Abstract
Driven by tissue engineering and regenerative medicine, endothelial cells are being used in combination with biomaterials in a number of applications for the purpose of improving blood compatibility and host integration. Endothelialized vascular grafts are beginning to be used clinically with some success in some centers, while endothelial seeding is being explored as a means of creating a vasculature within engineered tissues. The underlying assumption of this strategy is that when cultured on artificial biomaterials, a confluent layer of endothelial cells maintain their non-thrombogenic phenotype. In this review the existing knowledge base of endothelial cell thrombogenicity cultured on a number of different biomaterials is summarized. The importance of selecting appropriate endpoint measures that are most reflective of overall surface thrombogenicity is the focus of this review. Endothelial cells inhibit thrombosis through three interconnected regulatory systems (1) the coagulation cascade (2) the cellular components of the blood such as leukocytes and platelets and (3) the complement cascade, and also through effects on fibrinolysis and vascular tone, the latter which influences blood flow. Thus, in order to demonstrate the thromobgenic benefit of seeding a biomaterial with EC, the conditions under which EC surfaces are more likely to exhibit lower thrombogenicity than unseeded biomaterial surfaces need to be consistent with the experimental context. The endpoints selected should be appropriate for the dominant thrombotic process that occurs under the given experimental conditions.
Introduction
A vascular system to facilitate continuous nutrient delivery and waste removal is necessary to sustain tissue viability in organisms larger than 200 microns in size due to diffusion limitations. Furthermore enabling communication and transport between different tissues within the organism permits the development of specialized tissues that work in a cooperative manner without the requirement that they be in close proximity within the body. A circulatory system in which nutrients and waste enter and leave the blood within different regions of the circuit is efficient. However, this requires, strategies to control where and at what rate molecular exchange occurs within the circuit, processes to allow circuit growth, and repair mechanisms in the event of a break in the circuit i.e. a tissue wound. Endothelial cells (EC) are pivotal in the sophisticated mechanisms that have evolved to provide these system features. A monolayer of EC line the vasculature and through the expression and secretion of a spectrum of molecules regulate vascular tone and permeability, inflammation, thrombosis, and fibrinolysis. The relative expression levels of these molecules are determined by EC interactions with the surrounding blood, extracellular matrix (ECM) and peripheral cells. The endothelium is a dynamic interface which actively maintains blood circulation until tissue damage occurs, at which point the EC actively drive local thrombosis and inflammation enabling efficient wound repair.
The sensitivity of the circulatory system to disruption or alteration is one of the limits to which host integration of a biomaterial can be achieved. When artificial biomaterials come into contact with blood, a number of thrombotic and inflammatory reactions occur related to what occurs when blood is exposed to the ECM of a disrupted vessel. To prevent these reactions, EC are being combined with biomaterials in a number of applications to improve artificial device integration. For example, the lumens of vascular grafts have been seeded with endothelial cells as a strategy to create an interface that “hides” the underlying biomaterial, enabling blood contact without significant inflammation and thrombosis, and maintenance of graft patency [1]. In the case of tissue-engineered constructs, blood must be supplied to cells within the construct without contacting the biomaterial scaffold. Strategies have been developed to encourage blood vessel formation within biomaterial scaffolds involving either seeding the construct with EC prior to implantation [2] or encouraging EC in-growth from the host post-implantation [3], both of which involve EC contact with the construct biomaterial. The use of stents in the treatment of coronary or other artery disease also results in blood-biomaterial contact and the incidence of thromboembolic complications of stenting. A failure to re-endotheliaze is thought to be responsible for what is termed ”late stent thrombosis” (thrombosis occurring after 30 days), a particular issue with the new drug-eluting stents. [4]. The dynamic nature of EC and the complex control systems that govern their behaviour however, create challenging problems when attempting to combine EC with an artificial material in a controlled and precisely characterized manner.
This review briefly summarizes the reactions involved in thrombosis and the molecules expressed by EC that mediate these; highlights the molecular profiles expected on quiescent versus activated EC; and provides a brief overview of the mechanisms which regulate EC behaviour. The object is to identify the basic principles which guide EC phenotype relevant to the understanding of the effects seen in the presence of a biomaterial. In the latter half of this review, characterization of EC behaviour on biomaterials is addressed. To date this has primarily focused on: proliferation, relevant to vascular graft coverage and vessel development within a scaffold; cell adhesion, important for the maintenance of a non-thrombogenic layer on vascular grafts after implantation; extracellular matrix (ECM) secretion and substrate remodeling; and EC thrombogenicity. Of these properties, EC thrombogenicity is the least well studied and will be the focus here, with emphasis on a view of the dynamic and active nature of the endothelium that underlies the maintenance of its non-thrombogenic character. The other features of EC on biomaterials have been reviewed elsewhere [5–7]. Here a summary is provided of the methods used to characterize EC thrombogenicity on different biomaterials and the dominant experimental factors that have been found to influence thrombogenicity measurements.
EC mediation of thrombosis
Thrombosis involves the activation of three interconnected regulatory systems, the coagulation cascade, the complement cascade and the cellular components of the blood such as leukocytes and platelets (Figure 1) [see [8,9] for a comprehensive review]. The coagulation cascade involves a series of proteolytic reactions that convert zymogens into active enzymes, which then catalyze the activation of zymogens further down the cascade resulting in thrombin and finally fibrin formation. Thrombosis only occurs above a certain threshold of activated clotting factors in localized regions. However, under normal circumstances sub-threshold levels of cascade initiators, such as thrombin and Factors IXa and Xa, are found in the blood, prime the system for immediate cascade initiation on exposure to tissue factor [10]. In addition to flow (see below), several positive (e.g. thrombin, tissue factor) and negative (e.g. thrombomodulin, tissue factor pathway inhibitor) feedback molecular factors determine whether the “cascade switch” is on or off, characteristic of a threshold system that must be able to switch abruptly from one state to another when wounding occurs [11].
Figure 1.
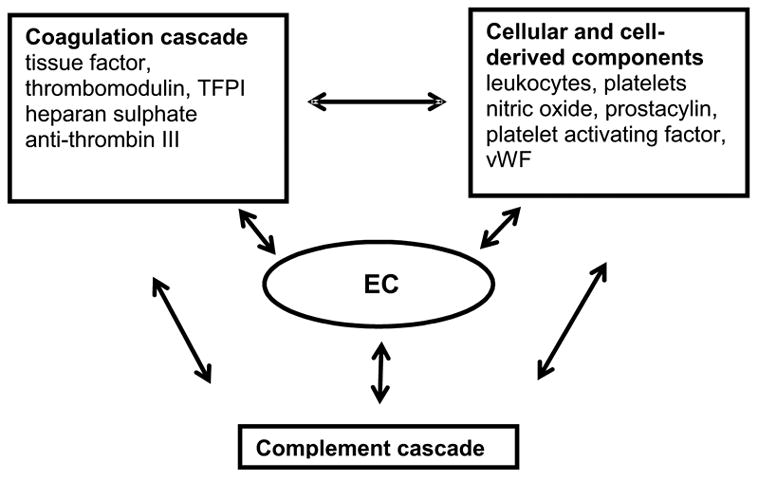
Systems with which EC interact to regulate thrombosis
Cellular components of the blood, such as platelets and leukocytes, contribute to maintenance of vessel integrity, and initiate wound healing. Platelets and leukocytes are activated by various components of the coagulation cascade and once activated they promote further local amplification of the thrombosis response by the generation of various factors in the coagulation cascade, such as tissue factor. Cellular components of the blood also help to contain thrombosis to the site of injury since for many coagulation reactions a multimolecular complex must be formed on an anionic phospholipid membrane surface (e.g. platelets) to achieve a significant reaction rate.
The complement system facilitates communication between the blood and the body’s immune elements. The complement cascade involves more than twenty plasma proteins and is initiated via the classical or alternative pathway [12], both of which result in terminal complement complex insertion into the lipid layer of the “foreign cell” and subsequent cell damage and or lysis. Various components of the cascade amplify inflammation and coagulation. For example, leukocytes can be activated by low amounts of complement factors such as C5b-9. Some coagulation factors (for example thrombin) also activate complement.
During thrombosis, flow offers a means of negative feedback by decreasing the localized concentration of some factors and removing activated material by dilution into a larger volume. Flow rates, which then influence thrombosis dynamics, are primarily controlled by vascular tone via molecules such as nitric acid, endothelin and prostacylin. Fibrinolysis, the process that enables removal of the fibrin plug after an injury, is another relevant process involving molecules such as tissue plasminogen activator, and plasminogen activator inhibitor.
The process of thrombosis has significant redundancy and complexity with the steady state resulting from a dynamic balance of all reactions. Such a high degree of control is necessary in a circulatory system where locally produced pro-thrombotic factors must be rapidly amplified yet systemic activation must not occur. Due to such interactions among each of the involved regulatory systems [8], activation of one system likely results in some level of disruption of the others thus creating a multifaceted system to facilitate wound healing. Endothelial cells play a central role in mediating thrombosis by interacting with each of these regulatory systems through several mechanisms (Table 1)[13], which are summarized briefly here.
Table 1.
Molecules expressed by endothelial cells for control of hemostasis.
| Molecule | Function | Location | Expression characteristics | Ref. |
|---|---|---|---|---|
| CoagulationThrombomodulin | Binds and inactivates thrombin
Catalyzes thrombin associated activation of protein C (APC); once activated APC enables inactivation of FVa, FVIIIa and PAI-1 |
Expressed on cell surface | Expressed constitutively and down regulated when cell activated | 21, 22 |
| Endothelial cell protein C receptor (EPCR) | Increases interaction between protein C and thrombin bound to thrombomodulin to increase anti-coagulant effect of APC | Membrane bound (present in much greater concentration on large vessel EC) | 210, 211 | |
| Tissue Factor (thromboplastin) | Receptor for FVII to enable conversion of prothrombin to thrombin | Membrane bound | Expressed in response to agonist | 16, 17, 18, 19, 20 |
| Tissue factor pathway inhibitor | Inhibits TF activity | Secreted by EC and membrane bound | Constitutively expressed | 23, 212 |
| Antithrombin III | Inactivates thrombin | Plasma protein, bound to EC surface via GAG molecules | Constitutively expressed | 9 |
| Heparan sulphate | Binds antithrombin III | Membrane bound | Constitutively expressed | 24 |
| Blood cellsEctonucleotidase enzymes | Terminate pro-aggregatory action of ADP on platelets | Luminal surface of EC | 26 | |
| Von Willebrand factor | Enables platelet adhesion to exposed ECM
Stabilizes FVIII in coagulation cascade |
Stored in Weibel Palade bodies and secreted when required | Secreted on activation in response to agonist | 13, 27 |
| I-CAM, V-CAM, PECAM | Enable binding and diapedesis of leukocytes to and through endothelium | Surface bound | Expression increases in response to agonist | 31, 32, 213 |
| Nitric oxide (NO) | Inhibits platelet aggregation and adhesion to the vessel wall.
Vasodilator |
Secreted | Expression increases in response to agonist | 29, 214, |
| Prostacylin (PGI2) | Inhibits platelet aggregation
Vasodilator |
Secreted | Expression increases in response to agonist | 28, 36 |
| Platelet activating factor (PAF) | Stimulates platelets and leukocytes
Vasodilator |
On EC mainly membrane bound with small portion secreted. Also secreted by other cell types. | Expressed in response to agonist | 25, 30 |
| Complement | ||||
| C1 inhibitor, factors H and I | Complement inhibitors | Secreted | Constitutively expressed | 33, 34, 35 |
| CD46, CD55, CD59 | Inhibit complement activation by favouring the decay of the C3 convertase (CD55), inhibiting the formation of the C3 convertase (CD46), or preventing the assembly of MAC (CD59). | Surface bound | Constitutively expressed | 33, 34, 35 |
| C3, C1 s, factor B and all the terminal complex components | Involved in complement activation | Secreted | Secreted from activated EC | 33, 34, 35 |
| Fibrinolysis | ||||
| Tissue plasminogen activator (tPA) | tPA activated by binding to fibrin causing plasmin generation enabling local thrombus fibrinolysis | Stored and secreted | Constitutively secreted and rapid secretion in response to agonist | 44, 45, 46 |
| Plasminogen activator inhibitor-1 (PAI-1) | Inhibits action of tPA preventing fibrinolysis | Secreted into blood and basement membrane matrix | Constitutively secreted at low levels and increased when activated. | 44, 45, 46 |
| Vascular tone | ||||
| Nitric oxide (NO) | See blood cell section above | |||
| Prostacylin (PGI2) | See blood cell section above | |||
| Platelet activating factor (PAF) | See blood cell section above | |||
| Endothelin-1 | Acts on smooth muscle cells to produce
vasoconstriction |
Secreted into basolateral compartment | Secreted in response to an agonist or increased shear | 40, 41 |
EC and coagulation
EC influence coagulation by the expression of several molecules, which initiate and regulate the coagulation cascade [14,15], and by providing the phospholipid surface necessary for many coagulation reactions to occur. The key initiation molecule, tissue factor (TF), is expressed on the surface of activated EC [16–19] and is present in intracellular pools, which are released if the integrity of the EC is disrupted [20]. EC also contribute to the three major negative feedback mechanisms that regulate the coagulation cascade; Thrombomodulin (TM) is expressed constitutively on the surface of un-activated EC [21,22]; tissue factor pathway inhibitor (TFPI) is predominantly synthesized by EC and both secreted into the circulating plasma and constitutively bound to the EC surface [23]; and heparan sulphate molecules bound to the EC surface support antithrombin iii binding [24]. On normal, un-activated, confluent EC, TF expression is low or absent, and TM expression is high. Activation causes a reversal of this profile.
EC and platelets, leukocytes and complement
EC interact with both platelets and leukocytes. EC synthesize molecules such as platelet activating factor (PAF) [25], ectonucleotidases [26] and von Willebrand factor [27] to facilitate interactions with platelets. Although platelets contain some von Willebrand Factor (vWF), virtually all plasma vWF is derived from the endothelium [13]. Nitric Oxide (NO, produced by EC) and prostacyclin (PGI2,), regulate vascular tone (see below), but also prevent platelet aggregation [28] and, in the case of NO, also prevent platelet adhesion [29]. Leukocyte adhesion to the EC surface is mediated by platelet activating factor [30], and cell adhesion molecules on the EC surface [31,32] such as PECAM, ICAM-1, P-selectin, E-selectin and VCAM-1. In addition EC express molecules involved in complement regulation and receptors for a number of complement system proteins [33–35]. On normal, un-activated, confluent EC, PGI2 secretion is low or absent depending on the EC location [36], NO secretion is present but low, vWF is present within the EC but not secreted, and the number of cell adhesion molecules expressed on the EC surface is also low. Activation causes increased secretion of NO, PGI2, vWF and increased expression of cell surface adhesion molecules.
EC mediation of vascular tone and fibrinolysis
Flow is an important factor in regulating thrombosis and is in turn influenced by vascular tone, which controls blood vessel diameter. Vascular tone is regulated by the release from EC of a combination of vasodilators [37], such as nitric oxide (NO) [38,39] and prostacyclin (PGI2), and vasoconstrictors, such as endothelins [40,41] and platelet activating factor (PAF) [42], which enable interaction with the underlying smooth muscle cell layer [43] and constriction or dilation of the vessel walls. NO is secreted constitutively at low levels to maintain basal tone but is synthesized and released locally and transiently in response to agonists such as ATP and ADP secreted from activated platelets and molecules involved in the coagulation cascade such as thrombin and bradykinin. PGI2 is also secreted by some EC at low basal levels but secretion is increased transiently in response to agonists. Mediators such as endothelins and PAF are secreted only in response to agonists.
Fibrinolysis, removal of a thrombus once formed to facilitate wound healing, is another important event associated with thrombosis in which EC play an important role. EC secrete both tPA and PAI-1 to control fibrinolysis [44]. Activation of the EC during thrombosis alters the relative balance [45] of tPA and PAI-1 to initiate thrombus dissolution and wound healing. On normal, un-activated, confluent EC, secretion of PAI is higher than tPA to achieve the several fold higher concentration of PAI seen in plasma [46].
EC sources for tissue engineering
The endothelium comprises heterogeneous, metabolically active cells and the activation state of the endothelium depends on the precise balance among the various molecules described above. Furthermore this balance varies depending on species and anatomical location. The cell source is therefore an important consideration when designing biomaterials/EC combination studies. There are a number of species from which EC are readily available for tissue engineering. Bovine, porcine, human, and rat EC are commercially available from Clonetics, Cell Applications Inc. and others, while protocols have been established to harvest canine [47], mouse [48], and sheep EC [49]. EC derived from hemotopoeitic stem cells [50] have been isolated and are another cell source option. Historically bovine EC (BAEC) and human umbilical vein EC (HUVEC) are most common for fundamental studies into EC biology while canine cells, human saphenous vein EC (HSVEC), and human omentum microvascular (HOTMEC) proved most popular in the vascular graft community in combination with dog models to test graft patency. A new and currently topical EC is the circulating endothelial progenitor cell (EPC). While EPC have only just begun to be used as a source for seeding vascular grafts or depositing on biomaterials [51], EPC are of great interest for cardiac and other tissue regeneration goals, since it is thought that if they can be made to “home” or otherwise concentrate at sites of tissue damage, the resulting vasculature and perfusion could drive a positive healing/remodeling response [52]. There has been great interest also in the stenting community with anti-C34 antibodies immobilized on a stent surface being used to facilitate the deposition of these EPC and corresponding re-endothelization of the stent [53].
The choice of EC source for a particular application is important as there is considerable phenotypic variation among EC from different sources [54,55], different locations within the same organ [56], different locations within the same vessel [57], and different vessel sizes [58,59]. For example, un-stimulated human umbilical vein endothelial cells (HUVEC), unlike omentum and atrium cells do not spontaneously express TF in culture and are considered the most useful cells for achieving a non-coagulant surface on vascular grafts [36,60,61]. For different EC sources, variations have been reported in the expression levels of vasconstrictors [62,63], cell specific markers [32,36] neutrophil adhesion after EC activation [64], coagulation mediators such as protein C receptor [65] and TF [36,60,61], fibrinolysis mediators [66,67], and ECM secreted in culture [68,69]. Differences have also been reported between sparse and confluent cells [32] and variations in the gene expression for different EC [70,71] and between EC in vivo versus in vitro [72] have also been observed. These differences highlight the diversity of EC function (for example large vessel endothelium controls vasoconstriction and vasodilatation while microvascular endothelial cells are involved in neovascularisation, blood-tissue exchange of oxygen and nutrients) and reflect local microenvironment variations in the extracellular matrix, paracrine/autocrine factors, cell-cell contact, and biomechanical factors [73] (for example large vessels are surrounded by smooth muscle cells while small vessels are surrounded by pericytes [74]). In comparing studies of EC on different biomaterials cells source is therefore a critical consideration.
Regulation of endothelial cell phenotype
To provide a comparison for the effects of biomaterials, we first discuss briefly the factors that influence the behaviour of EC in their natural state. In vivo, in the absence of a biomaterial, EC growth [75], differentiation [76], barrier function [77], migration, and survival [78] are regulated in a complex manner by a combination of the surrounding ECM [79], cell-cell contacts [80,81], growth factors [82] and mechanical cues [76,83]. In particular fluid shear stress resulting from blood flow over the endothelial surface, sensed via a number of mechanosensor mechanisms [84], produces junction [85], cytoskeleton [86] and integrin [87] re-organization which alters ECM organization [88,89], EC morphology [90], and EC gene expression [91–93]. This further affects the expression and secretion of proteins associated with EC function, such as regulation of vascular tone [94–96], fibrinolysis [97–99], surface adhesion [100] and coagulation proteins such as tissue factor and its inhibitor [101,102], thrombomodulin [103,104], prostacyclin [105,106] and von Willebrand factor [107]. Of interest here, is that KLF-2 [108] (Kruppel-like factor), a flow responsive transcription factor is a primary candidate as a central “switch” between quiescent and activated adult EC. The appropriate flow conditions for the particular location of a given EC in the vasculature are necessary to ensure correct EC function in that location. The nature of the interconnected network of cells and matrix created when EC are cultured on biomaterials and the flow/stress conditions of the experimental system are therefore important when considering how the biomaterial influences cell behaviour. As will become apparent to the reader below, not all these considerations have been carefully controlled for in most studies involving biomaterials and EC and therefore comparing the data reported from different sources is problematic.
Endothelial cell phenotype on biomaterials
Studies combining EC and biomaterials have characterized the resulting EC “phenotype” in a number of ways including proliferation, adhesion, viability, and to a lesser extent thrombogencity. Each of these measures of phenotype is considered below with a focus on thrombogenicity since this has been the least studied to date although it offers a measure of more specialized EC functions than the more general cell properties more commonly reported on.
EC proliferation and adhesion on biomaterials
Static in vitro studies on tissue culture polystyrene (TCPS) coated in various ECM proteins have focused on the effect of ECM substrate on EC proliferation, adhesion, migration and matrix secretion. In terms of adhesion, human EC adhered to collagens I, III and IV but only formed tight junctions on type IV [109], while fibronectin coatings provided the greatest level of initial adhesion with significant differences disappearing after 1 h in culture [110]. Cell proliferation was higher on collagen type III and laminin and lower on collagen type IV [111–113]. Capillary EC cultured on interstitial collagens tended to secrete more interstitial collagen, while on basement membrane more basement membrane collagen was secreted [111. The composition of the underlying substrate therefore appears to be very influential in determining EC behaviour.
A study by Underwood et al. [114] however, highlighted the difficulty of separating the effects of ECM proteins from the effects of cell shape on observed cell behaviour. The influence of cell shape on cell behaviour was first proposed by Folkman and Moscona [115] and subsequently it has been shown that the shape and extent of cell spreading on a surface controls cell behaviour [116]. Cell spreading/shape and cytoskeleton arrangement, which influence intracellular signaling and hence behaviour, has also been shown to differ depending on the ECM on which the cells are cultured [117]: for example the distribution of spectrin (fodrin), a high molecular weight heterodimer that appears to link surface receptors to actin filaments, was different in EC cultured on fibronectin compared to collagen [118]. Cell size/shape has also been shown to correlate with the extent of cell attachment and spreading to a particular substrate and was inversely correlated with proliferation and cell migration [119,120]. Underwood et al., however removed the cell shape effects from their experiments by demonstrated that optimal cell spreading can be reached on all ECM protein substrates but different concentrations are required. When different concentrations of each ECM were used (each concentration used to achieve optimum spreading) no differences in cell morphology or growth rate were observed among different coatings. However the type and amount of ECM deposited by the cells still differed with differences in ECM substrate and time in culture [114]. This suggests that ECM composition alters cell shape, cytoskeletal organization and integrin binding. Such changes in turn control the ECM deposited around the EC and their proliferative and migratory behaviour. Hence it is possible that the effects previously documented as matrix chemistry effects on cell behaviour were more related to differences in the extent of cell spreading at the ECM compositions tested. It is difficult, therefore to draw definitive conclusions from data involving ECM coated TCPS.
Variation in EC adhesion has also been characterized on biomaterials, other than TCPS. Introducing biomaterials other than TCPS into culture with EC is of particular interest in the development of vascular grafts. When biomaterials are coated with components of ECM, a common strategy that improves the adhesiveness of EC on to vascular grafts, trends, similar to ECM coatings on TCPS, were seen. Assuming surface topography and porosity are similar for all surfaces, initial adhesion is determined by the conformation and amount of protein intentionally or unintentionally (from serum), adsorbed on the material surface (which is in turn dependent on underlying material chemistry). Fibronectin produced the highest initial adhesion [121], while at later time points, when cell confluence was attained, no difference was seen on different ECM component coatings [122–125]. It is worth noting here that many studies on polymer grafts were confounded by variations in graft porosity or surface roughness, which alter the local stress state sensed by the EC. Moreover most studies assess the number of EC retained on the surface either after 1 h or after a few days. This, in our opinion, gives more of a measure of EC retention on a surface, rather than the adhesion force attaching the confluent monolayer to the surface; hence, conclusions about the effect of underlying chemistry on the adhesion of a final monolayer to the synthetic material are not clear.
EC thrombogenicity on biomaterials
Despite the numerous studies into EC adhesion and proliferation on biomaterials there have been far fewer which consider the function of EC when cultured in vitro. The vascular tone related properties of EC have been studied primarily in the context of in vivo pathology and the treatment of vascular disease, with a focus on signaling pathways, or in co-culture studies addressing the effect of vasoconstriction molecules on SMC behaviour. Vascular tone properties are not considered further here, but reviews are available [126,127]. Here we focus on the thrombogenic function of EC in vitro.
EC thrombogencity has been characterized under static conditions using a number of approaches both in terms of anti-thrombotic function and anti-fibrinolytic function. The term thrombogenicity has been used loosely and it is perhaps misleading to use this term to cover both thrombosis and fibrinolysis considering one relates to the ability of the EC to prevent thrombus formation while the other relates to the ability of the cells to dissolve a thrombus once formed. This is an important distinction. In the context of the devices being designed using EC, in which the function of the layer is to provide a non-thrombogenic surface, the former of these functions is likely more relevant.
Thrombogenicity can be probed in three ways, with increasing degree of complexity and experimental sophistication; the expression and activity of individual molecules which influence thrombosis; studies exposing EC to components of blood, the outcome of which is dependent on the combination of molecules being expressed by the EC; or studies exposing EC to whole blood, the outcome of which again depends on the balance of EC molecules expressed and the interactions among the different processes which occur in thrombosis. While the first two strategies are useful diagnostic tools (eg to probe mechanisms), only whole blood studies give an overall measure of biomaterial substrate associated EC thrombogenicity. Whole blood studies (and some of the other approaches) can be compromised by loss of EC (eg due to poor adhesion) so that the underlying thrombogenic substrate can contribute to the experimental outcome. This may explain why experimental studies in the past have focused on cell adhesion and biomaterial coverage. Despite the experimental difficulties in assessing EC thrombogenicity some data has been reported using each of the 3 experimental strategies described above.
Molecular expression studies
Overview of key trends
Analysis of different molecules provides information on different aspects of the EC thrombogenicity. Table 2 compares the different molecular tests that have been typically used to assess EC thrombogenicity on biomaterials and distinguishes what aspect of thrombogenicity each measures. Tissue factor (TF) and thrombomodulin (TM) are commonly characterized molecules as they are key players in the initiation of the coagulation cascade. Most TF studies have been performed on EC cultured on tissue culture polystyrene using ELISA, to characterize expression, or chromogenic substrate assays, to characterize activity (ability to convert FX to FXa), and have found low or undetectable levels of TF in non-stimulated confluent layers of HOTMEC [128], HUVEC [129–131], and BAEC [132]. TF was also observed to be low or undetectable in un-stimulated HUVEC on gelatin coated TCPS [133] and fibronectin coated TCPS [134]. Thrombomodulin expression (ELISA) and activity (ability to activate protein C in the presence of thrombin) was high on non-stimulated HUVEC [130,135–137] and BAEC [132] on TCPS and fibronectin coated TCPS [134] and decreased significantly on activation with an agonist. Thrombomodulin gene expression was also found to be high in HUVEC on TCPS and fibronectin coated TCPS [134,137]. Very few studies on the molecular expression profiles of EC in porous scaffolds, for tissue-engineering applications, have been reported. Work done in our lab measured the TF and TM expression and activity on HUVEC cultured on the surface of endotoxin-free collagen modules. These modules were subsequently assembled into a modular tissue-engineered porous construct [138,139]. TF was found to be low and TM was found to be high consistent with the previous studies described above. The combination of high TM and low TF suggests the EC exhibit a non-thrombogenic phenotype on these biomaterials.
Table 2.
Methods for characterization of particular molecules
| Molecule | Function | Expression in non-activated EC | Common Test Method |
|---|---|---|---|
| Prostacyclin (PGI2) | anti-platelet aggregation | Low | Radio-immunoassay or enzyme immunoassay |
| Von Willebrand factor (vWF) | platelet adhesion | High storage but low secretion of active vWF | Immunostaining (expression and location)
ELISA on supernatant (amount secreted) |
| I-CAM, V-CAM, PECAM | blood cell adhesion | Low | Western blot (total expression)
Confocal, ELISA, flow cytometry (expression on cell surface) |
| Thrombomodulin (TM) | anti-fibrin generation | High | Immunostaining, ELISA (expression on cell surface)
Chromogenic assay (activity) |
| Endothelial cell protein C receptor (EPCR) | anti-fibrin generation | High | Western blot (total expression)
Immunostaining, ELISA (expression on cell surface) |
| Tissue Factor (TF) | fibrin generation | Low | Immunostaining, ELISA (expression on cell surface)
Chromogenic assay (activity) |
| Tissue factor pathway inhibitor (TFPI) | anti-fibrin generation | High | Chromogenic assay (activity) |
| Tissue plasminogen activator (tPA) | fibrinolysis | Low | Western blot (total expression)
ELISA (amount secreted) Chromogenic assay (activity) |
| Plasminogen activator inhibitor-1 (PAI-1) | anti-fibrinolysis | High | Western blot (total expression)
ELISA (amount secreted) Chromogenic assay (activity) |
As an alternative strategy to characterize thrombogenicity, Von Willebrand Factor (vWF), prostacylin (PGI2) and the cell adhesion molecules VCAM-1, ICAM-1, PECAM and E-selectin have been measured as an indicator of endothelium adhesiveness towards platelets and leukocytes. Measurements suggested EC in culture exhibited a non-activated (non-thrombogenic) phenotype. vWF secretion was found to be low in non-stimulated HUVEC on TCPS [140] and on fibronectin coated polystyrene sodium sulfonate microcarriers [141]. Similarly PGI2 secretion, measured using immuno- or radio-immuno- assays, was low in non-stimulated HUVEC and BAEC cultured on plastic microspheres (Biosilon®) and dishes [142,143] and carbodiimide crosslinked gelatin and albumin gels [144]. HUVEC, analyzed by flow cytometry after removal from TCPS, Dacron, and Teflon showed no E-selectin and low levels of VCAM-1, independent of substrate, and HOTMEC showed no VCAM-1 and low levels of E-selectin. PECAM and ICAM-1 levels were similar in both cell types [145]. The process required to remove the cells from the initial culture substrate however complicates drawing conclusions from this flow cytometry study. Treatments of the cells to enable molecular measurement have the potential to alter the phenotype of the EC and distort results. Nonetheless the trends implied from the combination of all these studies suggest that non-stimulated EC in culture are non-adhesive to platelets and leukocytes.
Finally another alternative set of molecules to assess thrombogenicity is tPA and PAI-1. tPA and PAI-1 secretion are commonly measured to assess the fibrinolytic capacity of EC on biomaterials. It is important to note that tPA levels in un-activated EC are expected to be low and PAI-1 levels high, mimicking what is seen in vivo where only 20% of the tPA present in plasma is active due to the large excess of PAI-1. In addition it is interesting to note that tPA and PAI are usually measured by assessing their secretion into the culture medium. However, on some substrates PAI is released into the ECM and not into the culture medium. The ECM bound PAI still remains active [146] however. In vitro levels of secreted PAI-1 are in significant excess of tPA levels in human retinal EC [147] and HUVEC [148–150]as seen in vivo, suggesting a non-fibrinolytic and un-activated EC phenotype in culture.
Taken together, these molecular characterization studies suggest that on certain biomaterials, certain EC appear to have a non-thrombogenic phenotype. Activity studies, when possible, are likely more useful than expression values since it is the relative amounts of many different molecules that determine the thrombogenic state of the EC. Furthermore it is important to be aware of the sensitivity of the measurement technique. Measuring zero activity or expression of a particular molecule may result from a lack of sensitivity in the measurement technique used to quantify it. Regardless, the main difficultly in drawing conclusions from this sort of data is how “low” or “high” molecular expression/activity on the cultured EC relates to the in vivo non-thrombogenic or activated situation respectively. It is thus not possible to rule out the conclusion that cultured EC may be partially activated in the presence of the biomaterial. It is thus not possible to rule out the conclusion that cultured EC may be partially activated in the presence of the biomaterial. Systematic studies comparing molecular expression profiles on different materials are therefore necessary to rigorously quantify what is “low” and what is “high” and to determine the effect of an underlying biomaterial.
Effect of biomaterial substrate on molecular expression
A limited number of studies have systematically investigated the effects of biomaterial substrates on EC thrombogenicity (all under static conditions). Some reports have concluded there is no effect of substrate (Table 3) while others suggested that the substrate is influential (Table 4). Drawing definitive conclusions about the effect of surface composition on thrombogenicity from many of these studies is troublesome, however, as often a number of confounding factors have not been controlled.
Table 3.
Molecular based studies suggesting substrate does not affect EC thrombogenicity.
| Molecules tested | Cell type | Substrate material | Results | Ref. |
|---|---|---|---|---|
| TF and PGI2 | Human umbilical vein | Dacron, PET graft | No difference between materials but surface roughness had an effect | 153 |
| TF, TM, MCP-1 | Human aortic
Human pulmonary Human dermal microvascular |
Fibronectin and two plasma treated polymers* of different hydrophobicity | No difference among materials but cell source had an effect | 171 |
| TM | Human umbilical vein
Human omentum microvascular |
ePTFE
TCPS |
No difference between materials but cell source had an effect | 154 |
| TM | Human saphenous vein | ePTFE
TCPS |
No difference between materials but surface roughness had an effect | 155 |
| tPA, PAI-1, vWF | Human aortic | Polymers of different hydrophilicity with or without Fn coating | Differences seen on Fn substrates only at early time points but no differences at confluence | 164 |
| vWF and TF | Human umbilical vein | EDC-NHS crosslinked heparinized collagen
TCPS |
No differences | 165 |
| vWF | Human umbilical vein | Fn coated heparin like microcarriers
TCPS |
No differences | 141 |
| tPA, PAI-1 PGI2 | Human umbilical vein | EDC-NHS crosslinked heparinized collagen with different concentration of crosslinks | No differences | 150 |
| PGI2 | Human umbilical vein | Carbodiimide crosslinked gelatin and albumin gels | Reduction in PGI2 over time in culture with no differences between materials by day 5 | 144 |
| VCAM, ICAM, PECAM, ELAM | Human umbilical vein | Dacron
TCPS |
No differences | 151, 163 |
gamma-butyrolactone and N-vinyl-2-pyrrolidone
Fn = fibronectin, PS = polystyrene, TCPS = tissue culture PS, EDC = N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide, NHS = N-hydroxysuccinimide
Table 4.
Molecular based studies suggesting substrate affects EC thrombogenicity.
| Molecule tested | Cell type | Substrate material | results | Ref |
|---|---|---|---|---|
| TM, vWF PECAM VE-cadherin | Human umbilical vein | TCPS
Decellularised fibroblast matrix |
On decellularised matrix more PECAM, VE-cadherin and lower vWF. Also TM higher and constant over time compared to TCPS on which TM decreased over time. | 156 |
| TM, vWF | Human umbilical vein | albumin and sulphated chitosan coated polyester, TCPS | On polyester TM lower and vWF secretion higher | 157 |
| PGI2 | Bovine aortic | Fn, Laminin (of varying concentration) and gelatin coated TCPS | PGI2 increased post confluence on Fn and laminin coatings but not gelatin. Laminin conc. controlled pre and post confluence secretion levels | 173 |
| PGI2 | Bovine aortic
Porcine aortic |
TCPS coated with different concentration of poly-HEMA to control “adhesivity” | On higher “adhesivity” surface more distinct F-actin structure and higher PGI2 secretion | 166 |
| PGI2, vWF | Human umbilical vein | Fn-TCPS, crosslinked and non-crosslinked collagen | Small but significant difference in PGI2 and vWF secretion on TCPS versus crosslinked collagens | 140 |
| tPA, PGI2 | Human saphenous vein | ePTFE with or without coating of Fn, collagen-1 or mixture of both | Higher levels of PGI2 and tPA on Fn-ePTFE and ePTFE versus samples with collagen or collagen-Fn | 167 |
| PGI2, PAI-1, tPA | Human saphenous vein | Plastic, gelatin, collagen-1 gel, Fn, fibrin glue, de-endothelized porcine aorta, ePTFE precoated with collagen-I | No difference in basal levels of PGI2. tPA secretion significantly higher on collagen-1 gels but not on the collagen-1 precoated ePTFE. PAI higher on the collagen 1, gelatin, fibrin glue and porcine aorta. | 158 |
| PGI2, PAI-1, tPA | Human umbilical vein | PET, polypropylene | On PET significant less PGI2 and PAI-1 compared to polypropylene but tPA no different. | 168 |
| PGI2, PAI-1, tPA | Human umbilical vein | Ammonia plasma modified PTFE coated with Fn, collagen-1 or gelatin | Different levels of PGI2, PAI-1 and tPA between coatings and unmodified versus modified PTFE. | 148 |
| tPA and PAI-1 | Human umbilical vein | TCPS, PTFE and polyurethanes all coated with Fn | On Fn coated PTFE and polyurethanes higher secretion of PAI and tPA than on fn –TCPS, More PAI-1 deposited in underlying matrix on PTFE | 149 |
| PGI2, PAI-1, tPA | Human umbilical vein | Woven Dacron, double velour Dacron, Dacron-urethane and carbon coated Dacron urethane | Variations seen in PGI2 among surfaces. tPA secretion higher on Dacron-urethane and PAI-1 levels similar on all surfaces | 159 |
| tPA, PAI-1, vWF | Human umbilical vein | TCPS and PVC coated with gelatin, collagen and Fn | On PVC substrates, tPA increased, vWF secretion decreased and PAI-1increased (only PVC coated with collagen or gelatin). | 169 |
| ICAM | Human umbilical vein
Human saphenous vein |
Fn-ePTFE, PTFE, Fn-TCPS, TCPS | ICAM expression increased on ePTFE surface | 160,161, 162 |
| ELAM-1 | Human umbilical vein | PET with or without layer or pyrolytic carbon | ELAM-1 lower on PET with carbon layer | 215,216 |
| P-selectin | Human umbilical vein | Knitted PET, TCPS | P-selectin increased on knitted PET | 189 |
| ICAM, VCAM | Human umbilical vein | PS or TCPS both coated with Fn | No difference in ICAM but VCAM lower on PS. | 174 |
Fn = fibronectin, PS = polystyrene, TCPS = tissue culture PS, PET = poly(ethylene terephthalate)
Surface topography has been shown to be a key influence on EC behaviour. For example, different levels of ELAM are expressed on HUVEC cultured on knitted versus woven Dacron [151] and different neutrophil adhesion [152], tissue factor and prostacyclin levels have been observed on Dacron grafts versus smooth films [153]. The goal of many of the studies listed [154–163] was to compare particular vascular graft materials, with or without different ECM coatings, to TCPS, and thus differences can potentially be attributed to topography rather than surface chemistry.
EC function has also been shown to depend on cell density and level of confluence [140]. A number of studies have noted significant differences in initial EC function dependent on the underlying substrate, which become insignificant when confluence is reached [140,144,164,165]. For example, bovine and porcine EC cultured sparsely on TCPS, coated with different concentrations of poly(HEMA) to vary adhesiveness, showed variations in F-actin and cell shape which correlated with levels of prostacyclin [166]. This is consistent with data reporting an initial dependence of prostacyclin secretion on substrate material. Initially cell spreading, and hence prostacyclin secretion, was dictated by the surface properties of the biomaterial, while post confluence, when cell density influences shape more than the underlying material, differences in secretion levels among substrates became insignificant. This observed dependence of EC function on the level of cell confluence introduces uncertainty into much of the reported data in the literature. Many investigators have reported substrate dependent differences in pre-confluent samples without normalizing secretion levels for the different cell densities reported for each of the test substrates [167–169]. Even in cases where enough time is given to allow confluence to be reached on all the materials [149] being compared, changes in EC junction composition [149] that occur after confluence may result in differences which confound any effects seen among different substrates.
The absence or presence of serum in the culture medium to perform the experiments can also confound the molecular measurements. ECM controls EC function in part through a balance of alterations in cell shape, cytoskeletal organization and integrin binding. Available EC binding sites on a biomaterial substrate are dependent on the layer of proteins that adsorbs on the surface either from serum in the culture medium or if the surface is coated prior to use with a particular ECM component. Most biomaterial studies are performed in medium containing serum, resulting in deposition of vitronectin (Vn) [170]. This could account for the lack of substrate dependence seen in some studies where no other ECM coating layer was deposited on the biomaterial and binding sites on the surface are provided only by the protein adsorbed from the culture medium. For example, when human aortic and pulmonary artery EC and dermal microvascular EC were cultured on 2 different plasma polymers (gamma-butyrolactone and N-vinyl-2-pyrrolidone) of varying hydrophilicity no differences in levels of TM, TF or monocyte chemotactic protein-1 were reported [171].
In cases where an intentional ECM coating was deposited on the biomaterial surface to provide binding sites for cell adhesion, differences in EC thrombogenicity have been attributed to the availability and type of binding sites, presented by the particular ECM coating and adsorbed protein surface layer, for cells, growth factors and other mediating molecules. The surface properties of the underlying biomaterial determines the conformation of the adsorbed ECM coating, and hence the available binding sites and EC behaviour, at least up until a time point when the ECM is remodeled by the EC. For example, confluent HUVEC seeded on ammonia plasma modified PTFE coated with fibronectin, collagen I or gelatin exhibited different levels of PGI2, PAI-1 and tPA among coatings and on unmodified versus modified PTFE [148]. In a separate study HUVEC cultured on fibronectin coated PTFE and polyurethanes secreted more PAI and tPA than on fibronectin coated TCPS [149]. The general explanation of these kinds of observations is that the particular ECM coating composition defines the potential binding sites available. In another example, HUVEC seeded on vitronectin or fibronectin coated TCPS, cultured in medium depleted of both factors to prevent alterations of the culture surfaces by adsorption, showed significant differences in PAI-1 secretion. On vitronectin PAI-1 was bound within the ECM, while on fibronectin PAI-1 was secreted into the culture medium [172]. In yet another example, BAEC seeded on fibronectin, gelatin or laminin coated TCPS produced variable levels of prostacyclin (PGI2) both pre- and post-confluence. Varying the laminin concentration controlled sub- and post-confluence secretion levels while antibody blocking of the fibronectin receptor resulted in a 43% reduction in post confluent secretion levels [173]. Furthermore, consistent with these kinds of findings is the result that collagen cross-link density, which should not significantly alter the available binding sites at the surface, did not alter the secretion of PGI2, tPA or PAI-1 [150]. Not all molecules were sensitive to the underlying substrate. VCAM-1 was different in HUVEC cultured on fibronectin coated polystyrene and TCPS compared to uncoated polystyrene or TCPS. ICAM-1 however, was not dependent on the substrate. The authors attributed this difference to the integrin sensitive nature of VCAM-1 compared to the nature of ICAM-1, which is more dependent on complement products in the culture medium serum [174]. The sensitivity of different molecules to ECM substrate composition likely varies, depending on the signal transduction pathways that control their expression.
Overall molecular studies suggested that confluent EC display a non-thrombogenic profile of molecules on the majority of biomaterials tested. At sub-confluent densities however, EC appeared to be more thrombogenic. Different substrate chemistries did not produce significantly different EC behaviour when using a biomaterial with no ECM and serum-containing medium. In light of the studies into cell adhesion and growth discussed previously, it is likely that biomaterial chemistry dictates the composition and conformation of the protein layer deposited on the surface from the culture medium. This in turn dictates cell shape, cytoskeletal organization, integrin binding and ultimately cell thrombogenicity. Biomaterials coated in different ECM did show some variation in the resulting EC thrombogenicity to some extent, at least at some time points. However, the underlying mechanisms that result in one ECM/biomaterial combination producing slightly different levels of a particular molecule to another ECM/biomaterial combination have not been elucidated. The utility of this information in terms of principles of biomaterial design is therefore limited. Furthermore, despite these somewhat systematic studies, the issue of “low” and “high” measured molecular expression/activity values are on the cultured EC relative to the in vivo situation and the question of how much these measured values reflect the EC behaviour when in contact with blood remains unclear.
Studies exposing EC to components of blood
While the study of individual molecules gives a reasonable prediction of EC thrombogenicity, tests considering the consequences on blood, or components of the blood, in contact with EC seeded biomaterials reflect the combination effect of several molecules. On the other hand, molecular studies, especially those based on microscopy, are less compromised by incomplete substrate coverage issues. They enable prediction of expected molecular behaviour were there no defects in coverage, but do not account for the complexities of thrombosis. Simplified test systems, incorporating only selected components of blood, are useful for diagnosing the isolated contribution to thrombosis of the different reactions which can occur, while whole blood studies more closely reflect overall thrombogenicity resulting from the contribution of all thrombosis reactions and the interplay among them. In this section we consider data from experiments based on selected blood components; in the next section, we discuss whole blood studies.
Overview of key trends
Table 5 lists the typical blood component studies that have been used to characterize EC thrombogenicity. The most popular methods were platelet and leukocyte adhesion and activation studies performed under static conditions. Static conditions reduces the chance of losing attached EC, which could lead to confounding results from the exposed underlying substrate material. Surprisingly, plasma recalcification times, commonly used to characterize the thrombogenicity of biomaterials, have been rarely used in studies involving EC. Table 6 summarizes blood component studies performed on different biomaterials and indicates the flow conditions used for each study. These studies suggested overall that EC exhibit a relatively non-thrombogenic phenotype on a number of common polymer substrates with or without ECM coatings. Limited platelet adhesion and activation (serotonin release and spreading) occurs on non-stimulated HUVEC cultured on TCPS [175–177], fibrillar collagen [178], crosslinked albumin heparin gels with [179] and without heparin [180], thermanox coverslips [181] and fibronectin coated polyethylene [182]. Similar trends were also observed for BAEC cultured on TCPS [183,184], and for sheep pulmonary artery EC cultured on gelatinized polycarbonate or TCPS [185]. In a study by Dekker et al. similar, low platelet adhesion was observed on both HUVEC and human smooth muscle cells in a perfusion flow experiment [182], however on activation of the platelets, prior to perfusion through the circuit, significant deposition was induced only on the smooth muscle surface suggesting that inhibiting platelet-deposition is a property unique to endothelial cells. Low neutrophil adhesion was reported on non-stimulated ovine pulmonary artery EC cultured on TCPS [186] and BAEC cultured on microcarrier beads [187] (polymer unknown).
Table 5.
Characterization methods using blood components
| Blood component | Function characterized | Result for non-activated EC | Combination of molecules dominating response |
|---|---|---|---|
| Recalcified plasma | Fibrin generation | Long recalcification time | TF, TFPI, TM, AT |
| Platelets | Platelet adhesion Platelet activation | Low platelet adhesion and spreading and low (serotonin) release | Prostacyclin, vWF, NO, PECAM |
| Leukocytes | WBC adhesion WBC activation | Low adhesion and activation | V-CAM, I-CAM, E-selectin, PECAM |
| Fibrin dissolution | Fibrinolysis | Functional EC will lyse clot | tPA, PAI-1 |
AT Antithrombin
TFPI Tissue factor pathway inhibitor
TM Thrombomodulin
TF Tissue factor
NO Nitric oxide
tPA Tissue plasminogen actiator
PAI-1 Plasminogen activator inhibitor type 1
Table 6.
Studies using blood components (platelets, neutrophils, fibrinogen/fibin or plasma) to characterize EC thrombogenicity
| Cell type | Substrate material | Test system | Measurements | Result | Ref |
|---|---|---|---|---|---|
| Human umbilical vein | TCPS | Citrate PRP under flow conditions within cone and plate device. | platelet adhesion, release and fibrin deposition | Limited platelet adhesion observed on un-stimulated endothelial cells | 175 |
| Human umbilical vein | Type I collagen | Pre and post confluent EC incubated with platelet suspension for 40 mins. | platelet adhesion and morphology | Low platelet adhesion to EC but adhesion to collagen and pre-confluent EC surfaces | 178 |
| Bovine EC and fibroblasts | TCPS | Incubated with PRP | platelet adhesion and morphology | Low platelet adhesion on EC versus fibroblasts and no evidence of granule release. | 183 |
| Human umbilical vein | Glass coverslips | Pre and post confluent EC incubated with platelet suspension for 15 mins with shaking | Platelet adhesion and morphology | Platelet adhesion low on confluent EC and glass but high on pre-confluent EC. In pre-confluent EC platelets adhere to EC not exposed extracellular matrix | 176 |
| Sheep pulmonary artery | Gelatin coated polycarbonate | Incubation with washed platelet suspension for 60 mins | Platelet adhesion, morphology and serotonin release | Platelet adhesion and spreading on unactivated EC. | 185 |
| Bovine aortic | TCPS | Incubated with washed platelet suspension for 15 mins | Platelet adhesion and morphology | <3% platelet adhesion on EC similar to native aortic segment. | 184 |
| Human umbilical vein | Crosslinked albumin gels with and without heparin | Incubation with washed platelet suspension for 60 mins. (tests performed 4 h after seeding cells) | Platelet adhesion and morphology | Presence of EC reduced platelet adhesion on all places with least adhesion on albumin-heparin gels. | 179 |
| Human umbilical vein | CO2 treated PS and albumin-heparin gels with and without Fn coating | Incubation with washed platelet suspension for 60 mins. (tests performed 4h after seeding cells) | Platelet adhesion and morphology | Platelet adhesion low on EC surfaces. Surfaces seeded at higher EC density have higher platelet adhesion. No platelet spreading | 180 |
| Human umbilical vein | Fn coated PE | Perfusion of platelets, RBC and fibrinogen perfused at shear of 300 s−1 or 600 s−1 for 5 min. | Platelet adhesion and morphology | Low platelet adhesion on confluent EC. Increased adhesion in pre-confluent cells which varies with shear rate. | 182 |
| Bovine aortic and bovine corneal | TCPS uncoated and coated in Fn, coll-I, coll-III, coll-IV, coll-V | Incubation with PRP for 30 mins with shaking | Platelet adhesion and morphology, serotonin release, thromboxane B2 release. | Low platelet adhesion and activation by EC monolayer. Exposed ECM caused platelet aggregation | 188 |
| Human umbilical vein | Themanox | Incubation with PRP for 15 min. to 60 min. | Platelet adhesion and serotonin release | Similar platelet adhesion was observed on un-activated EC and themanox, with number dependent on time. | 190 |
| Canine aortic | TCPS and Fn coated fluoropore substrate | Incubation with labeled fibrinogen for30 min. | Fibrin deposition | Fibrin deposition dependent on level of cell confluence. | 191 |
| Ovine pulmonary artery | TCPS | Incubation with neutrophil suspension | Neutrophil adhesion | Low neutrophil adhesion on unactivated EC | 186 |
| Human umbilical vein | Fn coated Knitted PET and TCPS | Incubation with neutrophil suspension for 1h | Neutrophil adhesion | Neutrophil adhesion higher on PET than TCPS but both could be increased with TNF activation of the EC | 189 |
| Human retinal | TCPS | Fibrin lysis assay | Clot dissolution time | Clot lysis occurred fasted in TGF-β treated cells | 147 |
| Human umbilical vein and ometum microvascular | Fn coated TCPS | Fibrin lysis assay | Clot dissolution time | HOTMEC lysed clot in 2–3h but HUVEC caused no lysis. HOTMEC produce 100 × more tPA than HUVEC when activated. | 217 |
| Human umbilical vein and bovine aortic | Thermanox | Incubation with platelet or leukocyte suspensions for 30 min. | Leukocyte and platelet adhesion | Low platelet adhesion to both EC types. Low leukocyte adhesion to HUVEC but higher levels on BAEC. | 181 |
| Human omentum microvascular and umbilical vein | TCPS | Recalcified plasma added to 96 well plate | Plasma recalcification time(turbidity measurement) | Plasma clotting initiated faster on EC than on plastic but the rate of clotting was slower. Clotting rate on cells increased when protein C deficient plasma was used. | 154 |
Fn = fibronectin, coll = collagen, PRP = platelet rich plasma
In our lab, plasma recalcification time on HUVEC seeded on collagen modules was compared to that on lipopolysacharide activated HUVEC-collagen modules and on collagen modules alone (unpublished data, manuscript in preparation). Such modules can be used to form modular tissue-engineered constructs. Activated HUVEC modules consistently showed shorter recalcification times for both platelet rich and platelet poor plasma. No difference in recalcification times was observed for collagen only and non-activated HUVEC-collagen modules. We attribute this latter result to the test surface:background material surface ratio that results from performing this in a 96 well plate and to the inappropriateness of the assay to compare a material surface versus a cellular surface (see discussion below on ”experimental complexities” in whole blood section).
As in the molecular characterization studies, a similar problem exists of how “low” adhesion/activation levels on the non-activated EC are relative to the in vivo situation. In most studies the control to which non-activated EC are compared is lipopolysaccharide or tumour necrosis factor alpha activated EC. Such comparisons allow only limited conclusions regarding the biomaterial effect to be made from the already limited data.
Dominant factors influencing thrombogenicity measurement
The composition of the underlying biomaterial does not appear to have a significant effect on EC thrombogenicity based on the limited data available from studies using blood components. For example BAEC and corneal EC cultured on TCPS coated with fibronectin, collagen I, III, IV and V showed similar, low platelet adhesion with any exposure of the ECM producing platelet aggregation [188]. Low platelet adhesion and activation is characteristic of EC from a number of sources on a number of common polymer substrates. Some differences were observed in polymorphonuclear neutrophil adhesion to HUVEC cultured on knitted Dacron coated with fibronectin compared to TCPS, however, EC coverage on the materials differed [189]. Systematic studies on different biomaterials, controlling for the level of cell confluence, coverage and the roughness of the substrate, are necessary to confirm any conclusions regarding the effect of a biomaterial on EC behaviour in contact with blood components. Various reports highlight the importance of carefully controlling a number of factors to ensure comparisons among different experimental groups are valid (see below).
Cell density is a critical factor in determining thrombogenicity, as was also suggested by the molecular characterization studies. For example non-confluent HUVEC cultured on thermanox, showed a linear relation between EC numbers seeded and adherent platelets [190]. In a different kind of assay measuring fibrin deposition from blood plasma, canine aortic EC on both TCPS and microporous polymer (fluropore filter) with and without a fibronectin coating showed reduced fibrin deposition, that was dependent on the proliferative state of the cells [191]. Moreover in pre-confluent EC cultures platelet adhesion has been observed not just in regions of the exposed underlying matrix but also on the non-confluent EC themselves [176,178,179,183,185] suggesting that the EC are thrombogenic at non-confluent densities.
Experimental set-up is a critical factor influencing the thrombogenicity measurement. Most experiments described above are static, involving the incubation of an EC surface with a mixture of blood components, which does not mimic the transport dynamics of in vivo thrombosis. Since platelet adhesion dominates at high shear, a static system measuring platelet adhesion may not be an accurate measure of EC thrombogenicity. For example, different results on the same EC have been observed using rocking versus laminar flow experimental systems [192]. Furthermore alterations in platelet adhesion with shear level have been seen in a capillary flow circuit using HUVEC cultured on fibronectin coated polyethylene [182]. Another influential component of experimental set-up is blood source and anticoagulant used. Some studies have suggested differential adhesion of human leukocytes on human and non-human EC [190,193]. Although trends are not clear, immune reactions between EC and leukocytes from different species also have the potential to alter EC behaviour in response to any other blood components in the test system. For example the importance of leukocytes in EC response was highlighted by Rose et al. who demonstrated significantly decreased plasma recalcification times for EC treated with activated leukocytes [194].
Overall studies measuring EC function using blood component studies suggest that EC exhibit a non-thrombogenic phenotype, at least relative to activated EC, and that the underlying biomaterial, assuming 100% coverage of the cells, does not influence the EC thrombogenicity. However, systematic studies are necessary to confirm these latter conclusions due to the limited data available. Furthermore in our experience it is not clear that the resolution of the current experimental assays is sufficient to elucidate significant differences among different substrates. More precise blood component assays to quantify thrombogenicity may therefore be necessary to observe such a relationship.
Studies exposing EC to whole blood
Experimental studies using whole blood offer the most realistic measurements of overall thrombogenicity. There are a number of possible experimental systems and endpoint measurements, of varying logistical difficulty, which have been used to assess different aspects of the thrombosis process. Blood can be supplied from a fresh reservoir, directly from the blood donor, from an ex vivo shunt, or from a direct connection, in vivo, to a blood vessel. A fresh reservoir is logistically easier but requires the addition of an anticoagulant to prevent blood thrombosis in the reservoir container. A blood supply directly from the donor removes the need for an anticoagulant but requires a blood draw from the donors for the duration of the experiment and there is limited control of blood flow rate into the test system. Ex vivo and in vivo experimental systems are likely more clinically relevant but are experimentally more complex and require animal models.
Exposure of the endothelial cells to the blood can be done under static conditions, with gentle rocking or stirring, or under continuous flow. Furthermore the time of blood exposure to the endothelial cells can be continuous, flow with recirculation or flow with a one-time pass only. Static conditions are experimentally simpler but do not recapitulate the dynamics of thrombosis. Rocking or shaking setups enable some motion of the blood while remaining relatively simple to perform. Flow studies, while most accurately recapitulate the dynamics of thrombosis, are complicated to develop and perform and risk loss of EC from the surface resulting in exposure of the underlying substrate material.
There are also a variety of endpoint measurements. Table 7 describe the characteristics and endpoint measurements of different systems and highlight the advantages and difficulties of each. Ideally an experimental system, while maximizing logistical simplicity, should incorporate some element of flow to mimic the transport dynamics that occur during a natural thrombotic event, use minimal or no anticoagulant, and minimize the time that blood is out of contact with an endothelium surface.
Table 7.
Endpoint measurement options in whole blood experiments
| End point | Related characteristic | Measurement methods |
|---|---|---|
| Clot time/flow rate | Thrombus formation | Observation and timed collection |
| Blood viscosity | Thrombosis initiation and progression | Rheometer |
| Analysis of exposed surface | ||
| Platelet adhesion and spreading | Platelet adhesion and activation | SEM, radio-labeling |
| Leukocyte adhesion and spreading | Leukocyte adhesion and activation | SEM, radio-labeling |
| Fibrin/thrombus deposition | Thrombus accumulation | SEM, radio-labeling |
| Analysis of exposed blood | ||
| Fibrinopeptide A | Fibrin production | Chromogenic substrate |
| Thrombin-antithrombin complex (TAT) | Thrombin production | Chromogenic substrate |
| Prothrombin fragment (F1+2) | Prothrombin conversion (i.e. thrombin generation) | Chromogenic substrate |
| Thrombin activity | Thrombin activity | Chromogenic or fibrinogen substrate |
| Platelet activation | Platelet activation | Flow cytometry, serotonin secretion |
| Microparticle formation | Platelet activation | Flow cytometry |
| Leukocyte activation | Leukocyte activation | Flow cytometry |
Overview of key trends
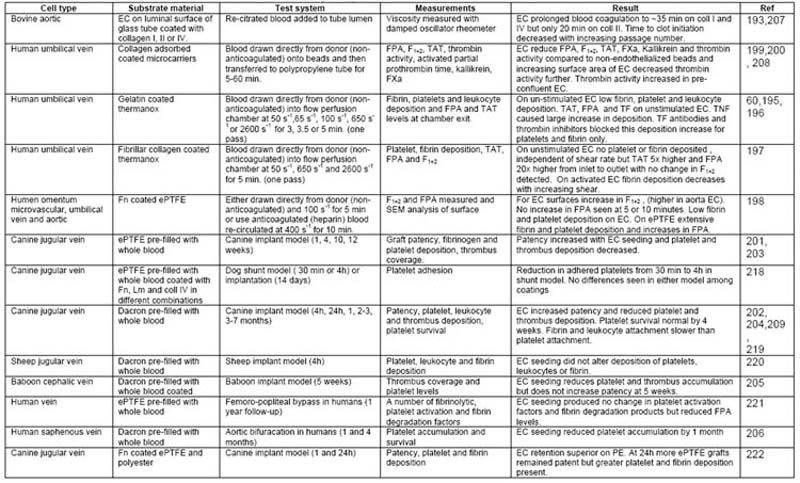
Table 8 outlines whole blood studies of different complexity performed on EC cultured on a variety of substrates. In general these studies suggested that non-stimulated EC provide a less thrombogenic surface than their underlying ECM substrate. HUVEC cultured on gelatin [60,195,196] or fibrillar collagen [197] coated thermanox slides connected directly to an antecubital vein (non-anticoagulated blood source) exhibited low levels of fibrin (< 1% coverage), platelet (<2% coverage) and leukocyte (< 5 cells/10mm cross section) deposition; and TAT (<5 μg/L), F1+2 (<1.5 nmol/L) and FPA (<10 μg/L) generation. Similarly human umbilical, human omentum microvascular, and human aortic EC cultured on fibronectin coated ePTFE connected directly to an antecubital vein (non-anticoagulated blood supply), showed reduced platelet aggregation, fibrin deposition and FPA (<700 ng/mL after 5 minutes min) compared to unseeded grafts [198]. The presence of HUVEC cultured on Biosilon microcarriers, with a high EC surface to blood volume ratio, reduced levels of TAT, F1+2, FPA, by 88, 47 and 81% respectively and reduced thrombin activity (to almost 0 u.mL), FXa (to almost 0 μg/mL) and kallikrein (to almost 0 PEU/mL) [199,200] compared to uncoated microcarriers. Moreover, in vivo models using canine [201–204], baboon [205] and human [206] EC seeded grafts report reduced platelet adhesion, prolonged platelet survival times and reduced thrombus deposition. In activated EC, fibrin, leukocyte and platelet deposition and TAT levels increased as expected. TF antibodies have been shown to block fibrin and platelet deposition and decrease TAT levels [60,61] but not block leukocyte adhesion [196], consistent with molecular characterization studies which indicate that EC activation results in up-regulation of tissue factor and leukocyte specific receptors on the EC surface.
Table 8.
Whole blood studies used to assess EC thrombogenicity.
Few whole blood studies have been performed on porous tissue-engineered constructs. Studies performed in our lab, involving rocking HUVEC covered collagen modules or collagen only modules in slightly heparanized whole blood, demonstrated significantly longer thrombosis times when HUVEC were present [138]. Furthermore perfusion with slightly heparinized whole blood of a modular construct assembled from HUVEC covered collagen modules resulted in minimal platelet loss relative to background (no construct present) [138]. This indicates that the seeded HUVEC were behaving in a non-thrombogenical fashion (at least relative to the control) within the modular construct.
There have been few systematic studies investigating the effect of EC substrate on EC thrombogenicity using whole blood testing. Most studies have been conducted in the context of vascular graft development whereby EC are seeded in serum containing medium, on fibronectin or collagen coated polymers or vascular grafts pre-clotted with blood. Few studies have characterized EC phenotype on other ECM components, likely due to the logistical difficulty involved in performing reproducible whole blood experiments. Moreover most experiments that incorporate flow are conducted over a 5–10 min duration, which provides but a limited indication of EC thrombogenicity.
Despite the experimental complexities of whole blood studies, these data are the most compelling evidence that a confluent layer of EC cultured on a biomaterial displays a non-thrombogenic phenotype. As with the other types of study, comparative measures are generally not made between the in vitro and in vivo situations (unless in vivo implantation studies are being used to assess EC function) but comparing performance with and without the presence of EC is likely a better control than comparing activated and non-activated EC. In order to draw the correct conclusions from the data reported it is important to appreciate the experimental complexities involved in designing a useful whole blood study that accurately reflects EC thrombogenicity.
Experimental complexities
From the limited data available, thrombosis initiation has been found to depend on passage number [207], for example thrombosis initiation time decreased from passage 5 to 12; and the EC surface area to blood volume ratio [208], for example increasing the ratio of EC area to blood volume by a factor of 2 resulted in a 5 fold reduction in thrombin generation. EC coverage is also important, for example, in dogs with an implanted Dacron vascular graft seeded with canine eternal jugular vein EC, platelet survival times were only completely restored to normal levels in animals with grafts exhibiting 100% cell coverage [209]. These observations are consistent with trends observed in molecular characterization and blood component studies.
The flow regime implemented in the experimental set up is perhaps the most dominant factor shown to influence whole blood thrombogenicity measurements. On activated EC or exposed ECM, at lower flow rates (100 s−1), thrombi tend to be rich in fibrin [198]. On the other hand at high flow rates (>400 s−1) fibrin deposition is reduced [60,61], perhaps due to a reduced residence time at the endothelium surface, and platelet adhesion is increased [197]. The end point measurement must be appropriate to the type of thrombotic response expected under the flow regime of the test system. For example, in a static system, comparing platelet adhesion to EC cultured on different substrates is less reflective of thrombogenicity than measures of fibrin generation.
The appropriate choice of endpoint is dependent is also on the surfaces being compared. For example, if EC are being compared to activated EC, measurements of platelet activation (under high flow conditions) or measurements of different components of the coagulation cascade, which reflect fibrin generation, (under low flow conditions) are both appropriate. However, if EC are being compared to unseeded biomaterial surfaces not all measurements are appropriate. On unseeded biomaterials thrombosis initiation is driven primarily by the activation of platelets and leukocytes, as opposed to tissue factor driven fibrin generation that occurs on the EC surface. Therefore, under experimental conditions that favour platelet rich thrombi over fibrin rich thrombi, EC are more likely to perform better. Specifically high flow rate platelet studies will generate the most significant differences between seeded and unseeded surfaces, while static studies measuring fibrin generation are less likely to demonstrate significant differences in thrombogenicity, particularly if endpoint measurements are made after only a short period of blood exposure. This is consistent with our observations (see above) that static plasma recalcification studies show no difference in the thrombogenicity of HUVEC covered collagen modules relative to collagen only modules, while whole blood flow studies do show a significant difference in thrombogenicity.
In most experimental studies, the presence of an EC monolayer on a biomaterial surface almost completely prevented platelet adhesion but only reduced, rather than fully suppressed, fibrin generation i.e. fibrin generation eventually occurred within the system even when the EC were present. This was perhaps due to the low EC surface-to-blood volume ratios used for many experiments. In vivo the average EC surface-to-blood volume ratio is between 560 and 1440 cm2/mL [199]. Initiators of the coagulation cascade are present in low concentrations within the blood priming the system. EC must therefore, actively prevent initiation of fibrin generation by the secretion and expression of several molecules discussed in earlier sections. A sufficient number of anti-thrombogenic molecules must be available per mL of blood to maintain the balance of factors in the blood and prevent initiation of the coagulation cascade. Platelet adhesion and activation is likely much less sensitive to this ratio however. Platelet activation is not actively prevented by EC under non-stimulated conditions. Therefore, when the EC surface area-blood volume ratio is low, experiments using platelet related end point measurements are more likely to demonstrate low EC thrombogenicity. Alternatively high surface area-volume ratios should be used. For example EC cultured on microspheres produced complete fibrin suppression [208].
EC also adapt to flow while biomaterial surfaces do not. For example, decreased thrombin generation is observed at high shear (650 – 2600 s−1) on EC surfaces but not material surfaces, likely due to changes in TFPI and TM. Experiments performed at a shear stress, which optimizes the anti-thrombogenic properties of the EC, are therefore more likely to demonstrate low EC thrombogenicity.
Considering each of these issues, the experimental conditions under which EC surfaces are more likely to exhibit lower thrombogenicity than unseeded biomaterial surfaces, can be identified. In experiments using platelet associated endpoint measurements, performed at high flow rates, where platelets deposition is more dominant than fibrin deposition, EC are more likely to appear less thrombogenic than unseeded biomaterial surfaces. The key point is that the endpoint measurements selected should be appropriate for the dominant thrombotic process that occurs under the given experimental conditions. If the appropriate endpoint measure is not selected the utility of the conclusions may be somewhat limited.
Summary and Conclusions
The endothelium is a dynamic interface which when quiescent actively prevents thrombosis and when activated drives thrombosis by mediating each of the underlying thrombosis processes. Because thrombosis must be actively inhibited in the un-activated state, the system is primed such that faster local amplification is achieved when activation occurs. Different processes dominate the thrombosis response at different flow rates appropriate to the extent of repair necessary for the injury. For example in large arteries, under high pressure and flow, a greater platelet component is required to seal the wound than in a small capillary. Furthermore, because platelet deposition and fibrin generation are controlled by a different combination of molecules, EC in different vascular locations are specialized to mediate the thrombotic processes that occur under the flow regime of their particular location.
EC seeding of biomaterial vascular grafts and scaffolds to improve device thrombogenicity is a popular strategy, which presumes that EC cultured on biomaterials will express a non-thrombogenic phenotype. In vivo EC behaviour is controlled by mechanical and chemical cues, generated by the ECM, peripheral cells, growth factors and other signaling molecules, and forces imposed by the flowing, pressurized blood. On a biomaterial surface, the number and type of cell binding sites on the material surface, created using ECM coatings or by protein adsorption from the culture medium, initially mediate the chemical and mechanical cues which determine EC thrombogenicity. Over time the secretion of ECM from the EC layer and the attainment of cell confluence alter the relevant mechanical and chemical cues and the resulting cell thrombogenicity. Once cell confluence has been reached it is not clear whether the underlying biomaterial has any significant effect on the seeded EC phenotype. Indeed, there is no unequivocal data in the literature which clearly indicates that the biomaterial substrate has an effect on confluent EC phenotype and particularly EC thrombogenicity. The biomaterial substrate may influence the time to reach confluence, if confluence is reached at all, or it may influence the retention of the cells under flow, but not the phenotype of the attached confluent cells, at least in the absence of activating blood components. In the literature, characterization of EC phenotype has focused primarily on cell adhesion and spreading. The underlying assumption that EC will display a non-thrombogenic phenotype when cultured on a biomaterial seems to have been assumed, perhaps due to early success observed with seeded vascular grafts. The task of generating a confluent layer to provide 100% coverage of the material thus became the focus of experimental efforts. Much of the data from cell adhesion studies is however inconclusive and difficult to compare due to the use of different experimental systems, and to a lack of control over experimental variable that are now know to be important. It is critical that studies in the future take account of these parameters and that the community decide on common quantitative measurements to report such that different studies are easily compared.
EC thrombogenicity has been measured in only a limited number of studies with different degrees of complexity. Molecular studies provide information on individual molecules. Blood component studies allow the different processes that occur during thrombosis to be studied separately under simplified conditions. Whole blood studies provide a more global picture, which results from a combination of all molecules and the interactions among the different processes that contribute to thrombosis. The advantage of studies using blood components or whole blood is the potential to incorporate flow into the experimental test system. Studies performed under static conditions have been popular for EC thrombogenicity characterization due to their logistical simplicity, but are not necessarily reflective of the surface thrombogenicity under flow conditions where reactant and product transport is highly relevant. An important conclusion is that the endpoint parameter selected should be appropriate for the process of thrombosis occurring under the flow regime used in the experiment. Inappropriate endpoints may result in conclusions of limited utility.
Molecular characterization studies suggest that confluent EC exhibit a non-thrombogenic phenotype when cultured on a number of biomaterials. These are consistent with the general trends reported for blood component and whole blood studies, which indicate that EC display a surface non-adhesive to platelets and leukocytes; and that minimal fibrin deposition occurs on EC seeded surfaces. Few studies however, have considered the phenotype of cultured EC relative to EC in vivo and therefore it is unclear how non-thrombogenic EC actually are. A few studies have attempted to compare EC thrombogenicity on different biomaterials. While some molecular studies have suggested an influence of the underlying polymer substrate, the precision of the quantification methods used in blood studies are unlikely to show significant differences among EC cultured on different polymers and ECM. Furthermore, the significance of the underlying polymeric substrate properties when confluence has been reached remains unclear as many studies are confounded by variations in surface texture and cell density. Methods of quantification and standardized formats of reporting data to enable true comparison both among different biomaterials (with different surface structure) within a single study, and among different studies, remains an issue that must be addressed before such relationships can be determined. Furthermore an understanding of the underlying molecular mechanisms that produce particular EC phenotypes will be critical if specialized biomaterial surfaces, enabling a specific EC phenotype, are to be rationally designed. For example studies of EC gene expression on different biomaterials at different cell densities and under different states of activation may prove insightful.
Nothwithstanding the experimental complexities, the most compelling evidence that EC display a non-thrombogenic phenotype on artificial materials comes from the whole blood data. Unfortunately, significant experimental infrastructure and personnel is necessary to perform blood experiments to a high standard and to observe subtle differences in results. Careful in vivo studies of blood vessel thrombogenicity, after initial molecular and blood component studies, may therefore be the most appropriate strategy for the future.
Taken together, the general trends reported suggest that the formation of a confluent non-thrombogenic EC layer on the surface of an artificial biomaterial is possible. This is critical to the success of EC seeding strategies for the development of clinically effective vascular grafts and tissue-engineered constructs. However, non-thrombogenic is measured relative to a control consisting of activated cells or the unseeded biomaterial. It is likely that there is a spectrum of possible EC activation states, ranging from fully non-thrombogenic through to varying degress of pro-thrombogenic. It therefore remains unclear from the current literature if the EC are non-thrombogenic enough for the clinical applications envisioned. Ultimately, an understanding of artificial tissue integration and tissue and material remodeling during the process of wound healing will be necessary to achieve the level of product integration likely required to produce normal tissue function.
Acknowledgments
We acknowledge the financial support of the National Institute of Health (EB001013, co-investigators, E. Yeo, A. Gotlieb) and the Natural Sciences and Engineering Research Council for funding, AM acknowledges the fellowship support of the Province of Ontario, the Canadian Institutes of Health Research Training Program in Regenerative Medicine and the Canadian Rhodes Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams SK. Endothelial cell transplantation. Cell Transplant. 1995;4(4):401–10. doi: 10.1177/096368979500400411. [DOI] [PubMed] [Google Scholar]
- 2.Kaihara S, Kim S, Kim BS, Mooney DJ, Tanaka K, Vacanti JP. Survival and function of rat hepatocytes cocultured with nonparenchymal cells or sinusoidal endothelial cells on biodegradable polymers under flow conditions. J Pediatr Surg. 2000;35(9):1287–90. doi: 10.1053/jpsu.2000.9298. [DOI] [PubMed] [Google Scholar]
- 3.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 4.Ong AT, Serruys PW. Drug-eluting stents: current issues. Tex Heart Inst J. 2005;32(3):372–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 6.Salacinski HJ, Tiwari A, Hamilton G, Seifalian AM. Cellular engineering of vascular bypass grafts: role of chemical coatings for enhancing endothelial cell attachment. Med Biol Eng Comput. 2001;39(6):609–18. doi: 10.1007/BF02345431. [DOI] [PubMed] [Google Scholar]
- 7.Tassiopoulos AK, Greisler HP. Angiogenic mechanisms of endothelialization of cardiovascular implants: a review of recent investigative strategies. J Biomater Sci Polym Ed. 2000;11(11):1275–84. doi: 10.1163/156856200744200. [DOI] [PubMed] [Google Scholar]
- 8.Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25(26):5681–703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Colman RW. In: Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Colman VJM RW, Clowes AW, George JN, Goldhaber SZ, editors. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 10.Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81(3):734–44. [PubMed] [Google Scholar]
- 11.Jesty J, Beltrami E, Willems G. Mathematical analysis of a proteolytic positive-feedback loop: dependence of lag time and enzyme yields on the initial conditions and kinetic parameters. Biochemistry. 1993;32(24):6266–74. doi: 10.1021/bi00075a021. [DOI] [PubMed] [Google Scholar]
- 12.Blajchman MA, Ozge-Anwar AH. The role of the complement system in hemostasis. Prog Hematol. 1986;14:149–82. [PubMed] [Google Scholar]
- 13.Pearson JD. Endothelial cell function and thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12(3):329–41. doi: 10.1053/beha.1999.0028. [DOI] [PubMed] [Google Scholar]
- 14.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30(43):10363–70. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 15.Esmon CT. Coagulation inhibitors in inflammation. Biochem Soc Trans. 2005;33(Pt 2):401–5. doi: 10.1042/BST0330401. [DOI] [PubMed] [Google Scholar]
- 16.Ryan J, Brett J, Tijburg P, Bach RR, Kisiel W, Stern D. Tumor necrosis factor-induced endothelial tissue factor is associated with subendothelial matrix vesicles but is not expressed on the apical surface. Blood. 1992;80(4):966–74. [PubMed] [Google Scholar]
- 17.Maynard JR, Dreyer BE, Stemerman MB, Pitlick FA. Tissue-factor coagulant activity of cultured human endothelial and smooth muscle cells and fibroblasts. Blood. 1977;50(3):387–96. [PubMed] [Google Scholar]
- 18.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Muller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80(6):1008–14. [PubMed] [Google Scholar]
- 19.Schorer AEMCF. Production of tissue factor. In: Ryan US, editor. Endothelial cells. CRC Press; 1988. [Google Scholar]
- 20.Mulder AB, Hegge-Paping KS, Magielse CP, Blom NR, Smit JW, van der Meer J, Hallie MR, Bom VJ. Tumor necrosis factor alpha-induced endothelial tissue factor is located on the cell surface rather than in the subendothelial matrix. Blood. 1994;84(5):1559–66. [PubMed] [Google Scholar]
- 21.Esmon CT. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. Faseb J. 1995;9(10):946–55. doi: 10.1096/fasebj.9.10.7615164. [DOI] [PubMed] [Google Scholar]
- 22.Esmon CT. Regulation of blood coagulation. Biochim Biophys Acta. 2000;1477(1–2):349–60. doi: 10.1016/s0167-4838(99)00266-6. [DOI] [PubMed] [Google Scholar]
- 23.Sandset PM. Tissue factor pathway inhibitor (TFPI)--an update. Haemostasis. 1996;26 (Suppl 4):154–65. doi: 10.1159/000217293. [DOI] [PubMed] [Google Scholar]
- 24.Marcum JA, McKenney JB, Rosenberg RD. Acceleration of thrombin-antithrombin complex formation in rat hindquarters via heparinlike molecules bound to the endothelium. J Clin Invest. 1984;74(2):341–50. doi: 10.1172/JCI111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntyre TM, Zimmerman GA, Satoh K, Prescott SM. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985;76(1):271–80. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman HPJD. Extracellular metabolism of nucleotides and adenosine in the cardio- vascular system. In: Burnstock GDJ, Liang BT, Linden J, editors. Cardiovascular Biology of Purines. Boston: Kluwer; 1998. pp. 342–358. [Google Scholar]
- 27.Jaffe EA, Hoyer LW, Nachman RL. Synthesis of von Willebrand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974;71(5):1906–9. doi: 10.1073/pnas.71.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moncada S. Eighth Gaddum Memorial Lecture. University of London Institute of Education, December 1980. Biological importance of prostacyclin. Br J Pharmacol. 1982;76(1):3–31. doi: 10.1111/j.1476-5381.1982.tb09186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radomski MW, Palmer RM, Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987;148(3):1482–9. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman GA, McIntyre TM, Mehra M, Prescott SM. Endothelial cell-associated platelet-activating factor: a novel mechanism for signaling intercellular adhesion. J Cell Biol. 1990;110(2):529–40. doi: 10.1083/jcb.110.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114(5–6):447–53. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers Arterioscler. Thromb Vasc Biol. 1997;17(7):1193–202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 33.Tedesco F, Fischetti F, Pausa M, Dobrina A, Sim RB, Daha MR. Complement-endothelial cell interactions: pathophysiological implications. Mol Immunol. 1999;36(4–5):261–8. doi: 10.1016/s0161-5890(99)90054-8. [DOI] [PubMed] [Google Scholar]
- 34.Lidington EA, Haskard DO, Mason JC. Induction of decay-accelerating factor by thrombin through a protease-activated receptor 1 and protein kinase C-dependent pathway protects vascular endothelial cells from complement-mediated injury. Blood. 2000;96(8):2784–92. [PubMed] [Google Scholar]
- 35.Hamilton KK, Ji Z, Rollins S, Stewart BH, Sims PJ. Regulatory control of the terminal complement proteins at the surface of human endothelial cells: neutralization of a C5b-9 inhibitor by antibody to CD59. Blood. 1990;76(12):2572–7. [PubMed] [Google Scholar]
- 36.Zetter BR. Endothelial heterogeneity: influence of vessel size, organ location and species specificity on the properties of cultured endothelial cells. In: Ryan US, editor. Endothelial Cells. CRC press; 1988. [Google Scholar]
- 37.Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20(11 Suppl 2):II-3–10. [PubMed] [Google Scholar]
- 38.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 39.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–42. [PubMed] [Google Scholar]
- 40.Ortega Mateo A, de Artinano AA. Highlights on endothelins: a review. Pharmacol Res. 1997;36(5):339–51. doi: 10.1006/phrs.1997.0246. [DOI] [PubMed] [Google Scholar]
- 41.Bhatt AD. Endothelins and anti-endothelins. J Assoc Physicians India. 1997;45(11):868–72. [PubMed] [Google Scholar]
- 42.Lopes-Martins R, Catelli M, Araujo C, Estato V, Cordeiro R, Tibirica E. Pharmacological evidence of a role for platelet activating factor as a modulator of vasomotor tone and blood pressure. Eur J Pharmacol. 1996;308(3):287–94. doi: 10.1016/0014-2999(96)00310-x. [DOI] [PubMed] [Google Scholar]
- 43.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 44.Hajjar KA. Cellular receptors in the regulation of plasmin generation. Thromb Haemost. 1995;74(1):294–301. [PubMed] [Google Scholar]
- 45.Schleef RR, Bevilacqua MP, Sawdey M, Gimbrone MA, Jr, Loskutoff DJ. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J Biol Chem. 1988;263(12):5797–803. [PubMed] [Google Scholar]
- 46.Rijken DC, Hoylaerts M, Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982;257(6):2920–5. [PubMed] [Google Scholar]
- 47.Ford JW, Burkel WE, Kahn RH. Isolation of adult canine venous endothelium for tissue culture. In Vitro. 1981;17(1):44–50. doi: 10.1007/BF02618029. [DOI] [PubMed] [Google Scholar]
- 48.Dong QG, Bernasconi S, Lostaglio S, De Calmanovici RW, Martin-Padura I, Breviario F, Garlanda C, Ramponi S, Mantovani A, Vecchi A. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants Arterioscler. Thromb Vasc Biol. 1997;17(8):1599–604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- 49.Knedler A, Eckel RH, Kern PA, Ham RG. Microvascular endothelial cell cultures from human omental adipose tissue. In Vitro Cell Dev Biol. 1989;25(10):863–4. doi: 10.1007/BF02623995. [DOI] [PubMed] [Google Scholar]
- 50.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8(4):498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirota T, He H, Yasui H, Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9(1):127–36. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 52.Szmitko PE, Fedak PW, Weisel RD, Stewart DJ, Kutryk MJ, Verma S. Endothelial progenitor cells: new hope for a broken heart. Circulation. 2003;107(24):3093–100. doi: 10.1161/01.CIR.0000074242.66719.4A. [DOI] [PubMed] [Google Scholar]
- 53.Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol. 2005;45(10):1574–9. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 54.Speiser W, Anders E, Preissner KT, Wagner O, Muller-Berghaus G. Differences in coagulant and fibrinolytic activities of cultured human endothelial cells derived from omental tissue microvessels and umbilical veins. Blood. 1987;69(3):964–7. [PubMed] [Google Scholar]
- 55.Imbert E, Poot AA, Figdor CG, Feijen J. Different growth behaviour of human umbilical vein endothelial cells and an endothelial cell line seeded on various polymer surfaces. Biomaterials. 1998;19(24):2285–90. doi: 10.1016/s0142-9612(98)00137-9. [DOI] [PubMed] [Google Scholar]
- 56.Lang I, Pabst MA, Hiden U, Blaschitz A, Dohr G, Hahn T, Desoye G. Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. Eur J Cell Biol. 2003;82(4):163–73. doi: 10.1078/0171-9335-00306. [DOI] [PubMed] [Google Scholar]
- 57.Antonov AS, Key NS, Smirnov MD, Jacob HS, Vercellotti GM, Smirnov VN. Prothrombotic phenotype diversity of human aortic endothelial cells in culture. Thromb Res. 1992;67(2):135–45. doi: 10.1016/0049-3848(92)90133-u. [DOI] [PubMed] [Google Scholar]
- 58.Hewett PW, Murray JC. Human microvessel endothelial cells: isolation, culture and characterization. In Vitro Cell Dev Biol Anim. 1993;29A(11):823–30. doi: 10.1007/BF02631356. [DOI] [PubMed] [Google Scholar]
- 59.Kumar S, West DC, Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987;36(1):57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 60.Diquelou A, Dupouy D, Gaspin D, Constans J, Sie P, Boneu B, Sakariassen KS, Cadroy Y. Relationship between endothelial tissue factor and thrombogenesis under blood flow conditions. Thromb Haemost. 1995;74(2):778–83. [PubMed] [Google Scholar]
- 61.Zwaginga JJ, de Boer HC, MJ IJ, Kerkhof A, Muller-Berghaus G, Gruhlichhenn J, Sixma JJ, de Groot PG. Thrombogenicity of vascular cells. Comparison between endothelial cells isolated from different sources and smooth muscle cells and fibroblasts. Arteriosclerosis. 1990;10(3):437–48. doi: 10.1161/01.atv.10.3.437. [DOI] [PubMed] [Google Scholar]
- 62.Glassberg MK, Nolop KB, Jackowski JT, Abraham WM, Wanner A, Ryan US. Microvascular and macrovascular endothelial cells produce different constrictor substances. J Appl Physiol. 1992;72(5):1681–6. doi: 10.1152/jappl.1992.72.5.1681. [DOI] [PubMed] [Google Scholar]
- 63.Geiger M, Stone A, Mason SN, Oldham KT, Guice KS. Differential nitric oxide production by microvascular and macrovascular endothelial cells. Am J Physiol. 1997;273(1 Pt 1):L275–81. doi: 10.1152/ajplung.1997.273.1.L275. [DOI] [PubMed] [Google Scholar]
- 64.Pohlman TH, Stanness KA, Beatty PG, Ochs HD, Harlan JM. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986;136(12):4548–53. [PubMed] [Google Scholar]
- 65.Laszik Z, Mitro A, Taylor FB, Jr, Ferrell G, Esmon CT. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation. 1997;96(10):3633–40. doi: 10.1161/01.cir.96.10.3633. [DOI] [PubMed] [Google Scholar]
- 66.Wojta J, Hoover RL, Daniel TO. Vascular origin determines plasminogen activator expression in human endothelial cells. Renal endothelial cells produce large amounts of single chain urokinase type plasminogen activator. J Biol Chem. 1989;264(5):2846–52. [PubMed] [Google Scholar]
- 67.Levin EG, Santell L, Osborn KG. The expression of endothelial tissue plasminogen activator in vivo: a function defined by vessel size and anatomic location. J Cell Sci. 1997;110 ( Pt 2):139–48. doi: 10.1242/jcs.110.2.139. [DOI] [PubMed] [Google Scholar]
- 68.Sage H, Pritzl P, Bornstein P. Secretory phenotypes of endothelial cells in culture: comparison of aortic, venous, capillary, and corneal endothelium. Arteriosclerosis. 1981;1(6):427–42. doi: 10.1161/01.atv.1.6.427. [DOI] [PubMed] [Google Scholar]
- 69.Canfield AE, Wren FE, Schor SL, Grant ME, Schor AM. Aortic endothelial cell heterogeneity in vitro. Lack of association between morphological phenotype and collagen biosynthesis. J Cell Sci. 1992;102 ( Pt 4):807–14. doi: 10.1242/jcs.102.4.807. [DOI] [PubMed] [Google Scholar]
- 70.Ho M, Yang E, Matcuk G, Deng D, Sampas N, Tsalenko A, Tabibiazar R, Zhang Y, Chen M, Talbi S, et al. Identification of endothelial cell genes by combined database mining and microarray analysis. Physiol Genomics. 2003;13(3):249–62. doi: 10.1152/physiolgenomics.00186.2002. [DOI] [PubMed] [Google Scholar]
- 71.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100(19):10623–8. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liaw L, Schwartz SM. Comparison of gene expression in bovine aortic endothelium in vivo versus in vitro. Differences in growth regulatory molecules. Arterioscler Thromb. 1993;13(7):985–93. doi: 10.1161/01.atv.13.7.985. [DOI] [PubMed] [Google Scholar]
- 73.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–61. [PubMed] [Google Scholar]
- 74.Risau W. Differentiation of endothelium. Faseb J. 1995;9(10):926–33. [PubMed] [Google Scholar]
- 75.Underwood PA, Bean PA, Gamble JR. Rate of endothelial expansion is controlled by cell:cell adhesion. Int J Biochem Cell Biol. 2002;34(1):55–69. doi: 10.1016/s1357-2725(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 76.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91(10):877–87. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 77.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L419–22. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 78.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81(3):173–82. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schnaper HW, Kleinman HK. Regulation of cell function by extracellular matrix. Pediatr Nephrol. 1993;7(1):96–104. doi: 10.1007/BF00861587. [DOI] [PubMed] [Google Scholar]
- 80.Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999;112 ( Pt 12):1915–23. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- 81.Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron MO, Sulpice E, Scaife R, Alemany M, Vernet T. Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem. 1998;273(45):29786–93. doi: 10.1074/jbc.273.45.29786. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz SM, Heimark RL, Majesky MW. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70(4):1177–209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- 83.Ingber D. Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J Cell Biochem. 1991;47(3):236–41. doi: 10.1002/jcb.240470309. [DOI] [PubMed] [Google Scholar]
- 84.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L529–33. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 85.Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85(6):504–14. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- 86.Girard PR, Nerem RM. Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J Cell Physiol. 1995;163(1):179–93. doi: 10.1002/jcp.1041630121. [DOI] [PubMed] [Google Scholar]
- 87.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. Embo J. 2001;20(17):4639–47. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thoumine O, Nerem RM, Girard PR. Changes in organization and composition of the extracellular matrix underlying cultured endothelial cells exposed to laminar steady shear stress. Lab Invest. 1995;73(4):565–76. [PubMed] [Google Scholar]
- 89.Chiquet M, Matthisson M, Koch M, Tannheimer M, Chiquet-Ehrismann R. Regulation of extracellular matrix synthesis by mechanical stress. Biochem Cell Biol. 1996;74(6):737–44. doi: 10.1139/o96-080. [DOI] [PubMed] [Google Scholar]
- 90.Ives CL, Eskin SG, McIntire LV. Mechanical effects on endothelial cell morphology: in vitro assessment. In Vitro Cell Dev Biol. 1986;22(9):500–7. doi: 10.1007/BF02621134. [DOI] [PubMed] [Google Scholar]
- 91.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31(1 Pt 2):162–9. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 92.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2001;98(16):8955–60. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patrick CW, Jr, McIntire LV. Shear stress and cyclic strain modulation of gene expression in vascular endothelial cells. Blood Purif. 1995;13(3–4):112–24. doi: 10.1159/000170194. [DOI] [PubMed] [Google Scholar]
- 94.Knudsen HL, Frangos JA. Role of cytoskeleton in shear stress-induced endothelial nitric oxide production. Am J Physiol. 1997;273(1 Pt 2):H347–55. doi: 10.1152/ajpheart.1997.273.1.H347. [DOI] [PubMed] [Google Scholar]
- 95.Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun. 1989;161(2):859–64. doi: 10.1016/0006-291x(89)92679-x. [DOI] [PubMed] [Google Scholar]
- 96.Chan BP, Reichert WM, Truskey GA. Effect of streptavidin-biotin on endothelial vasoregulation and leukocyte adhesion. Biomaterials. 2004;25(18):3951–61. doi: 10.1016/j.biomaterials.2003.10.077. [DOI] [PubMed] [Google Scholar]
- 97.Grabowski EF, Lam FP. Endothelial cell function, including tissue factor expression, under flow conditions. Thromb Haemost. 1995;74(1):123–8. [PubMed] [Google Scholar]
- 98.Diamond SL, Sharefkin JB, Dieffenbach C, Frasier-Scott K, McIntire LV, Eskin SG. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J Cell Physiol. 1990;143(2):364–71. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- 99.Diamond SL, Eskin SG, McIntire LV. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science. 1989;243(4897):1483–5. doi: 10.1126/science.2467379. [DOI] [PubMed] [Google Scholar]
- 100.Sampath R, Kukielka GL, Smith CW, Eskin SG, McIntire LV. Shear stress-mediated changes in the expression of leukocyte adhesion receptors on human umbilical vein endothelial cells in vitro. Ann Biomed Eng. 1995;23(3):247–56. doi: 10.1007/BF02584426. [DOI] [PubMed] [Google Scholar]
- 101.Westmuckett AD, Lupu C, Roquefeuil S, Krausz T, Kakkar VV, Lupu F. Fluid flow induces upregulation of synthesis and release of tissue factor pathway inhibitor in vitro. Arterioscler Thromb Vasc Biol. 2000;20(11):2474–82. doi: 10.1161/01.atv.20.11.2474. [DOI] [PubMed] [Google Scholar]
- 102.Grabowski EF, Reininger AJ, Petteruti PG, Tsukurov O, Orkin RW. Shear stress decreases endothelial cell tissue factor activity by augmenting secretion of tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2001;21(1):157–62. doi: 10.1161/01.atv.21.1.157. [DOI] [PubMed] [Google Scholar]
- 103.Malek AM, Jackman R, Rosenberg RD, Izumo S. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ Res. 1994;74(5):852–60. doi: 10.1161/01.res.74.5.852. [DOI] [PubMed] [Google Scholar]
- 104.Takada Y, Shinkai F, Kondo S, Yamamoto S, Tsuboi H, Korenaga R, Ando J. Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem Biophys Res Commun. 1994;205(2):1345–52. doi: 10.1006/bbrc.1994.2813. [DOI] [PubMed] [Google Scholar]
- 105.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227(4693):1477–9. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 106.Bhagyalakshmi A, Frangos JA. Mechanism of shear-induced prostacyclin production in endothelial cells. Biochem Biophys Res Commun. 1989;158(1):31–7. doi: 10.1016/s0006-291x(89)80172-x. [DOI] [PubMed] [Google Scholar]
- 107.Galbusera M, Zoja C, Donadelli R, Paris S, Morigi M, Benigni A, Figliuzzi M, Remuzzi G, Remuzzi A. Fluid shear stress modulates von Willebrand factor release from human vascular endothelium. Blood. 1997;90(4):1558–64. [PubMed] [Google Scholar]
- 108.Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107(11):4354–63. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 109.Baker KS, Williams SK, Jarrell BE, Koolpe EA, Levine E. Endothelialization of human collagen surfaces with human adult endothelial cells. Am J Surg. 1985;150(2):197–200. doi: 10.1016/0002-9610(85)90118-7. [DOI] [PubMed] [Google Scholar]
- 110.Macarak EJ, Howard PS. Adhesion of endothelial cells to extracellular matrix proteins. J Cell Physiol. 1983;116(1):76–86. doi: 10.1002/jcp.1041160112. [DOI] [PubMed] [Google Scholar]
- 111.Madri JA, Williams SK. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983;97(1):153–65. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kirkpatrick CJ, Kampe M, Fischer EG, Rixen H, Richter H, Mittermayer C. Differential expansion of human endothelial monolayers on basement membrane and interstitial collagens, laminin and fibronectin in vitro. Pathobiology. 1990;58(4):221–5. doi: 10.1159/000163588. [DOI] [PubMed] [Google Scholar]
- 113.Form DM, Pratt BM, Madri JA. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986;55(5):521–30. [PubMed] [Google Scholar]
- 114.Underwood PA, Bennett FA. The effect of extracellular matrix molecules on the in vitro behavior of bovine endothelial cells. Exp Cell Res. 1993;205(2):311–9. doi: 10.1006/excr.1993.1091. [DOI] [PubMed] [Google Scholar]
- 115.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273(5661):345–9. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 116.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 117.Berge N, Loganadane LD, Vassy J, Monnet E, Legrand C, Fauvel-Lafeve F. Adhesion-induced intracellular signalling in endothelial cells depends on the nature of the matrix. Cell Adhes Commun. 1999;7(1):29–41. doi: 10.3109/15419069909034390. [DOI] [PubMed] [Google Scholar]
- 118.Pratt BM, Harris AS, Morrow JS, Madri JA. Mechanisms of cytoskeletal regulation. Modulation of aortic endothelial cell spectrin by the extracellular matrix. Am J Pathol. 1984;117(3):349–54. [PMC free article] [PubMed] [Google Scholar]
- 119.Madri JA, Pratt BM, Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988;132(1):18–27. [PMC free article] [PubMed] [Google Scholar]
- 120.Ingber DE, Madri JA, Folkman J. Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol. 1987;23(5):387–94. doi: 10.1007/BF02620997. [DOI] [PubMed] [Google Scholar]
- 121.Thomson GJ, Vohra RK, Carr MH, Walker MG. Adult human endothelial cell seeding using expanded polytetrafluoroethylene vascular grafts: a comparison of four substrates. Surgery. 1991;109(1):20–7. [PubMed] [Google Scholar]
- 122.Pompe T, Kobe F, Salchert K, Jorgensen B, Oswald J, Werner C. Fibronectin anchorage to polymer substrates controls the initial phase of endothelial cell adhesion. J Biomed Mater Res A. 2003;67(2):647–57. doi: 10.1002/jbm.a.10130. [DOI] [PubMed] [Google Scholar]
- 123.Burmeister JS, Vrany JD, Reichert WM, Truskey GA. Effect of fibronectin amount and conformation on the strength of endothelial cell adhesion to HEMA/EMA copolymers. J Biomed Mater Res. 1996;30(1):13–22. doi: 10.1002/(SICI)1097-4636(199601)30:1<13::AID-JBM3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 124.van Wachem PB, Hogt AH, Beugeling T, Feijen J, Bantjes A, Detmers JP, van Aken WG. Adhesion of cultured human endothelial cells onto methacrylate polymers with varying surface wettability and charge. Biomaterials. 1987;8(5):323–8. doi: 10.1016/0142-9612(87)90001-9. [DOI] [PubMed] [Google Scholar]
- 125.van Wachem PB, Beugeling T, Mallens BW, Dekker A, Feijen J, Bantjes A, van Aken WG. Deposition of endothelial fibronectin on polymeric surfaces. Biomaterials. 1988;9(1):121–3. doi: 10.1016/0142-9612(88)90083-x. [DOI] [PubMed] [Google Scholar]
- 126.Nadar S, Blann AD, Lip GY. Endothelial dysfunction: methods of assessment and application to hypertension. Curr Pharm Des. 2004;10(29):3591–605. doi: 10.2174/1381612043382765. [DOI] [PubMed] [Google Scholar]
- 127.Marsden PA, Goligorsky MS, Brenner BM. Endothelial cell biology in relation to current concepts of vessel wall structure and function. J Am Soc Nephrol. 1991;1(7):931–48. doi: 10.1681/ASN.V17931. [DOI] [PubMed] [Google Scholar]
- 128.Rubens FD, Labow RS, Meek E, Dudani AK, Ganz PR. Tissue factor expression by cells used for sodding of prosthetic vascular grafts. J Surg Res. 1997;72(1):22–8. doi: 10.1006/jsre.1997.5149. [DOI] [PubMed] [Google Scholar]
- 129.Andoh K, Pettersen KS, Filion-Myklebust C, Prydz H. Observations on the cell biology of tissue factor in endothelial cells. Thromb Haemost. 1990;63(2):298–302. [PubMed] [Google Scholar]
- 130.Moore KL, Andreoli SP, Esmon NL, Esmon CT, Bang NU. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987;79(1):124–30. doi: 10.1172/JCI112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mulder AB, Blom NR, Smit JW, Ruiters MH, van der Meer J, Halie MR, Bom VJ. Basal tissue factor expression in endothelial cell cultures is caused by contaminating smooth muscle cells. Reduction by using chymotrypsin instead of collagenase. Thromb Res. 1995;80(5):399–411. doi: 10.1016/0049-3848(95)00192-t. [DOI] [PubMed] [Google Scholar]
- 132.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163(3):740–5. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Crossman DC, Carr DP, Tuddenham EG, Pearson JD, McVey JH. The regulation of tissue factor mRNA in human endothelial cells in response to endotoxin or phorbol ester. J Biol Chem. 1990;265(17):9782–7. [PubMed] [Google Scholar]
- 134.Scarpati EM, Sadler JE. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem. 1989;264(34):20705–13. [PubMed] [Google Scholar]
- 135.Hackeng TM, Tans G, Koppelman SJ, de Groot PG, Rosing J, Bouma BN. Protein C activation on endothelial cells by prothrombin activation products generated in situ: meizothrombin is a better protein C activator than alpha-thrombin. Biochem J. 1996;319 ( Pt 2):399–405. doi: 10.1042/bj3190399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moore KL, Esmon CT, Esmon NL. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989;73(1):159–65. [PubMed] [Google Scholar]
- 137.Conway EM, Rosenberg RD. Tumor necrosis factor suppresses transcription of the thrombomodulin gene in endothelial cells. Mol Cell Biol. 1988;8(12):5588–92. doi: 10.1128/mcb.8.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci U S A. 2006;103(31):11461–6. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.She M, McGuigan AP, Sefton MV. Tissue factor and thrombomodulin expression on endothelial cell-seeded collagen modules for tissue engineering. J Biomed Mater Res A. 2007;80(2):497–504. doi: 10.1002/jbm.a.31083. [DOI] [PubMed] [Google Scholar]
- 140.Wissink MJ, Beernink R, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Relation between cell density and the secretion of von Willebrand factor and prostacyclin by human umbilical vein endothelial cells. Biomaterials. 2001;22(16):2283–90. doi: 10.1016/s0142-9612(00)00417-8. [DOI] [PubMed] [Google Scholar]
- 141.Najab-Benhayoun M, Serne H, Jozefowicz M, Fischer AM, Brisson C, Sultan Y. Human umbilical vein endothelial cell culture on heparin-like microcarriers. J Biomed Mater Res. 1993;27(4):511–20. doi: 10.1002/jbm.820270412. [DOI] [PubMed] [Google Scholar]
- 142.Davies PF. Microcarrier cultures in vascular endothelial research. Dev Biol Stand. 1981;50:125–36. [PubMed] [Google Scholar]
- 143.Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977;74(9):3922–6. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Penhoat J, Sigot-Luizard MF, Warocquier-Clerout R. Prostacyclin secretion by human endothelial cells grown on carbodiimide cross-linked proteins: an assessment of the cytocompatibility of a substratum. Biomaterials. 1993;14(7):503–6. doi: 10.1016/0142-9612(93)90237-v. [DOI] [PubMed] [Google Scholar]
- 145.Imbert E, Poot AA, Figdor CG, Feijen J. Expression of leukocyte adhesion molecules by endothelial cells seeded on various polymer surfaces. J Biomed Mater Res. 2001;56(3):376–81. doi: 10.1002/1097-4636(20010905)56:3<376::aid-jbm1106>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 146.Schleef RR, Podor TJ, Dunne E, Mimuro J, Loskutoff DJ. The majority of type 1 plasminogen activator inhibitor associated with cultured human endothelial cells is located under the cells and is accessible to solution-phase tissue-type plasminogen activator. J Cell Biol. 1990;110(1):155–63. doi: 10.1083/jcb.110.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wileman SM, Booth NA, Moore N, Redmill B, Forrester JV, Knott RM. Regulation of plasminogen activation by TGF-beta in cultured human retinal endothelial cells. Br J Ophthalmol. 2000;84(4):417–22. doi: 10.1136/bjo.84.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lu A, Sipehia R. Antithrombotic and fibrinolytic system of human endothelial cells seeded on PTFE: the effects of surface modification of PTFE by ammonia plasma treatment and ECM protein coatings. Biomaterials. 2001;22(11):1439–46. doi: 10.1016/s0142-9612(00)00302-1. [DOI] [PubMed] [Google Scholar]
- 149.Zhang JC, Wojta J, Binder BR. Growth and fibrinolytic parameters of human umbilical vein endothelial cells seeded onto cardiovascular grafts. J Thorac Cardiovasc Surg. 1995;109(6):1059–65. doi: 10.1016/S0022-5223(95)70188-5. [DOI] [PubMed] [Google Scholar]
- 150.Wissink MJ, van Luyn MJ, Beernink R, Dijk F, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Endothelial cell seeding on crosslinked collagen: effects of crosslinking on endothelial cell proliferation and functional parameters. Thromb Haemost. 2000;84(2):325–31. [PubMed] [Google Scholar]
- 151.Cenni E, Granchi D, Ciapetti G, Verri E, Cavedagna D, Gamberini S, Cervellati M, Di Leo A, Pizzoferrato A. Expression of adhesion molecules on endothelial cells after contact with knitted Dacron. Biomaterials. 1997;18(6):489–94. doi: 10.1016/s0142-9612(96)00160-3. [DOI] [PubMed] [Google Scholar]
- 152.Tunstall A, Eberhart RC, Prager MD. Endothelial cells on Dacron vascular prostheses: adherence, growth, and susceptibility to neutrophils. J Biomed Mater Res. 1995;29(10):1193–9. doi: 10.1002/jbm.820291006. [DOI] [PubMed] [Google Scholar]
- 153.Tunstall A, Eberhart RC, Prager MD. Comparison of tissue factor and prostacyclin production by human umbilical vein endothelial cells on Dacron vascular prostheses and Dacron smooth films. J Biomed Mater Res. 1994;28(10):1233–8. doi: 10.1002/jbm.820281013. [DOI] [PubMed] [Google Scholar]
- 154.Hedeman Joosten PP, Verhagen HJ, Heijnen-Snyder GJ, Sixma JJ, de Groot PG, Eikelboom BC, Elisen MG. Thrombomodulin activity of fat-derived microvascular endothelial cells seeded on expanded polytetrafluorethylene. J Vasc Res. 1999;36(2):91–9. doi: 10.1159/000025630. [DOI] [PubMed] [Google Scholar]
- 155.Gillis-Haegerstrand C, Frebelius S, Haegerstrand A, Swedenborg J. Cultured human endothelial cells seeded on expanded polytetrafluoroethylene support thrombin-mediated activation of protein C. J Vasc Surg. 1996;24(2):226–34. [PubMed] [Google Scholar]
- 156.Remy-Zolghadri M, Laganiere J, Oligny JF, Germain L, Auger FA. Endothelium properties of a tissue-engineered blood vessel for small-diameter vascular reconstruction. J Vasc Surg. 2004;39(3):613–20. doi: 10.1016/j.jvs.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 157.Remy M, Bareille R, Villars F, Rouais F, Gorodkov A, Bordenave L. Endothelial cells lining polyester fabric express pro-coagulant phenotype in vitro. Med Biol Eng Comput. 1998;36(2):256–7. doi: 10.1007/BF02510755. [DOI] [PubMed] [Google Scholar]
- 158.Gillis C, Bengtsson L, Wilman B, Haegerstrand A. Secretion of prostacyclin, tissue plasminogen activator and its inhibitor by cultured adult human endothelial cells grown on different matrices. Eur J Vasc Endovasc Surg. 1996;11(2):127–33. doi: 10.1016/s1078-5884(96)80040-2. [DOI] [PubMed] [Google Scholar]
- 159.Cenni E, Ciapetti G, Stea S, Di Leo A, Cavedagna D, Pizzoferrato A. Functional alterations of endothelial cells cultured in vitro in contact with biomaterials. ALTA. 1992;20:61. [Google Scholar]
- 160.Margiotta MS, Benton L, Greco RS. Endothelial cells adherent to expanded polytetrafluoroethylene express the intercellular adhesion molecule-1. J Am Coll Surg. 1995;181(3):215–9. [PubMed] [Google Scholar]
- 161.Margiotta M, Benton L, Robertson F, Greco RS. Role of adhesion molecules in leukocyte binding to endothelial cells adherent to vascular grafts. J Am Coll Surg. 1994;179(6):689–95. [PubMed] [Google Scholar]
- 162.Margiotta MS, Robertson FS, Greco RS. The adherence of endothelial cells to Dacron induces the expression of the intercellular adhesion molecule (ICAM-1) Ann Surg. 1992;216(5):600–4. doi: 10.1097/00000658-199211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Granchi D, Cenni E, Verri E, Ciapetti G, Gori A, Gamberini S, Di Leo A, Pizzoferrato A. Adhesive protein expression on human endothelial cells after in vitro contact with woven Dacron. Biomaterials. 1998;19(1–3):93–8. doi: 10.1016/s0142-9612(97)00161-0. [DOI] [PubMed] [Google Scholar]
- 164.Kottke-Marchant K, Veenstra AA, Marchant RE. Human endothelial cell growth and coagulant function varies with respect to interfacial properties of polymeric substrates. J Biomed Mater Res. 1996;30(2):209–20. doi: 10.1002/(SICI)1097-4636(199602)30:2<209::AID-JBM11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 165.Wissink MJ, Beernink R, Scharenborg NM, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Endothelial cell seeding of (heparinized) collagen matrices: effects of bFGF pre-loading on proliferation (after low density seeding) and pro-coagulant factors. J Control Release. 2000;67(2–3):141–55. doi: 10.1016/s0168-3659(00)00202-9. [DOI] [PubMed] [Google Scholar]
- 166.Dubose DA, Shepro D, Hechtman HB. Correlation among endothelial cell shape, F-actin arrangement, and prostacyclin synthesis. Life Sci. 1987;40(5):447–53. doi: 10.1016/0024-3205(87)90109-3. [DOI] [PubMed] [Google Scholar]
- 167.Li JM, Menconi MJ, Wheeler HB, Rohrer MJ, Klassen VA, Ansell JE, Appel MC. Precoating expanded polytetrafluoroethylene grafts alters production of endothelial cell-derived thrombomodulators. J Vasc Surg. 1992;15(6):1010–7. [PubMed] [Google Scholar]
- 168.Cenni E, Ciapetti G, Cavedagna D, Di Leo A, Pizzoferrato A. Production of prostacyclin and fibrinolysis modulators by endothelial cells cultured in the presence of polyethylene terephthalate. J Biomed Mater Res. 1993;27(9):1161–4. doi: 10.1002/jbm.820270906. [DOI] [PubMed] [Google Scholar]
- 169.Storck J, Del Razek A, Zimmermann ER. Effect of polyvinyl chloride plastic on the growth and physiology of human umbilical vein endothelial cells. Biomaterials. 1996;17(18):1791–4. doi: 10.1016/0142-9612(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 170.Underwood PA, Bennett FA. A comparison of the biological activities of the cell-adhesive proteins vitronectin and fibronectin. J Cell Sci. 1989;93 ( Pt 4):641–9. doi: 10.1242/jcs.93.4.641. [DOI] [PubMed] [Google Scholar]
- 171.Murugesan G, Rani MR, Ransohoff RM, Marchant RE, Kottke-Marchant K. Endothelial cell expression of monocyte chemotactic protein-1, tissue factor, and thrombomodulin on hydrophilic plasma polymers. J Biomed Mater Res. 2000;49(3):396–408. doi: 10.1002/(sici)1097-4636(20000305)49:3<396::aid-jbm13>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 172.Underwood PA, Bean PA, Cubeddu L. Human endothelial cells grow poorly on vitronectin: role of PAI-1. J Cell Biochem. 2001;82(1):98–109. doi: 10.1002/jcb.1112. [DOI] [PubMed] [Google Scholar]
- 173.Balcells M, Edelman ER. Effect of pre-adsorbed proteins on attachment, proliferation, and function of endothelial cells. J Cell Physiol. 2002;191(2):155–61. doi: 10.1002/jcp.10087. [DOI] [PubMed] [Google Scholar]
- 174.van der Zijpp YJ, Poot AA, Feijen J. ICAM-1 and VCAM-1 expression by endothelial cells grown on fibronectin-coated TCPS and PS. J Biomed Mater Res A. 2003;65(1):51–9. doi: 10.1002/jbm.a.10327. [DOI] [PubMed] [Google Scholar]
- 175.Dardik R, Varon D, Tamarin I, Zivelin A, Salomon O, Shenkman B, Savion N. Homocysteine and oxidized low density lipoprotein enhanced platelet adhesion to endothelial cells under flow conditions: distinct mechanisms of thrombogenic modulation. Thromb Haemost. 2000;83(2):338–44. [PubMed] [Google Scholar]
- 176.Solberg S, Larsen T, Jorgensen L. Differences in reactivity of confluent and nonconfluent cultures of human endothelial cells toward thrombin-stimulated platelets or heparinized salt solution. In Vitro Cell Dev Biol. 1985;21(11):612–6. doi: 10.1007/BF02623292. [DOI] [PubMed] [Google Scholar]
- 177.Jorgensen L, Grothe AG, Larsen T, Kinlough-Rathbone RL, Mustard JF. Injury to cultured endothelial cells by thrombin-stimulated platelets. Lab Invest. 1986;54(4):408–15. [PubMed] [Google Scholar]
- 178.Chazov EI, Alexeev AV, Antonov AS, Koteliansky VE, Leytin VL, Lyubimova EV, Repin VS, Sviridov DD, Torchilin VP, Smirnov VN. Endothelial cell culture on fibrillar collagen: model to study platelet adhesion and liposome targeting to intercellular collagen matrix. Proc Natl Acad Sci U S A. 1981;78(9):5603–7. doi: 10.1073/pnas.78.9.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bos GW, Scharenborg NM, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Endothelialization of crosslinked albumin-heparin gels. Thromb Haemost. 1999;82(6):1757–63. [PubMed] [Google Scholar]
- 180.Bos GW, Scharenborg NM, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Blood compatibility of surfaces with immobilized albumin-heparin conjugate and effect of endothelial cell seeding on platelet adhesion. J Biomed Mater Res. 1999;47(3):279–91. doi: 10.1002/(sici)1097-4636(19991205)47:3<279::aid-jbm1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 181.Gimbrone MA, Jr, Buchanan MR. Interactions of platelets and leukocytes with vascular endothelium: in vitro studies. Ann N Y Acad Sci. 1982;401:171–83. doi: 10.1111/j.1749-6632.1982.tb25716.x. [DOI] [PubMed] [Google Scholar]
- 182.Dekker A, Poot A, Beugeling T, Bantjes A, van Aken WG. The effect of vascular cell seeding on platelet deposition in an in vitro capillary perfusion model. Thromb Haemost. 1989;61(3):402–8. [PubMed] [Google Scholar]
- 183.Wechezak AR, Holbrook KA, Way SA, Mansfield PB. Platelet adherence in endothelial cell cultures. Blood Vessels. 1979;16(1):35–42. doi: 10.1159/000158188. [DOI] [PubMed] [Google Scholar]
- 184.Sneddon JM, Vane JR. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988;85(8):2800–4. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kaplan JE, Moon DG, Weston LK, Minnear FL, Del Vecchio PJ, Shepard JM, Fenton JW., 2nd Platelets adhere to thrombin-treated endothelial cells in vitro. Am J Physiol. 1989;257(2 Pt 2):H423–33. doi: 10.1152/ajpheart.1989.257.2.H423. [DOI] [PubMed] [Google Scholar]
- 186.Bizios R, Lai LC, Cooper JA, Del Vecchio PJ, Malik AB. Thrombin-induced adherence of neutrophils to cultured endothelial monolayers: increased endothelial adhesiveness. J Cell Physiol. 1988;134(2):275–80. doi: 10.1002/jcp.1041340214. [DOI] [PubMed] [Google Scholar]
- 187.Zwahlen RD, Holden WJ, Wyder-Walther M, Holub M, Moiola F. Influence of anti-inflammatory drugs on adhesion of neutrophils to endothelial cells cultured on microcarriers: a novel in vitro system as an alternative to animal experimentation. Zentralbl Veterinarmed A. 1994;41(9):671–82. doi: 10.1111/j.1439-0442.1994.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 188.Vlodavsky I, Eldor A, HyAm E, Atzom R, Fuks Z. Platelet interaction with the extracellular matrix produced by cultured endothelial cells: a model to study the thrombogenicity of isolated subendothelial basal lamina. Thromb Res. 1982;28(2):179–91. doi: 10.1016/0049-3848(82)90260-2. [DOI] [PubMed] [Google Scholar]
- 189.Guidollet J, Chignier E, Pillot R, Gayet O, MacGregor J, Louisot P. Enhanced expression of P–selectin (CD62P) by endothelial cells seeded onto synthetic arterial prostheses (PET, Dacron) is correlated with leukocyte interactions. J Biomed Mater Res. 1999;44(2):156–61. doi: 10.1002/(sici)1097-4636(199902)44:2<156::aid-jbm5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 190.Curwen KD, Kim HY, Vazquez M, Handin RI, Gimbrone MA., Jr Platelet adhesion to cultured vascular endothelial cells. A quantitative monolayer adhesion assay. J Lab Clin Med. 1982;100(3):425–36. [PubMed] [Google Scholar]
- 191.Scott WJ, Mann P. Proliferation and substrate effects on endothelial cell thrombogenicity. ASAIO Trans. 1990;36(3):M737–8. [PubMed] [Google Scholar]
- 192.Parsons TJ, Haycraft DL, Hoak JC, Sage H. Interaction of platelets and purified collagens in a laminar flow model. Thromb Res. 1986;43(4):435–43. doi: 10.1016/0049-3848(86)90088-5. [DOI] [PubMed] [Google Scholar]
- 193.Iwata H, Kaibara M, Suzuki Y, Nakajima H. Antithrombogenicity of cultured endothelial cell-detached surface. Colloids Surf B Biointerfaces. 2000;19(3):219–226. doi: 10.1016/s0927-7765(00)00159-4. [DOI] [PubMed] [Google Scholar]
- 194.Rose SL, Babensee JE. Procoagulant phenotype of endothelial cells after coculture with biomaterial-treated blood cells. J Biomed Mater Res A. 2005;72(3):269–78. doi: 10.1002/jbm.a.30222. [DOI] [PubMed] [Google Scholar]
- 195.Kirchhofer D, Sakariassen KS, Clozel M, Tschopp TB, Hadvary P, Nemerson Y, Baumgartner HR. Relationship between tissue factor expression and deposition of fibrin, platelets, and leukocytes on cultured endothelial cells under venous blood flow conditions. Blood. 1993;81(8):2050–8. [PubMed] [Google Scholar]
- 196.Kirchhofer D, Tschopp TB, Hadvary P, Baumgartner HR. Endothelial cells stimulated with tumor necrosis factor-alpha express varying amounts of tissue factor resulting in inhomogenous fibrin deposition in a native blood flow system. Effects of thrombin inhibitors. J Clin Invest. 1994;93(5):2073–83. doi: 10.1172/JCI117202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Diquelou A, Lemozy S, Dupouy D, Boneu B, Sakariassen K, Cadroy Y. Effect of blood flow on thrombin generation is dependent on the nature of the thrombogenic surface. Blood. 1994;84(7):2206–13. [PubMed] [Google Scholar]
- 198.Hedeman Joosten PP, Verhagen HJ, Heijnen-Snyder GJ, van Vroonhoven TJ, Sixma JJ, de Groot PG, Eikelboom BC. Thrombogenesis of different cell types seeded on vascular grafts and studied under blood-flow conditions. J Vasc Surg. 1998;28(6):1094–103. doi: 10.1016/s0741-5214(98)70036-9. [DOI] [PubMed] [Google Scholar]
- 199.Biedermann B, Rosenmund A, Muller M, Kohler HP, Haeberli A, Straub PW. Human endothelial cells suppress prothrombin activation in nonanticoagulated whole blood in vitro. J Lab Clin Med. 1994;124(3):339–47. [PubMed] [Google Scholar]
- 200.Bombeli T, Muller M, Straub PW, Haeberli A. Cyclosporine-induced detachment of vascular endothelial cells initiates the intrinsic coagulation system in plasma and whole blood. J Lab Clin Med. 1996;127(6):621–34. doi: 10.1016/s0022-2143(96)90153-5. [DOI] [PubMed] [Google Scholar]
- 201.Koveker GB, Burkel WE, Graham LM, Wakefield TW, Stanley JC. Endothelial cell seeding of expanded polytetrafluoroethylene vena cava conduits: effects on luminal production of prostacyclin, platelet adherence, and fibrinogen accumulation. J Vasc Surg. 1988;7(4):600–5. [PubMed] [Google Scholar]
- 202.Allen BT, Long JA, Clark RE, Sicard GA, Hopkins KT, Welch MJ. Influence of endothelial cell seeding on platelet deposition and patency in small-diameter Dacron arterial grafts. J Vasc Surg. 1984;1(1):224–33. [PubMed] [Google Scholar]
- 203.Budd JS, Allen KE, Hartley G, Bell PR. The effect of preformed confluent endothelial cell monolayers on the patency and thrombogenicity of small calibre vascular grafts. Eur J Vasc Surg. 1991;5(4):397–405. doi: 10.1016/s0950-821x(05)80171-9. [DOI] [PubMed] [Google Scholar]
- 204.Clagett GP, Burkel WE, Sharefkin JB, Ford JW, Hufnagel H, Vinter DW, Kahn RH, Graham LM, Stanley JC, Ramwell PW. Platelet reactivity in vivo in dogs with arterial prostheses seeded with endothelial cells. Circulation. 1984;69(3):632–9. doi: 10.1161/01.cir.69.3.632. [DOI] [PubMed] [Google Scholar]
- 205.Shepard AD, Eldrup-Jorgensen J, Keough EM, Foxall TF, Ramberg K, Connolly RJ, Mackey WC, Gavris V, Auger KR, Libby P, et al. Endothelial cell seeding of small-caliber synthetic grafts in the baboon. Surgery. 1986;99(3):318–26. [PubMed] [Google Scholar]
- 206.Ortenwall P, Wadenvik H, Kutti J, Risberg B. Reduction in deposition of indium 111–labeled platelets after autologous endothelial cell seeding of Dacron aortic bifurcation grafts in humans: a preliminary report. J Vasc Surg. 1987;6(1):17–25. doi: 10.1067/mva.1987.avs0060017. [DOI] [PubMed] [Google Scholar]
- 207.Kaibara M, Kawamoto Y. Rheological measurement of blood coagulation in vascular vessel model tube consisting of endothelial cells monolayer. Biorheology. 1991;28(3–4):263–74. doi: 10.3233/bir-1991-283-416. [DOI] [PubMed] [Google Scholar]
- 208.Kohler HP, Muller M, Bombeli T, Straub PW, Haeberli A. The suppression of the coagulation of nonanticoagulated whole blood in vitro by human umbilical endothelial cells cultivated on microcarriers is not dependent on protein C activation. Thromb Haemost. 1995;73(4):719–24. [PubMed] [Google Scholar]
- 209.Sharefkin JB, Latker C, Smith M, Cruess D, Clagett P, Rich NM. Early normalization of platelet survival by endothelial seeding of Dacron arterial prostheses in dogs. Surgery. 1982;92(2):385–93. [PubMed] [Google Scholar]
- 210.Esmon CT. The endothelial protein C receptor. Curr Opin Hematol. 2006;13(5):382–5. doi: 10.1097/01.moh.0000239712.93662.35. [DOI] [PubMed] [Google Scholar]
- 211.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24(8):1374–83. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 212.Lwaleed BA, Bass PS. Tissue factor pathway inhibitor: structure, biology and involvement in disease. J Pathol. 2006;208(3):327–39. doi: 10.1002/path.1871. [DOI] [PubMed] [Google Scholar]
- 213.Petri B, Bixel MG. Molecular events during leukocyte diapedesis. Febs J. 2006;273(19):4399–407. doi: 10.1111/j.1742-4658.2006.05439.x. [DOI] [PubMed] [Google Scholar]
- 214.Fish JE, Marsden PA. Endothelial nitric oxide synthase: insight into cell-specific gene regulation in the vascular endothelium. Cell Mol Life Sci. 2006;63(2):144–62. doi: 10.1007/s00018-005-5421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cenni E, Granchi D, Ciapetti G, Verri E, Cavedagna D, Gamberini S, Di Leo A, Pizzoferrato A. Cytokine production and adhesive protein expression by endothelial cells after contact with polyethylene terephthalate. Biomaterials. 1996;17(21):2071–6. doi: 10.1016/0142-9612(96)00037-3. [DOI] [PubMed] [Google Scholar]
- 216.Cenni E, Granchi D, Arciola CR, Ciapetti G, Savarino L, Stea S, Cavedagna D, Di Leo A, Pizzoferrato A. Adhesive protein expression on endothelial cells after contact in vitro with polyethylene terephthalate coated with pyrolytic carbon. Biomaterials. 1995;16(16):1223–7. doi: 10.1016/0142-9612(95)98128-2. [DOI] [PubMed] [Google Scholar]
- 217.Speiser W, Anders E, Binder BR, Muller-Berghaus G. Clot lysis mediated by cultured human microvascular endothelial cells. Thromb Haemost. 1988;60(3):463–7. [PubMed] [Google Scholar]
- 218.Koveker GB, Graham LM, Burkel WE, Sell R, Wakefield TW, Dietrich K, Stanley JC. Extracellular matrix preparation of expanded polytetrafluoroethylene grafts seeded with endothelial cells: influence on early platelet deposition, cellular growth, and luminal prostacyclin release. Surgery. 1991;109(3 Pt 1):313–9. [PubMed] [Google Scholar]
- 219.Jensen N, Lindblad B, Ljungberg J, Leide S, Bergqvist D. Early attachment of leucocytes, platelets and fibrinogen in endothelial cell-seeded Dacron venous conduits. Br J Surg. 1997;84(1):52–7. doi: 10.1046/j.1365-2168.1997.02489.x. [DOI] [PubMed] [Google Scholar]
- 220.Jensen N, Lindblad B, Ljungberg J, Leide S, Bergqvist D. Early attachment of platelets, leukocytes, and fibrinogen in endothelial cell seeded Dacron grafts. Ann Vasc Surg. 1996;10(6):530–6. doi: 10.1007/BF02000441. [DOI] [PubMed] [Google Scholar]
- 221.Smyth JV, Welch M, Carr HM, Dodd PD, Eisenberg PR, Walker MG. Fibrinolysis profiles and platelet activation after endothelial cell seeding of prosthetic vascular grafts. Ann Vasc Surg. 1995;9(6):542–6. doi: 10.1007/BF02018827. [DOI] [PubMed] [Google Scholar]
- 222.Kesler KA, Herring MB, Arnold MP, Park HM, Baughman S, Glover JL. Short-term in vivo stability of endothelial-lined polyester elastomer and polytetrafluoroethylene grafts. Ann Vasc Surg. 1986;1(1):60–5. doi: 10.1016/S0890-5096(06)60704-8. [DOI] [PubMed] [Google Scholar]