Abstract
Clinical data suggests a strong negative impact of traumatic attachments on adult mental illness, presumably through organizing brain development. To further explore this clinical issue, a mammalian model of imprinting was developed to characterize the neural basis of attachment in both healthy and traumatic attachments. The altricial neonatal rat must learn the mother’s odor for nipple attachment, huddling, and orienting to the mother, all of which are required for pup survival. While it appears maladaptive to depend upon learning for attachment, the unique learning system of neonatal pups greatly enhances odor-preference learning and attachment while pups are confined to the nest. This heightened learning is expressed behaviorally as an enhanced ability to acquire learned odor preferences and a decreased ability to acquire learned odor aversions. Specifically, both odor-milk and odor-shock (0.5 mA) conditioning result in odor-preference acquisition. It appears as though there are at least three brain structures underlying the neonatal rat’s sensitive period for heightened odor learning: (1) odor learning is encoded in the olfactory bulb; (2) the hyperfunctioning noradrenergic locus coeruleus (LC) appears to support preference conditioning through release of NE; and (3) the hypofunctioning amygdala appears to underlie pups’ difficulty in learning odor aversions. Overall, this suggests that the CNS of altricial infants is specialized for optimizing attachments to their caregiver.
Keywords: mother-infant interactions, olfactory bulb, classical conditioning, norepinephrine, attachment, imprinting, locus coeruleus, amygdala, learning, abuse, stress, corticosterone, fear conditioning
INTRODUCTION
A loving, caring family should be the main source of a child’s sense of safety and security. However, according to a 1999 report by the Administration for Children and Families of the U.S. Dept. of Health and Human Services, 11.8 of every 1000 American children are abused or neglected, most often by parents or significant caregiver. Children aged 0–3 years are more likely to be abused than any other age group. Thus, during the critical years for brain development, basic emotional and social learning, and attachment, these children experience mixed messages concerning their safety within the attachment dyad. However, most of these children still form an attachment to their abuser (Zeanah, Connor, and Dahl chapters in this volume).1,2 While it is difficult to define safety as it relates to a young child, it is reasonable to say that the abused child develops an attachment within the context of a situation that is not entirely safe.
The infant attachment neural circuitry has been elusive, perhaps because of difficulty in characterizing terms such as love, security, and comfort. In clinical terms, a secure attachment is one in which the child–caregiver relationship provides pleasure, security, and safety for the child, and results in psychological well-being. While these are useful clinical terms, they are difficult terms to place in the developmental neurobehavioral approach required for an animal model. While an animal attachment model ultimately needs to accommodate the clinical literature, we have begun our approach to this issue by simply documenting the unique pathways and structures used by the infant brain while learning about the attachment figure.
HUMAN ATTACHMENT
The study of attachment began in the 1950s, partly in reaction to the distant and nonnurturing approach to child-rearing typical of the day, when mothers were advised to limit contact with their babies to avoid spreading germs and spoiling the child.3 A notable figure in promoting mother–infant contact was John Bowlby, a psychiatrist treating disturbed adolescents, all of whom had suffered from poor maternal care. The poor mental health of his patient population led Bowlby to conclude that a strong mother–infant attachment was necessary for adult mental health and the quality of all adult relationships. In an attempt to better understand the attachment process, Bowlby characterized four components of attachment: (1) the infant rapidly forms an attachment to the caregiver, (2) the infant seeks caregiver proximity, (3) the caregiver provides a safe haven, and (4) the infant undergoes considerable abuse while remaining in contact with the caregiver. Bowlby’s framework for human infant attachment continues to permit the assessment of attachment in an experimentally refined protocol and has provided the foundation for much of the current attachment literature.
The child’s attachment to the mother appears to begin before birth, when the baby learns about the mother’s voice and odors. Attachment continues afterbirth when the infant learns the mother’s face, and additional qualities of her odors and voice appear to bridge the pre- and postnatal environments.4–7 The maternal odor produces orienting responses and mouthing and soothes a crying infant,6,8 and novel odors quickly acquire at least some of these properties through classical conditioning.9 These odors may have qualities of “safety” or comfort (attenuates crying, orienting) as described in Bowlby’s characterization of attachment. It is quite possible that this neonatal learning about the caregiver is the first postnatal expression of learning within the attachment system and perhaps one of the first ways in which our sense of safety is constructed.
ATTACHMENT IN OTHER SPECIES
Remarkably, Bowlby’s characterization of human attachment is relevant throughout the animal kingdom. Imprinting in chicks illustrates rapidity of attachment formation, proximity seeking, and willingness to undergo considerable abuse while remaining in contact with the caregiver. Specifically, during imprinting, chicks will continue to follow their mother even while being shocked, although just hours later, in postsensitive period naïve chicks, this treatment results in avoidance.10 Similar work in young dogs showed that puppies quickly learn a strong attachment to a handler providing either petting, electric shock, or rough treatment. This phenomenon extends to nonhuman primates and abused children, who also exhibit a strong attachment to their abusive caregiver (Dahl, Zeanah, and Settles, and Connor chapters in this volume).11–14 We hypothesized that this attachment system evolved to ensure altricial animals easily form a repertoire of proximity-seeking behaviors to the caregiver, regardless of the quality of care-giving received.15
ANIMAL ATTACHMENT MODEL
To assess the neurobiology of infant attachment, we have developed an infant rat model that conforms to the characteristics of attachment initially described by Bowlby. Rats are an altricial species that must learn about their mother’s odor for attachment. Until walking emerges at 10 days of age, pups remain in the nest and spend most of their time nursing.16 With sensory functioning limited to the somatosensory and chemical senses of taste and smell, pups rely on their olfactory system to orient and seek proximity to the mother. Neonatal rats very rapidly and easily learn this proximity-seeking behavior both within the nest with natural odors and in more controlled learning experiments.17–20 Pups will also undergo considerable abuse while forming the odor preference that underlies the proximity-seeking behavior: pairing a novel odor with pain (tailpinch or 0.5-mA shock) results in pups learning an odor preference.21–23 Specifically, although neonatal rats show clear pain responses to stimuli such as shock and tail pinch during acquisition, the odor paired with these stimuli is approached during testing. This learning is limited to the neonatal period when pups are confined to the nest. While this attenuated aversive conditioning appears paradoxical, it is possible that this attachment system developed to prevent pups from learning an aversion to the mother during the occasional rough handling typical in the nest.15 Indeed, considering the importance of proximity-seeking behaviors in procuring mother’s milk, warmth, and protection, pups’ survival may depend upon pups only learning approach responses to the mother.
As the sensitive period ends, walking develops and the probability of leaving the nest greatly increases.16 Pups then express the more complex “adult” learning system to deal with the increasingly complex extranest environment. However, with maturation, attachments continue to be formed, especially in behaviors important to survival, such as reproduction (Insel’s chapter in this volume).24–27
INFANT ATTACHMENT NEURAL CIRCUITRY
Neonatal rats can be easily classically conditioned, yet brain areas important in adult learning are not yet functional (e.g., amygdala, hippocampus, frontal cortex). 23,28–32 Therefore, the neonatal rat must use a different neural pathway to support learning, presumably one designed through evolution to ensure caregiver attachment, and hence survival.15 Currently, three brain areas appear critical for attachment in the neonatal rat: the olfactory bulb, locus coeruleus, and amygdala (Fig. 1).
FIGURE 1.
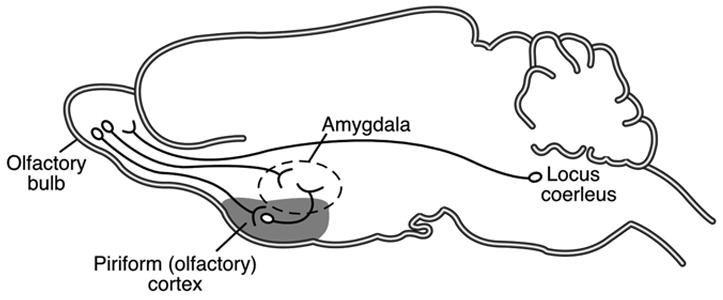
Schematic representation of circuitry important for neonatal learning during the sensitive period. To learn an odor preference, an odor must be paired with NE from the locus coeruleus. Odor aversion learning from odor-shock conditioning appears to be prevented due to lack of amygdala participation in this learning.
Encoding Attachment Odors in the Olfactory Bulb
The first relay station for olfactory information within the brain is the olfactory bulb. During the sensitive period, odor learning produces changes in the olfactory bulb that are odor-specific and retained into adulthood (c-fos, 2-DG, mitral cellmitral cell single-cell recordings, glomerular anatomical changes; see review).19 The olfactory bulb learning-induced changes appear to be due to the unique responses of the neonatal olfactory bulb to odors paired with reward. Specifically, while the primary-output mitral cells quickly habituate to repeated odor-only presentation, mitral cells fail to habituate if that odor is presented along with a reward,33 due to the reward activating the release of norepinephrine (NE) from the locus coeruleus (LC, discussed below). Continued activation of mitral cells results in a cascade of metabolic changes within mitral cells that is characteristic of those previously identified following learning in adults.34,35 Since the information leaving the olfactory bulb is modified following neonatal learning, additional brain areas may also encode information about the attachment odor. Following the sensitive period, odor-learning fails to produce any of the olfactory bulb neural changes,36 presumably because of the greatly reduced levels of NE released by the mature LC.37
The Locus Coeruleus (LC) and Rapid Odor Preference Learning
Olfactory bulb and behavioral changes associated with sensitive-period learning require NE, with the LC as the bulb’s sole source.38 Depleting the olfactory bulb of NE by chemically lesioning the LC or blocking NE receptors in the olfactory bulb prevents sensitive-period learning.39,40 Moreover, during the sensitive period, presenting an odor while increasing olfactory bulb NE (by chemically stimulating the LC or infusing NE into the bulb) is sufficient to produce an odor preference.34,39–42 Thus, the NE system appears necessary and sufficient to control pups’ odor preference learning during the sensitive period.
The role of NE during the sensitive period is in sharp contrast to the role of NE in post-sensitive-period or adult learning. NE is not necessary for post-sensitive-period learning, although it enhances or attenuates consolidation of memories.43 We believe this developmental change in NE-dependent learning is due to the dramatic reduction in LC NE release around the end of the sensitive period. Specifically, a 1-s stimulation with either shock or a puff of air results in a 20- to 30-s response in the sensitive-period LC, but only a ms response in the post-sensitive-period LC.44 According to Rangel and Leon,37 learning during the post-sensitive period yields a dramatic reduction of olfactory bulb NE as compared to sensitive-period release. The specific mechanisms for enhancing the sensitive-period LC’s responsiveness appear to be due to greatly reduced LC α2 autoinhibition, enhanced LC α1 autoexcitation, and increased LC electrotonic coupling.44,45 Based on these data, we suggest that the large release of NE from the sensitive-period LC is responsible for enhanced odor-preference learning, and that maturation of the LC signals the termination of rapid NE-dependent preference learning. In support of this, we have successfully reinstated the sensitive-period NE-dependent learning in post-sensitive-period pups by functionally reinstating the neonatal distribution of LC autoreceptors.46 Specifically, while stimulating the LC, we also reinstated the reduced α2 autoinhibition by blocking LC α2 receptors and reinstated the enhanced α1 autoexcitation through activation of LC α1 receptor agonists.
Amygdala Attenuated Odor-Avoidance Learning
The amygdala is a brain area important for both natural fear and fear conditioning (Amaral, Davis chapters in this volume).29 Our data suggest that failure of the amygdala to participate in odor-shock conditioning during the sensitive period prevents odor-avoidance learning. Specifically, in sensitive-period pups, when odor-shock conditioning produces an odor preference, the amygdala does not participate in learning.23 Neonatal lesion of the amygdala supports the nonfunctional role of the amygdala in neonatal learning,47 a procedure that greatly attenuates an adult animal’s ability to learn.48 However, in post-sensitive-period pups, when odor–shock conditioning easily produces odor avoidance, the amygdala participates in fear conditioning. It should be noted that neonatal pups can learn an odor avoidance if that odor is paired with malaise. According to Haroutunian and Campbell,49 only interoceptive cues (illness), but not exteroceptive cues (shock), support aversive conditioning and are thought not to require the amygdala. This suggests that the developmental emergence of amygdala participation in odor–shock conditioning may underlie the developmental delay in post-sensitive-period emergence of odor–shock-induced fear conditioning.
It is possible that immaturity of the amygdala or lack of functional amygdala connections underlies its lack of participation in odor–shock learning.32,50–54 Behavioral data are consistent with this interpretation, since other amygdala-based olfactory behaviors also emerge around 10 days of age: fear to predator odor,55,56 inhibitory conditioning,57 and passive avoidance.58,59 However, work from our lab by Stephanie Moriceau, as well as from other labs, has shown that some amygdala-based behaviors can be induced to emerge at a developmentally earlier age through manipulation of corticosterone (CORT).60 Normally, at this early stage of development, pups have a hyporesponsive stress period (HRSP) that defines an age range in which stress-induced CORT release is greatly attenuated (Plotsky chapter in this volume).61,62 Thus, increasing normally low levels of CORT with exogenous CORT injected minutes earlier permits 8-day-old (sensitive period) pups to express fear to predator odor,55 learn inhibitory conditioning,63 and fear conditioning.60 The sites of CORT action need to be determined, since receptors are abundant in many neonatal brain areas, most notably the limbic system and the two stress systems: the LC-amygdala and hypothalamus–pituitary–adrenal (HPA) (Plotsky chapter in this volume).
While NE and CORT appear critically important in neonatal learning, other neurotransmitters are also important. For example, dopamine, NE, and opioids modulate acquisition,64–67 and Tania Roth in my lab has shown that opioids are critical for neonatal consolidation of the odor-shock-induced odor preference.68 Other neurotransmitters work directly in the olfactory bulb, with serotonin potentiating the excitatory role of NE on mitral cells41,69 and GAB A modulating the inhibitory control of mitral cells by the granule cells.35 These neurotransmitter systems have been implicated in other adult attachment behaviors such as mating and infant care (Insel chapter in this volume).24–27
ADULT CONSEQUENCES
Clinically, development of a disordered attachment to the caregiver results in a variety of maladaptive behaviors and an increased incidence of many mental illnesses (Kaslow, Ferris, Pollak chapters in this volume).12,71–73 In our animal model, odors learned during the sensitive period continue to be preferred by pups into adulthood (see review).19 However, similar to visual stimuli in chick imprinting,74 the odor’s role changes with maturation into enhancement of sexual and maternal behaviors.26,75,76 The learning-induced changes in the neonatal olfactory bulb still present in the adult bulb are believed to underlie these adult behavioral effects.
Do Human Children Use This Attachment Neural Circuitry?
While the human attachment literature fits well with our animal abuse model, there is insufficient information on human brain development to discuss the neural circuitry of human attachment (Peterson, Skuse chapters in this volume).77 There has been speculation that the human infant’s large NE surge at birth and continued high NE level during early childhood is important for attachment in the newborn, although no causal relationship has been established. Evidence for CORT’s role in human attachment is also vague, although considerable data have been gathered on fluctuating CORT levels during childhood (Gunner chapter in this volume). However, regardless of the existence of a homologous neural circuitry for attachment, this work suggests that the brain of newborns is uniquely designed to optimize attachment to their caregivers and suggests a new conceptual framework in which to explore human attachments.
Acknowledgments
This work was supported by grants NICHD-HD33402 and NSF-IBN0117234.
References
- 1.Perry BD, Pollard R. Homeostasis, stress, trauma, and adaptation. A neurodevelopmental view of childhood trauma. Child Adolesc Psychiatry Clin N Am. 1998;7:33–51. [PubMed] [Google Scholar]
- 2.Siegel DJ. Toward an interpersonal neurobiology of the developing mind: attachment relationships, “mindsight,” and neural integration. Infant Mental Health J. 2001;22:67–94. [Google Scholar]
- 3.Bowlby J. Attachment. Basic Books; New York: 1965. [Google Scholar]
- 4.Hepper PG. Human fetal olfactory learning. Int J Prenatal Perinatol Psychol Med. 1995;7:147–151. [Google Scholar]
- 5.Mennella JA, Johnson A, Beauchamp GK. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem Senses. 1995;20:207–209. doi: 10.1093/chemse/20.2.207. [DOI] [PubMed] [Google Scholar]
- 6.Schaal B, Marlier L, Soussignan R. Human foetuses learn odours from their pregnant mother’s diet. Chem Senses. 2000;25:729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- 7.Moon CM, Fifer WP. Evidence of transnatal auditory learning. J Perinatol. 2000;20:37–44. doi: 10.1038/sj.jp.7200448. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan RM, Toubas P. Clinical usefulness of maternal odor in newborns: soothing and feeding preparatory responses. Biol Neonate. 1998;74:402–408. doi: 10.1159/000014061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan RM, et al. Olfactory classical conditioning in neonates. Pediatrics. 1991;87:511–518. [PMC free article] [PubMed] [Google Scholar]
- 10.Hess EH. Ethology: an approach to the complete analysis of behavior. In: Brown R, Galanter E, Hess EH, Mendler G, editors. New Directions in Psychology. Holt, Rinehart and Winston; New York: 1962. [Google Scholar]
- 11.Harlow HF, Harlow MK. The affectional systems. In: Schrier A, Harlow HF, Stollnitz F, editors. Behavior of Nonhuman Primates. Vol. 2. Academic Press; New York: 1965. [Google Scholar]
- 12.Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopath. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 13.Helfer ME, Kempe RS, Krugman RD. The Battered Child. University of Chicago Press; Chicago: 1997. [Google Scholar]
- 14.Rajecki DW, Lamb ME, Obmascher P. Towards a general theory of infantile attachment; a comparative review of aspects of the social bond. Behav Brain Sci. 1978;3:417–464. [Google Scholar]
- 15.Hofer MA, Sullivan RM. Toward a neurobiology of attachment. In: Nelson CA, Luciana M, editors. Hand-book of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 599–616. [Google Scholar]
- 16.Bolles RC, Woods PJ. The ontogeny of behavior in the albino rat. Anim Behav. 1965;12:427–441. [Google Scholar]
- 17.Campbell BA. Reflections on the ontogeny of learning and memory. In: Kail R, Spear NE, editors. Comparative Perspectives on the Development of Memory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1984. pp. 23–35. [Google Scholar]
- 18.Spear NE, Rudy JW. Tests of the ontogeny of learning and memory: issues, methods, and results. In: Shair HN, Barr GA, Hofer MA, editors. Developmental Psychobiology: New Methods and Changing Concepts. Oxford University Press; New York: 1991. pp. 84–113. [Google Scholar]
- 19.Sullivan RM. Unique characteristics of neonatal classical conditioning: the role of the amygdala and locus coeruleus. Integ Physiol Behav Sci. 2001;36:293–307. doi: 10.1007/bf02688797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan RM, et al. Modified behavioral olfactory bulb responses to maternal odors in preweanling rats. Dev Brain Res. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- 21.Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by conditioned change in behavioral state. Dev Psychobiol. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan RM, et al. Good memories of bad events in infancy: ontogeny of conditioned fear and the amygdala. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennen PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:451–457. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- 25.Insel TR, Young LJ. The neurobiology of attachment. Nature Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 26.Fleming AS, et al. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol Biochem Behav. 2002;73:61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- 27.Terrazas A, et al. Twenty-four-hour-old lambs rely more on maternal behavior than on the learning of individual characteristics to discriminate between their own and an alien mother. Dev Psychobiol. 2002;40:408–418. doi: 10.1002/dev.10041. [DOI] [PubMed] [Google Scholar]
- 28.Crain B, et al. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. 1973;63:195–204. doi: 10.1016/0006-8993(73)90088-7. [DOI] [PubMed] [Google Scholar]
- 29.Fanselow MS, Rudy JW. Convergence of experimental and developmental approaches to animal learning and memory processes. In: Carew TJ, Menzel R, Shatz CJ, editors. Mechanistic Relationships Between Development and Learning. Wiley; New York: 1998. pp. 15–28. [Google Scholar]
- 30.Sananes CB, Campbell BA. Role of the central nucleus of the amygdala in olfactory heart rate conditioning. Behav Neurosci. 1989;103:519–525. [PubMed] [Google Scholar]
- 31.Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- 32.Verwer RW, et al. Prefrontal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J Comp Neurol. 1996;376:75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Wilson DA, Sullivan RM. Olfactory associative conditioning in infant rats with brain stimulation as reward. II. Norepinephrine mediates a specific component of the bulb response to reward. Behav Neurosci. 1991;105:843–849. doi: 10.1037//0735-7044.105.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan Q, et al. Mitral cell β1 and 5-HT2A receptor co-localization and camp co-regulation: a new model of norepinephrine-induced learning in the olfactory bulb. Learn Mem. 2003;10:5–15. doi: 10.1101/lm.54803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okutani F, et al. Non-specific olfactory aversion induced by intrabulbar infusion of the GABA(A) receptor antagonist bicuculline in young rats. Neuroscience. 2002;112:901–906. doi: 10.1016/s0306-4522(02)00117-3. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan RM, Wilson DA. Neural correlates of conditioned odor avoidance in preweanling rats. Behav Neurosci. 1991;105:307–312. doi: 10.1037//0735-7044.105.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rangel S, Leon M. Early odor preference training increases olfactory bulb norepinephrine. Dev Brain Res. 1995;85:187–191. doi: 10.1016/0165-3806(94)00211-h. [DOI] [PubMed] [Google Scholar]
- 38.Shipley MT, Halloran FJ, De la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res. 1985;239:294–299. doi: 10.1016/0006-8993(85)90537-2. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan RM, et al. Bilateral 6-OHDA lesions of the locus coeruleus impair associative olfactory learning in newborn rats. Brain Res. 1994;643:306–309. doi: 10.1016/0006-8993(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan RM, et al. The role of olfactory bulb norepinephrine in early olfactory learning. Dev Brain Res. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- 41.McLean JH, et al. Serotonergic influences on olfactory learning in the neonatal rat. Behav Neural Biol. 1993;60:152–162. doi: 10.1016/0163-1047(93)90257-i. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan RM, et al. Association of an odor with activation of olfactory bulb noradrenergic p-receptors or locus coeruleus stimulation is sufficient to produce learned approach response to that odor in neonatal rats. Behav Neurosci. 2000;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGaugh JL, Roozendaal B. The role of adrenal stress hormone in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura ST, Sakaguchi T. Development and plasticity of the locus coeruleus. A review of recent physiological and pharmacological experimentation. Prog Neurobiol. 1990;34:505–526. doi: 10.1016/0301-0082(90)90018-c. [DOI] [PubMed] [Google Scholar]
- 45.Marshall KC, et al. Developmental aspects of the locus coeruleus-noradrenaline system. Prog Brain Res. 1991;88:173–185. doi: 10.1016/s0079-6123(08)63807-8. [DOI] [PubMed] [Google Scholar]
- 46.Moriceau S, Sullivan RM. Reinstating the neonatal sensitive period for olfactory learning. J Neurosci. 2003 Accepted pending revisions. [Google Scholar]
- 47.Sullivan RM, Wilson DA. Role of the amygdala complex in early olfactory associative learning. Behav Neurosci. 1993;107:254–263. doi: 10.1037//0735-7044.107.2.254. [DOI] [PubMed] [Google Scholar]
- 48.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not performance of conditioned fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- 50.Bayer SA. Quantitative 3H-thymidine radiographic analysis of neurogenesis in the rat amygdala. J Comp Neurol. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- 51.Berdel B, Morys J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int J Dev Neurosci. 1997;15:755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 52.Mizukawa K, Tseng I-Ming, Otsuka N. Quantitative electron microscopic analysis of postnatal development of zinc-positive nerve endings in the rat amygdala using Timm’s sulphide silver technique. Dev Brain Res. 1989;50:197–203. doi: 10.1016/0165-3806(89)90195-8. [DOI] [PubMed] [Google Scholar]
- 53.Hunt P, Campbell BA. Developmental dissociation of the components of conditioned fear. In: Bouton ME, Fanselow MS, editors. Learning, Motivation, and Cognition: The Functional Behaviorism of Robert C. Bolles. American Psychological Association; Washington, DC: 1999. [Google Scholar]
- 54.Nair HP, Gonzalez-Lima F. Extinction of behavior in infant rats: development of functional coupling between septal, hippocampal, and ventral tegmental regions. J Neurosci. 1999;19:8646–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi LK. Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Dev Brain Res. 1994;81:121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 56.Wiedenmayer CP, BARR GA. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behav Brain Res. 2001;126:147–157. doi: 10.1016/s0166-4328(01)00260-1. [DOI] [PubMed] [Google Scholar]
- 57.Myslivecek J. Inhibitory learning and memory in newborn rats. Prog Neurobiol. 1997;53:399–430. doi: 10.1016/s0301-0082(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 58.Blozovski D, Cudennec A. Passive avoidance learning in the young rat. Dev Psychobiol. 1980;13:513–518. doi: 10.1002/dev.420130510. [DOI] [PubMed] [Google Scholar]
- 59.Collier AC, et al. Approach-avoidance conflict in preweanling rats: a developmental study. Anim Learn Behav. 1979;7:514–520. [Google Scholar]
- 60.Moriceau S, Sullivan RM. Corticosterone influences on mammalian imprinting. Behav Neurosci. doi: 10.1037/0735-7044.118.2.274. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine S. Plasma-free corticosterone response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- 62.Levine S. Primary social relationships influence the development of the hypo-thalamic–pituitary–adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- 63.Bialik RJ, Pappas BA, Roberts DC. Neonatal 6-hydroxydopamine prevents adaptation to chemical disruption of the pituitary-adrenal system in the rat. Horm Behav. 1984;18:12–21. doi: 10.1016/0018-506x(84)90046-1. [DOI] [PubMed] [Google Scholar]
- 64.Barr GA, Rossi G. Conditioned place preference from ventral tegmental injections of morphine in neonatal rats. Dev Brain Res. 1992;66:133–136. doi: 10.1016/0165-3806(92)90149-q. [DOI] [PubMed] [Google Scholar]
- 65.Kehoe P, Blass E. Central nervous system mediation of positive and negative reinforcement in neonatal albino rats. Dev Brain Res. 1986;27:69–75. doi: 10.1016/0165-3806(86)90233-6. [DOI] [PubMed] [Google Scholar]
- 66.Roth T, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Dev Psychobiol. 2001;39:188–198. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weldon DA, Travis ML, Kennedy DA. Posttraining D1 receptor blockade impairs odor conditioning in neonatal rats. Behav Neurosci. 1991;105:450–458. doi: 10.1037//0735-7044.105.3.450. [DOI] [PubMed] [Google Scholar]
- 68.Roth T, Sullivan RM. Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiol Behav. 2003;78:135–142. doi: 10.1016/s0031-9384(02)00961-7. [DOI] [PubMed] [Google Scholar]
- 69.McLean JH, et al. pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learn Mem. 1999;6:608–618. doi: 10.1101/lm.6.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okere CO, Kaba H. Increased expression of neuronal nitric oxide synthase mRNA in the accessory olfactory bulb during the formation of olfactory recognition memory in mice. Eur J Neurosci. 2000;12:4552–4556. doi: 10.1046/j.0953-816x.2000.01325.x. [DOI] [PubMed] [Google Scholar]
- 71.Bernet CZ, Stein MB. Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depress Anxiety. 1999;9:169–174. [PubMed] [Google Scholar]
- 72.Read J, et al. The contribution of early traumatic events to schizophrenia in some patients: a traumagenic neurodevelopmental model. Psychiatry. 2001;64:319–345. doi: 10.1521/psyc.64.4.319.18602. [DOI] [PubMed] [Google Scholar]
- 73.Teicher MH, et al. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann NY Acad Sci. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- 74.Bolhuis JJ. The development of animal behavior: from Lorenz to neural nets. Naturwissenschaften. 1999;86:101–111. doi: 10.1007/s001140050582. [DOI] [PubMed] [Google Scholar]
- 75.Moore CL, Jordan L, Wong L. Early olfactory experience, novelty and choice of sexual partner by male rats. Physiol Behav. 1996;60:1361–1367. doi: 10.1016/s0031-9384(96)00249-1. [DOI] [PubMed] [Google Scholar]
- 76.Fillion TJ, Blass EM. Infantile experience with suckling odors determined adult sexual behavior in male rats. Science. 1986;231:729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- 77.Shore AN. Effect of secure attachment relationship on right brain development, affect regulation and infant mental health. Infant Ment Health J. 2001;22:7–66. [Google Scholar]


