Abstract
Neutral sphingomyelinase 2 (nSMase2), which has two hydrophobic segments at its NH2-terminus, plays important roles in ceramide-mediated cell regulation. Here, we investigated the membrane topology of nSMase2. When a double-tagged nSMase2 at both the NH2 and COOH termini, was overexpressed in MCF-7 cells, the signals from both tags were detected in the inner leaflet of the plasma membrane. Furthermore, insertion of a tag into the internal sequence and GFP-fused deletion mutants revealed that the entire catalytic region of the protein was located on the cytosolic face of the membranes and each hydrophobic segment is integrated into the membranes, but likely to span the entire membrane. These results indicate the presence of the enzyme in the inner leaflet of plasma membrane.
Keywords: sphingomyelinase, neutral sphingomyelinase, membrane topology, sphingomyelin, ceramide, sphingolipids
Introduction
Ceramide, the product of sphingomyelin hydrolysis, has been shown to play important roles as a bioactive molecule in cell regulatory pathways inducing cell growth, differentiation, and apoptosis [1]. The numerous effects of ceramide are probably due to multiple factors including the magnitude of ceramide generation, the enzymatic source of ceramide, and the subcellular localization of the generated ceramide. Thus, the study and characterization of enzymes that regulate ceramide levels have become essential areas of study.
Sphingomyelinase (SMases) produce ceramide from the hydrolysis of sphingomyelin and they have been implicated as a major pathway of stress-induced ceramide production [2]. Multiple forms of SMases (acid, neutral, and alkaline) have been cloned from mammals [2, 3]. Acid and alkaline SMases contribute to the hydrolysis of sphingomyelin in the lumenal border of cells, in the extracellular space, or in the endosomal system [3]. In contrast, it has been suggested that the magnesium-dependent neutral SMase (nSMase) functions in the inner leaflet of plasma membrane [4]. However, there is little information concerning the membrane topology and orientation of nSMase. nSMase2, which is distributed in Golgi apparatus and plasma membranes, has been suggested to be involved in various ceramide-mediated pathways, including regulation of confluence, cytokine signaling, and the action of toxic agents and stress [5, 6]. Here, we investigated plasma membrane topology of nSMase2. The results show that the entire protein faces the inner leaflet of plasma membrane. The implications of this orientation for sphingolipid metabolism and cell regulation are discussed.
Materials and methods
Cell Culture and cDNA Transfection
MCF-7 cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum at 37°C in a humidified 5% CO2 incubator. cDNA transfection was done using Effectene® transfection reagent (QIAGEN) as described previously [7].
SDS-PAGE, and Western Blotting
SDS-PAGE and Western blotting were performed as described previously [7] using anti-GFP (0.4 μg/ml; Invitrogen) or anti-calnexin antibody (0.2 μg/ml; Santa Cruz Biotechnology).
nSMase activity
nSMase activity was measured using [14C]sphingomyelin as a substrate as described previously [7].
Preparation of Soluble and Membrane Fractions
MCF-7 cells transfected with plasmids were washed twice with PBS, suspended in buffer A (20 mM Tris-HCl (pH7.4), 0.25 M sucrose, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1x protease inhibitor mixture (Roche Diagnostics Co.)), and sonicated. After removal of cell debris by centrifugation at 500 × g for 5 min at 4°C, cell lysates were centrifuged at 100,000 × g for 90 min at 4°C. Occasionally, the cell lysates were treated with an equal volume of buffer A containing 2 M NaCl, 5 M Urea, or 1% Triton X-100 for 1 h on ice before centrifugation. The resulting supernatant and pellet were used as soluble and membrane fractions, respectively.
Immunofluorescence and Confocal Microscopy
Transfected cells were cultured on cover glass and then fixed with 3% paraformaldehyde in PBS for 15 min. After being rinsed with PBS and 50 mM NH4Cl in PBS, cells were permeabilized, if necessary, by 0.1% Triton X-100 in PBS for 5 min at room temperature or 5 μg/ml digitonin for 10 min on ice. After treatment with blocking buffer (2% FBS in PBS) for 15 min, the samples were incubated with anti-V5 (3 μg/ml; Invitrogen), anti-GFP (8 μg/ml; Invitrogen), anti-KDEL (1 μg/ml; Stressgen) or anti-giantin (1:300 dilution; Abcam) antibody with blocking buffer at room temperature for 2 h followed by anti-mouse or rabbit IgG-Alexa FluorR 555 secondary antibody (Invitrogen) at room temperature for 1 h. All confocal images were taken with a laser-scanning confocal microscope (LSM 510 Meta; Carl Zeiss, Thornwood, NY).
Plasmid Construction
the GFP-fused mouse nSMase2 (NSM2GFP) was constructed as described previously [7]. A double-tagged mouse nSMase2 (V5NSM2GFP), with a V5 epitope (GKPIPNPLLGLDST) at the NH2 terminus and a GFP at the COOH terminus, was amplified with a 5′ primer with a XhoI restriction site (5′-TCTCGAGAATGGGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACGGTTTTGTACACGACCCCCTTTCCT-3′) and a 3′ primer (5′-CGCCTCCTCTTCCCCTGCAGACA-3′) using Pfx50™ DNA polymerase (Invitrogen). The fragment was subcloned into the vector pEGFP-N2. The V5 epitope-inserted nSMase2 (V5in1 and V5in2) were constructed by fusing the NH2-terminal fragment of Met1-Thr42 (for V5in1) or Met1-Ser123 (for V5in2) with V5 epitope sequence and the COOH-terminal fragment of Tyr43-Ala655 (for V5in1) or Phe124-Ala655 (for V5in2) with V5 epitope sequence. The NH2-terminal fragments were amplified by PCR using a 5′ primer with XhoI restriction site (5′-GCCGCTCGAGAATGGTTTTGTACACGAC-3′) and a 3′ primer (5′-GACCGAGGAGAGGGTTAGGGATAGGCTTACCGGTGGTGGGTATGAAAGAGGC-3′) for V5in1 or (5′-GACCGAGGAGAGGGTTAGGGATAGGCTTACCGCTTTTGCCTGCCCCCGT-3′) for V5in2. The COOH-terminal fragments were amplified by PCR using a 5′ primer (5′-CCCTAACCCTCTCCTCGGTCTCGATTCTACGTATGAGAAGCGCCAGAGGG-3′) for V5in1 or (5′-CCCTAACCCTCTCCTCGGTCTCGATTCTACGTTTTGCTTCGCCACGGCC-3′) for V5in2, and a 3′ primer (5′-CGCCTCCTCTTCCCCTGCAGACA-3′). These fragments were extended by PCR and subcloned into pEGFP-N2. The GFP fused with NH2-terminal region of nSMase2 (GFPm1 and GFPm3) were designed using two sequences of the NH2-terminal fragments of the enzyme, Met1-Pro52 and Met1-Lys302, respectively. Each sequence was amplified by PCR using a 5′ primer with XhoI restriction site (5′-GCCGCTCGAGAATGGTTTTGTACACGAC-3′) and a 3′-primer (5′-GGGGTCATCTGCCCTCTGGCGCTT-3′) for GFPm1 or (5′-CTTCACCAAGGACTCCCTGGAGG-3′) for GFPm3, and subcloned into pEGFP-N2. The hydrophobic segment 2 (HS2)-fused GFP (GFPm2) was constructed by fusing the NH2-terminal fragment of Met1-Ser11 and the COOH-terminal fragment of Thr60-Ser123. The NH2-terminal fragment was amplified using a 5′ primer with XhoI restriction site (5′-GCCGCTCGAGAATGGTTTTGTACACGAC-3′) and a 3′ primer (5′-GCGTGAAGAGGACCGTGCTATTAGGAAAGGGGGTCG-3′). The COOH-terminal fragment was amplified using a 5′ primer (5′-CCCCTTTCCTAATAGCACGGTCCTCTTCACGCCAGT-3′) and a 3′ primer (5′-GCTTTTGCCTGCCCCCGTGCC-3′). These fragments were extended and subcloned into pEGFP-N2. The sequences of all constructs were verified with an ABI377 DNA sequencer.
Results
Orientation of the NH2 and COOH termini of nSMase2 in the plasma membrane
To clarify the membrane topology of nSMase2, a double-tagged protein (V5NSM2GFP), with a V5 tag at the NH2 terminus and a GFP tag at the COOH terminus, was constructed (Fig. 1A). As reported previously [8], nSMase2 was localized at the plasma membrane when overexpressed in the confluent culture of MCF-7 cells (Fig. 1B). The orientation of the NH2 and COOH termini was determined by antibody accessibility in permeabilized and unpermeabilized cells. The V5 signal was observed on the plasma membrane when cells were permeabilized with 0.1% Triton X-100 after fixation (Fig. 1B, panel b). However, the signal was very weak and hardly detectable in unpermeabilized cells although the protein was clearly detected by GFP fluorescence itself (Fig. 1B, panels c and d). Thus, while the protein itself was at the plasma membrane, the V5 signal was not accessible from the exterior of the cell. Likewise, the COOH-terminal GFP was stained by anti-GFP antibody only when cells were permeabilized with Triton X-100 (Fig. 1B, f versus h). To confirm that the addition of the V5 tag at the NH2 terminus does not disturb the protein functionally, nSMase activity was measured in lysates of V5NSM2GFP or GFP-tagged nSMase2 at the COOH terminus [7] overexpressing cells. As shown in Fig. 1C, both constructs exhibited robust and significant nSMase activity. The immunostaining of the outer leaflet of plasma membrane in unpermeabilized condition was confirmed by overexpressed human full-length neutral ceramidase, which is reported to be localized, in part, at the plasma membrane as a type II transmembrane protein [9] (see supplemental Fig). Taken together, these results indicate that both NH2 and COOH termini of nSMase2 reside on the cytoplasmic side of the plasma membrane.
Fig. 1. Topology of NH2 and COOH termini of nSMase2 at the plasma membrane.

A, schematic diagram of NH2- and COOH-terminus tagged nSMase2 (V5NSM2GFP). B, immunofluorescence of overexpressed V5NSM2GFP with anti-V5 and GFP antibodies under permeabilized or unpermeabilized condition in MCF-7 cells. Cells overexpressing V5NSM2GFP were fixed, permeabilized with 0.1% TritonX-100 (a, b, e, and f) or not (c, d, g and h), and immunostained with anti-V5 (b and d) or GFP (f and h) antibody. C, nSMase activity in lysates of MCF-7 cells overexpressing V5NSM2GFP or GFP-tagged nSMase2 at the COOH terminus (NSM2GFP) was measured using [14C]sphingomyelin as a substrate. Details are described in “Materials and methods”.
Orientation of internal sequences of nSMase2 in the plasma membrane
The hydropathy profile of nSMase2 shows that there are two highly hydrophobic segments (HS1 and HS2) near the NH2-terminal region (Fig. 2A), which have been proposed as transmembrane regions. To investigate the orientation of each hydrophobic domain in membranes, a V5 tag was inserted into the internal sequence of the protein. Two constructs were generated as shown in figure 2B; insertion of the V5 tag between HS1 and HS2 (V5in1) or after the sequence of HS2 (V5in2). Both constructs were tagged with GFP at the COOH terminus. When these constructs were overexpressed in MCF-7 cells, the GFP fluorescence signal was detected on the plasma membrane, which corresponded to the staining of a plasma membrane marker FM4-64FX (data not shown). In addition, both constructs exhibited robust nSMase activity when transfected (data not shown). In V5in1-overexpressing cells, a strong signal for anti-V5 antibody (Fig. 2C, b versus d) or anti-GFP antibody (Fig. 2C, f versus h) was detected on the plasma membrane in permeabilized cells much more strongly than in unpermeabilized cells. Similar results were obtained when cells were transfected with V5in2 (Fig. 2D). These results indicated that the sequence between HS1 and HS2 and the sequence after HS2 both reside on the cytoplasmic side of the plasma membrane.
Fig. 2. Insertion of V5 tag into internal sequences of nSMase2.

A, Hydrophobicity profile of nSMase2. The deduced amino acid sequence of mouse nSMase2 was analyzed by the method of Kyte and Doolittle [19] for hydrophobicity plotting. Two highly hydrophobic segments (HS1 and HS2) were marked. B, schematic diagram of V5 tag-inserted mutants. C and D, immunofluorescence of overexpressed V5in1 (C) and V5in2 (D) with anti-V5 (b and d) or GFP antibody (f and h) under permeabilized (a, b, e, and f) or unpermeabilized (c, d, g and h) condition. Details are described in “Materials and methods”.
The role of hydrophobic segments of nSMase2 in membrane binding
The preceding results on the orientation of the NH2 and COOH termini and the internal sequence of nSMase2 suggested that the two hydrophobic segments may not span the plasma membranes from the outer to inner leaflets or from inner to outer leaflets, but reside on the cytoplasmic side of the plasma membrane. To investigate whether the two hydrophobic segments function as a transmembrane region, GFP was used as a reporter molecule (Fig. 3A) whereby the HS1- or HS2-fused proteins (GFPm1 and GFPm2) were constructed and expressed in MCF-7 cells. We also used GFP-tagged nSMase2 (NSM2GFP) and a chimeric protein (GFPm3), in which the whole putative catalytic region was replaced with GFP. Both GFPm1 and GFPm2 showed two bands, 27 kDa and 33 kDa, and 27 kDa and 36 kDa, respectively (Fig. 3B). The significant amount of the 27-kDa GFP found in cells expressing both chimeric proteins can be explained by a translation from the internal GFP initiation codon. These fusion proteins were recovered in the membrane fraction of cell lysates of the overexpressed cells, whereas normal GFP was observed in the soluble fraction (Fig. 4A). The membrane association of both GFPm1 and GFPm2 proteins was not disturbed by urea or by high salt treatment (Fig. 4B). On the other hand, the association was almost completely disrupted in the presence of 0.5% Triton X-100, suggesting that this is not caused by aggregation due to misfolding of the proteins. These results were similar to those for NSM2GFP and the integral membrane protein marker calnexin (Fig. 4B). These data suggest that both hydrophobic segments are integrated into membranes.
Fig. 3. Construction of chimeric proteins of GFP and nSMase2.
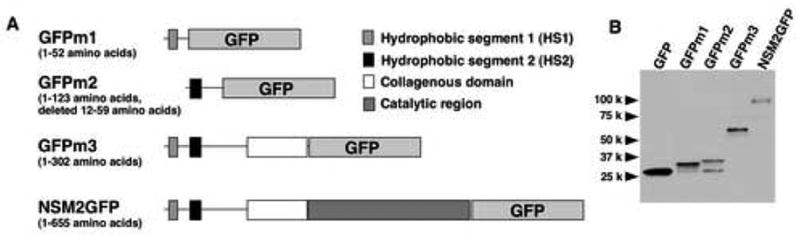
A, schematic diagram of chimeric GFP proteins. B, Western blotting of the overexpressed proteins. MCF-7 cells were transfected with plasmid vector containing GFP, GFPm1, GFPm2, GFPm3, or NSM2GFP. Cell lysates were analyzed by Western blotting using anti-GFP antibody.
Fig. 4. Function of HS1 and HS2 of nSMase2 in membrane binding.

A, total cell lysates prepared from GFP, GFPm1, GFPm2, GFPm3 or NSM2GFP-overexpressing cells were fractionated by ultracentrifugation into the soluble (S) and membrane fractions (M), then examined by Western blotting using anti-GFP or calnexin antibody as described in “Materials and methods”. B, total cell lysates prepared from NSM2GFP, GFPm1, or GFPm2-overexpressing cells were treated with buffer containing 2.5 M urea, 1 M NaCl, or 0.5% Triton X-100 for 1 h on ice, then fractionated by ultracentrifugation, and examined by Western blotting.
Membrane topology of GFP-fused proteins
Unlike wild-type nSMase2, the GFPm1 and GFPm3 proteins localized in the ER/Golgi compartments when overexpressed in MCF-7 cells whereas normal GFP was found in the cytosol and in nuclei (Fig. 5A). The chimeric proteins were partly co-localized with giantin, a marker protein for the Golgi apparatus (Fig. 5A). To determine the membrane topology of these fusion proteins, the orientation of the GFP moiety in GFPm1 and GFPm3 was examined through the accessibility of antibody in appropriately permeabilized cells. Digitonin treatment of fixed cells selectively permeabilizes the plasma membrane whereas Triton X-100 treatment permeabilizes all intracellular membranes [10]. The effectiveness of each detergent was confirmed by anti-KDEL antibody, which allows the detection of ER luminal proteins, and anti-GFP antibody in normal GFP-overexpressing cells. The signal of normal GFP by anti-GFP antibody was observed in both digitonin and Triton X-100-treated cells (Fig. 5B, panels d and f) whereas the KDEL signal was observed in only Triton X-100-treated cells (Fig. 5B, panels j and l), demonstrating that digitonin does not allow access to a luminal signal. Importantly, the signal for anti-GFP antibody in GFPm1- or GFPm3-expressing cells was detected by treatment with either Triton X-100 or digitonin (Fig. 5C and D, panels d and f). These results indicate that the GFP moiety of each chimeric protein resides on the cytoplasmic leaflet of intracellular membranes. These results coincided well with those of V5 and GFP-tagged wild type/full length nSMase2 (Fig. 1 and 2).
Fig. 5. Localization and membrane topology of GFP-fused proteins.

A, subcellular localization of overexpressed GFP, GFPm1, and GFPm3 in MCF-7 cells. Cells were fixed, permeabilized, and immunostained with anti-giantin. Images of GFP (left panels) and giantin (center panels) were merged (right panels). B, effectiveness of permeabilization by digitonin and Triton X-100 in fixed cells. MCF-7 cells overexpressing GFP were fixed and treated with 5 μg/ml digitonin (panels c, d, i, and j), 0.1% Triton X-100 (panels e, f, k, and l) or not (a, b, g, and h). The cells were stained with anti-GFP or anti-KDEL antibody. C and D, orientation of the GFP moiety of GFPm1 (C) and GFPm3 (D). MCF-7 cells overexpressing GFPm1 or GFPm3 were fixed and treated with 5 μg/ml digitonin (panels c and d), 0.1% Triton X-100 (panels e and f) or not (a and b). The cells were stained with anti-GFP antibody. Details are described in “Materials and methods”.
Discussion
The results from the present study provide information on the membrane orientation of nSMase2. Two hydrophobic segments at the NH2 terminus are integrated into membranes, but both NH2 and COOH termini of these segments are cytoplasmically located (Fig. 6). Taken together, these results suggest the localization of the entire protein at the inner leaflet of the plasma membrane and a hairpin-like structure of the two hydrophobic segments of nSMase2 in the membrane. Similar structures were also suggested for a few membrane and membrane-associated proteins, such as caveolin-1 and S2P (site-2 protease for SREBPs) which have one and four hairpin-like transmembrane domains, respectively [11, 12]. Very recently, it has been shown that nSMase2 is S-palmitoylated in two Cys clusters; one cluster was located between the two hydrophobic segments, and the second one located in the middle of catalytic region of the protein [7]. Since S-palmitoylation is mostly restricted to the cytoplasmic side of the membranes [13], this posttranslational modification strongly supports the model presented in this study (proposed membrane topology and palmitoylation sites are shown in Fig. 6).
Fig. 6. Predicted membrane topology of nSMase2.

The scheme illustrates the predicted membrane topology of nSMase2 which is also supported by the presence of the two palmitoylation clusters as indicated.
The present study clearly indicates that the catalytic region of nSMase2 faces to the inner leaflet of plasma membrane, suggesting that the enzyme hydrolyzes sphingomyelin at this site. It has already been suggested that the hydrolysis of sphingomyelin occurs at the inner leaflet of the plasma membrane in response to activation of nSMases [4]. Furthermore, potential direct target molecules of ceramide, such as protein phosphatase2A, protein phosphatase1, and protein kinase Cζ, are localized at the cytoplasmic region of cells [14, 15]. However, this notion remained somewhat difficult to accept, because sphingomyelin is mostly localized at the outer leaflet of the plasma membrane and its synthesis occurs primarily in the lumenal side of the Golgi [16, 17]. On the other hand, the presence of a sphingomyelin pool at the inner leaflet of the plasma membrane has been demonstrated [18]. Thus, this study lends significant molecular support for the presence of a major sphingomyelin-metabolizing enzyme at the inner leaflet. These results also raise questions as to how sphingomyelin may reach the inner leaflet. As such, the elucidation of site specific synthesis of sphingomyelin vs. flipping from the outer leaflet to the inner leaflet become important areas for further study.
Importantly, the membrane orientation of nSMase2 would be critical for understanding regulatory mechanisms of this enzyme at a molecular level, such as cytoplasm-specific posttranslational modifications (acylation, phosphorylation, and ubiquitination etc) or direct interaction with other cytosolic proteins. Finally, the molecular characterization of nSMase2 will facilitate our understating of signaling pathways mediated by the enzyme.
Supplementary Material
Acknowledgments
This work is supported in part by NIH grant GM43825 and the Japan Society for the Promotion of Science.
Abbreviations
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- PBS
phosphate-buffed saline
- PCR
polymerase chain reactions
- SMase
sphingomyelinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 3.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007;19:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Andrieu-Abadie N, Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta. 2002;1585:126–134. doi: 10.1016/s1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci U S A. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 7.Tani M, Hannun YA. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues: role of palmitoylation in subcellular localization. J Biol Chem. 2007 doi: 10.1074/jbc.M611249200. In press. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem. 2004;279:25101–25111. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- 9.Hwang YH, Tani M, Nakagawa T, Okino N, Ito M. Subcellular localization of human neutral ceramidase expressed in HEK293 cells. Biochem Biophys Res Commun. 2005;331:37–42. doi: 10.1016/j.bbrc.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda S, Nishijima M, Hanada K. Localization, topology, and function of the LCB1 subunit of serine palmitoyltransferase in mammalian cells. J Biol Chem. 2003;278:4176–4183. doi: 10.1074/jbc.M209602200. [DOI] [PubMed] [Google Scholar]
- 11.Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000;275:21605–21617. doi: 10.1074/jbc.M002558200. [DOI] [PubMed] [Google Scholar]
- 12.Zelenski NG, Rawson RB, Brown MS, Goldstein JL. Membrane topology of S2P, a protein required for intramembranous cleavage of sterol regulatory element-binding proteins. J Biol Chem. 1999;274:21973–21980. doi: 10.1074/jbc.274.31.21973. [DOI] [PubMed] [Google Scholar]
- 13.Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 14.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Sliva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 16.Barenholz Y, Thompson TE. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta. 1980;604:129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- 17.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. EMBO J. 2003;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linardic CM, Hannun YA. Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle. J Biol Chem. 1994;269:23530–23537. [PubMed] [Google Scholar]
- 19.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


