Abstract
The present series of experiments assessed how information from the whiskers controls and modulates infant rat behavior during early learning and attachment. Passive vibrissal stimulation can elicit behavioral activity in pups throughout the first two postnatal weeks, although orienting to the source of stimulation is evident only after ontogenetic emergence of whisking. In addition, while pups were capable of demonstrating learning in a classical conditioning paradigm pairing vibrissa stimulation with electric shock, no corresponding changes were detected in the anatomy of the barrel cortex as determined by cytochrome oxidase (CO) staining. Finally, the role of whiskers in a more naturalistic setting was determined in postnatal day (PN)3–5 and PN11–12 pups. Our results showed that both nipple attachment and huddling were disrupted in whisker-clipped PN3–5 pups but only marginally altered in PN11–12 pups. Together, these results suggest that the neonatal whisker system is behaviorally functional and relevant for normal mother–infant interactions, though it lacks the sophistication of a mature whisker system that evokes very specific and directed responses.
Keywords: mother-infant interactions, nipple attachment, huddling, vibrissae, whiskers, development, plasticity, cortical barrels
Introduction
Neonatal rat pups rely primarily on tactile and olfactory sensory cues to orient in their environment to receive milk and warmth required for survival (reviewed in Hofer and Sullivan, 2001). Although the role of olfaction in early behavioral development has been well described (Tobach, 1971; Singh and Hofer, 1978; Brunjes and Alberts, 1979; Galef and Kaner, 1980; Hofer et al., 1981; Alberts and May, 1984; Campbell, 1984; Sullivan et al., 1990; Terry and Johanson, 1996; Polan and Hofer, 1998; Sullivan, 2001), complementary information is not available for the vibrissal system. Thus, the present study examined the role of vibrissal stimulation in modulating neonatal behavior.
The neonatal ages assessed here (the first two postnatal weeks) overlap with critical periods in whisker somatosensory system anatomical development. This sensory system begins to develop during gestation but continues development postnatally in a peripheral-to-central manner (Woolsey and Van der Loos, 1970; Woolsey, 1990). Fine whiskers are present in specialized cells that function as follicle sensory mechanoreceptors by embryonic day 20 (Yamakato and Yohro, 1979; English et al., 1980). The accompanying neural pathway matures sequentially, with the trigeminal barrellette pattern present at birth and thalamic barrelloids maturing at postnatal day (PN)1, with the thalamocortical afferents quickly invading the upper tier of cortical layer IV (Taber, 1963; Killackey and Belford, 1979; Forbes and Welt, 1981). Cortical barrels first appear at PN3–5 (Rice et al., 1985; Rice, 1995), and continue development for at least the next week (Stern et al., 2001).
Rat pups can survive and develop without whiskers (Van der Loos and Woolsey, 1973; Weller and Johnson, 1975; Harris and Woolsey, 1979; Killackey and Belford, 1979; Simons and Land, 1987; Henderson et al., 1991; Schlaggar and O’Leary, 1994; Rhoades et al., 1996). However, at least two pieces of evidence suggest a functional importance for whiskers during development. First, neonatal rats can learn an association to passive whisker stimulation during the first postnatal week (Landers and Sullivan, 1999a,b). Second, whisker clipping during the first postnatal month (PN1–40) impairs adult performance on difficult texture discriminations in animals whose whiskers have been allowed to regrow (Carvell and Simons, 1996).
These data suggest that the vibrissal system is functional during the neonatal period and capable of supporting associative learning, although the neural correlates of this behavioral plasticity remain unclear. Within the neonatal olfactory system, which is the only other functioning sensory system in the neonate, one day of neonatal odor learning produces enhanced neural activity and increased glomerular size within the olfactory bulb (reviewed in Sullivan, 2001). Therefore, it is possible that whisker learning during the first days of life may result in an enhancement of cortical barrel size.
The present study was designed to (1) identify specific behaviors elicited by whisker stimulation, (2) determine the effect of neonatal manipulations on the development of barrel dimensions, and (3) explore the adaptive value of whisker stimulation in a “naturalistic” postnatal environment. In these experiments, pups were stimulated and tested at ages chosen to span the established milestones in whisker behavioral and barrel development noted above. Since the developing rodent whisker system is a widely used model system for neurobehavioral plasticity, this information may be valuable for understanding the role of behavioral ontogeny in central nervous system (CNS) development (Erzurumlu and Kind, 2001).
Materials and methods
Subjects
Subjects were Long Evans male and female rat pups born in vivariums at the University of Oklahoma and Columbia University College of Physicians and Surgeons. No more than one male and one female from a litter were used in an experimental condition. Dams were housed in rectangular polypropylene cages (34 × 29 × 17 cm) lined with wood chips in a temperature and light controlled room (20°C, 12 h/12 h light/dark cycle). Ad libitum food and water were available at all times. Births were checked twice daily, with the day of birth considered PN0. Litters were culled on PN0–1 to five males and five females.
Whisker stimulation
Pups were removed from their home cage and placed in a thermoneutral environment (27°C) in plastic Petri dishes (10 cm diameter, 15 mm height). After a 10-min acclimation period (to allow recuperation from experimental handling) pups received eight presentations of unilateral whisker stimulation. All whiskers on one side of the snout were stimulated manually every 3 min for 30 s (about 50 sweeps cycling back and forth across the entire whisker field) using a wooden rod about 1 mm in diameter. Stimulation consisted of repeated flexion of all mystacial vibrissa, without stimulating the intervibrissal hair or skin on the snout.
Behavioral assessment protocol for passive whisker stimulation and learning
Activity was recorded 10 s prior to stimulation as a baseline control and during the whisker stimulation, using a five-point scale originally designed by Hall (1979) and widely used as a measure of behavior in the motorically immature rat pup. This scale assigns a score for the number of major body parts or regions that moved for at least 2 s (Sullivan et al., 1986; Landers and Sullivan, 1999a, b). Lack of movement receives a value of 0; movement of one limb or the head receives a value of 1; movements of two limbs, such as facewiping, receives a value of 2; movements of two limbs and the head, such as pivoting, receives a value of 3; movements of all four limbs, such as locomotion or lying on the side or supine and treading, receives a value of 4; and movements involving five body parts or regions, such as locomotion with head movements, rolling over, or wall climbing, receives a value of 5. The highest valued behavior occurring during the 30-s test determines that pup’s score, with scores ranging from 0 to 5. Pups also exhibit specific movements such as mouthing, head movements and crawling, and these behaviors were counted during the observation period. Furthermore, the specific behaviors of head turned toward and away from the stimulation, head-up and mouthing were noted. Pups were immediately returned to the litter following the experiment.
Classical conditioning
To maximize conditioning efficacy, the conditioning paradigm was performed daily for 8 days from PN1 through PN8 (Landers and Sullivan, 1999a). These ages were chosen to overlap with cortical barrel anatomical development. Pups were removed from the mother, placed in Petri dishes (10 cm diameter), and allowed to acclimate for 10 min. Training lasted 21 min with the following groups: (1) PAIRED—every 3 min, pups received a 30-s vibrissae stimulation (as described above) paired with shock (0.5 mA for 0.5 s to the hind trunk) overlapping with the last second of the vibrissae stimulation; (2) VIBRISSAE stimulation only—every 3 min, pups received a 30-s vibrissae stimulation; (3) SHOCK only—every 3 min, pups received a shock (0.5 mA for 0.5 s to the hind trunk); and (4) RANDOM presentation of the vibrissae stimulation and shock—shock was delivered every 3 min, but the 30-s vibrissae stimulation occurred randomly within the 3-min intertrial interval and did not overlap with the shock or its observed motor response. The RANDOM condition is a standard classical conditioning control, in which pups receive the same amount of whisker stimulation and shock as PAIRED pups, but it does not support learning because of the lack of temporal pairing. Behavior was monitored throughout conditioning using the same behavioral observation regime (0–5) as described above, and acquisition curves were used to assess learning. Consistent with adult learning studies, pups’ acquisition curves during training reliably predicted test performance (Landers and Sullivan, 1999b). Specifically, behavior was recorded during a 10-s baseline immediately preceding whisker stimulation and during 30 s of whisker stimulation. Pups were immediately returned to the mother at the end of each 21-min training session.
Cortical barrel assessment
Four hours after the last conditioning session (PN8) pups were killed, perfused, and the somatosensory cortex removed and flattened for simultaneous viewing of barrels. The cortex was tangentially sectioned on a microtome (40 μm) and stained with cytochrome oxidase (CO) (Wong-Riley, 1979). Area and perimeter measurement of each barrel and row was carried out by an individual blind to the experimental condition, using the public domain NIH Image program.
Nipple attachment—effects of whisker removal
Two types of nipple attachment test were performed: mother-onside (University of Oklahoma—Hofer et al., 1976) and mother-above, in which the mother was placed above the pups on a Plexiglas platform with the exposed ventrum facing downward (Columbia University—Polan et al., 2001). Both are normal nursing postures but required different pup behaviors. Mothers were anesthetized and therefore did not provide pups with milk (ip urethane, 1.5 ml/kg body weight used at University of Oklahoma, and a mixture of 7 mg ketamine/100 g and 0.6 mg xylazine HCl/100 g body weight at Columbia University). Some tests were videotaped.
Before testing at either university, pups were removed from the nest and half the pups had their whiskers removed with surgical microscissors under a light with a 3-diopter glass lens. Control pups, whose whiskers remained intact, were handled by the experimenter for a similar amount of time as the dewhiskered pups, and the whisker area was stimulated with a small wooden probe using motions similar to those used during whisker cutting. Whisker cutting (and sham cutting) took approximately 1 min per pup. Only four pups were separated from the mother at a time and housed in an incubator (30°C) to maintain pups at thermoneutral between testing sessions. Pups were continuously observed and their behavior recorded every 30 s. Tests at the University of Oklahoma lasted 3 min, while the nipple attachment test at Columbia University lasted 10 min.
Pups in the mother-on-side test version were initially placed perpendicular to the mother’s ventrum with forepaws in contact with the mother, whereas pups in the mother-above test were placed under the mother with their back in light contact with the dam’s ventrum. The latter test required pups to roll over on to their backs to grasp a nipple. Pups were given access to only two adjacent nipples and a nipple was used only once. The dam was the pups’ mother about half the time, and a different, similarly aged postpartum dam from the same colony the other half, although own-mother was represented similarly across experimental conditions. In both behavior tests, latency of nipple attachment was specifically measured but all pup behaviors were continuously monitored.
Huddling test—effects of whisker removal
The huddling test assessed the ability of pups to locate and maintain contact with another stationary pup referred to as the “target” pup (anesthetized, urethane 1.5 ml/kg). The warm (34°C) target pup was placed in a prone position in the center of an enclosure (142 × 25 cm) on the wire mesh floor. The test pup was then placed perpendicular to the target pup with its forepaws touching the target pup. Time spent in contact with the target pup was measured and all pup behaviors were monitored continuously.
Statistics
All results were analyzed with ANOVA followed by post hoc Fisher tests.
Results
Pups’ responsiveness to passive whisker stimulation
At all ages tested, pups responded to passive whisker stimulation with an increase in activity (Fig. 1; F(1, 66) = 171.761, p < 0.001). Activity significantly changed with age (F(4, 66) = 9.927, p < 0.001), with the youngest and oldest pups exhibiting significantly higher activity levels (post hoc Fisher tests p < 0.05). Moreover, prior to the emergence of whisking, whisker stimulation elicited horizontal head movements (not specifically directed towards or away from the stimulation source) (F(4, 66) = 2.879, p < 0.05), head-up (F(4,66) = 3.872, p < 0.01), and mouthing (F(4, 66) = 3.587, p < 0.01). However, in older whisking pups (PN13–15), whisker stimulation did not produce mouthing, continued to elicit head-up responses, and pups showed head turns towards source of stimulation (post hoc Fisher tests p < 0.05).
Figure 1.
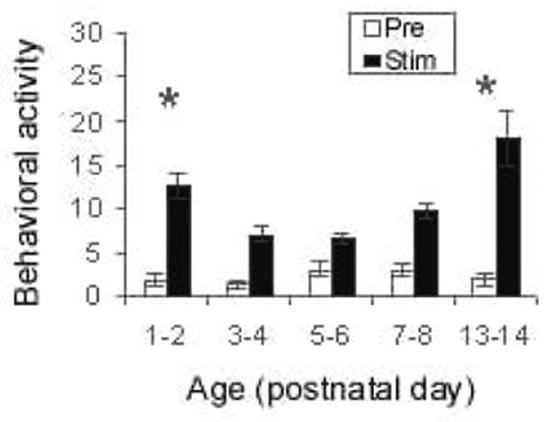
Whisker stimulation produced an increase in behavioral activity in pups as indicated by cumulative response of eight 30-s whisker stimulations (N = 10–16 pups/group). Asterisk represents significant age differences (p < 0.05).
Barrel cortex
Pups used for assessment of the barrel cortex had undergone 8 days of somatosensory conditioning. While the PAIRED pups showed robust acquisition curves (Fig. 2A), no accompanying change in barrel size was detected in the barrel cortex (Fig. 2B, C: both barrel area and perimeter; data analyses for individual barrels, rows and entire barrel field were nonsignificant, ANOVA).
Figure 2.
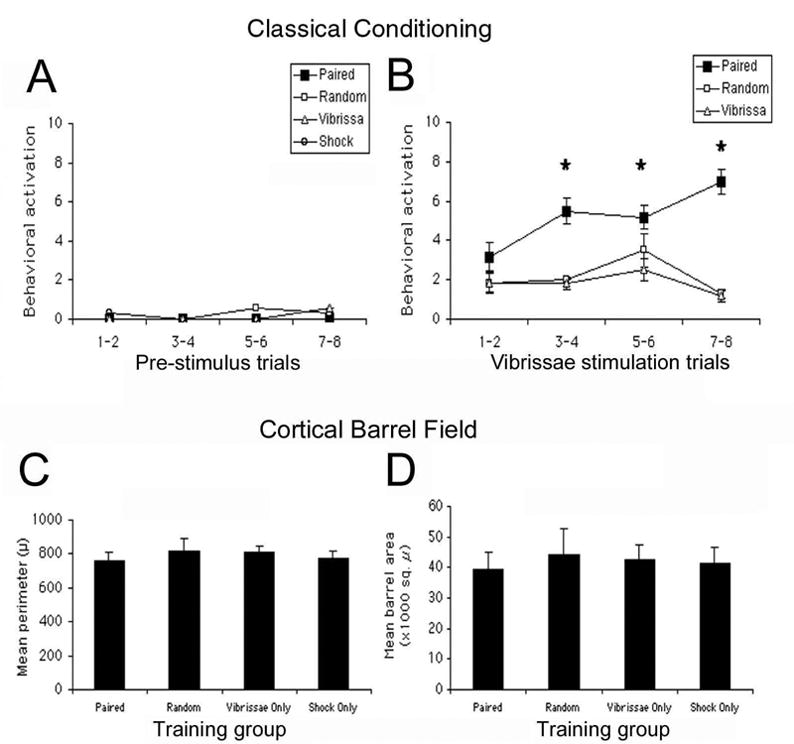
(A) Neonatal rat pups given a whisker learning paradigm (whisker stimulation paired with 0.5 mA shock) for the first 8 days of life exhibited conditioned behavioral activity as measured by acquisition curves during training (N = 6–8 pups/training group). Acquisition curves represent the first 29 s of whisker stimulation, and nonwhisker stimulated conditions such as SHOCK only are not shown. No corresponding plastic changes were found in the anatomy of the barrel cortex for (B) area and (C) perimeter of the barrels (N = 3–4 pups/group). Additional analysis by cortical barrel row yielded consistent nonsignificant results. Asterisk represents a significant difference between the experimental PAIRED group and each of the control groups (p < 0.05).
Nipple attachment and huddling
Removing pups’ whiskers disrupted nipple attachment in the mother-on-side test (Fig. 3A; F(1, 28) = 13.181, p < 0.01), nipple attachment in the mother-above test (F(1, 7) = 10.334, p < 0.01) and huddling (Fig. 3C; F(1, 24) = 5.91, p < 0.05). Post hoc analysis indicates that most of the effect was due to disruption of behavior in PN4–6 pups (post hoc Fisher tests p < 0.05). Moreover, videotape analysis suggests that the delay in nipple attachment reflects the fact that dewhiskered pups exhibited more crawling/rolling (F(1, 8) = 10.638, p < 0.02) and passing over the nipple when it was encountered (ANOVA; F(1, 7) = 9.228, p < 0.05). On the other hand, the reduction in huddling behavior seems to reflect attenuated response to the test situation, with dewhiskered pups showing less activity (ANOVA; F(1, 8) = 6.32, p < 0.05), specifically reduced crawling/pivoting (ANOVA; F(1, 8) = 10.0, p < 0.02).
Figure 3.
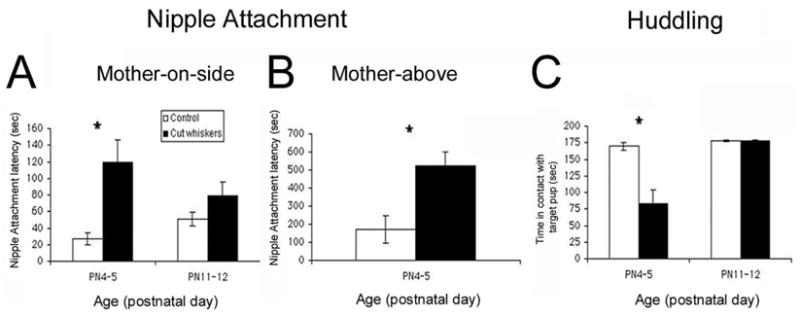
Removing pups’ whiskers disrupted (A) nipple attachment when the mother was on her side (mother-on-side, N = 8 pups/group), (B) nipple attachment when the mother was suspended above the pup (mother-above, N = 4–5 pups/group) and (C) huddling responses to a target pup (N = 7 pups/group). Asterisk represents a significant difference between groups (p < 0.05).
Discussion
The present results suggest that, prior to the emergence of active whisking, passive stimulation of rat pups’ whiskers has at least two important behavioral consequences: (1) modulation of general behavioral activity levels in a context-dependent manner, and (2) focusing of behaviors towards encountered targets. In addition to these behavioral regulatory functions, neonatal whisker stimulation can serve as a conditioned stimulus in an associative conditioning paradigm. Both whisker-dependent behavioral regulation and whisker-associated classical conditioning emerge prior to cortical barrel field maturation, and thus appear to be sub-cortically mediated.
Prior to the emergence of whisking, passive stimulation of rat pups’ whiskers results in generalized behavioral activation that includes increased mouthing and head movements. This evoked activity is similar to behaviors pups use during nipple search and attachment, as well as interactions with other pups. Moreover, the disruptive effect of whisker removal on nipple attachment and huddling suggests that pups’ information from the whiskers enhances behaviors critical for survival by increasing the probability of pups encountering and detecting an important target object (i.e., a nipple or a sibling). For example, the mouthing elicited by whisker stimulation may increase the probability of grasping a nipple when it is encountered. Moreover, dewhiskered pups were more likely to bypass the “target” nipple or littermate after contact, suggesting that information from the whiskers must also have a role in focusing the pup’s behaviors on encountered targets. This suggests that whiskers may provide very specific information about the target. While whiskers may be used to detect a stimulus, whisker input may also modulate responses following contact. Specifically, dewhiskered pups were overactive in the nipple attachment task but underactive in the huddling test, suggesting that the direction of behavioral control by whisker input may depend on the particular conspecific cues or context. Our findings are consistent with previous research on the peri-oral area in neonatal rats, cats, and pigs (Hofer et al., 1981; Larson and Stein, 1984; Morrow-Tesch and McGlone, 1990), but given the effects of dewhiskering, specifically implicate the vibrissal system.
Whisker stimulation following the emergence of whisking (PN13–15) also elicited behavioral activity but failed to elicit mouthing. Moreover, by contrast with the “undirected” movements of younger animals, head movements elicited by stimulation were directed towards the source of stimulation. Such directed movements are characteristic of the effects of whisker stimulation in adults (Simons and Tees, 1990).
In adults, passive whisker stimulation can serve as a conditioned stimulus in a classical conditioning paradigm associated with changes in barrels (2-DG; Siucinska and Kossut, 1996). In the olfactory system of neonatal rat pups, associative odor learning in pups (< PN9) is correlated with both enhanced odor-evoked 2-deoxyglucose uptake in odor-specific glomeruli of the olfactory bulb (Sullivan and Leon, 1986) and an increase in glomerular size (Woo et al., 1987) and number of juxta-glomerular neurons (Woo and Leon, 1991). It therefore seemed possible that similar changes might occur in the neonatal somatosensory system, since the barrel cortex appears responsive in 1-week-old pups (2-DG and surface potentials; Seo-Hiraiwa et al., 1995; Wu and Gonzales, 1997; Landers and Sullivan, 1999a). However, as in our earlier study (2-DG; Landers and Sullivan, 1999a), this early learning is not associated with detectable anatomical changes in the neonatal cortical barrel field. Therefore, we propose that whisker associated conditioning and whisker modulation of behavioral activity is subcortically mediated, perhaps through thalamic sites of sensorimotor contact (Stepniewska et al., 2003). Although learning is usually associated with cortical function, sub-cortical sites for neural correlates of learning are not unusual (Edeline, 1999; McAlonan and Brown, 2002).
In summary, the neonatal whisker system is functional in the nest and exhibits a rapid behavioral plasticity that does not appear to involve the somatosensory cortex. Furthermore, these data suggest that information from the neonatal whiskers aids in the behavioral synchrony and reciprocity necessary to sustain mother–infant interactions.
Acknowledgments
The work was funded by grants NIH NICHD33402, NSF IBN9814837, NSF IBN0117234 (R.M.S.) and NIH MH01569 (H.J.P.). Additional funds were provided by the University of Oklahoma undergraduate research program to J.F. and the Oklahoma Center for Neuroscience to M.L. We would like to thank Jeremy Phelps and Cara Smith for assistance in the cortical barrel analysis, the Woolsey and Jacquin laboratories for guidance in removal and histological preparation of the somatosensory cortex and to Lucy Eljuga for help performing the nipple attachment tests at Columbia University’s Department of Psychiatry. Additional thanks go to D. A. Wilson and H. P. Zeigler for comments on an earlier draft of this paper.
References
- Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Dev Psychobiol. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Alberts JR. Olfactory stimulation induces filial preferences for huddling in rat pups. J Comp Physiol Psychol. 1979;93:548–555. doi: 10.1037/h0077571. [DOI] [PubMed] [Google Scholar]
- Campbell BA. Reflections on the ontogeny of learning and memory. In: Kail R, Spear NE, editors. Comparative Perspectives on the Development of Memory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1984. pp. 23–35. [Google Scholar]
- Carvell GE, Simons DJ. Abnormal tactile experience early in life disrupts active touch. J Neurosci. 1996;16:2750–2757. doi: 10.1523/JNEUROSCI.16-08-02750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Edeline JM. Learning-induced physiological plasticity in the thalamo-cortical sensory systems: a critical evaluation of receptive field plasticity, map changes and their potential mechanisms. Prog Neurobiol. 1999;57:165–224. doi: 10.1016/s0301-0082(98)00042-2. [DOI] [PubMed] [Google Scholar]
- English KB, Burgess PR, Kavka-van Norman D. Development of rat Merkel cells. J Comp Neurol. 1980;194:475–496. doi: 10.1002/cne.901940212. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of “barrels” in the neocortex. Trends Neurosci. 2001;10:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes DJ, Welt C. Neurogenesis in the trigeminal ganglion of the albino rat: a quantitative autoradiographic study. J Comp Neurol. 1981;199:133–137. doi: 10.1002/cne.901990111. [DOI] [PubMed] [Google Scholar]
- Galef GG, Kaner HC. Establishment and maintenance of preference for natural and artificial olfactory stimuli in juvenile rats. J Comp Physiol Psychol. 1980;94:588–595. doi: 10.1037/h0077693. [DOI] [PubMed] [Google Scholar]
- Hall WG. Feeding and behavioral activation in infant rats. Science. 1979;205:206–209. doi: 10.1126/science.451591. [DOI] [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Morphology of Golgi-impregnated neurons in mouse cortical barrels following vibrissae damage at different post-natal ages. Brain Res. 1979;161:143–149. doi: 10.1016/0006-8993(79)90201-4. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Woolsey TA, Jacquin MF. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Dev Brain Res. 1991;66:146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- Hill DL, Almli CR. Olfactory bulbectomy in infant rats: survival, growth and ingestive behaviors. Physiol Behav. 1981;27:811–817. doi: 10.1016/0031-9384(81)90047-0. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Fisher A, Shair H. Effects of infraorbital nerve section on survival, growth, and suckling behaviors of developing rats. J Comp Physiol Psychol. 1981;95:123–133. doi: 10.1037/h0077758. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair H, Singh H. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiol Behav. 1976;17:131–136. doi: 10.1016/0031-9384(76)90279-1. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Sullivan RM. Toward a neurobiology of attachment. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA.: 2001. pp. 599–616. [Google Scholar]
- Killackey HP, Belford GR. The formation of afferent patterns in the somatosensory cortex of the neonatal rat. J Comp Neurol. 1979;183:285–304. doi: 10.1002/cne.901830206. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. Norepinephrine and associative conditioning in the neonatal rat somatosensory system. Dev Brain Res. 1999a;114:261–264. doi: 10.1016/s0165-3806(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. Vibrissae evoked behavior and conditioning before functional ontogeny of the somatosensory vibrissae cortex. J Neurosci. 1999b;19:5131–5137. doi: 10.1523/JNEUROSCI.19-12-05131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MA, Stein BE. The use of tactile and olfactory cues in neonatal orientation and localization of the nipple. Dev Psychobiol. 1984;17:423–436. doi: 10.1002/dev.420170408. [DOI] [PubMed] [Google Scholar]
- McAlonan K, V, Brown J. The thalamic reticular nucleus: more than a sensory nucleus? The Neuroscientist. 2002;8:302–305. doi: 10.1177/107385840200800405. [DOI] [PubMed] [Google Scholar]
- Morrow-Tesch J, McGlone JJ. Sensory systems and nipple attachment behavior in neonatal pigs. Physiol Behav. 1990;47:1–4. doi: 10.1016/0031-9384(90)90034-2. [DOI] [PubMed] [Google Scholar]
- Polan HJ, Eljuga L, Lee J, Thomas S, Hofer MA. Competence of nipple attachment at birth: importance of doing it the hard way. Dev Psychobiol. 2001;38:211. [Google Scholar]
- Polan HJ, Hofer MA. Olfactory preference for mother over home nest shavings by newborn rats. Dev Psychobiol. 1998;33:5–20. [PubMed] [Google Scholar]
- Polan HJ, Hofer MA. Maternally directed orienting behaviors of newborn rats. Dev Psychobiol. 1999;34:269–279. doi: 10.1002/(sici)1098-2302(199905)34:2<269::aid-dev3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Polan HJ, Milano D, Eljuga L, Hofer MA. Development of rats’ maternally-directed orienting from birth to day 2. Dev Psychobiol. 2002;40:81–103. [PubMed] [Google Scholar]
- Rema V, Armstrong-James M, Ebner FF. Experience-dependent plasticity is impaired in adult rat barrel cortex after whiskers are unused in early postnatal life. J Neurosci. 2003;23:358–366. doi: 10.1523/JNEUROSCI.23-01-00358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Crissman RS, Bennett-Clarke CA, Killackey HP, Chiaia NI. Development and plasticity of local intracortical projections within the vibrissae representation of the rat primary somatosensory cortex. J Comp Neurol. 1996;370:524–535. doi: 10.1002/(SICI)1096-9861(19960708)370:4<524::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rice FL. Comparative aspects of barrel structure and development. In: Jones EG, Diamond I, editors. Cerebral Cortex, The Barrel Cortex of Rodents. Vol. 11. Plenum Press; New York: 1995. pp. 1–76. [Google Scholar]
- Rice FL, Gomez C, Barstow C, Burnet A, Sands P. A comparative analysis of the development of the primary somatosensory cortex: interspecies similarities during barrel and laminar development. J Comp Neurol. 1985;236:477–495. doi: 10.1002/cne.902360405. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, O’Leary DM. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994;346:80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- Seo-Hiraiwa ML, Seto-Ohshima A, Kato K. The surface evoked potential and parvalbumin-immunoreactivity in the somatosensory cortex of the developing rat. Dev Psychobiol. 1995;28:337–351. doi: 10.1002/dev.420280604. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987;326:694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Singh PJ, Hofer MA. Oxytocin reinstates maternal olfactory cues for nipple orientation and attachment in rat pups. Physiol Behav. 1978;20:385–389. doi: 10.1016/0031-9384(78)90317-7. [DOI] [PubMed] [Google Scholar]
- Singh PJ, Tobach E. Olfactory bulbectomy and nursing behavior in rat pups. Dev Psychobiol. 1975;8:151–164. doi: 10.1002/dev.420080207. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Short lasting classical conditioning induces reversible changes of representational maps of vibrissae in mouse SI cortex: a 2-DG study. Cerebral Cortex. 1996;96:506–513. doi: 10.1093/cercor/6.3.506. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Sakai ST, Qi HX, Kaas JH. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: a potential substrate for parkinsonian tremor. J Comp Neurol. 2003;455:378–395. doi: 10.1002/cne.10499. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Syoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Unique characteristics of neonatal classical conditioning: the role of the amygdala and locus coeruleus. Integr Physiol Behav Sci. 2001;36:293–307. doi: 10.1007/bf02688797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake S, Williams CL. Huddling and independent feeding of neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986;19:625–635. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Dev Brain Res. 1986;27:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral olfactory bulb responses to maternal odors in preweanling rats. Dev Brain Res. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Symons LA, Tees RC. An examination of the intramodal and intermodal behavioral consequences of long-term vibrissae removal in rats. Dev Psychobiol. 1990;23:849–867. doi: 10.1002/dev.420230807. [DOI] [PubMed] [Google Scholar]
- Taber E. Histogenesis of brain stem neurons studied autoradiographically with thymidine-H3 in the mouse. Anat Rec. 1963;145:291. [Google Scholar]
- Terry LM, I, Johanson B. Effects of altered olfactory experiences on the development of infant rats’ responses to odors. Dev Psychobiol. 1996;29:353–377. doi: 10.1002/(SICI)1098-2302(199605)29:4<353::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tobach E. Photoreception and chemoreception: questions for the evolution and development of orientation. Ann NY Acad Sci. 1971;188:194–201. doi: 10.1111/j.1749-6632.1971.tb13098.x. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;1796:395–397. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Welker W. Analysis of sniffing of the albino rat. Behav. 1964;12:223–244. [Google Scholar]
- Weller LW, Johnson JI. Barrels in cerebral cortex altered by receptor disruption in newborn but not in 5 day old mice. Brain Res. 1975;83:504–508. doi: 10.1016/0006-8993(75)90844-6. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Woo CC, Coopersmith R, Leon M. Localized changes in olfactory bulb morphology associated with early olfactory learning. J Comp Neurol. 1991;263:113–125. doi: 10.1002/cne.902630110. [DOI] [PubMed] [Google Scholar]
- Woo CC, Leon M. Increases in focal population of juxtaglomerular cells in the olfactory bulb associated with early learning. J Comp Neurol. 1991;305:49–56. doi: 10.1002/cne.903050106. [DOI] [PubMed] [Google Scholar]
- Woolsey TA. Peripheral alteration and somatosensory development. In: Coleman JR, editor. Development of Sensory Systems in Mammals. Wiley; New York: 1990. pp. 461–516. [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (S1) of mouse cerebral cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Wu CC, Gonzalez MF. Functional development of the vibrissae somatosensory system of the rat: (14C) 2-deoxyglucose metabolic mapping study. J Comp Neurol. 1997;384:323–336. doi: 10.1002/(sici)1096-9861(19970804)384:3<323::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakato M, Yohro T. Subdivision of mouse vibrissae on an embryological basis with descriptions of variations in the number and arrangement of sinus hairs and cortical barrels. Am J Anat. 1979;155:153–174. doi: 10.1002/aja.1001550202. [DOI] [PubMed] [Google Scholar]


