Abstract
Canonical Wnt signaling is essential for bone formation. Activation involves binding of secreted members of the Wnt family of proteins with a membrane receptor Frizzled on osteoblasts, an interaction that is facilitated by LRP5/LRP6 co-receptors. LRP5 is known to play a particularly important role in bone formation such that the loss of this protein results in a reduction in osteoblast number, a delay in mineralization and a reduction in peak BMD. During the course of a VDR ChIP-chip analysis we found that 1,25(OH)2D3 could induce binding of the VDR to sites within the Lrp5 gene locus. Importantly, this interaction between 1,25(OH)2D3-activated VDR and the Lrp5 gene led to both a modification in chromatin structure within the Lrp5 locus and the induction of LRP5 mRNA transcripts in vivo as well as in vitro. One site within Lrp5 was discovered to confer 1,25(OH)2D3 response to a heterologous promoter in osteoblastic cells, permitting both the identification and characterization of the component VDRE. While the regulatory region in Lrp5 was highly conserved in the human genome, the VDRE was not. Our studies show that 1,25(OH)2D3 can enhance the expression of a critical component of the Wnt signaling pathway which is known to impact osteogenesis.
Keywords: osteoblasts, Wnt signaling pathway, vitamin D response element, ChIP on chip, vitamin D receptor, coregulators
1. Introduction
The actions of 1,25(OH)2D3 are vital to the maintenance of calcium and phosphorus homeostasis, and are critical to the integrity of the vertebrate skeleton [1–3]. Central to these actions is the ability of 1,25(OH)2D3q to diffuse into cells and to interact specifically with the vitamin D receptor (VDR). In turn this protein binds directly to regulatory regions of target genes altering their transcriptional output. Selective VDR DNA binding, therefore, enables the cell to direct its transcriptional machinery to that subset of genes whose products orchestrate the specific biological response to 1,25(OH)2D3.
Early studies have shown that 1,25(OH)2D3 plays a significant role in controlling mature osteoblast function, particularly as it relates to the secretion of bone matrix and the capacity to promote bone mineralization [4–6]. These actions are complex and impact such events as early osteoblast precursor cell progression as well as osteoblast proliferation and differentiation. Furthermore, these effects are highly cell stage-specific, and have produced an array of diverse and sometimes conflicting observations with respect to osteoblast biology. While 1,25(OH)2D3 modulates the expression of functional genes involved in osteoblast differentiation the hormone is also able to influence the expression of 1) secreted factors that activate cellular differentiation pathways, 2) components that represent key transducing factors within those pathways, and 3) the transcription factor targets that serve as mediators of these pathways at the level of gene expression. Therefore, the hormone is likely capable of triggering a broad range of effects on osteoblast proliferation, differentiation and function [5].
The canonical Wnt signaling pathway also plays a crucial role in osteoblast proliferation and differentiation, and appears to exert a significant developmental role in bone formation [7–9]. This pathway is initiated through the interaction of members of the Wnt family with the cell surface receptor Frizzled and co-receptor LRP5 [10]. Stimulation leads to inhibition of the activity of glycogen synthase kinase-3β (GSK3β) which results in stabilization and redistribution of the transcription factor β-catenin to the nucleus where it activates LEF/TCF mediated gene transcription (Fig. 1a). Activation of Frizzled and its co-receptor (LRP5 & 6) is also regulated by numerous Wnt antagonists several of which involve additional membrane proteins [7].
Fig. 1.
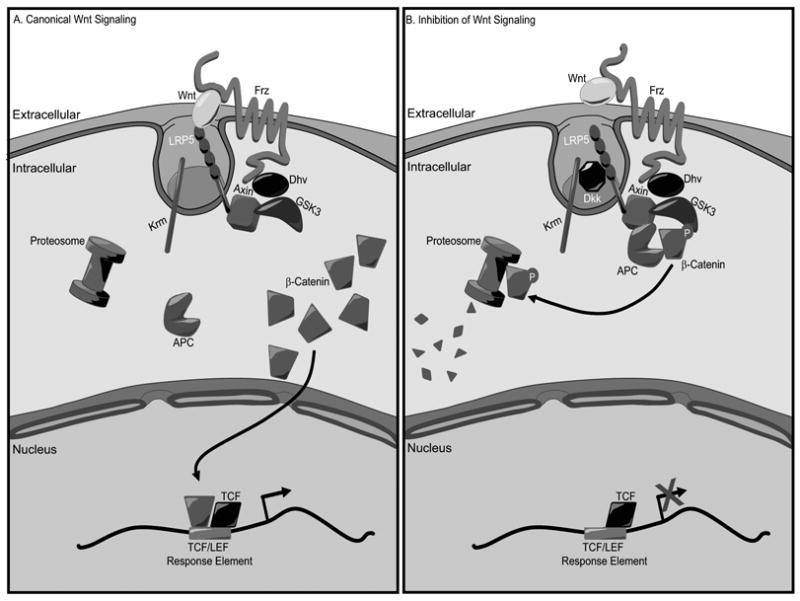
Canonical Wnt Signaling Pathway. A, Binding of Wnt to Frizzled and LRP5 results in stabilization of β-catenin and consequently activation of TCF/LEF-responsive gene transcription. B, Inhibition of Wnt signaling occurs when LRP5 is shunted away through interactions with antagonistic cytokines.
Many of the osteogenic effects of Wnt signaling were identified through discovery of the biological consequences associated with aberrant expression of LRP5; hypermorphic alleles of LRP5 were associated with high bone mass [11, 12] and loss-of-function mutations were associated with osteoporosis-pseudoglioma syndrome [13]. The Lrp5 gene has subsequently been inactivated in mice resulting in a phenotype of low bone mass similar to that seen in humans [14]. Importantly, a thorough investigation of the phenotype of this mouse model revealed that the primary effect of Wnt activation was to influence both osteoblast proliferation and function, particularly as it relates to both the secretion of bone matrix and subsequent timely mineralization.
During a ChIP-chip screen of genes capable of influencing osteoblast function, we found potential binding sites for VDR on the Lrp5 gene. 1,25(OH)2D3 activation and VDR binding was correlated directly to subsequent modifications that occurred on chromatin within this locus as well as to increased levels of expression of LRP5 both in vitro and in vivo. Cloning of each of these potential regulatory regions revealed that the major regulatory site could confer 1,25(OH)2D3 response to a heterologous promoter, allowing for identification an characterization of the active VDRE. Interestingly, this VDRE was not conserved in the human gene despite significant conservation in the surrounding region. Our studies identify mouse Lrp5 as a target gene for 1,25(OH)2D3 and provide a mechanism whereby this hormone can influence the expression of a regulatory component influential in modulating bone formation.
2. Materials and Methods
2.1 Tiled oligonucleotide microarray analysis
ChIP-chip analysis was carried out as described by others [15]. In brief, DNA was isolated by ChIP and then subjected to ligation mediated PCR. The resulting amplicon pools were labeled with Cy3 or Cy5 dyes through indirect labeling and then mixed in the presence of CoT-1 DNA, denatured, and co-hybridized to custom oligonucleotide microarray (Nimblegen Systems Inc, Madison, WI). The microarrays were scanned using an Axon 4000B scanner. Custom arrays included maskless in situ-synthesized 50-mers at 2 bp intervals representing a 132 kb screen of the mouse Lrp5 gene from 20 kb upstream of the gene’s TSS to 10 kb downstream of the final 3′ non-coding exon. After sample co-hybridization, the logarithmic enrichment ratio of Cy5 to Cy3 intensities (log2) were plotted as a function of chromosome nucleotide position (Dec.2004 Assembly) and scored with a peak finding algorithm [16].
2.2 Animal studies
Eight week-old C57BL6 wildtype mice were dosed by intraperitoneal injection of 1,25(OH)2D3 (10 ng/g body weight). Groups of animals (n = 3) were sacrificed at 0–6 hours and calvarial tissue was collected for RNA isolation. Experimental protocols were reviewed and approved by the Research Animal Resources Center (University of Wisconsin-Madison, Madison, WI).
2.3 RNA isolation and analysis
Total RNA was isolated from homogenized animal tissue or cells using Triazol reagent obtained from MRC (Cincinnati, OH). The isolated RNA was reverse transcribed using the Superscript III RNase H Reverse Transcriptase kit (Invitrogen) and then subjected to PCR analysis using Real Time or standard PCR methods.
2.4 Transfection analysis
MC3T3-E1, ST2, mOB and/or MG63 cells were seeded into 24-well plates (5.0 × 104 cells/well) in the appropriate complete media. Cells were transfected 24 hrs later with Lipofectamine Plus in serum and antibiotic-free media. Cells were harvested 24 hours after stimulation and the lysates were assayed for luciferase and β-galactosidase activities as previously described [17].
2.5 Statistical Analysis
All values are expressed as mean ± SEM. We evaluated differences between groups through one way analysis of variance (ANOVA) or student’s one tailed t-test.
3. Results and Discussion
Our investigation was initiated by exploring the capacity of 1,25(OH)2D3 to promote VDR and RXR binding to genes with the potential to influence the osteoblast. Lrp5 was one such candidate. We utilized a scanning method wherein DNA sequences across genes of interest were tiled on a DNA microarray at 50 bp resolution and then screened with DNA labeled fragments obtained from ChIP using antibodies to either VDR or RXR. This approach yielded three potential binding sites for the VDR/RXR on Lrp5 located both proximal to the TSS and at extended distances (Fig 2). Independent ChIP analysis confirmed variable VDR/RXR binding at these intronic sites in ST2, MC3T3-E1, and primary mOB cells, although the mLrp5-P2 region was the most consistent in all target cells examined. The locations of the vitamin D regulatory regions within Lrp5 introns were surprising, since the bulk of the VDREs thus far identified have been located within the first kilobase or so upstream of the TSS. Other studies, however, have identified other hormone regulatory elements within downstream introns [18, 19], suggesting that our results do not establish a new paradigm.
Fig. 2.
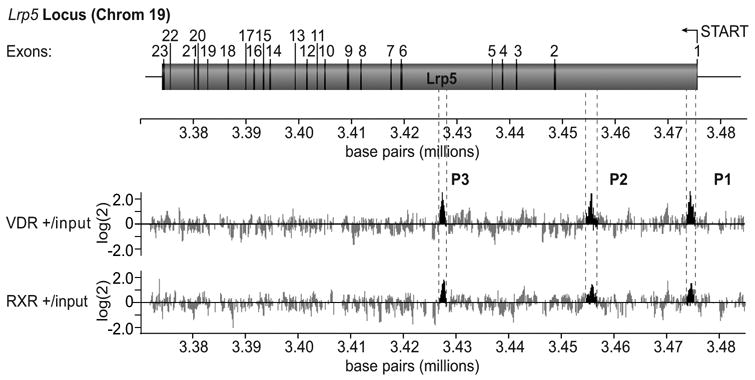
ChIP-Chip analysis of identifies VDR-binding regions in the mouse Lrp5 locus. ChIP-chip analysis of the mouse Lrp5 candidate target gene. Upper panel: schematic diagram of the mouse Lrp5 gene and its 23 exons. Base pair numbering indicates nucleotide location on chromosome 19 (December 2004 Assembly). The arrow indicates the direction of transcription on the reverse strand. Lower panel: Individual data tracks representing the enrichment ratios of Cy5 to Cy3 of hybridization intensity (log2) for VDR plus hormone versus input DNA and RXR plus hormone versus input DNA. Base pair numbering indicates nucleotide location on chromosome 19 (December 2004 Assembly). The highlighted areas indicate peaks of interest that are designated mLrp5-P1, mLrp5-P2, and mLrp5-P3.
We extended our findings by demonstrating that 1,25(OH)2D3 activation and VDR/RXR binding was associated with functional consequences to the chromatin located within these regions of the Lrp5 gene. As expected, both VDR as well as RXR bound to the P1-P3 regions of the Lrp5 gene, although binding at P2 was clearly predominant (Fig 3). The ChIP analysis depicted also shows both a strong residual as well as 1,25(OH)2D3-inducible histone 4 acetylation, which was particularly evident at mLrp5-P1 and mLrp5-P2. Since histone 4 modification is also seen in the Ctrl region, it suggests that the acetylation pattern on the Lrp5 gene is potentially very broad. Perhaps most interesting is the observation that RNA pol II levels are enhanced in response to 1,25(OH)2D3 at all three regions. While the mLrp5-P1 region is located proximal to the TSS, and therefore likely to represent RNA pol II recruitment to a bona fide pre-initiation complex, the appearance of RNA pol II at mLrp5-P2 and mLrp5-P3 was surprising. Taken together, these results suggest that mLrp5-P2 and mLrp5-P3, and perhaps mLrp5-P1 represent regulatory enhancer regions capable of both modifying chromatin in response to 1,25(OH)2D3 induction and VDR/RXR binding, and acting as potential recruitment centers for increasing the RNA pol II levels necessary for enhanced transcription.
Fig. 3.
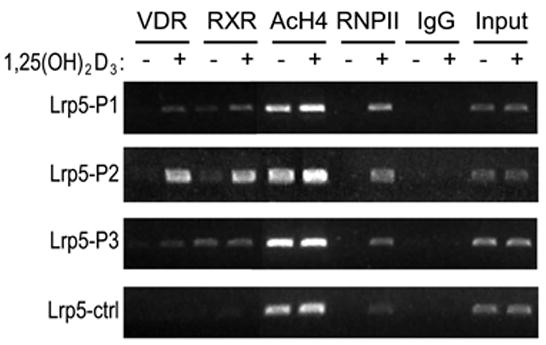
VDR/RXR heterodimer binding to the mLrp5-P1- mLrp5-P3 regions of Lrp5 enhances chromatin modification at histone 4 and induces the recruitment of RNA polymerase II. A, ChIP analysis of 1,25(OH)2D3 induced VDR and RXR binding, histone acetylation and RNA pol II presence at the P1–P3 and Ctrl regions of the Lrp5 gene. MC3T3-E1 cells were treated with either vehicle or 1,25(OH)2D3 (10−7 M) for 3 hrs and then subjected to ChIP using antibodies specific to VDR, RXR, tetra-acetylated histone 4, RNA pol II or an irrelevant IgG antibody. DNA precipitates were isolated and then subjected to PCR.
Although all three regions of Lrp5 bound VDR and RXR, mLrp5-P2 was the only fragment active in transcriptional activation in vitro and therefore was the only fragment for which direct mapping studies could be conducted. These studies identified a functional VDRE located 19,241/19,255 bp downstream of the TSS (Fig. 4a). Interestingly, while there is conservation of a large region of Lrp5 P2 between species, the VDRE is not maintained in the human genome due to a 3 bp substitution in the downstream halfsite.
Fig. 4.
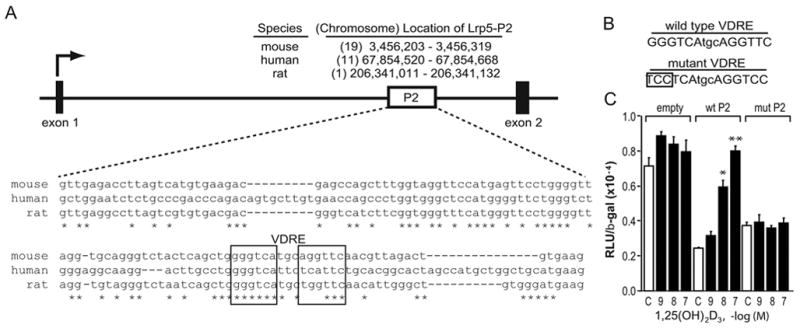
The mouse mLrp5-P2 region contains a functional VDRE. A, Conservation of P2 within the mouse, human and rat Lrp5 genes. Upper panel: The diagram depicts the boxed position of the Lrp5-P2 region located within the intron between exons 1 and 2. The nucleotide and chromosomal position of an analogous region in the human and rat genes are indicated. Lower panel: Nucleotide sequence of the P2 region with the mouse, human and rat Lrp5 gene wherein half-sites of a putative VDRE identified by the CONSITE algorithm is indicated. B, The panel illustrates the VDRE constructs used to evaluate the transcriptional activity of the potential VDRE. C, Transcriptional activity of wildtype and mutant forms of mLrp5-P2. MC3T3-E1 cells were co-transfected with pCH110-βgal, pcDNA-hVDR, and either ptk-luc control vector, ptk-mLrp5-P2 or the mutant version of ptk- mLrp5-P2. Cells were treated with either vehicle or increasing concentrations of 1,25(OH)2D3 (10−7 M) for 24 hrs and then evaluated for both luciferase and β-gal activity. Each point represents the normalized RLU average ± SEM for a triplicate set of transfections. * indicates p<0.01 compared to vehicle treated sample (one way ANOVA).
Having established a 1,25(OH)2D3-stimulated interaction between the VDR and the Lrp5 gene, we next examined whether this interaction was capable of inducing Lrp5 expression. 1,25(OH)2D3 induced Lrp5 not only in vitro, but also in vivo. A single dose of 1,25(OH)2D3 (10 ng/g bw) for periods up to 6 hours displayed a time-dependent upregulation in LRP5 mRNA from wildtype calvarial tissue (Fig. 5). This suggests that 1,25(OH)2D3-mediated induction of Lrp5 expression is mediated by an interaction of VDR and RXR with the P1–P3 regions of Lrp5.
Fig. 5.
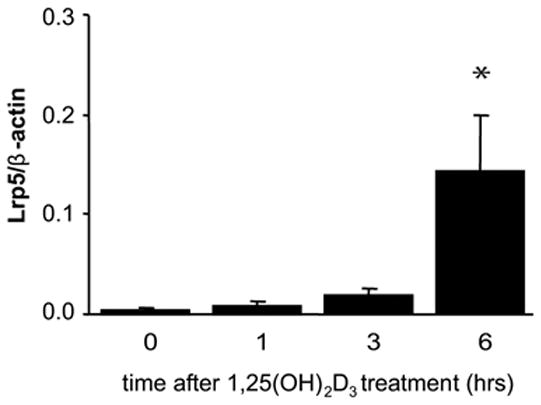
1,25(OH)2D3 induces Lrp5 mRNA levels in vivo via a VDR-dependent mechanism. Female C57Bl6 mice (n = 3/group) were treated with a single dose of 1,25(OH)2D3 (10 ng/g bw) via IP injection. The calvaria were then harvested after 0, 1, 3 and 6 hr and the isolated RNA subjected to qRT-PCR analysis using β-actin and Lrp5 primers as described in Materials and Methods. Lrp5 measurements were normalized to β-actin levels. Each point represents the mean ± SEM. Differences were determined by student’s one tailed t-test (*, p<0.05 in comparison to vehicle).
Vitamin D is a bone remodeling agent. Thus, its overall effects on bone formation as well as bone resorption via direct regulation of gene expression are well established [5, 20]. That many of these effects of 1,25(OH)2D3 in bone are redundant with other regulatory factors is also well recognized and highlighted most recently by the finding that the dramatic skeletal phenotype observed in the VDR null mouse can be rescued through a diet rich in calcium and phosphorous [21]. It is now believed, however, that high levels of calcium and phosphorus cannot fully normalize the skeletal defects associated with genetic ablation of the VDR or 1,25(OH)2D3 deficiency, manifest as reductions in osteoblast numbers, mineral apposition rate, and bone volume compared to that seen in wildtype mice. The potential anabolic effects of 1,25(OH)2D3 both on osteoblast differentiation as well as osteoblastic bone formation are consistent with that of many of the activities induced by the Wnt family of regulators. Whether the upregulation of Lrp5 by 1,25(OH)2D3 contributes significantly to the overall actions of 1,25(OH)2D3 on bone remains to be determined. If so, the effects of 1,25(OH)2D3 would be similar to that observed with the BMPs, which induce a similar rapid and sustained upregulation of Lrp5 during the first 6 days or so of osteoblast proliferation and differentiation [13].
Interestingly, studies in other systems have suggested that vitamin D might inhibit Wnt signaling. Accordingly, recent observations have shown that VDR, and other nuclear receptors, can bind directly to β-catenin and repress wnt/β-catenin/LEF-TCF signaling [22]. The bulk of this activity, however, has been observed in colon cancer cells, wherein the actions of Wnt signaling are largely proliferative and where β-catenin activation has been dis-regulated. The exact relationships connecting each of these pathways in different tissues and cells are not fully understood at this stage and remain to be resolved.
In summary, although the similarity of effects of vitamin D could be fortuitous and entirely independent of key extracellular signaling pathway activity, it is also possible that the related effects arise through a direct regulatory action by the steroid hormone on one or more components of these key control pathways. Indeed, vitamin D is known to regulate both signal pathway components as well as many of the transcription factors they target, making this hypothesis a plausible one. In support of this type of activity by vitamin D, we show that 1,25(OH)2D3 is able to upregulate the expression of Lrp5 transcripts in osteoblasts. Mechanistically, Lrp5 upregulation is due to the presence of three control regions located within the gene, at least one of which contains a specific VDRE. Interestingly, while the Lrp5 P2 region was generally conserved within the human locus, critical differences were apparent within the VDRE sequence itself. Despite this, our results in the mouse provide a possible secondary mechanism whereby 1,25(OH)2D3 may impact osteoblast biology.
Acknowledgments
We thank the members of the Pike Laboratory for their individual contributions to this work. We thank Adam Steinberg for preparation of the second figure. This work was supported by NIH grants to J.W.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutton AL, MacDonald PN. Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17(5):777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 2.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 3.Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2(2):203–216. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- 4.Ishida H, Bellows CG, Aubin JE, Heersche JN. Characterization of the 1,25-(OH)2D3-induced inhibition of bone nodule formation in long-term cultures of fetal rat calvaria cells. Endocrinology. 1993;132(1):61–66. doi: 10.1210/endo.132.1.8419147. [DOI] [PubMed] [Google Scholar]
- 5.Aubin JE, Heersche JNM. Vitamin D and Osteoblasts. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D 2. Elsevier Academic Press; Burlington, MA: 2005. pp. 649–663. [Google Scholar]
- 6.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006 doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104(3):341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 9.Perrimon N, McMahon AP. Negative feedback mechanisms and their roles during pattern formation. Cell. 1999;97(1):13–16. doi: 10.1016/s0092-8674(00)80710-2. [DOI] [PubMed] [Google Scholar]
- 10.Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407(6803):527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 11.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 12.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 14.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157(2):303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberley MJ, Farnham PJ. Probing chromatin immunoprecipitates with CpG-island microarrays to identify genomic sites occupied by DNA-binding proteins. Methods Enzymol. 2003;371:577–596. doi: 10.1016/S0076-6879(03)71043-X. [DOI] [PubMed] [Google Scholar]
- 16.Glynn EF, Megee PC, Yu HG, Mistrot C, Unal E, Koshland DE, DeRisi JL, Gerton JL. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol. 2004;2(9):E259. doi: 10.1371/journal.pbio.0020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto H, Shevde NK, Warrier A, Plum LA, DeLuca HF, Pike JW. 2-Methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 potently stimulates gene-specific DNA binding of the vitamin D receptor in osteoblasts. J Biol Chem. 2003;278(34):31756–31765. doi: 10.1074/jbc.M304737200. [DOI] [PubMed] [Google Scholar]
- 18.Slater EP, Rabenau O, Karin M, Baxter JD, Beato M. Glucocorticoid receptor binding and activation of a heterologous promoter by dexamethasone by the first intron of the human growth hormone gene. Mol Cell Biol. 1985;5(11):2984–2992. doi: 10.1128/mcb.5.11.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zella LA, Kim S, Shevde NK, Pike JW. Enhancers Located within Two Introns of the Vitamin D Receptor Gene Mediate Transcriptional Autoregulation by 1,25-Dihydroxyvitamin D3. Mol Endocrinol. 2006 doi: 10.1210/me.2006-0015. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda H, Kanji Higashio, Tatsuo Suda. Vitamin D and Osteoclastogenesis. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D 2. Elsevier Academic Press; Burlington, MA: 2005. pp. 665–685. [Google Scholar]
- 21.Balsan S, Garabedian M, Larchet M, Gorski AM, Cournot G, Tau C, Bourdeau A, Silve C, Ricour C. Long-term nocturnal calcium infusions can cure rickets and promote normal mineralization in hereditary resistance to 1,25-dihydroxyvitamin D. J Clin Invest. 1986;77(5):1661–1667. doi: 10.1172/JCI112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah S, Pishvaian MJ, Easwaran V, Brown PH, Byers SW. The role of cadherin, beta-catenin, and AP-1 in retinoid-regulated carcinoma cell differentiation and proliferation. J Biol Chem. 2002;277(28):25313–25322. doi: 10.1074/jbc.M203158200. [DOI] [PubMed] [Google Scholar]


