Abstract
Recent work in the area of stroke and brain ischemia has demonstrated the significance of the inflammatory response accompanying necrotic brain injury. Acutely, this response appears to contribute to ischemic pathology, and anti-inflammatory strategies have become popular. This chapter will discuss the current knowledge of the contribution of systemic and local inflammation in experimental stroke. It will review the role of specific cell types including leukocytes, endothelium, glia, microglia, the extracellular matrix and neurons. Intracellular inflammatory signaling pathways such as nuclear factor kappa beta and mitogen-activated protein kinases, and mediators produced by inflammatory cells such as cytokines, chemokines, reactive oxygen species and arachidonic acid metabolites will be reviewed as well as the potential for therapy in stroke and hypoxic-ischemic injury.
1. Introduction
Stroke is one of the most frequent causes of death and disability worldwide, and has significant clinical and socioeconomic impact. Although different mechanisms are involved in the pathogenesis of stroke, there is increasing evidence showing that inflammation accounts for its progression, at least acutely (Barone and Feuerstein, 1999; Chamorro and Hallenbeck, 2006; Samson et al., 2005). A robust inflammatory reaction characterized by peripheral leukocyte influx into the cerebral parenchyma and activation of endogenous microglia follows focal cerebral ischemia (Becker, 1998; Davies et al., 1998; Hallenbeck, 1996; Morioka et al., 1993; Zheng and Yenari, 2004). Cessation of cerebral blood flow leads to energy depletion and necrotic neuron death, which can trigger immune responses ultimately leading to inflammatory cell activation and infiltration. Reperfusion of the occluded vessel, either due to compensation by the collateral circulation, or spontaneous or therapeutic recanalization leads to the generation of reactive oxygen species (ROS) either by reperfusion with oxygenated blood or production within brain and immune cells. ROS can then stimulate ischemic cells, even ischemic neurons, to secrete inflammatory cytokines and chemokines that cause, among other things, adhesion molecule upregulation in the cerebral vasculature and peripheral leukocyte recruitment, respectively. Once activated, inflammatory cells can release a variety of cytotoxic agents including more cytokines, matrix metalloproteinases (MMPs), nitric oxide (NO) and more ROS (Figure 1). These substances may induce more cell damage as well as disruption of the blood-brain barrier (BBB) and extracellular matrix ((Danton and Dietrich, 2003; Emsley and Tyrrell, 2002). BBB disruption can further potentiate brain tissue injury and contribute to secondary ischemic brain damage by permitting serum elements and blood to enter the brain (Rosenberg, 1999; Siesjo and Siesjo, 1996). Secondary damage develops as a consequence of brain edema, post-ischemic microvascular stasis and vasomotor/hemodynamic deficits leading to hypoperfusion and post-ischemic inflammation, thus involving activation of microglia and brain infiltration of peripheral inflammatory cells (Dirnagl et al., 1999; Siesjo and Siesjo, 1996). This type of migration of peripheral circulating leukocytes into the brain could produce an amplification of inflammatory signal cascades, which will enhance the damage. These processes are especially pronounced during reperfusion when previously occluded vessels are opened and lead to massive influx of ROS and leukocytes into injured brain. Blocking various aspects of the inflammatory cascade has shown to ameliorate injury from experimental stroke (Han and Yenari, 2003), although this has yet to be demonstrated at the clinical level (Becker et al., 2001).
Figure 1. Inflammation following stroke.
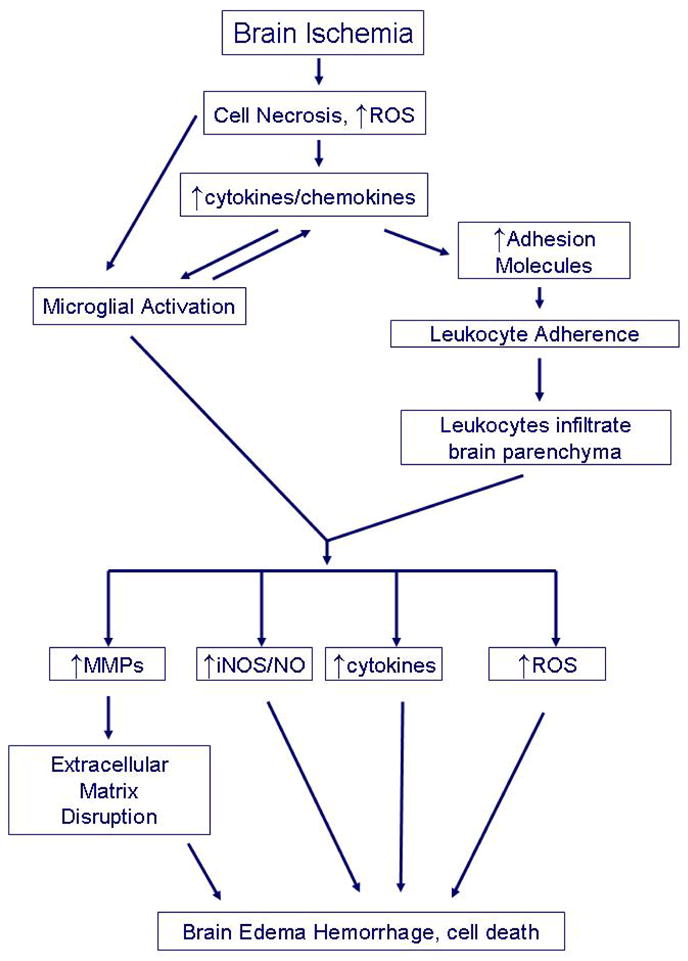
Brain ischemia is triggers inflammatory responses due to the presence of necrotic cells, generation of reactive oxygen species (ROS) and production of inflammatory cytokines even within neurons. These initiators lead to microglial activation which produce more cytokines causing upregulation of adhesion molecules in the cerebral vasculature. Chemokines lead to inflammatory cell chemotaxis to ischemic brain. Adhesion molecules mediate adhesion of circulating leukocytes to vascular endothelia and infiltration into the brain parenchyma. Once in the brain, activated leukocytes and microglia produce a variety of inflammatory mediators such as matrix metalloproteinases (MMPs), inducible nitric oxide synthase (iNOS) which generates nitric oxide (NO), cytokines and more ROS which lead to brain edema, hemorrhage and ultimately, cell death. MMPs are thought to mediate extracellular matrix disruption, a key event in brain edema and hemorrhage.
During the past few years, progress has been made towards identifying the roles of important inflammatory signaling molecules, cells and proteins in the process of initiation and development of post-ischemic inflammation. This review focuses on current findings and provides an update on the understanding of post-ischemic inflammation.
2. Cellular response to ischemic stroke
Inflammation is characterized by the accumulation of inflammatory cells and mediators in the ischemic brain. After ischemia onset, inflammatory cells such as blood-derived leukocytes and microglia are activated and accumulate within the brain tissue subsequently leading to inflammatory injury. Increasing evidence shows that astrocytes may also act as inflammatory cells responding to ischemic stroke.
2.1. Leukocytes
4–6 h hours after ischemia onset, circulating leukocytes adhere to vessel walls, leading to migration and accumulation into ischemic brain tissue with subsequent release of proinflammatory mediators. These mediators lead to secondary injury of potentially salvageable tissue within the penumbra surrounding the infarct core. Neutrophils are generally the first leukocyte subtype recruited to the ischemic brain, and may potentiate injury by directly secreting deleterious substances or other inflammatory mediators (Hallenbeck, 1996). In transient ischemia, several studies have shown that infarct volume is significantly reduced when neutrophil infiltration is inhibited (Bowes et al., 1995; Chopp et al., 1996; Clark et al., 1995; Connolly et al., 1996; Garau et al., 2005; Yenari et al., 1998). Some mediators, while not directly cytotoxic, may be involved in the destruction of necrotic and neighboring viable tissue. Evidence that neutrophils potentiate ischemic injury include numerous studies documenting improved neurological outcome following neutrophil depletion and inhibition of adhesion molecules which facilitate neutrophil entry into injured brain (see reviews (Hartl et al., 1996; Zheng and Yenari, 2004). The roles of lymphocytes are generally intended to play a negative role in ischemic brain pathogenesis even though there is also conflicting data. Following permanent middle cerebral artery occlusion (MCAO) in rats, lymphocytes were elevated in the ischemic lesion after neutrophils (Li et al., 2005; Stevens et al., 2002). Preventing lymphocyte trafficking into ischemic brain ameliorated injury, suggesting that like neutrophils, lymphocytes also play a deleterious role (Becker et al., 2001). Clinical studies also show that lymphocytes have a strong pro-inflammatory and tissue-damaging properties, and the upregulation of circulating lymphocytes are correlated to an increased risk of stroke recurrence and death (Nadareishvili et al., 2004). However, in a study of cultured primary neurons, isolated neutrophils, but not lymphocytes potentiated neuronal injury due to excitotoxin exposure (Dinkel et al., 2004).
2.2. Microglia/Macrophages
Microglia, the resident macrophages of the brain, play a critical role as resident immunocompetent and phagocytic cells in the CNS (Kreutzberg, 1996), and serve as scavenger cells in the event of infection, inflammation, trauma, ischemia, and neurodegeneration (El Khoury et al., 1998; Thomas, 1992). Once activated, microglia can undergo morphologic transformation into phagocytes, making them virtually indistinguishable from circulating macrophages.
Microglial activation may be induced by cerebral ischemia, causing a release of a variety of substances many of which are cytotoxic and/or cytoprotective (Wood, 1995). Via CD14, microglia are activated, followed by stimulation of toll-like receptor 4 (TLR4) (Guha and Mackman, 2001; Saito et al., 2000). How microglia are activated following ischemia is not completely clear, but CD14 receptors have been documented in monocytes and activated microglia in brains of stroke patients (Beschorner et al., 2002). Furthermore, work in neonatal mice indicated that TLR4 was necessary for microglial activation following hypoxia/ischemia (Lehnardt et al., 2003).
Whether microglia/macrophages are necessarily damaging following brain ischemia is unclear, but a few lines of evidence suggest that activated microglia may contribute to injury. Edaravone, a novel free radical scavenger, significantly reduced the infarct volume and improved the neurological deficit scores for ischemic mice by reducing microglial activation (Zhang et al., 2005a). In spontaneously hypertensive rats with permanent MCAO, Gunther et al. (Gunther et al., 2005) found that repetitive hyperbaric oxygen (HBO) treatment obviously reduced the infarct volume by suppressing microglia activation. In transient MCAO, phagocytic microglial were documented in the cerebral cortex of the ischemic hemisphere (Yu et al., 2005; Zhang et al., 1997). Minocycline, a tetracycline family antibiotic, was shown to provide significant protection against brain ischemia by inhibiting of microglial activation and proliferation (Yrjanheikki et al., 1998; Yrjanheikki et al., 1999). Direct evidence supporting a damaging role following ischemic insults were demonstrated when microglia/macrophages were applied to neuron or oligodendrocyte cultures. Injury from various ischemia-like insults was increased in the presence of microglia/macrophages (Giulian et al., 1993; Lehnardt et al., 2003; Zhang et al., 1997). However, some studies indicate that microglia/macrophages or their secreted factors may actually protect cells (Watanabe et al., 2000). No effect on infarct size was seen after depleting peripheral macrophages using liposome-encapsulated clodronate (Schroeter et al., 1997). However, this latter study did not deplete brain macrophages and may suggest that brain, rather than circulating macrophages are important in ischemic pathogenesis.
2.3. Astrocytes
Aside from traditional inflammatory cells, astrocytes are known to express different kinds of inflammatory mediators (Benveniste, 1998; Che et al., 2001). Following ischemia, brain astrocytes are activated resulting in increased glial fibrillary acidic protein (GFAP) expression and a so-called “reactive gliosis,” characterized by specific structural and functional changes (Pekny and Nilsson, 2005). Astrocytes also participate in brain inflammation by expressing major histocompatibility complex (MHC) and costimulatory molecules, developing Th2 (anti-inflammatory) immune responses and suppressing interleukin-12 (IL-12) expression, though this has yet to be demonstrated in ischemia models (reviewed by(Dong and Benveniste, 2001). Astrocytes are also capable of secreting inflammatory factors such as cytokines, chemokines and inducible nitric oxide synthase (iNOS) (Dong and Benveniste, 2001). Following 10 minutes of transient global ischemia, iNOS expression was found in reactive astrocytes of hippocampus but not in uninjured hippocampal astrocytes (Endoh et al., 1994). Furthermore, inducible nitric oxide synthase (iNOS) in astrocytes has been shown to potentiate ischemia-like injury to neurons (Hewett et al., 1996). Recently studied protein, tumor necrosis factor-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis factor superfamily, is thought to be produced by neurons, astrocytes and endothelial cells (Donohue et al., 2003; Yepes et al., 2005), and can stimulate proinflammatory molecule production by interaction with its Fn14 receptor found on astrocytes(Saas et al., 2000). Expression of TWEAK and Fn14 has been documented in a murine model of stroke, and a soluble decoy to Fn14 markedly reduced infarct volume (Yepes et al., 2005). These data may suggest that while astrocytes normally play important roles in neuron maintenance and function, activated astrocytes have the potential to pose harm to ischemic brain.
3. ADHESION MOLECULES
Adhesion molecules play a pivotal role in the infiltration of leukocytes into the brain parenchyma after stroke and may represent important therapeutic targets (see recent review by (Sughrue et al., 2004). Three major steps, rolling and adhesion and transendothelial migration of leukocytes, are involved in the access of leukocytes to the brain through the endothelial wall. Activated leukocytes, especially neutrophils, result in further damage of ischemic lesions through reperfusion or secondary injury mechanisms (Guha and Mackman, 2001). The interaction between leukocytes and the vascular endothelium is mediated by three main groups of cell adhesion molecules: selectins (P-selectin, E-selectin, and L-selectin), the immunoglobulin superfamily (intercellular adhesion molecules, e.g. ICAM-1, 2 and vascular cell adhesion molecule-1, or VCAM-1) and integrins (CD11a–c) (DeGraba, 1998; Emsley and Tyrrell, 2002). Several reports have shown that inhibiting leukocyte adhesion by targeting various adhesion molecules, thus preventing leukocytes from entering ischemic brain, results in reduced neurologic injury (Clark et al., 1995; Clark et al., 1997). Furthermore, animals deficient in adhesion molecules have reduced infarct volume after transient focal cerebral ischemia (Connolly et al., 1997; Kitagawa et al., 1998; Soriano et al., 1999). Although several adhesion molecules have been documented in both permanent and transient MCAO, (Haring et al., 1996; Suzuki et al., 1998; Zhang et al., 1996), inhibiting these targets only appears to be effective when reperfusion occurs (Clark et al., 1997; Prestigiacomo et al., 1999; Zhang et al., 1995a).
3.1. Selectins
Selectins mediate cell-cell adhesion, and rolling of leukocytes on the endothelium of postcapillary venules. Three kinds of selectins have been identified: E-selectin, P-selectin and L-selectin (Carlos and Harlan, 1994; Hallenbeck, 1996; Kim, 1996). They are expressed on the outer cell membrane immediately upon cell activation by stimulants such s thrombin or histamine. While E- and P-selectin are involved in leukocyte rolling and recruitment during the early stages of activation, L-selectin acts as a guide for unstimulated leukocytes (Bargatze et al., 1994).
The expression of P- and E-selectins have been documented in different experimental stroke models and their upregulation appears to be involved in promoting ischemic inflammatory responses and increases injury due to ischemic stroke (Huang et al., 2000b; Huang et al., 1999). In animal studies, mice overexpressing P-selectin had exacerbation of infarcts, whereas treatment with antibodies or inhibitors against P- and E-selectin was associated with improved neurological outcome (Huang et al., 2000b; Mocco et al., 2002). The effects of P-selectin in ischemic stroke appear different in focal and global ischemia. In focal cerebral ischemia, neutrophils accumulated in the ischemic cortex of wildtype mice more abundantly than in P-selectin knockout mice (Connolly et al., 1997). Moreover, P-selectin deficient mice demonstrated smaller infarct volumes and improved survival compared with wildtype mice. Functional blockade of P-selectin in using a monoclonal antibody also improved early reflow and stroke outcome, with reduced cerebral infarction even when the blocking antibody was administered after ischemia onset. However, P-selectin may play a different role in global cerebral ischemia. Antibody blockade of P-selectin, while reducing leukocyte rolling, paradoxically reduced survival (Lehmberg et al., 2006). The reasons for these contrasting outcomes remain unclear, but suggests that the inflammatory response and its significance after focal and global ischemia may be quite different.
The role of L-selectin in brain ischemia is less clear. Although L-selectin mediates leukocyte transmigration, does not appear to significantly influence stroke outcome. Treating rabbits exposed to transient focal brain ischemia with an L-selectin antibody did not affect stroke outcome (Yenari et al., 2001). However, fucoidin, an inhibitor of both P- and L-selectin, significantly reduced infarct size and improved neurological function in experimental stroke in rats, but these observations could have been due to inhibition of P-selectin rather than L-selectin (Ruehl et al., 2002).
Recent work has also shown that exposing animals to E-selectin intranasally can induce immune tolerance to brain antigens, and consequently reduce the extent of injury (Chen et al., 2003b) and even prevent their occurrence (Takeda et al., 2002). Since E-selectin is exclusively upregulated in stimulated endothelium, tolerance to E-selectin could lead to suppression of immune responses and prevent peripheral leukocyte trafficking into the brain. Thus, it is conceivable that intranasal E-selectin might lead to the development of a vaccine against stroke.
3.2. Immunoglobulin superfamily
Members of the immunoglobulin superfamily include 5 molecules: ICAM-1 and ICAM-2, VCAM-1, platelet-endothelial cell adhesion molecule-1 (PECAM-1), and the mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1). ICAM-1 (also referred to as CD54) is constitutively present in low levels on cell membranes of endothelial cells, leukocytes, epithelial cells, and fibroblasts. Its expression increases upon stimulation by cytokines. ICAM-2 (CD102) is an endothelial cell membrane receptor that does not increase after stimulation, whereas VCAM-1 (CD106) is induced by TNF-α and IL-1. PECAM-1 (CD31) is involved in the attachment of endothelial cells to each other, and leukocyte transmigration across the endothelium. MAdCAM-1, acting as an endothelial cell ligand for leukocyte homing receptors L-selectin and alpha4beta7 integrin, is an adhesion protein expressed on endothelium in mucosal tissues that has been shown to play an important role in the selective homing of lymphocytes to intestinal mucosa and associated lymphoid tissue.
Among all of the 5 immunoglobulin members, ICAM-1 and VCAM-1 have been the most extensively investigated in cerebral ischemia. Prior work has shown increased expression of ICAM-1 in ischemic brain within hours after stroke onset, peaking at about 12–24 h, and precedes leukocyte infiltration (Wang and Feuerstein, 1995; Wang et al., 1994; Zhang et al., 1995b). Several studies have now shown that blocking ICAM-1 with antibodies (Bowes et al., 1993; Chopp et al., 1996; Kanemoto et al., 2002) or inhibiting ICAM-1 mRNA with antisense oligonucleotides (Vemuganti et al., 2004) improves outcome from experimental stroke. Similarly, mice deficient in ICAM-1 had smaller infarcts compared to wildtype mice (Connolly et al., 1996; Kitagawa et al., 1998). Not only inhibitors of ICAM-1 but also nitric oxide donors prevented the ischemia/reperfusion (I/R)-induced increase in ICAM-1 mRNA after ischemia/reperfusion and also led to neuroprotection (Khan et al., 2006). In diabetic rats, ICAM-1 expression was higher after ischemia compared to non-diabetic rats, suggesting that ICAM-1 may, in part, explain why stroke is exacerbated under conditions of hyperglycemia (Ding et al., 2005).
The role of VCAM-1 in stroke is less clear. While increases in VCAM-1 mRNA after cerebral ischemia have been observed (Blann et al., 1999), others have failed to observe such significant changes (Vemuganti et al., 2004). In a study of global cerebral ischemia in rats, ONO-1078, a potent leukotriene receptor antagonist, improved neurological deficits and reduced neuron death by inhibiting the ischemia-induced upregulation of VCAM-1 in the hippocampus of ischemic rats (Zhang and Wei, 2003). One study showed that unfractionated heparin led to reduced infarct size in experimental stroke, and was associated with a reduced inflammatory response including decreased VCAM-1 expression (Cervera et al., 2004). However, another study showed that treatment with anti-VCAM-1 antibodies did not have any effect on stroke outcome suggesting that VCAM-1 may not play a significant role in ischemic brain injury (Justicia et al., 2006).
At the clinical level, increased sICAM-1 and sVCAM-1 have been documented in the plasma and cerebral spinal fluid of subjects with recent cerebral ischemic patients, and correlated to stroke severity (Ehrensperger et al., 2005; Selakovic et al., 2003; Simundic et al., 2004). VCAM-1 expression has been observed in autopsied brains of stroke victims within cerebral vessels and astrocytes (Blann et al., 1999). However, a phase III clinical trial of anti-ICAM therapy for stroke, indicated that anti-ICAM therapy with enlimomab was not an effective treatment for ischemic stroke. In fact, treatment significantly worsened outcome(Enlimomab, 2001). However, the interpretation of this study may have been confounded by the use of a murine antibody in humans, with subsequent neutrophil and complement activation (Vuorte et al., 1999).
3.3. Integrins
As a family of adhesion molecules, integrins consist of a common β subunit and a variable α subunit (Albelda, 1991). There are three subfamilies for the β subunits, denoted β1–3. Members of the β1 subfamily bind collagen, laminin and fibronectin and are involved in the structure of the extracellular matrix, whereas β2 integrins (CD18) are involved in leukocyte cell adhesion. β3 integrins, also known as the cytoadhesins, include the platelet glycoprotein IIb/IIIa (αIIb/β3) and the vitronectin receptor (αv/β3), factors involved in clot generation and stabilization.
Leukocyte integrins, the transmembrane cell surface proteins, are activated by chemokines, cytokines, and other chemoattractants. In order for leukocytes to bind to activated endothelium, integrins must be expressed on the cell surface so as to recognize endothelial cell adhesion molecules (Smith, 1993). Binding to receptors of the immunoglobulin gene superfamily, β2 integrins contain a common β2 chain with one of 3 distinct α chains (CD11a, CD11b, or CD11c). The CD11a/CD18 integrin is referred as LFA-1, whereas CD11b/CD18 is also called Mac-1. Of the α chains, CD11b has been the most studied in stroke models. Leukocytes and monocytes also express the α4β1 (very late antigen-4, VLA-4, CD49d) integrin, which binds to VCAM-1 and ligands of the subendothelial matrix (Frijns and Kappelle, 2002).
In an in vitro study, hypoxia caused an increase of neutrophil CD11b expression compared to normoxia, and this injury was protected by aprotinin by reducing the upregulation of neutrophil CD11b (Harmon et al., 2004). Treatment with 3-aminobenzamide (3-AB), a PARP inhibitor, appeared to protect by reducing expression of CD11b and other pro-inflammatory molecules (Koh et al., 2004).
Blocking CD11b (Chen et al., 1994) as well as CD18 (Bowes et al., 1995) or both (Jiang et al., 1995; Yenari et al., 1998) reduces injury from experimental stroke and is associated with decreased neutrophil infiltration. Similarly, mice lacking CD18 exhibited reduced leukocyte adhesion to endothelial cell monolayers, and improved cerebral blood flow and less neurological injury and neutrophil accumulation when subjected to experimental stroke (Prestigiacomo et al., 1999). Blocking integrins essential for lymphocyte and monocyte trafficking may also limit damage due to reperfusion injury. Antibodies against VLA-4 given 2 h after a 3 h period of temporary MCAO followed by reperfusion decreased infarct size (Becker et al., 2001).
To date, few clinical studies examined the potential of anti-integrin therapies in acute stroke patients. In one study, a humanized CD11/CD18 antibody (LeukArrest) was given to patients within 12 h symptom onset (Becker, 2002). Another trial was a phase IIb dose escalation study of a non-antibody peptide, recombinant neutrophil inhibiting factor (rNIF) in stroke patients (Acute Stroke Therapy by Inhibition of Neutrophils or ASTIN) administered within 6 h of symptom onset (Krams et al., 2003). Both studies were terminated prematurely due to a lack of effect on predetermined endpoints. Although both compounds appeared to be effective in rodent stroke models (Jiang et al., 1995; Yenari et al., 1998), lack of an obvious effect in humans could be due to study design not in line with laboratory data (such as late treatment or lack of documented reperfusion in humans) or the inherent heterogeneity of clinical stroke. Another possibility is that changes in neutrophil integrins are different in acute ischemic stroke patients compared to rodents. For example, CD11b is actually decreased in human stroke (Caimi et al., 2001), but increased after experimental stroke in rats (Campanella et al., 2002). Therefore, some anti-adhesion approaches may not be appropriate in humans. Regardless, it is clear that more work and possibly improved trial design are needed.
4. INFLAMMATORY MEDIATORS
4.1. Cytokines
Cytokines are upregulated in the brain after a variety of insults including stroke, and are expressed not only in cells of the immune system, but production by resident brain cells, including glia and neurons, have been observed (Liu et al., 1994; Sairanen et al., 2001). The most studied cytokines related to inflammation in stroke are interleukin-1 (IL-1), TNF-α, interleukin-6 (IL-6), interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) (Han and Yenari, 2003). Among those cytokines, IL-1 and TNF-α appear to exacerbate cerebral injury; however, IL-6, IL-10 and TGF-β may be neuroprotective (Allan and Rothwell, 2001).
IL-1
IL-1’s two isoforms, IL-1α and IL-1β and its endogenous inhibitor, IL-1 receptor antagonist (IL-1ra) have been the most studied in experimental stroke. IL-1beta mRNA elevations have been documented within 15–30 min after ischemia (Buttini et al., 1994) with increased protein a few hours later (Davies et al., 1999). Follwoing 20 min transient global cerebral ischemia in rats followed, IL-1beta mRNA and protein expression were increased not only during early reperfusion (1 h), but also at later times (6–24 h) indicating biphasic expression (Haqqani et al., 2005). Consistent with a potential damaging effect, increased brain damage occurred when IL-1beta was administered to rats (Yamasaki et al., 1995), and mice deficient in IL-1 had smaller infarcts compared to wildtype. Overexpression or treatment with IL-1ra reduced infarct size (Mulcahy et al., 2003; Yang et al., 1997), while IL-1ra deficient mice exhibited a dramatic increase in ischemic damage (Pinteaux et al., 2006). Moreover, basal and NMDA- or AMPA-induced cells death was significantly higher in glial-neuronal co-cultures from IL-1ra KO than from WT mice, strongly suggesting that endogenous IL-1ra produced by microglia is neuroprotective in cerebral ischemia. IL-1 has two receptors, IL-1R1 and IL-1R2, but only the former is involved in signal transduction (Rothwell and Luheshi, 2000). Inactivating or knocking out the IL-1R1 decreased the extent of damage caused by a hypoxic-ischemic (H/I) insult and preservea neurological function (Basu et al., 2005).
TNF-α
TNF-α is also upregulated in the brain after ischemia with similar expression patterns as IL-1beta. Initial increases are seen 1–3 h after ischemia onset (Liu et al., 1994), and, like IL-1beta, has a biphasic pattern of expression with a second peak at 24–36 h (Murakami et al., 2005; Offner et al., 2006). TNF-α expression was initially observed in neurons (Liu et al., 1994), then later in microglia and some astrocytes (Uno et al., 1997) as well as in the peripheral immune system (Offner et al., 2006). TNF-α appears to have pleiotropic functions in the ischemic brain (Hallenbeck, 2002). Inhibition of TNF-α reduces ischemic brain injury (Yang et al., 1998), while administration of recombinant TNF-α protein after stroke onset worsens ischemic brain damage (Barone et al., 1997). However, TNF-α may also protect the brain under certain circumstances. TNF-α appears to be involved in the phenomenon of ischemic tolerance (Ginis et al., 2002), and mice deficient in TNF receptors have larger infarcts ((Bruce et al., 1996). The reasons for this disparity might be due to different pathways through which TNF-α signals. There are at least two TNF-α receptors: TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2). Most effects induced by TNF-α are mediated by TNFR1, which contains a death domain (DD) that interacts directly with TNFR1 and may act as a bifurcation point for signaling related to cell death or cell survival. Ischemic preconditioning caused upregulation of neuronal TNFR1, and intracerebral administration of TNFR1 antisense oligodeoxynucleotide, which caused a reduction in TNFR1 expression, inhibited the ischemic preconditioning-induced protective effect, suggesting that TNFR1 upregulation is implicated in ischemic tolerance (Pradillo et al., 2005). Increased expression of TNFR1 mRNA in the ipsilateral cortex was noted slightly during ischemia and was significantly increased at 12 h after reperfusion in tMCAO (Yin et al., 2004). A clinical study also showed that TNFR1 concentration increased in patients with acute ischemic stroke (Fassbender et al., 1997). By signaling through the Fas-associated death domain (FADD) and caspase-8, TNF- α may lead to apoptosis, whereas reacting with TNF-receptor associating factor 2 and receptor-interacting protein may lead to anti-inflammatory and anti-apoptotic functions (reviewed by (Hallenbeck, 2002). Whether and how this applies in brain ischemia has yet to be clearly elucidated.
Other inflammation related cytokines
IL-6 is largely thought of as a pro-inflammatory cytokine, but whether it plays a significant role in ischemic stroke is far from clear. IL-6 deficient mice have similar sized infarcts compared to wildtype suggesting that it does not participate in ischemic pathogenesis (Clark et al., 2000). However, other studies suggest either a beneficial (Herrmann et al., 2003) or detrimental role (Smith et al., 2004). Clinical studies in stroke patients showed that serum concentrations of IL-6 had the strongest independent predictive value for in-hospital mortality (Rallidis et al., 2005). In a double blind clinical trial on patients with acute stroke, IL-6 concentration was much lower in rhIL-1ra, a neuroprotective drug, treated patients, who showed a better outcome compared with placebo treated group (Emsley et al., 2005).
IL-10, an anti-inflammatory cytokine, acts by inhibiting IL-1 and TNF-α and also by suppressing cytokine receptor expression and receptor activation. It is synthesized in the central nervous system (CNS) and is upregulated in experimental stroke (Strle et al., 2001). Both exogenous administration (Spera et al., 1998) and gene transfer of IL-10 (Ooboshi et al., 2005) in cerebral ischemia models appear to have beneficial effects. Patients with acute ischemic stroke have an elevated numbers of peripheral blood mononuclear cells secreting IL-10 (Pelidou et al., 1999) and elevated concentrations in cerebrospinal fluid (Tarkowski et al., 1997). Furthermore, subjects with low IL-10 levels have an increased risk of stroke (van Exel et al., 2002).
Expression of TGF-β1 has been reported in microglia and astrocytes, with low levels in neurons (Flanders et al., 1998). Overexpression of TGF-β1 using an adenoviral vector protected mouse brains from ischemic stroke and reduced the accompanying inflammatory response (Pang et al., 2001). A recent study showed that cultured neurons may be protected from ischemia-like insults by microglia-secreted TGF-β1 (Lu et al., 2005). Because TGF-β exhibits prominently in the recovery phase of some CNS diseases, it is proposed that TGF-β may contribute to the recovery of ischemic stroke (Benveniste, 1998).
4.2. Chemokines
Chemokines are a family of regulatory polypeptides with roles in cellular communication and inflammatory cell recruitment in host defense, such as regulating the migration of leukocytes in inflammatory and immune responses. Expression of chemokines following focal ischemia is thought to have a deleterious role by increasing leukocyte infiltration (Emsley and Tyrrell, 2002). Based on the positions of cysteine residues (C), chemokines can be divided into four groups: C, CC, CXC, and CX3C, with which chemokines act through specific and shared receptors belonging to the superfamily of G-protein-coupled receptors (Bajetto et al., 2001). Increasing data showed that a variety of chemokines such as monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α) are induced in the animal models of focal cerebral ischemia (Chen et al., 2003a). Consistent with a deleterious role, their inhibition or deficiency is associated with reduced injury (Garau et al., 2005), whereas (Chen et al., 2003a) found that over-expression of MCP-1 in the brain obviously exacerbated ischemic injury, which is correlated with recruitment of inflammatory cells.
Fractalkine, a neuronally expressed chemokine, acts through its G-protein-coupled receptor CX3C. Following ischemia, its expression has been localized to viable neurons in the infarct periphery as well as some endothelial cells. Interestingly, expression of its receptor, CX3CR1 was observed only on microglia/macrophages suggesting that fractalkine is involved in neuron-microglial signaling (Tarozzo et al., 2002). Furthermore, fractalkine deficient mice have smaller infarct sizes and lower mortality after transient focal cerebral ischemia, suggesting that fractalkine somehow exacerbates cell death (Soriano et al., 2002).
In addition to chemotactic properties, chemokines were found to directly affect BBB permeability. The addition of MCP-1 enhanced 17-fold the permeability of an in vitro BBB (cocultures of endothelial cells and astrocytes) model and caused alterations in tight junction (TJ) proteins, suggesting that MCP-1 may play a role in ‘opening’ the BBB (Stamatovic et al., 2005).
With recent interest in the area of cell based therapy for stroke, chemokines may also play an important role in honing stem cells to regions of injury (Newman et al., 2005). MCP-1 and SDF-1 and/or their receptors have been observed at the interface of ischemic tissue and cell transplants (Kelly et al., 2004). MCP-1 and other chemokines seem to be involved in marrow derived stromal cell migration into ischemic brain (Wang et al., 2002a; Wang et al., 2002b). Manipulation of these signals may be important in the successful application of such therapies.
4.3. Arachidonic Acid metabolites
Downstream of immune cell activation, the arachidonic acid (AA) cascade is initiated via the release of phospholipase A2 (PLA2) (Stanimirovic and Satoh, 2000). Energy failure due to cessation of blood flow can result in calcium accumulation in brain cells. This high concentration calcium activates PLA2 which hydrolyses glycerophospholipids to release AA. Following transient MCAO, PLA2 activity significantly increased (Adibhatla et al., 2006). As potent mediators, AA metabolites contribute to post-ischemic brain inflammation and circulatory disorders (Sanchez-Moreno et al., 2004). Consistent with a damaging role of this pathway, PLA2 deficient mice had smaller infarcts and developed less brain edema with fewer neurological deficits than their wild type littermates (Bonventre et al., 1997). AA is metabolized through two different pathways via cyclooxygenase (COX) or lipoxygenase (LOX). (Figure 2).
Figure 2. Ischemic activation of the arachidonic acid cascade.
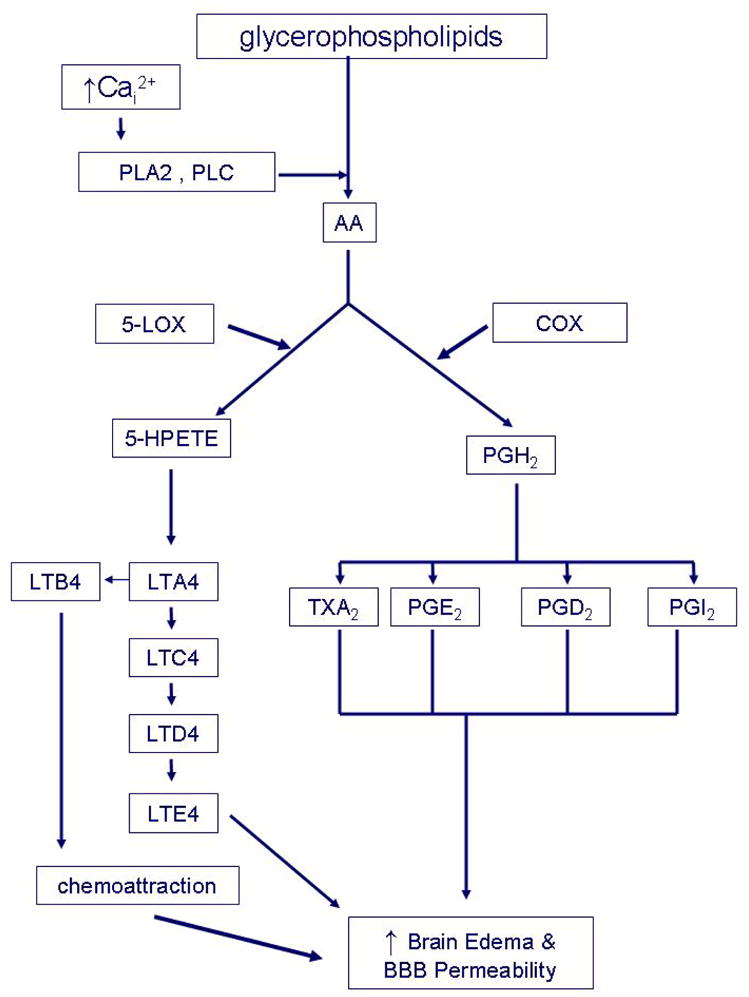
In the setting of cerebral ischemia, excess intracellular calcium (Cai2+) activates various lipases, including phospholipase A2 (PLA2) and phospholipase C (PLC) which breakdown both intracellular and membrane phospholipids and release arachidonic acid (AA). Both the cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) pathways are activated. The COX pathway converts AA to prostaglandin H2 (PGH2), a precursor prostaglandin. PGH2 is then metabolized to various eicosanoids, including prostacyclin (PGI2), thromboxane A2 (TXA2), prostaglandin E2 and prostaglandin D2. Eicosanoid family members have been shown to exert potent effect on brain vasomotor regulation and increase microvascular and BBB permeability. In the brain, they can act as neutrophil chemoattractants. AA can be also converted to 5-hydroperoxyeicosatetraenoic acid (5-HPETE) by LOX-5. 5-HPETE is then metabolized to leukotriene A4 (LTA4) and B4 (LTB4). LTA4 is a precursor of cysteinyl leukotrienes (cysLTs). cysLTs are converted sequentially to leukotriene C4 (LTC4), leukotriene D4 (LTD4), leukotriene E4 (LTE4). Leukotrienes play multiple roles to mediate events such as chemoattraction, brain edema and BBB permeability.
4.3.1 Cyclooxygenase pathway
Arachidonic acid released from brain phospholipids during ischemia/reperfusion is converted to prostaglandin H2 (PGH2) by cyclooxygenase (COX). There are two isoforms of COX. COX-1 is constitutively expressed in many cells types, including microglia and leukocytes during brain injury (Schwab et al., 2002). COX-1 deficient mice have increased vulnerability to brain ischemia, and would support a protective role possibly due to an effect on maintaining cerebral blood flow (Iadecola et al., 2001b). However, conflicting data exist. In transient global cerebral ischemia, pharmacologic inhibition of COX-1 with valeryl salicylate increased the number of healthy neurons in the hippocampal CA1 (Candelario-Jalil et al., 2003). These discrepancies may be due to differences in the immune responses between focal and global cerebral ischemia models.
COX-2, a rate-limiting enzyme for prostanoid synthesis, is upreglated and present at border of the ischemic territory following ischemia (Nogawa et al., 1997). In postmortem specimens of ischemic stroke patients, COX-2 is upregulated not only in regions of ischemic injury (Iadecola et al., 1999), but also regions remote from the infarct area (Sairanen et al., 1998). The roles of various COX metabolites are protean, but accumulated data suggest that those downstream of COX-2 are likely deleterious. Recent work has shown that prostaglandin E (2) EP1 receptors may be the downstream effectors responsible for neurotoxicity in ischemic stroke (Kawano et al., 2006). Several studies have now shown that treatment with COX-2 inhibitors improve neurological outcome after stroke (Nogawa et al., 1997; Sugimoto and Iadecola, 2003). Furthermore, COX-2 deficient mice have reduced injury after N-methyl-D-aspartate (NMDA) exposure (Iadecola et al., 2001a), whereas COX-2 overexpression exacerbates brain injury (Dore et al., 2003). Interestingly, COX-2 mediates its toxic effect through PGE2 rather than ROS, even though COX-2 can generate both (Manabe et al., 2004).
4.3.2 5-Lipoxygenase pathway
Compared to the COX pathway, there is limited knowledge about the role of the lipoxygenase pathway in brain ischemia. AA can be converted to 5-hydroperoxyeicosatetraenoic acid (5-HPETE) by 5-lipoxygenase (5-LOX) which is metabolized to leukotriene A4 (LTA4), a precursor of cysteinyl leukotrienes (cysLTs). LTC4 is a potent chemoattractant that has been implicated in the BBB dysfunction, edema and neuronal death after ischemia/reperfusion. During brain ischemia/reperfusion, biphasic AA and LTC4 elevations have been documented and appear to correspond to biphasic patterns of BBB disruption (Rao et al., 1999). 5-LOX has also been documented in autopsied ischemic human brains, with 5-LOX localizing to perivascular monocytes (Tomimoto et al., 2002). Pretreatment with a 5-LOX inhibitor, AA861 resulted in significant attenuation of LTC4 levels and reduction in brain edema and cell death (Baskaya et al., 1996). Furthermore, OGD-induced PC12 cell death was attenuated by 5-LOX inhibitor caffeic acid (Song et al., 2004). However, 5-LOX deficient mice had similar infarct sizes 6 days after both permanent and transient MCAO to wild type mice (Kitagawa et al., 2004). These conflicting observations may reflect the different times when assessments were made, but clearly, more work is needed in this area.
4.4. Nitric Oxide/Nitric Oxide Synthase
Nitric oxide (NO) is an important signaling molecule involved in physiological processes such as neuronal communication, host defense, and regulation of vascular tone. This relatively stable gas readily diffuses into cells and cell membranes where it reacts with molecular targets. Three nitric oxide synthases (NOS) isoforms exist; endothelial NOS (also known as type III, NOS-III and NOS-3), neuronal NOS (also known as type I, NOS-I and NOS-1), and inducible NOS (also known as type II, NOS-II and NOS-2). Among these three isoforms, iNOS is especially relevant to inflammatory cells and may contribute to ischemic injury via NO. In fact, iNOS expression is thought to be restricted to cells involved in inflammatory responses such circulating leukocytes, microglia and astrocytes. In the brain, ischemia-induced upregulation of iNOS mRNA and protein is associated with increases in iNOS enzymatic activity and NO production (Iadecola et al., 1995a; Iadecola et al., 1995b). NO may cause DNA damage in cerebral ischemia through the formation of peroxynitrite (Cui et al., 2000; Cui et al., 1999; Huang et al., 2000a). Pharmacological inhibition of iNOS reduces infarct volume by about 30% (Iadecola et al., 1995b), and iNOS-null mice have smaller infarcts and better neurologic outcomes than wild-type control animals (Zhao et al., 2000). Consistent with a damaging role, protection by hypothermia is associated with reduced microglial generation of both NO and iNOS (Han et al., 2002), and ischemic brain protection by estrogen and progesterone appears to be through modulating postischemic iNOS expression (Coughlan et al., 2005; Park et al., 2006)
4.5. Reactive Oxygen Species
Generation of reactive oxygen species (ROS) by inflammatory cells occurs via several enzyme systems. Superoxide is generated via COX, xanthine dehydrogenase, xanthine oxidase and NADPH oxidase, whereas myeloperoxidase (MPO) and monoamine oxidase (MAO) generate hypochlorous acid and H2O2. Among all the oxidants in the brain parenchyma after MCAO, superoxide anion is a major one, causing direct injury to ischemic brain or by reacting with NO to generate peroxynitrite (Chan, 2001).
NADPH oxidase (NOX) is a multicomponent enzyme that consists of two membrane bound subunits, gp91 and p22, and three cytosolic subunits, p67, p47 and p40 plus Rac, a small GTPase (Groemping and Rittinger, 2005). Other NOX isoforms have been recently characterized, but for the scope of this review, we will focus on the most studied NOX, NOX2 (phagocytic NOX) (Lambeth, 2004). With appropriate stimuli, the cytosolic subunits translocate to the membrane where they interact with the membrane bound subunits to transfer electrons from NADPH to oxygen to form superoxide. Largely thought to be exclusively produced by cells of the immune system, it is a major and significant source of inflammatory cell generated superoxide. Prior work has shown that mice deficient in the gp91 subunit of NOX2 have smaller infarcts than wild type mice (Walder et al., 1997). Recent work has shown that microglia potentiate injury to the blood brain barrier due to superoxide produced by NOX2 in brain ischemia models (Yenari et al., 2006).
Myeloperoxidase (MPO), an enzyme in leukocytes such as neutrophils and monocytes, is linked to inflammation, and is thought to mediate bactericidal killing through H2O2 and hypochlorous acid. MPO activity is normally used as a marker of polymorphonuclear neutrophil infiltration, and its upregulation in blood may predict the early risk of infarction. MPO has been documented in both permanent and transient MCAO (Garau et al., 2005). However, after focal cerebral ischemia, infarct size was increased in MPO deficient mice (Takizawa et al., 2002), suggesting a beneficial role. MPO deficient mice also had increased products of nitrosylation within the ischemic brain and suggested that MPO‘s protective effect may be due to its ability to scavenge nitrotyrosine (a by product of peroxynitrite reactions) in the presence of glutathione (Takizawa et al., 2002). Therefore, it is possible that MPO may actually limit the extent of ROS-mediated tissue injury.
4.6. Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are proteases that can break down extracellular proteins, such as collagen, and are involved in extracellular matrix remodeling as well as the neuroinflammatory response. Follwoing tPA treatedment, components of the BBB were disrupted following MMP-9 activation (Kelly et al., 2006). MMPs are normally found in the cytosol in a pro- or inactivated state, but are cleaved by proteases such as plasmin or other MMPs to their active state (Rosenberg, 2002). As a major source of the MMPs following ischemia, microglia are also necessary to stimulate astrocytes to generate active MMPs (Rosenberg et al., 2001).
In experimental stroke models, MMP inhibition reduces infarct size, brain edema and hemorrhage (Pfefferkorn and Rosenberg, 2003). Mice deficient in MMP-9 had smaller infarcts compared to wildtype controls (Asahi et al., 2000); however, such an effect was not observed in MMP-2 deficient mice (Asahi et al., 2001), suggesting that MMP-9 appears to play a more significant role in stroke compared to MMP-2. Peripheral inflammatory cell, rather than brain derived MMP-9 may contribute significantly to ischemic brain injury as mice transplanted with bone marrow from MMP-9 deficient mice suffer less injury and less BBB disruption than mice transplanted with marrow containing intact MMP-9 (Gidday et al., 2005). Current work also showed that MMPs may be involved in endogenous mechanisms of neurogenic migration because a broad spectrum MMP inhibitor GM6001 was found to significantly decrease the migration of doublecortin-positive cells that extend from the SVZ into the striatum in transient focal cerebral ischemia in mice (Lee et al., 2006).
Interestingly, MMPs seem to play a different role in the later phases of cerebral ischemia, and may participate in plasticity and recovery. For instance, MMPs are known to associate with factors involved in angiogenesis such as vascular endothelial growth factor (VEGF). In one study, treatment with the MMP inhibitor FN-439 7 d post MCAO suppressed neurovascular remodeling, increased ischemic brain injury and impaired functional recovery at 14 days. This was also associated with reduced VEGF signaling resulting from MMP inhibition (Zhao et al., 2006).
5. TRANSCRIPTIONAL REGULATION OF INFLAMMATION
It is now well recognized that cerebral ischemia upregulates gene expression. Activation of several transcription factors has been documented in experimental stroke models. Some of these transcription factors are particularly involved in the inflammatory response, and will be discussed here.
5.1. Nuclear factor κB (NF-κB)
NF-κB, involved in the regulation of inflammation (Baeuerle and Henkel, 1994), is a dimeric transcription factor consisting of subunits of the Rel family, which includes five Rel forms: Rel (cRel), RelA (p65), RelB, NF-κB1 (p50 and its precursor p105) and NF-κB. The most common form of NF-κB is a heterodimer composed of Rel A (p65) and p50. NF-κB is normally located in the cytoplasm bound to its endogenous inhibitor protein, known as IκB, a family of proteins consisting of IκB-α, IκB-β, IκB-γ and IκB-ε. Phosphorylation of IκB-α at serines 32 and 36 by an upstream IκB kinase (IKK) leads to IκB phosphorylation, ubiquitination and degradation in the 26S proteasome. This liberates NF-κB and allows it to translocate to the nucleus, and bind to κB sites, specific domains within the promoters of downstream genes to activate their transcription. Many genes involved in inflammation contain functional κB sites, such as tumor necrosis factor-α (TNF-α), intercellular adhesion molecule-1 (ICAM-1), cyclooxygenase-2 (COX-2), iNOS and interleukin-6 (IL-6). Han et al., (Han et al., 2002) found that IKK and NF-κB activation were correlated to the anti-inflammatory effect of mild hypothermia following experimental stroke. However, the function of NF-κB in stroke is still controversial (Cechetto, 2001). Mice deficient in NF-κB’s p50 subunit are protected from experimental stroke (Schneider et al., 1999), consistent with a death-promoting role of NF-κB in focal ischemia. Similar observations were made by inhibiting activation of NF-κB with the treatment of S-nitrosoglutathione (GSNO), an NO modulator (Khan et al., 2005). After deleting IkappaB kinase complex IKK, the central component of NF-kappaB activation, inhibition of IKK activity markedly reduced infarct size in a mouse model of stroke (Herrmann et al., 2005). In contrast, constitutive activation of IKK2 enlarged the infarct size (Herrmann et al., 2005). In global ischemia, neuronal damage was significantly attenuated by employing the nuclear factor-kappa B decoy oligodeoxynucleotides into rat brain neurons through the carotid artery (Ueno et al., 2001). However, rats given diethyldithiocarbamate (DDTC), a NF-κB inhibitor, had enhanced neuronal DNA fragmentation and larger infarct sizes compared to controls, suggesting a beneficial role (Hill et al., 2001), and. The reasons for these discrepancies are not clear, but could be due to the cell type in which NFkB is activated, the experimental model studied, or a lack of specificity of pharmacological inhibitors.
5.2. Mitogen-activated protein kinase (MAPK)
Mitogen-activated protein kinases play an important role in transducing stress-related signals by a cascade of intracellular kinase phosphorylation and transcription factor activation that regulate inflammatory gene production among other functions (For reviews, see (Barone and Feuerstein, 1999); (Irving and Bamford, 2002)). During cerebral ischemia, three interlinked signaling pathways have been documented: the stress-activated protein kinases/c-Jun N-terminal kinases (SAPK/JNK), the p38 MAPKs and extracellular signal-regulated kinases (ERKs) (Irving and Bamford, 2002; Irving et al., 2000; Sugino et al., 2000). p38 MAPK promotes the stabilization and enhanced translation of mRNAs encoding proinflammatory proteins (Kyriakis and Avruch, 2001). In forebrain ischemia, MLK3-MKK4-JNK activation was rapidly increased with peaks both at 30 min and 3 days of reperfusion (Zhang et al., 2005b). Intracerebroventricular infusion of POSH (plenty of SH3s) antisense oligodeoxynucleotides (AS-ODNs) not only significantly decreased POSH interactions with MLK3, MKK4 and phospho-JNKs, but also attenuated the activation of the JNK signalling pathway as well as significantly increased the neuronal density in the CA1 region. This type of protective effect of POSH AS-ODNs on ischemic injury might be through a mechanism of inhibition of the MLK3-MKK4-JNK signalling pathway and c-Jun activation, which strongly suggests the involvement of MAPK. After inducing ischemia in vitro with an anaerobic chamber, introduction of bone marrow stromal cells activated mitogen-activated protein kinase, subsequently exerted protection to post-ischmic injury (Gao et al., 2005). In permanent MCAO, CDP-choline, a major neuronal membrane lipid precursor, showed a key role in recovery after ischaemic stroke by a notable reduction in the phosphorylation of MAP-kinase family members, ERK1/2 and MEK1/2, as well as Elk-1 transcription factor. Following forebrain ischemia in rodents, phosphorylated p38 MAPK was detected in the hippocampus within neuron- (Sugino et al., 2000) and microglia-like (Walton et al., 1998) cells, suggesting its role in the endogenous inflammatory response. Furthermore, p38 MAPK inhibitors have been shown to reduce brain injury and neurological deficits in focal cerebral ischemia as well as ischemia-induced cytokine expression (Barone and Feuerstein, 1999).
5.3. Activator Protein-1 (AP-1)
AP-1 is a heterodimer comprised of bZIP transcription factors (e.g., c-Jun and JunD), activating transcription factor 2 (ATF2) and c-Fos. Upstream activation of AP-1 components is mediated through the JNK/SAPK cascade. c-Fos was found to be up regulated as early as 30 minutes after stroke onset (Lu et al., 2004). The Fos protein contains a DNA binding region and a leucine zipper. The leucine zipper forms an α-helix which can align with other proteins (such as Jun) containing like structures to form dimers. These dimers bind to specific DNA regions known as the AP-1 domain, which regulates the expression of a number of target genes (collectively referred to as late response genes). Combinations of c-Fos and c-Jun family proteins form different dimers consisting of various subunits depending on the circumstances. The composition of the dimer may determine whether the late response gene is turned on or off (Morgan and Curran, 1991). Inhibition of p38 MAP kinase resulted in the attenuation of c-fos and c-jun mRNA and AP-1 DNA binding by lipopolysaccharide (LPS) (Simi et al., 2000), and subsequently led to neuroprotection in cerebral ischemia. ATF2 is a member of the cAMP response element binding protein (CREB) subfamily of bZIP transcription factors. AP-1 heterodimers containing ATF transcription factors can bind both to the TPA (12-O-tetradecanoylphorbol-13-acetate) responsive element (TRE) and to the cAMP response element (CRE) (Karin et al., 1997). In human umbilical vein endothelial cells (HUVECs), a dose-dependent lowering of mRNA expression of VCAM-1 and ICAM-1 was observed by inhibiting AP-1 DNA binding activities (Zhou et al., 2005). Recent evidence also showed that selective induction of DeltaFosB, an AP-1 (activator protein-1) subunit, can promote the proliferation of quiescent neuronal precursor cells, thus enhancing neurogenesis after transient forebrain ischemia (Kurushima et al., 2005).
6. CONCLUSION
Inflammation is increasingly recognized to be the key element in pathological progression of ischemic stroke. Whether inflammation is destructive or beneficial may depend on how severe the ischemia is and the stages of ischemia in which inflammatory responses contribute. Likely, early inflammatory responses may potentiate ischemic injury, while late responses may be important in recovery and repair. Future work should address the optimal timing of inflammation modulating interventions as well as elucidating how the immune system moves from damaging to protective/restorative responses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao FH. CDP-choline significantly restores phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP: phosphocholine cytidylyltransferase after stroke. J Biol Chem. 2006;281:6718–6725. doi: 10.1074/jbc.M512112200. [DOI] [PubMed] [Google Scholar]
- Albelda SM. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4:195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Asahi M, Sumii T, Fini ME, Itohara S, Lo EH. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001;12:3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Baskaya MK, Hu Y, Donaldson D, Maley M, Rao AM, Prasad MR, Dempsey RJ. Protective effect of the 5-lipoxygenase inhibitor AA-861 on cerebral edema after transient ischemia. J Neurosurg. 1996;85:112–116. doi: 10.3171/jns.1996.85.1.0112. [DOI] [PubMed] [Google Scholar]
- Basu A, Lazovic J, Krady JK, Mauger DT, Rothstein RP, Smith MB, Levison SW. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J Cereb Blood Flow Metab. 2005;25:17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- Becker KJ. Inflammation and acute stroke. Curr Opin Neurol. 1998;11:45–49. doi: 10.1097/00019052-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18(Suppl 2):s18–22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunol. 2002;126:107–115. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Blann A, Kumar P, Krupinski J, McCollum C, Beevers DG, Lip GY. Soluble intercelluar adhesion molecule-1, E-selectin, vascular cell adhesion molecule-1 and von Willebrand factor in stroke. Blood Coagul Fibrinolysis. 1999;10:277–284. doi: 10.1097/00001721-199907000-00009. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- Bowes MP, Rothlein R, Fagan SC, Zivin JA. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45:815–819. doi: 10.1212/wnl.45.4.815. [DOI] [PubMed] [Google Scholar]
- Bowes MP, Zivin JA, Rothlein R. Monoclonal antibody to the ICAM-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp Neurol. 1993;119:215–219. doi: 10.1006/exnr.1993.1023. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Buttini M, Sauter A, Boddeke HW. Induction of interleukin-1 beta mRNA after focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 1994;23:126–134. doi: 10.1016/0169-328x(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Caimi G, Canino B, Ferrara F, Montana M, Musso M, Porretto F, Carollo C, Catania A, Lo Presti R. Granulocyte integrins before and after activation in acute ischaemic stroke. J Neurol Sci. 2001;186:23–26. doi: 10.1016/s0022-510x(01)00495-6. [DOI] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Alvarez D, Al-Dalain S, Martinez G, Leon OS, Springer JE. Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia. J Neurochem. 2003;86:545–555. doi: 10.1046/j.1471-4159.2003.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- Cechetto DF. Role of nuclear factor kappa B in neuropathological mechanisms. Prog Brain Res. 2001;132:391–404. doi: 10.1016/S0079-6123(01)32090-3. [DOI] [PubMed] [Google Scholar]
- Cervera A, Justicia C, Reverter JC, Planas AM, Chamorro A. Steady plasma concentration of unfractionated heparin reduces infarct volume and prevents inflammatory damage after transient focal cerebral ischemia in the rat. J Neurosci Res. 2004;77:565–572. doi: 10.1002/jnr.20186. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902:171–177. doi: 10.1016/s0006-8993(01)02328-9. [DOI] [PubMed] [Google Scholar]
- Chen H, Chopp M, Zhang RL, Bodzin G, Chen Q, Rusche JR, Todd RF., 3rd Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol. 1994;35:458–463. doi: 10.1002/ana.410350414. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003a;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ruetzler C, Pandipati S, Spatz M, McCarron RM, Becker K, Hallenbeck JM. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci U S A. 2003b;100:15107–15112. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Li Y, Jiang N, Zhang RL, Prostak J. Antibodies against adhesion molecules reduce apoptosis after transient middle cerebral artery occlusion in rat brain. J Cereb Blood Flow Metab. 1996;16:578–584. doi: 10.1097/00004647-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Clark WM, Lauten JD, Lessov N, Woodward W, Coull BM. The influence of antiadhesion therapies on leukocyte subset accumulation in central nervous system ischemia in rats. J Mol Neurosci. 1995;6:43–50. doi: 10.1007/BF02736758. [DOI] [PubMed] [Google Scholar]
- Clark WM, Lessov N, Lauten JD, Hazel K. Doxycycline treatment reduces ischemic brain damage in transient middle cerebral artery occlusion in the rat. J Mol Neurosci. 1997;9:103–108. doi: 10.1007/BF02736854. [DOI] [PubMed] [Google Scholar]
- Clark WM, Rinker LG, Lessov NS, Hazel K, Hill JK, Stenzel-Poore M, Eckenstein F. Lack of interleukin-6 expression is not protective against focal central nervous system ischemia. Stroke. 2000;31:1715–1720. doi: 10.1161/01.str.31.7.1715. [DOI] [PubMed] [Google Scholar]
- Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, Naka Y, Solomon RA, Pinsky DJ. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997;81:304–310. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan T, Gibson C, Murphy S. Modulatory effects of progesterone on inducible nitric oxide synthase expression in vivo and in vitro. J Neurochem. 2005;93:932–942. doi: 10.1111/j.1471-4159.2005.03068.x. [DOI] [PubMed] [Google Scholar]
- Cui J, Holmes EH, Greene TG, Liu PK. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. Faseb J. 2000;14:955–967. doi: 10.1096/fasebj.14.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Holmes EH, Liu PK. Oxidative damage to the c-fos gene and reduction of its transcription after focal cerebral ischemia. J Neurochem. 1999;73:1164–1174. doi: 10.1046/j.1471-4159.1999.0731164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- Davies CA, Loddick SA, Stroemer RP, Hunt J, Rothwell NJ. An integrated analysis of the progression of cell responses induced by permanent focal middle cerebral artery occlusion in the rat. Exp Neurol. 1998;154:199–212. doi: 10.1006/exnr.1998.6891. [DOI] [PubMed] [Google Scholar]
- Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1999;19:87–98. doi: 10.1097/00004647-199901000-00010. [DOI] [PubMed] [Google Scholar]
- DeGraba TJ. The role of inflammation after acute stroke: utility of pursuing anti-adhesion molecule therapy. Neurology. 1998;51:S62–68. doi: 10.1212/wnl.51.3_suppl_3.s62. [DOI] [PubMed] [Google Scholar]
- Ding C, He Q, Li PA. Diabetes increases expression of ICAM after a brief period of cerebral ischemia. J Neuroimmunol. 2005;161:61–67. doi: 10.1016/j.jneuroim.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Dinkel K, Dhabhar FS, Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci U S A. 2004;101:331–336. doi: 10.1073/pnas.0303510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Donohue PJ, Richards CM, Brown SA, Hanscom HN, Buschman J, Thangada S, Hla T, Williams MS, Winkles JA. TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity. Arterioscler Thromb Vasc Biol. 2003;23:594–600. doi: 10.1161/01.ATV.0000062883.93715.37. [DOI] [PubMed] [Google Scholar]
- Dore S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, Hurn PD, Traystman RJ, Andreasson K. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 2003;54:155–162. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- Ehrensperger E, Minuk J, Durcan L, Mackey A, Wolfson C, Fontaine AM, Cote R. Predictive value of soluble intercellular adhesion molecule-1 for risk of ischemic events in individuals with cerebrovascular disease. Cerebrovasc Dis. 2005;20:456–462. doi: 10.1159/000088985. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol Aging. 1998;19:S81–84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–1419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res. 1994;651:92–100. doi: 10.1016/0006-8993(94)90683-1. [DOI] [PubMed] [Google Scholar]
- Enlimomab AST. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Dempfle CE, Mielke O, Rossol S, Schneider S, Dollman M, Hennerici M. Proinflammatory cytokines: indicators of infection in high-risk patients. J Lab Clin Med. 1997;130:535–539. doi: 10.1016/s0022-2143(97)90131-1. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Ren RF, Lippa CF. Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol. 1998;54:71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136:123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, Mennini T, Ghezzi P, Villa P. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 2005;30:125–131. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab. 2002;22:142–152. doi: 10.1097/00004647-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res. 1993;36:681–693. doi: 10.1002/jnr.490360609. [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Gunther A, Kuppers-Tiedt L, Schneider PM, Kunert I, Berrouschot J, Schneider D, Rossner S. Reduced infarct volume and differential effects on glial cell activation after hyperbaric oxygen treatment in rat permanent focal cerebral ischaemia. Eur J Neurosci. 2005;21:3189–3194. doi: 10.1111/j.1460-9568.2005.04151.x. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl. 1996;66:27–31. doi: 10.1007/978-3-7091-9465-2_5. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. 2002;22:3921–3928. doi: 10.1523/JNEUROSCI.22-10-03921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs. 2003;4:522–529. [PubMed] [Google Scholar]
- Haqqani AS, Nesic M, Preston E, Baumann E, Kelly J, Stanimirovic D. Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLC-MS/MS. Faseb J. 2005;19:1809–1821. doi: 10.1096/fj.05-3793com. [DOI] [PubMed] [Google Scholar]
- Haring HP, Berg EL, Tsurushita N, Tagaya M, del Zoppo GJ. E-selectin appears in nonischemic tissue during experimental focal cerebral ischemia. Stroke. 1996;27:1386–1391; discussion 1391–1382. doi: 10.1161/01.str.27.8.1386. [DOI] [PubMed] [Google Scholar]
- Harmon D, Lan W, Shorten G. The effect of aprotinin on hypoxia-reoxygenation-induced changes in neutrophil and endothelial function. Eur J Anaesthesiol. 2004;21:973–979. doi: 10.1017/s0265021504000365. [DOI] [PubMed] [Google Scholar]
- Hartl R, Schurer L, Schmid-Schonbein GW, del Zoppo GJ. Experimental antileukocyte interventions in cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:1108–1119. doi: 10.1097/00004647-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, Malfertheiner M, Kohrmann M, Potrovita I, Maegele I, Beyer C, Burke JR, Hasan MT, Bujard H, Wirth T, Pasparakis M, Schwaninger M. IKK mediates ischemia-induced neuronal death. Nat Med. 2005;11:1322–1329. doi: 10.1038/nm1323. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, Vogel J, Prinz S, Schwab S, Monyer H, Brombacher F, Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- Hewett SJ, Muir JK, Lobner D, Symons A, Choi DW. Potentiation of oxygen-glucose deprivation-induced neuronal death after induction of iNOS. Stroke. 1996;27:1586–1591. doi: 10.1161/01.str.27.9.1586. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Carroll JE, Wakade CG, Howard EF, Chen Q, Cheng C, Martin-Studdard A, Waller JL, Beswick RA. The NF-kappaB inhibitor diethyldithiocarbamate (DDTC) increases brain cell death in a transient middle cerebral artery occlusion model of ischemia. Brain Res Bull. 2001;55:375–386. doi: 10.1016/s0361-9230(01)00503-2. [DOI] [PubMed] [Google Scholar]
- Huang D, Shenoy A, Cui J, Huang W, Liu PK. In situ detection of AP sites and DNA strand breaks bearing 3′-phosphate termini in ischemic mouse brain. Faseb J. 2000a;14:407–417. doi: 10.1096/fasebj.14.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES., Jr Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000b;31:3047–3053. [PubMed] [Google Scholar]
- Huang J, Kim LJ, Mealey R, Marsh HC, Jr, Zhang Y, Tenner AJ, Connolly ES, Jr, Pinsky DJ. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285:595–599. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Forster C, Nogawa S, Clark HB, Ross ME. Cyclooxygenase-2 immunoreactivity in the human brain following cerebral ischemia. Acta Neuropathol (Berl) 1999;98:9–14. doi: 10.1007/s004010051045. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci U S A. 2001a;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Sugimoto K, Niwa K, Kazama K, Ross ME. Increased susceptibility to ischemic brain injury in cyclooxygenase-1-deficient mice. J Cereb Blood Flow Metab. 2001b;21:1436–1441. doi: 10.1097/00004647-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu S, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab. 1995a;15:378–384. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995b;268:R286–292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Irving EA, Barone FC, Reith AD, Hadingham SJ, Parsons AA. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 2000;77:65–75. doi: 10.1016/s0169-328x(00)00043-7. [DOI] [PubMed] [Google Scholar]
- Jiang N, Moyle M, Soule HR, Rote WE, Chopp M. Neutrophil inhibitory factor is neuroprotective after focal ischemia in rats. Ann Neurol. 1995;38:935–942. doi: 10.1002/ana.410380615. [DOI] [PubMed] [Google Scholar]
- Justicia C, Martin A, Rojas S, Gironella M, Cervera A, Panes J, Chamorro A, Planas AM. Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb Blood Flow Metab. 2006;26:421–432. doi: 10.1038/sj.jcbfm.9600198. [DOI] [PubMed] [Google Scholar]
- Kanemoto Y, Nakase H, Akita N, Sakaki T. Effects of anti-intercellular adhesion molecule-1 antibody on reperfusion injury induced by late reperfusion in the rat middle cerebral artery occlusion model. Neurosurgery. 2002;51:1034–1041; discussion 1041–1032. doi: 10.1097/00006123-200210000-00033. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Shuaib A, Todd KG. Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Jatana M, Elango C, Singh Paintlia A, Singh AK, Singh I. Cerebrovascular protection by various nitric oxide donors in rats after experimental stroke. Nitric Oxide. 2006 doi: 10.1016/j.niox.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, Ayasolla K, Elango C, Singh AK, Singh I. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 2005;25:177–192. doi: 10.1038/sj.jcbfm.9600012. [DOI] [PubMed] [Google Scholar]
- Kim JS. Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci. 1996;137:69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Hori M. Cerebral ischemia in 5-lipoxygenase knockout mice. Brain Res. 2004;1004:198–202. doi: 10.1016/j.brainres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Mabuchi T, Yagita Y, Ohtsuki T, Hori M, Yanagihara T. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab. 1998;18:1336–1345. doi: 10.1097/00004647-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Koh SH, Park Y, Song CW, Kim JG, Kim K, Kim J, Kim MH, Lee SR, Kim DW, Yu HJ, Chang DI, Hwang SJ, Kim SH. The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci. 2004;20:1461–1472. doi: 10.1111/j.1460-9568.2004.03632.x. [DOI] [PubMed] [Google Scholar]
- Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kurushima H, Ohno M, Miura T, Nakamura TY, Horie H, Kadoya T, Ooboshi H, Kitazono T, Ibayashi S, Iida M, Nakabeppu Y. Selective induction of DeltaFosB in the brain after transient forebrain ischemia accompanied by an increased expression of galectin-1, and the implication of DeltaFosB and galectin-1 in neuroprotection and neurogenesis. Cell Death Differ. 2005;12:1078–1096. doi: 10.1038/sj.cdd.4401648. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmberg J, Beck J, Baethmann A, Uhl E. Effect of P-selectin inhibition on leukocyteendothelium interaction and survival after global cerebral ischemia. J Neurol. 2006;253:357–363. doi: 10.1007/s00415-005-0996-4. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GZ, Zhong D, Yang LM, Sun B, Zhong ZH, Yin YH, Cheng J, Yan BB, Li HL. Expression of interleukin-17 in ischemic brain tissue. Scand J Immunol. 2005;62:481–486. doi: 10.1111/j.1365-3083.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Lu XC, Williams AJ, Yao C, Berti R, Hartings JA, Whipple R, Vahey MT, Polavarapu RG, Woller KL, Tortella FC, Dave JR. Microarray analysis of acute and delayed gene expression profile in rats after focal ischemic brain injury and reperfusion. J Neurosci Res. 2004;77:843–857. doi: 10.1002/jnr.20218. [DOI] [PubMed] [Google Scholar]
- Lu YZ, Lin CH, Cheng FC, Hsueh CM. Molecular mechanisms responsible for microglia-derived protection of Sprague-Dawley rat brain cells during in vitro ischemia. Neurosci Lett. 2005;373:159–164. doi: 10.1016/j.neulet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, Iadecola C. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004;55:668–675. doi: 10.1002/ana.20078. [DOI] [PubMed] [Google Scholar]
- Mocco J, Choudhri T, Huang J, Harfeldt E, Efros L, Klingbeil C, Vexler V, Hall W, Zhang Y, Mack W, Popilskis S, Pinsky DJ, Connolly ES., Jr HuEP5C7 as a humanized monoclonal anti-E/P-selectin neurovascular protective strategy in a blinded placebo-controlled trial of nonhuman primate stroke. Circ Res. 2002;91:907–914. doi: 10.1161/01.res.0000042063.15901.20. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol. 1993;327:123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol. 2003;140:471–476. doi: 10.1038/sj.bjp.0705462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Saito K, Hara A, Zhu Y, Sudo K, Niwa M, Fujii H, Wada H, Ishiguro H, Mori H, Seishima M. Increases in tumor necrosis factor-alpha following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus. J Neurochem. 2005;93:1616–1622. doi: 10.1111/j.1471-4159.2005.03163.x. [DOI] [PubMed] [Google Scholar]
- Nadareishvili ZG, Li H, Wright V, Maric D, Warach S, Hallenbeck JM, Dambrosia J, Barker JL, Baird AE. Elevated pro-inflammatory CD4+CD28- lymphocytes and stroke recurrence and death. Neurology. 2004;63:1446–1451. doi: 10.1212/01.wnl.0000142260.61443.7c. [DOI] [PubMed] [Google Scholar]
- Newman MB, Willing AE, Manresa JJ, Davis-Sanberg C, Sanberg PR. Stroke-induced migration of human umbilical cord blood cells: time course and cytokines. Stem Cells Dev. 2005;14:576–586. doi: 10.1089/scd.2005.14.576. [DOI] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, Arakawa S, Sugimori H, Kamouchi M, Kitazono T, Iida M. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111:913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Pelidou SH, Kostulas N, Matusevicius D, Kivisakk P, Kostulas V, Link H. High levels of IL-10 secreting cells are present in blood in cerebrovascular diseases. Eur J Neurol. 1999;6:437–442. doi: 10.1046/j.1468-1331.1999.640437.x. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- Pinteaux E, Rothwell NJ, Boutin H. Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia. 2006;53:551–556. doi: 10.1002/glia.20308. [DOI] [PubMed] [Google Scholar]
- Pradillo JM, Romera C, Hurtado O, Cardenas A, Moro MA, Leza JC, Davalos A, Castillo J, Lorenzo P, Lizasoain I. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- Prestigiacomo CJ, Kim SC, Connolly ES, Jr, Liao H, Yan SF, Pinsky DJ. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke. 1999;30:1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- Rallidis LS, Vikelis M, Panagiotakos DB, Rizos I, Zolindaki MG, Kaliva K, Kremastinos DT. Inflammatory markers and in-hospital mortality in acute ischaemic stroke. Atherosclerosis. 2005 doi: 10.1016/j.atherosclerosis.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Rao AM, Hatcher JF, Kindy MS, Dempsey RJ. Arachidonic acid and leukotriene C4: role in transient cerebral ischemia of gerbils. Neurochem Res. 1999;24:1225–1232. doi: 10.1023/a:1020916905312. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis. 1999;42:209–216. doi: 10.1016/s0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Ruehl ML, Orozco JA, Stoker MB, McDonagh PF, Coull BM, Ritter LS. Protective effects of inhibiting both blood and vascular selectins after stroke and reperfusion. Neurol Res. 2002;24:226–232. doi: 10.1179/016164102101199738. [DOI] [PubMed] [Google Scholar]
- Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, Chicheportiche Y, Dietrich PY. TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia. 2000;32:102–107. [PubMed] [Google Scholar]
- Sairanen T, Carpen O, Karjalainen-Lindsberg ML, Paetau A, Turpeinen U, Kaste M, Lindsberg PJ. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke. 2001;32:1750–1758. doi: 10.1161/01.str.32.8.1750. [DOI] [PubMed] [Google Scholar]
- Sairanen T, Ristimaki A, Karjalainen-Lindsberg ML, Paetau A, Kaste M, Lindsberg PJ. Cyclooxygenase-2 is induced globally in infarcted human brain. Ann Neurol. 1998;43:738–747. doi: 10.1002/ana.410430608. [DOI] [PubMed] [Google Scholar]
- Saito S, Matsuura M, Tominaga K, Kirikae T, Nakano M. Important role of membrane-associated CD14 in the induction of IFN-beta and subsequent nitric oxide production by murine macrophages in response to bacterial lipopolysaccharide. Eur J Biochem. 2000;267:37–45. doi: 10.1046/j.1432-1327.2000.00956.x. [DOI] [PubMed] [Google Scholar]
- Samson Y, Lapergue B, Hosseini H. [Inflammation and ischaemic stroke: current status and future perspectives] Rev Neurol (Paris) 2005;161:1177–1182. doi: 10.1016/s0035-3787(05)85190-2. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno C, Dashe JF, Scott T, Thaler D, Folstein MF, Martin A. Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke. 2004;35:163–168. doi: 10.1161/01.STR.0000105391.62306.2E. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28:382–386. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Beschorner R, Meyermann R, Gozalan F, Schluesener HJ. Persistent accumulation of cyclooxygenase-1-expressing microglial cells and macrophages and transient upregulation by endothelium in human brain injury. J Neurosurg. 2002;96:892–899. doi: 10.3171/jns.2002.96.5.0892. [DOI] [PubMed] [Google Scholar]
- Selakovic V, Colic M, Jovanovic M, Raicevic R, Jovicic A. Cerebrospinal fluid and plasma concentration of soluble intercellular adhesion molecule 1, vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule in patients with acute ischemic brain disease. Vojnosanit Pregl. 2003;60:139–146. doi: 10.2298/vsp0302139s. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Siesjo P. Mechanisms of secondary brain injury. Eur J Anaesthesiol. 1996;13:247–268. [PubMed] [Google Scholar]
- Simi A, Ingelman-Sundberg M, Tindberg N. Neuroprotective agent chlomethiazole attenuates c-fos, c-jun, and AP-1 activation through inhibition of p38 MAP kinase. J Cereb Blood Flow Metab. 2000;20:1077–1088. doi: 10.1097/00004647-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Simundic AM, Basic V, Topic E, Demarin V, Vrkic N, Kunovic B, Stefanovic M, Begonja A. Soluble adhesion molecules in acute ischemic stroke. Clin Invest Med. 2004;27:86–92. [PubMed] [Google Scholar]
- Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CW. Leukocyte-endothelial cell interactions. Semin Hematol. 1993;30:45–53; discussion 54–45. [PubMed] [Google Scholar]
- Song Y, Wei EQ, Zhang WP, Zhang L, Liu JR, Chen Z. Minocycline protects PC12 cells from ischemic-like injury and inhibits 5-lipoxygenase activation. Neuroreport. 2004;15:2181–2184. doi: 10.1097/00001756-200410050-00007. [DOI] [PubMed] [Google Scholar]
- Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, Fairchild-Huntress V, Fang Q, Dunmore JH, Huszar D, Pan Y. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- Soriano SG, Coxon A, Wang YF, Frosch MP, Lipton SA, Hickey PR, Mayadas TN. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Stanimirovic D, Satoh K. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 2000;10:113–126. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- Sughrue ME, Mehra A, Connolly ES, Jr, D’Ambrosio AL. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. Inflamm Res. 2004;53:497–508. doi: 10.1007/s00011-004-1282-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Iadecola C. Delayed effect of administration of COX-2 inhibitor in mice with acute cerebral ischemia. Brain Res. 2003;960:273–276. doi: 10.1016/s0006-8993(02)03805-2. [DOI] [PubMed] [Google Scholar]
- Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, Nishida E. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci. 2000;20:4506–4514. doi: 10.1523/JNEUROSCI.20-12-04506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Abe K, Tojo S, Kimura K, Mizugaki M, Itoyama Y. A change of P-selectin immunoreactivity in rat brain after transient and permanent middle cerebral artery occlusion. Neurol Res. 1998;20:463–469. doi: 10.1080/01616412.1998.11740549. [DOI] [PubMed] [Google Scholar]
- Takeda H, Spatz M, Ruetzler C, McCarron R, Becker K, Hallenbeck J. Induction of mucosal tolerance to E-selectin prevents ischemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke. 2002;33:2156–2163. doi: 10.1161/01.str.0000029821.82531.8b. [DOI] [PubMed] [Google Scholar]
- Takizawa S, Aratani Y, Fukuyama N, Maeda N, Hirabayashi H, Koyama H, Shinohara Y, Nakazawa H. Deficiency of myeloperoxidase increases infarct volume and nitrotyrosine formation in mouse brain. J Cereb Blood Flow Metab. 2002;22:50–54. doi: 10.1097/00004647-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Rosengren L, Blomstrand C, Wikkelso C, Jensen C, Ekholm S, Tarkowski A. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol. 1997;110:492–499. doi: 10.1046/j.1365-2249.1997.4621483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarozzo G, Campanella M, Ghiani M, Bulfone A, Beltramo M. Expression of fractalkine and its receptor, CX3CR1, in response to ischaemia-reperfusion brain injury in the rat. Eur J Neurosci. 2002;15:1663–1668. doi: 10.1046/j.1460-9568.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992;17:61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Shibata M, Ihara M, Akiguchi I, Ohtani R, Budka H. A comparative study on the expression of cyclooxygenase and 5-lipoxygenase during cerebral ischemia in humans. Acta Neuropathol (Berl) 2002;104:601–607. doi: 10.1007/s00401-002-0590-0. [DOI] [PubMed] [Google Scholar]
- Ueno T, Sawa Y, Kitagawa-Sakakida S, Nishimura M, Morishita R, Kaneda Y, Kohmura E, Yoshimine T, Matsuda H. Nuclear factor-kappa B decoy attenuates neuronal damage after global brain ischemia: a future strategy for brain protection during circulatory arrest. J Thorac Cardiovasc Surg. 2001;122:720–727. doi: 10.1067/mtc.2001.115917. [DOI] [PubMed] [Google Scholar]
- Uno H, Matsuyama T, Akita H, Nishimura H, Sugita M. Induction of tumor necrosis factor-alpha in the mouse hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:491–499. doi: 10.1097/00004647-199705000-00002. [DOI] [PubMed] [Google Scholar]
- van Exel E, Gussekloo J, de Craen AJ, Bootsma-van der Wiel A, Frolich M, Westendorp RG. Inflammation and stroke: the Leiden 85-Plus Study. Stroke. 2002;33:1135–1138. doi: 10.1161/01.str.0000014206.05597.9e. [DOI] [PubMed] [Google Scholar]
- Vemuganti R, Dempsey RJ, Bowen KK. Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke. 2004;35:179–184. doi: 10.1161/01.STR.0000106479.53235.3E. [DOI] [PubMed] [Google Scholar]
- Vuorte J, Lindsberg PJ, Kaste M, Meri S, Jansson SE, Rothlein R, Repo H. Anti-ICAM-1 monoclonal antibody R6.5 (Enlimomab) promotes activation of neutrophils in whole blood. J Immunol. 1999;162:2353–2357. [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- Walton KM, DiRocco R, Bartlett BA, Koury E, Marcy VR, Jarvis B, Schaefer EM, Bhat RV. Activation of p38MAPK in microglia after ischemia. J Neurochem. 1998;70:1764–1767. doi: 10.1046/j.1471-4159.1998.70041764.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, Chen J, Gautam SC, Zhang Z, Lu M, Chopp M. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002a;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002b;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- Wang X, Feuerstein GZ. Induced expression of adhesion molecules following focal brain ischemia. J Neurotrauma. 1995;12:825–832. doi: 10.1089/neu.1995.12.825. [DOI] [PubMed] [Google Scholar]
- Wang X, Siren AL, Liu Y, Yue TL, Barone FC, Feuerstein GZ. Upregulation of intercellular adhesion molecule 1 (ICAM-1) on brain microvascular endothelial cells in rat ischemic cortex. Brain Res Mol Brain Res. 1994;26:61–68. doi: 10.1016/0169-328x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Abe H, Takeuchi S, Tanaka R. Protective effect of microglial conditioning medium on neuronal damage induced by glutamate. Neurosci Lett. 2000;289:53–56. doi: 10.1016/s0304-3940(00)01252-0. [DOI] [PubMed] [Google Scholar]
- Wood PL. Microglia as a unique cellular target in the treatment of stroke: potential neurotoxic mediators produced by activated microglia. Neurol Res. 1995;17:242–248. doi: 10.1080/01616412.1995.11740321. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. discussion 681. [DOI] [PubMed] [Google Scholar]
- Yang GY, Gong C, Qin Z, Ye W, Mao Y, Bertz AL. Inhibition of TNFalpha attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport. 1998;9:2131–2134. doi: 10.1097/00001756-199806220-00041. [DOI] [PubMed] [Google Scholar]
- Yang GY, Zhao YJ, Davidson BL, Betz AL. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751:181–188. doi: 10.1016/s0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, Whitaker S, Steinberg GK. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153:223–233. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Sun GH, Kunis DM, Onley D, Vexler V. L-selectin inhibition does not reduce injury in a rabbit model of transient focal cerebral ischemia. Neurol Res. 2001;23:72–78. doi: 10.1179/016164101101198154. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- Yepes M, Brown SA, Moore EG, Smith EP, Lawrence DA, Winkles JA. A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia. Am J Pathol. 2005;166:511–520. doi: 10.1016/S0002-9440(10)62273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Ohtaki H, Nakamachi T, Kudo Y, Makino R, Shioda S. Delayed expressed TNFR1 co-localize with ICAM-1 in astrocyte in mice brain after transient focal ischemia. Neurosci Lett. 2004;370:30–35. doi: 10.1016/j.neulet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YM, Kim JB, Lee KW, Kim SY, Han PL, Lee JK. Inhibition of the cerebral ischemic injury by ethyl pyruvate with a wide therapeutic window. Stroke. 2005;36:2238–2243. doi: 10.1161/01.STR.0000181779.83472.35. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Wei EQ. Neuroprotective effect of ONO-1078, a leukotriene receptor antagonist, on transient global cerebral ischemia in rats. Acta Pharmacol Sin. 2003;24:1241–1247. [PubMed] [Google Scholar]
- Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke. 2005a;36:2220–2225. doi: 10.1161/01.STR.0000182241.07096.06. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Wang RM, Yin XH, Pan J, Xu TL, Zhang GY. Knockdown of POSH expression is neuroprotective through down-regulating activation of the MLK3-MKK4-JNK pathway following cerebral ischaemia in the rat hippocampal CA1 subfield. J Neurochem. 2005b;95:784–795. doi: 10.1111/j.1471-4159.2005.03435.x. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, Anderson DC. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995a;26:1438–1442. doi: 10.1161/01.str.26.8.1438. discussion 1443. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, Tang WX, Tsang W, Anderson DC, Manning AM. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995b;682:182–188. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Phillips ML, Rosenbloom CL, Cruz R, Manning A. E-selectin in focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 1996;16:1126–1136. doi: 10.1097/00004647-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M, Powers C. Temporal profile of microglial response following transient (2 h) middle cerebral artery occlusion. Brain Res. 1997;744:189–198. doi: 10.1016/S0006-8993(96)01085-2. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. 2000;872:215–218. doi: 10.1016/s0006-8993(00)02459-8. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu Y, Miao AD, Wang SQ. Protocatechuic aldehyde suppresses TNF-alpha-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells. Eur J Pharmacol. 2005;513:1–8. doi: 10.1016/j.ejphar.2005.01.059. [DOI] [PubMed] [Google Scholar]


