Abstract
Our previous studies showed vitamin D deficiency results in increased cardiac contractility, hypertrophy and fibrosis and has profound effects on heart proteomics, structure and function in rat. In this study we found that the heart in vitamin D receptor knockout (VDR-KO) mice is hypertrophied. Six homozygous VDR knockout (−/−), 6 wild type (+/+) and 6 heterozygous (+/−) mice were fed a diet containing 2% Ca, 1.25% P and 20% lactose to maintain normal blood calcium and phosphate levels for 12 months. Tail-cuff blood pressure was performed on all mice. Blood pressure determinations showed no differences in systolic or mean blood pressure in WT (+/+), KO (−/−) or HETERO (+/−) mice at 3 and 6 months. However, decreased systolic BP in the KO mice relative to WT at 9 months of age was observed. ECG analysis showed no significant differences in the intact KO, HETERO or WT mice. The mice were killed at 12 months. Heart wt/body wt ratio was 41% (p<.003) greater in the KO mice vs. WT and HETERO was 19% (p<.05) increased vs. WT. Other VDR-KO tissues did not display hypertrophy. Cross sectional and longitudinal analysis of the heart myofibrils showed highly significant cellular hypertrophy in VDR-KO mice. Trichrome staining of heart tissue showed marked increase in fibrotic lesions in the KO mice. Analysis of plasma renin activity, angiotensin II (AII) and aldosterone levels showed elevated but not significantly different renin activity in KO vs. WT and no significant differences in AII or aldosterone levels. Our data do not support the concept that the renin-angiotensin system or hypertension are the factors that elicit these changes. Data presented here reveal that ablation of the VDR signaling system results in profound changes in heart structure. We propose that calcitriol acts directly on the heart as a tranquilizer by blunting cardiomyocyte hypertrophy.
Keywords: 1,25-dihydroxyvitamin D3; heart; cardiac hypertrophy
INTRODUCTION
Several laboratories have characterized the effects that modification of the vitamin D endocrine system has on cardiac muscle structure and function. We showed that vitamin D3 deficiency alters rat myocardial morphology, ECM and function.(1–3) Our initial studies revealed that large and statistically significant increases in ventricular pressure development (+dP/dt) are observed in perfused hearts from young (9week old) vitamin D3-deficient rats compared to hearts from vitamin D3-sufficient rats. A mechanism by which myocardial contractility can be increased is by raising intracellular calcium concentrations. We showed that 9-week-old vitamin D3-deficient rats had an increase of L-type calcium channels and post rest contraction response, a measure of sarcoplasmic reticulum calcium uptake (2). Heart failure affects nearly 5 million people in the United States alone and at present no single unifying hypothesis can account for the etiology of this disease. However, it is known that neuroendocrine factors and cytokines are associated with HF and that left ventricular remodeling is an important determinant in the progression to heart failure. Reduced levels of the most active vitamin D metabolite, 1,25-dihydroxyvitamin D3 are associated with an increased risk of heart failure and reports also indicate ventricular function is compromised and a dilated cardiomyopathy develops in pediatric patients with rickets caused by a vitamin D deficiency. Thus, the following study of the cardiovascular effects of VDR ablation in mice is important and relevant to human disease. A recent study by Xiang et. al. suggests that activation of both systemic and cardiac RAS plays an important role in the development of cardiac hypertrophy in VDR(−/−) mice. (5)
MATERIALS AND METHODS
Mouse breeding, housing and feeding
Wild-type [WT] VDR (+/+), Heterozygous (+/−) and VDRKO (−/−) mice were produced from VDR(+/−) originally the generous gift of Dr. Marie Demay, Mass General Hospitals. All mice, breeders and offspring were housed in the University of Michigan Laboratory Animal facility with a 12:12-h light-dark cycle and fed a rodent chow containing 2%. Ca, 1.25% Phosphorus and 20% Lactose. Age- matched WT(+/+), Heterozygous (+/−) and VDRKO(−/−) mice were genotyped and used in this study.
Measurement of myocardial heart weight/body weight ratio
Mice were weighed and then killed via cervical dislocation. The chest cavity was rapidly opened, and the heart removed and rinsed in two washes of ice-cold saline. Major blood vessels and connective tissue was removed, the heart blotted dry, weighed, and the heart weight/body weight ratio calculated. Other organ weights were determined and weight to total body weight ratios calculated.
Measurement of Serum Calcium, Phosphate, Magnesium
Blood calcium (Arsenazo III), ionized calcium (NOVA ISE), phosphate (Phosphomolybdate), and magnesium (Formazan Dye) were measured by the Special Chemistry Clinical Laboratories, University of Michigan Hospital.
Measurement of Ang II, aldosterone and renin activity
Blood angiotensin II and aldosterone levels were measured by radioimmunoassay from commercially available kits by the University Michigan Animal Diagnostic Laboratory in the Unit for Laboratory Animal Medicine. Plasma renin activity was measured assessing enzyme activity by the Special Chemistry Clinical Labs at the University of Michigan Hospitals.
Non-invasive Recording Electrocardiograms in Conscious Mice
The System used was the AnonyMOUSE ECG Screening System (Mouse Specifics, Boston, MA). The mice were placed on the instrument’s platform and acclimate for 10 minutes prior to ECG recordings. Data were analyzed by propriatal software and all mice ECG intervals analyzed by student’s two-tail t test.
Non-invasive blood pressure measurement by external tail pulse detection
To obtain an accurate blood pressure reading, mice remained still and unperturbed throughout the measurement period. Mice were conditioned to the restraint and the warming chamber for 10–20 min/day for at least 3 days before measurements. After this 3-day training period, mice typically remained relatively still and unperturbed when placed in the restrainer on the day of testing. The chamber (model 306 warming chamber, IITC Life Science; Woodland Hills, CA) was kept at 31–33°C, and a darkened nose cone helped calm and secure the mouse in the restraint (model 84 mouse restrainers, IITC Life Science). An integrated sensor-cuff occluder (model B60-1/4, IITC Life Science) operated to stop tail pulsation on inflation and to detect the return of tail pulsations (RTP) passing through the occluder cuff on each deflation cycle. The RTP-computerized blood pressure monitor (model 6M 229 6 channel mouse system, IITC Life Science) was set for desired sensitivity, number of cycles, the maximum tail-cuff inflation pressure, the rate of deflation, and the interval between cycles. The maximum pressure of inflation was set 20–40 mmHg above anticipated systolic pressure. The instrument was set for a maximum inflation pressure of 200 mmHg for mice that were expected to have pressures in the normal range and was increased to 250 or 300 mmHg for markedly hypertensive mice. After 5–10 min of stabilization in the chamber, a typical run involved 10 repetitions of the automated inflation-deflation cycle.
Statistical significance was determined by the two-tail student t test.
RESULTS
Figure 1 shows the heart weight body weight ratio of VDR wild type (+/+), knockout (−/−) and heterozygous (+/−) mice. There was a significant 41% [P<.005] increased HW/BW ratio for the VDRKO hearts relative to the VDRWT mice hearts. Heterozygous (+/−) mice had an increased 14% [P<.05] HW/BW ratio relative to WT. Figure 2 is data obtained from the analysis of liver, kidney, brain and tibia for organ weight/body weight ratios for the KO(−/−), WT(+/+) and HET(+/−) mice. The data show that for liver, kidney and tibia VDRWT, VDRKO or Heterozygous genotypes, no significant changes in organ weight/body weight ratios were observed. We have not investigated the significance of this observation. The data reveal specificity of organ hypertrophy. Figure 3 shows blood total calcium levels, phosphate levels and magnesium levels in the VDRKO, VDRWT and heterozygous mice. No significant changes were observed. Figure 4 shows systolic and mean arterial blood pressure at 3, 6 and 9 months for the VDRKO and VDRWT mice. At 3 and 6 months no significant changes were observed for VDRKO versus VDRWT in systolic or mean arterial pressure. However at 9 months systolic blood pressure was significantly lower in the VDRKO than the VDRWT mice [P<.05]. Figure 5 shows the angiotensin II and aldosterone levels in the blood from the VDRKO, VDRWT, and heterozygous mice. No significant changes in angiotensin II or aldosterone level were observed. Figure 6 shows the plasma renin activity of 3 pooled [2–4 mice per pool] mice plasma samples of VDRKO and 3 pooled plasma samples from VDRWT mice. An increased level of renin activity was observed in the VDRKO mice but this ~50% increase was not significantly different. Significance was assessed for n=3 assays for VDRKO and VDRWT. ECG analysis in the intact KO, HETERO or WT mice was performed. ECG analysis showed no significant differences in the intact KO, HETERO or WT mice at 3,6 9 months.[DATA NOT SHOWN] The absence of QRS elongation in the VDRKO mice supports the measured normal serum calcium levels.
FIGURE 1.
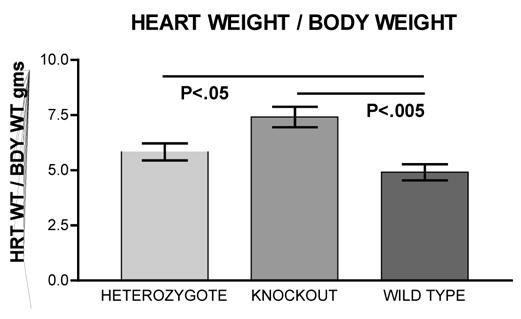
Heart weight/body weight ratios for VDR heterozygous [(+/−) n=6], knockout [(−/−) n=6] and wild type [(+/+) n=5] mice. Mice were bred from mothers maintained on a diet containing 2% Calcium, 1.25% Phosphate and 20% Lactose (Teklad # 96348) and remained on this diet for 12 months.
FIGURE 2.
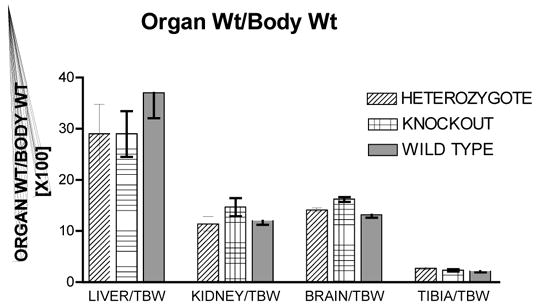
Organ weight/body weight ratios are for VDR heterozygous [(+/−) n=6], knockout [(−/−) n=6] and wild type [(+/+) n=5] mice. Body weight were: (+/−) = 30.7+/−4.9gm; (−/−)= 27.7 +/− 4.8gm and (+/+)= 34.3+/− 5.9 [X+/−SD]. Liver, kidney, brain and tibia were removed, dried, cleaned of other tissues and weighed from the 12-month-old mice.
FIGURE 3.
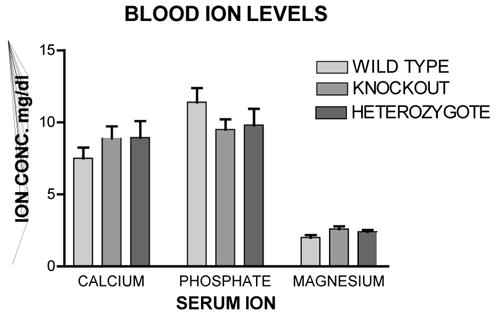
Blood ion levels from the 12 month old mice maintained on the 2% Calcium, 1.25% Phosphate and 20% Lactose diet. Veinous blood was obtained from the inferior vena cava and plasma analyzed for total calcium, phosphate and magnesium at the Special Chemistry Labs at the University of Michigan Hospitals
FIGURE 4.
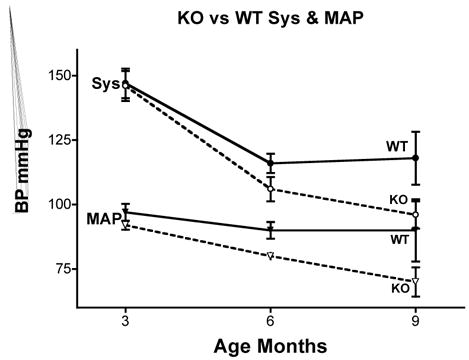
Age-dependant and phenotype dependant changes in systolic blood pressure and calculated mean arterial blood pressure of knockout (open circles) and wild type (closed circles) mice on the high calcium diet. Non-invasive blood pressures were measured by tail cuff occlusion using an eMouse Specifics Inc. apparatus. Ten measurements were taken for each mouse at each time point and averaged. The group average was then calculated and data presented as Mean +/− Standard Deviation.
FIGURE 5.
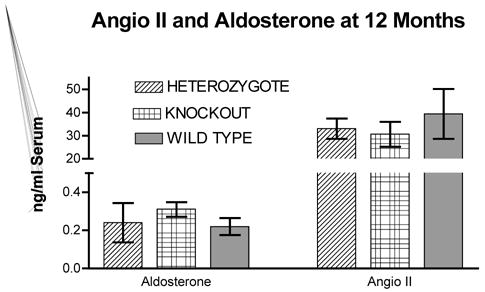
Blood angiotensin II and aldosterone levels were measured by radioimmunoassay methods from commercially available kits at the University Michigan Animal Diagnostic Laboratory in the Unit for Laboratory Animal Medicine. N=6 for heterozygous, N=5 for wild type and N= 6 for knockout mice. Data displayed as Mean+/− Standard Deviation.
FIGURE 6.
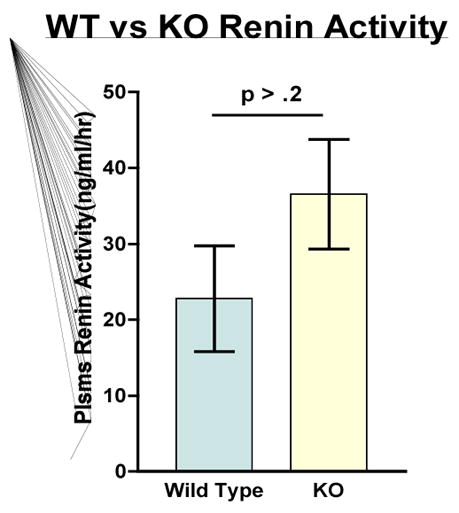
Plasma renin activity was measured Special Chemistry Clinical Labs at the University of Michigan Hospitals. Mouse plasma pooled sample with N=3 for knockout and N=3 wild type mice. No significance differences were observed P> .02.
DISCUSSION
Results from the present study demonstrate the effects of ablation of the vitamin D receptor and signaling on adult mouse heart structure. The data reveal that profound cardiac hypertrophy is apparent at 12 months of age for the VDRKO mouse. Relative to our previous studies with vitamin D deficient rats the increase in heart weight to body weight was much greater in the VDRKO mouse (41%) relative to the level of hypertrophy in 9 or 18 month old vitamin D deficient rat (~18%) initially reported by my laboratory. (1–3) This increase in hypertrophy (> 2 fold) as a result of VDR ablation could be a result of incomplete depletion of vitamin D in the rats on vitamin D deficient diets in our previous studies or the age of the animals. Recent reports have suggested that the observed cardiac hypertrophy seen in VDRKO mice is a consequence of activation of both the systemic and cardiac RAS, and support the notion that 1,25-dihydroxyvitamin D3 regulates cardiac function, at least in part, through the RAS. Our data do not directly contradict this possibility but do show that in the absence of significant increases in renin, angiotensin II, aldosterone, or blood pressure, cardiac hypertrophy as a result of VDR knockout occurs and is significantly greater (41% versus 20%) at 12 months than reported at 3 months.(5) This prior study used VDRKO mice transferred to the high calcium and lactose diet for a period of 5 weeks. In the present study mice were bred from mothers on the high calcium diet and maintained on it for the entire 12 months of the study. This is the most apparent difference in the two studies. Interestingly, it is recognized that high calcium diets decrease plasma renin activity, reduce angiotensin II receptors and increase sodium excretion. (Reviewed in 6). Recently, high calcium diets were shown to down regulate kidney angiotensin converting enzyme (ACE) and thus decrease blood pressure. (6) Thus, our observation of increased renin level but normal blood pressure, angiotensin II and aldosterone levels could be a result of the length of time (12 months) high calcium diets were fed to mice in our study and the concomitant decreased expression of ACE. This possibility has not been examined.
Footnotes
THIS WORK WAS SUPPORTED BY THE NATIOAL INSTITUES OF HEALTHGRANT # HL074894 AND THE NIH FUNDED DIABETES, CANCER, ORGANOGENESIS AND BONE CENTERS AT THE UNIVERSITY OF MICHIGAN MEDICAL SCHOOL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simpson RU. Evidence for a specific 1,25-dihydroxyvitamin D3 receptor in rat heart. Circulation. 1983;68:239. [Google Scholar]
- 2.Weishaar RE, Simpson RU. The involvement of endocrine system in regulating cardiovascular function: emphasis on vitamin D3. Endocr Rev. 1989;10:351–365. doi: 10.1210/edrv-10-3-351. [DOI] [PubMed] [Google Scholar]
- 3.Weishaar RE, Kim SN, Saunders D, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258:E134–42. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 4.Zittermann A, Schulze Schleithoff S, Tenderich G, Berthold HK, Körfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41(2003):105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 5.Xiang W, Kong J, Chen S, Cao L, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice:role of the systemic and cardiac renin-angiotensin systems. Amer J Pysiol. 2005;288:E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 6.Porsti I, Fan M, Koobi P, Jolma P, Kalliovalkama J, Vehmas TI, Helin H, Holthofer H, Mervaala E, Nyman T, Tikkanen I. High calcium diet down-regulates kidney angiotensin-converting enzyme in experimental renal failure. Kidney Int. 2004;66(6):2155–66. doi: 10.1111/j.1523-1755.2004.66006.x. [DOI] [PubMed] [Google Scholar]


