997; Takeda, 2003). However, the basis for the use of MnSO4 and MnPO4 in this study lies in the fact that they are the two major combustion products of MMT, a gasoline additive used in certain countries and approved for use in the U.S. (Lynam et al., 1999). Consequently, the present study attempted to assess the neurotoxic potential, in particular the effects on striatal dopamine levels, of MMT’s combustion products and compare them to the more commonly used MnCl2. We have performed studies on the differential effects of MnCl2, MnSO4, and MnPO4, using a striatal tissue culture system and a cell-free system. Our major findings demonstrate that levels of DA in striatal tissue and culture medium are significantly decreased following exposure to all three compounds. Furthermore, levels of Dopac and L-DOPA (in the presence of the AAAD inhibitor NSD-1015) were also decreased by all three compounds; however, MnPO4 was up to 100-fold more potent than the other two compounds. The decreases in DA and DOPAC levels occurred most extensively in the culture media. Our results are consistent with previous studies showing that Mn can decrease DA levels. For example, Poirier et al., (1985) and Donaldson et al., (1982) demonstrated that Mn2+ increases the rate of DA autooxidation in vitro, whereas others have reported that in vivo MnCl2 treatment resulted in a decrease in striatal DA in rats (Autissier et al., 1982; Tran et al., 2002).
The greater potency of MnPO4 was specific for Mn and it was not due to the presence of the PO4 moiety, as exposures to ZnPO4 or CoPO4 did not affect DA and Dopac levels the way MnPO4 exposure did. Interestingly, exposure to 100 μM ZnPO4 resulted in a significant decrease of tissue DA and a corresponding increase in media DA levels. Part of this response may be due to cytotoxicity as the LDH levels in the media were also increased by the 100 μM ZnPO4 exposure. Alternatively, the increase in media DA levels by ZnPO4 may have been caused by the simultaneous inhibition of DA uptake and an enhancement of DA efflux as already demonstrated (Meinild et al., 2004).
The exact mechanisms of manganese neurotoxicity are likely complex and not fully understood. However, the basal ganglia are oxygen-rich and, potentially, Mn could be oxidized to a species with high valence states (Donaldson et al., 1982). One hypothesis put forth to explain Mn neurotoxicity is that Mn2+ can undergo redox cycling reactions with Mn3+ leading to dopaminergic toxicity, with Mn3+ being the reactive species responsible for toxicity (Archibald and Tyree, 1987). The increased toxicity of Mn3+can be partially explained by the four unpaired d orbital electrons which results in a highly unstable atom with an elevated redox potential compared to the more thermodynamically stable Mn2+ (HaMai and Bondy, 2004). Archibald and Tyree (1987) demonstrated that when L-DOPA was added to a physiological buffer containing Mn3+-pyrophosphate, a discernable color change was produced. Parallel studies using Mn2+-pyrophosphate or a 1000-fold molar excess of MnCl2 (Mn2+) or MnSO4 (Mn2+) resulted in no reaction with L-DOPA. However, the time scale of this study was less than 5 sec, whereas Mn2+-driven oxidation of catecholamines apparently occurs much slower (Donaldson et al., 1982), as was the case in our studies. In fact, when known amounts of DA were incubated with Mn (Fig. 8), the oxidation of DA was time-dependent and had not plateaued after 1 h. Thus, in the 4-h striatal slice experiments (Figs. 2–3), the decreases in media DA and, probably, Dopac are at least in part the cumulative result of Mn-enhanced catecholamine oxidation.
The present study also looked at the effects of the sulfhydryl compounds glutathione (GSH) and N-acetylcysteine (NAC) on MnPO4-induced catecholamine depletion in cultured striatal slices. NAC is an antioxidant and a precursor of GSH (Peristeris et al., 1992). NAC is also capable of crossing cellular membranes (Stredrick et al., 2004). On the other hand, GSH does not cross the cell membrane as readily as NAC (Deneke, 2000). Despite this fact, both of these compounds partially protected DA and Dopac in the culture medium from degradation by MnPO4, which suggests that the detrimental effects of MnPO4 are primarily extracellular. It can also be inferred that MnPO4 does not have to be taken into the DA neuronal terminals in order to exert its detrimental effects. In support of this, when known amounts of DA and Dopac were incubated with MnPO4 in the presence of either GSH or NAC, similar results were obtained (data not shown). The association between the redox environment and striatal DA levels has also been demonstrated before (Liccione and Maines, 1988). GSH content was markedly depleted in the striatum of rats exposed to Mn, but only modestly decreased in the whole brain. Importantly, the decrease in GSH was accompanied by depletion of DA and Dopac in the striatum (Liccione and Maines, 1988). Moreover, similar to our findings, exposure of PC12 cells to MnCl2 resulted in marked depletion of extracellular Dopac, whereas intracellular DA was relatively spared (Vescovi et al., 1991).
The finding that Mn phosphate accelerates the oxidation of catecholamines more potently than MnCl2 or MnSO4 is significant because engines powered by gasoline containing MMT produce MnPO4 as a byproduct. To further assess the possibility of a direct interaction between MnPO4 and DA/Dopac, the present study tested the protective effects of several metal chelators. EDTA and EGTA are chelators of divalent metals such as Mn2+ and when co-administered with MnPO4, both chelators had considerable protective effects on DA. Desferioxamine (DES), a highly selective chelator of trivalent metals (HaMai and Bondy, 2004), provided protection at 1 and 10 μM against media Dopac/DA degradation by MnCl2 that was present in 100- or 1000-fold molar excess. Thus, this provided evidence that trace amounts of Mn3+ were present in the MnCl2-containin exposure medium. While DES exhibited substantial protective effects for MnCl2 even at a concentration of 1μM, only 10μM DES had protective effects against 10μM MnPO4 with little to no protective ability when incubated with 100μM MnPO4. Previous studies indicate that the pro-oxidant activity of MnCl2 is exhausted in the presence of 500-fold lower concentrations of desferioxamine (HaMai and Bondy, 2004). Data from the present study appears to support the presence of trace amounts of Mn3+ in MnCl2 solutions and corrobate this finding. The apparent lack of protection against MnPO4 with DES indicates that, either, little Mn in the MnPO4 formulation we used is found in the Mn3+ valence state, or, that DES cannot bind effectively to Mn3+ in the solubilized MnPO4 complex.
More than one theory exists to account to the formation of Mn3+ and the oxidation of catecholamines. An earlier theory (Donaldson et al., 1982) suggested that regardless of the valence state of Mn that enters the brain, spontaneous oxidation and dismutation, peroxidatic activity, or O2• − mediated oxidation, will give rise to Mn3+. Mn2+ scavenges O2•− effectively and is in turn oxidized to Mn3+(Donaldson et al., 1982). Furthermore, the presence of two hydroxyl groups at adjacent 3- and 4- positions on DA and Dopac will allow Mn2+ and Mn3+ to redox cycle and generate semiquinones and orthoquinone by the sequential oxidation of catecholamines via several one electron transfer reactions (Donaldson, 1987). According to a more recent mechanism proposed by HaMai and Bondy (2004), the pro-oxidant activity of Mn2+ is dependent on trace amounts of Mn3+, which may facilitate a small portion of Mn2+ to oxidize to Mn3+. This synergistic relationship between Mn2+ and Mn3+ results in continuous redox cycling (HaMai and Bondy, 2004). During our own study, evidence for the formation of ortho-quinones was shown by monitoring the production of aminochrome spectrophotometrically. Again, we observed that MnPO4 was the most potent Mn compound tested. Consistent with the above mechanism, Lloyd (1995) provided evidence for a redox mechanism in which Mn2+ forms a complex with DA (and other 3,4-hydroxylated catecholamines) that is oxidized to a DA-Mn complex with a Mn3+ valence state (see Fig. 9). Subsequent oxidation of DA to a semiquinone and transfer of one electron from the semiquinone to Mn3+ allows for the reformation of Mn2+. Thus, it is possible that MnPO4 is more efficient at forming the DA-Mn complex, and perhaps, the Dopac-Mn complex, (i.e., has a greater affinity for 3,4-hydroxylated catecholamines) than MnCl2 or MnSO4; hence, oxidizing catecholamines more effectively. Figure 9, which is modified from Lloyd (1995), highlights the proposed mechanism of DA oxidation catalyzed by Mn and the interaction of different Mn ions with DA.
Figure 9.
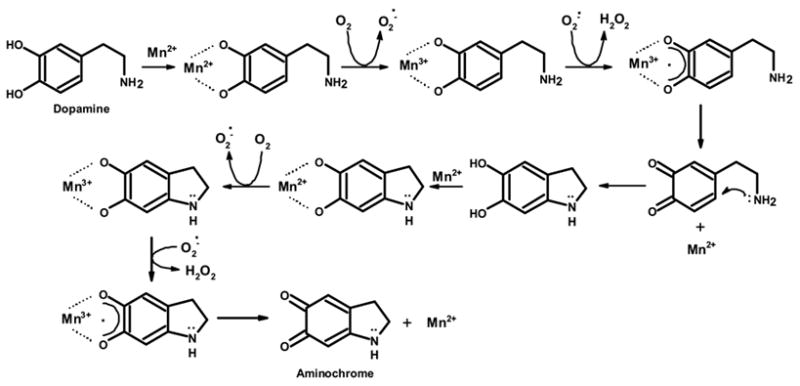
Proposed mechanism of dopamine oxidation by manganese (Adapted from Lloyd, 1995).
According to Lynam et al. (1999), the two major combustion products of MMT are particles of MnPO4 and MnSO4 that have a mass median aerodynamic diameter of 1–2 microns. In rats, MnSO4 accumulated at a higher level in the olfactory bulb and the striatum following inhalation exposure (Dorman et al., 2001), and, MnSO4 exposure was also associated with locomotor deficits (Tapin et al., 2006). Similarly, human data indicates that Mn accumulates primarily in the basal ganglia and other brain regions that have high iron and neuromelanin-binding capacity (Aschner et al., 1999). However, MnPO4 does not appear to gain access to or accumulate in the basal ganglia as selectively as MnSO4 in rats (Vitarella et al., 2000). Apparently, the distribution of the different forms of Mn to various brain regions depends, in part, on the solubility of each Mn compound (Roels et al., 1997; Dorman et al., 2001) and MnPO4 is much less soluble than the chloride and sulfate forms of Mn (Vitarella et al., 2000). This is important to take into account when considering MMT exposure. Although MnPO4 is more potent than MnSO4 in oxidizing DA/Dopac, a greater percentage of MnSO4 will likely accumulate in DA-rich brain regions as a result of exposure to the combustion products of MMT. Thus, due to the greater accumulation of MnSO4 in the basal ganglia and the greater potency, at least in terms of catecholamine oxidation, of MnPO4, these two MMT combustion products may be equally detrimental to the basal ganglia in cases of excessive exposure. These pharmacokinetic and pharmacodynamic considerations should be taken into account when evaluating the potential neurotoxicity risk associated with MMT use and subsequent exposure to its combustion products.
Acknowledgments
This project was supported by research grant from the National Institute of Environmental Health Sciences (NIH), ES011654. Infrastructure support was provided, in part, by a P20 RR017661 grant from the National Center for Research Resources (NIH). The assistance provided by Mr. E. Meek (Center for Environmental Health Sciences) is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W., Jr Manganese-induced reactive oxygen species: comparison between Mn+2 and Mn+3. Neurodegeneration. 1995;4:329. doi: 10.1016/1055-8330(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Archibald FS, Tyree C. Manganese poisoning and the attack of trivalent manganese upon catecholamines. Arch Biochem Biophys. 1987;256:638. doi: 10.1016/0003-9861(87)90621-7. [DOI] [PubMed] [Google Scholar]
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS. Neurotoxicology. 1999;20:173. [PubMed] [Google Scholar]
- Autissier N, Rochette L, Dumas P, Beley A, Loireau A, Bralet J. Dopamine and norepinephrine turnover in various regions of the rat brain after chronic manganese chloride administration. Toxicology. 1982;24:175. doi: 10.1016/0300-483x(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Barbeau A, Inoue N, Cloutier T. Role of manganese in dystonia. Adv Neurol. 1976;14:339. [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. Polychlorinated biphenyls and methylmercury act synergistically to reduce rat brain dopamine content in vitro. Environ Health Perspect. 1999;107:879. doi: 10.1289/ehp.99107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird ED, Anton AH, Bullock B. The effect of manganese inhalation on basal ganglia dopamine concentrations in rhesus monkey. Neurotoxicology. 1984;5:59. [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477. [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences [comment] [see comments] Neurology. 1994;44:1583. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Cano G, Suarez-Roca H, Bonilla E. Alterations of excitatory amino acid receptors in the brain of manganese-treated mice. Mol Chem Neuropathol. 1997;30:41. doi: 10.1007/BF02815149. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Fisher JP, Seegal RF. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology. 1996;17:653. [PubMed] [Google Scholar]
- Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151. doi: 10.1016/s0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Weber S, Dorman DC, Lash LK, Erikson KM, Aschner M. Oxidative stress is induced in the rat brain following repeated inhalation exposure to manganese sulfate. Biol Trace Elem Res. 2003;93:113. doi: 10.1385/BTER:93:1-3:113. [DOI] [PubMed] [Google Scholar]
- Donaldson J. The physiopathologic significance of manganese in brain: its relation to schizophrenia and neurodegenerative disorders. Neurotoxicology. 1987;8:451. [PubMed] [Google Scholar]
- Donaldson J, McGregor D, LaBella F. Manganese neurotoxicity: a model for free radical mediated neurodegeneration? Can J Physiol Pharmacol. 1982;60:1398. doi: 10.1139/y82-208. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, James RA, Marshall MW, Parkinson CU, Wong BA. Influence of particle solubility on the delivery of inhaled manganese to the rat brain: manganese sulfate and manganese tetroxide pharmacokinetics following repeated (14-day) exposure. Toxicol Appl Pharmacol. 2001;170:79. doi: 10.1006/taap.2000.9088. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M. Persistent alterations in biomarkers of oxidative stress resulting from combined in utero and neonatal manganese inhalation. Biol Trace Elem Res. 2005;104:151. doi: 10.1385/BTER:104:2:151. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Tedroff J, Thuomas KA, Aquilonius SM, Hartvig P, Fasth KJ, Bjurling P, Langstrom B, Hedstrom KG, Heilbronn E. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol. 1992;66:403. doi: 10.1007/BF02035130. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Yan CY, Greene LA. N-acetylcysteine (D- and L-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci. 1995;15:2857. doi: 10.1523/JNEUROSCI.15-04-02857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipov NM, Lawrence DA, Seegal RF. Influence of polychlorinated biphenyls and turning preference on striatal dopamine metabolism. J Toxicol Environ Health A. 2005;68:167. doi: 10.1080/15287390590890563. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Miller LM, Gavin CE, Eliseev R, Salter J, Buntinas L, Alexandrov A, Hammond S, Gunter KK. Determination of the oxidation states of manganese in brain, liver, and heart mitochondria. J Neurochem. 2004;88:266. doi: 10.1046/j.1471-4159.2003.02122.x. [DOI] [PubMed] [Google Scholar]
- HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:129. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- HaMai D, Campbell A, Bondy SC. Modulation of oxidative events by multivalent manganese complexes in brain tissue. Free Radic Biol Med. 2001;31:763. doi: 10.1016/s0891-5849(01)00639-6. [DOI] [PubMed] [Google Scholar]
- Hollrah DP, Roos JW. The role of MMT in producing environmentally clean fuels. International Journal of Hydrocarbon Engineering. 1997;2:38. [Google Scholar]
- Iregren A. Manganese neurotoxicity in industrial exposures: proof of effects, critical exposure level, and sensitive tests. Neurotoxicology. 1999;20:315. [PubMed] [Google Scholar]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64:2021. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Wagner JR, Kirby ML, Anantharam V, Kanthasamy AG. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J Pharmacol Exp Ther. 2002;302:26. doi: 10.1124/jpet.302.1.26. [DOI] [PubMed] [Google Scholar]
- Liccione JJ, Maines MD. Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J Pharmacol Exp Ther. 1988;247:156. [PubMed] [Google Scholar]
- Lioy PJ. Air pollution emission profiles of toxic and trace elements from energy related sources: status and needs. Neurotoxicology. 1983;4:103. [PubMed] [Google Scholar]
- Lloyd RV. Mechanism of the manganese-catalyzed autoxidation of dopamine. Chem Res Toxicol. 1995;8:111. doi: 10.1021/tx00043a015. [DOI] [PubMed] [Google Scholar]
- Loranger S, Zayed J. Environmental contamination and human exposure to airborne total and respirable manganese in Montreal. J Air Waste Manag Assoc. 1997;47:983. doi: 10.1080/10473289.1997.10463954. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Roos JW, Pfeifer GD, Fort BF, Pullin TG. Environmental effects and exposures to manganese from use of methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline. Neurotoxicology. 1999;20:145. [PubMed] [Google Scholar]
- Meinild AK, Sitte HH, Gether U. Zinc potentiates an uncoupled anion conductance associated with the dopamine transporter. J Biol Chem. 2004;279:49761. doi: 10.1074/jbc.M407660200. [DOI] [PubMed] [Google Scholar]
- Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology. 1967;17:128. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, Osame M. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurol Sci. 1999;162:102. doi: 10.1016/s0022-510x(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Parenti M, Flauto C, Parati E, Vescovi A, Groppetti A. Manganese neurotoxicity: effects of L-DOPA and pargyline treatments. Brain Res. 1986;367:8. doi: 10.1016/0006-8993(86)91571-4. [DOI] [PubMed] [Google Scholar]
- Peristeris P, Clark BD, Gatti S, Faggioni R, Mantovani A, Mengozzi M, Orencole SF, Sironi M, Ghezzi P. N-acetylcysteine and glutathione as inhibitors of tumor necrosis factor production. Cell Immunol. 1992;140:390. doi: 10.1016/0008-8749(92)90205-4. [DOI] [PubMed] [Google Scholar]
- Poirier J, Donaldson J, Barbeau A. The specific vulnerability of the substantia nigra to MPTP is related to the presence of transition metals. Biochem Biophys Res Commun. 1985;128:25. doi: 10.1016/0006-291x(85)91639-0. [DOI] [PubMed] [Google Scholar]
- Pomier-Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet. 1995;345:735. doi: 10.1016/s0140-6736(95)90909-5. [DOI] [PubMed] [Google Scholar]
- Ressler T, Wong J, Roos J. Manganese speciation in exhaust particulates of automobiles using MMT-containing gasoline. J Synchrotron Radiat. 1999;6:656. doi: 10.1107/S0909049598015623. [DOI] [PubMed] [Google Scholar]
- Roels H, Meiers G, Delos M, Ortega I, Lauwerys R, Buchet JP, Lison D. Influence of the route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats. Arch Toxicol. 1997;71:223. doi: 10.1007/s002040050380. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Goncharova EI. Spontaneous mutagenesis in mammalian cells is caused mainly by oxidative events and can be blocked by antioxidants and metallothionein. Mutat Res. 1998;402:103. doi: 10.1016/s0027-5107(97)00287-x. [DOI] [PubMed] [Google Scholar]
- Roth JA, Feng L, Walowitz J, Browne RW. Manganese-induced rat pheochromocytoma (PC12) cell death is independent of caspase activation. J Neurosci Res. 2000;61:162. doi: 10.1002/1097-4547(20000715)61:2<162::AID-JNR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Bush B. High-performance liquid chromatography of biogenic amines and metabolites in brain, cerebrospinal fluid, urine and plasma. J Chromatogr. 1986;377:131. doi: 10.1016/s0378-4347(00)80768-9. [DOI] [PubMed] [Google Scholar]
- Sloot WN, van der Sluijs-Gelling AJ, Gramsbergen JB. Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus. J Neurochem. 1994;62:205. doi: 10.1046/j.1471-4159.1994.62010205.x. [DOI] [PubMed] [Google Scholar]
- Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, Lash LH, Aschner M, Vrana KE. Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology. 2004;25:543. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Nishiyama K, Fujii N. Study of subacute toxicity of manganese dioxide in monkeys. Tokushima J Exp Med. 1975;22:5. [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41:79. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Tapin D, Kennedy G, Lambert J, Zayed J. Bioaccumulation and locomotor effects of manganese sulfate in Sprague-Dawley rats following subchronic (90 days) inhalation exposure. Toxicol Appl Pharmacol. 2006;211:166. doi: 10.1016/j.taap.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Crinella FM, Chicz-DeMet A, Lonnerdal B. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology. 2002;23:635. doi: 10.1016/s0161-813x(02)00091-8. [DOI] [PubMed] [Google Scholar]
- Verity MA. Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology. 1999;20:489. [PubMed] [Google Scholar]
- Vescovi A, Facheris L, Zaffaroni A, Malanca G, Parati EA. Dopamine metabolism alterations in a manganese-treated pheochromocytoma cell line (PC12. Toxicology. 1991;67:129. doi: 10.1016/0300-483x(91)90137-p. [DOI] [PubMed] [Google Scholar]
- Vitarella D, Wong BA, Moss OR, Dorman DC. Pharmacokinetics of inhaled manganese phosphate in male Sprague-Dawley rats following subacute (14-day) exposure. Toxicol Appl Pharmacol. 2000;163:279. doi: 10.1006/taap.1999.8874. [DOI] [PubMed] [Google Scholar]
- Wedler FC, Denman RB, Roby WG. Glutamine synthetase from ovine brain is a manganese(II) enzyme. Biochemistry. 1982;21:6389. doi: 10.1021/bi00268a011. [DOI] [PubMed] [Google Scholar]
- Zheng W, Ren S, Graziano JH. Manganese inhibits mitochondrial aconitase: a mechanism of manganese neurotoxicity. Brain Res. 1998;799:334. doi: 10.1016/s0006-8993(98)00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yase Y. The pathogenesis of amyotrophic lateral sclerosis. Lancet. 1972;2:292. doi: 10.1016/s0140-6736(72)92903-0. [DOI] [PubMed] [Google Scholar]


