Abstract
AMD3100 inhibits the interaction between SDF-1 and CXCR4, and rapidly mobilizes hematopoietic progenitors for clinical transplantation. However, the repopulating function of human cells mobilized with AMD3100 has not been characterized in comparison to cells mobilized with G-CSF in the same donor. Therefore, healthy donors were leukapheresed 4 hours after injection with AMD3100, after 10 days drug clearance the same donor was mobilized with G-CSF, allowing a paired comparison of repopulation by mobilized cells. Transplantation of mononuclear cells (MNC) or purified CD34+ cells was compared at limiting dilution into NOD/SCID mice. Human AMD3100-mobilized MNC possessed enhanced repopulating frequency in comparison to G-CSF-mobilized MNC from paired donors, and purified CD34+ progenitors were at least as efficient as the G-CSF mobilized cells. The frequencies of NOD/SCID repopulating cells (SRC) were 1 SRC in 8.7×106 AMD3100-mobilized MNC compared to 1 SRC in 29.0×106 G-CSF-mobilized MNC, and 1 SRC in 1.2×105 AMD3100-mobilized CD34+ cells compared to 1 SRC in 1.8×105 G-CSF-mobilized CD34+ cells. Hematopoietic differentiation of transplanted progenitors was similar after AMD3100 or G-CSF-mobilization. Thus, AMD3100 mobilized peripheral blood represents a rapidly obtained, highly repopulating source of hematopoietic progenitors for clinical transplantation.
Keywords: stem cell mobilization, AMD3100, GCSF, CD34, hematopoiesis, NOD, SCID Transplantation
Introduction
Mobilized peripheral blood (MPB) hematopoietic progenitor cell (HPC) transplantation has emerged as the preferred strategy for the treatment of hematological malignancies. Granulocyte-colony stimulating factor (G-CSF) is the “gold standard” to mobilize HPC from the BM to the peripheral blood for leukapheresis and transplantation.1,2 After 4–5 days G-CSF effectively increases the total yield of CD34+ cells available for transplantation. In comparison to bone marrow transplantation, the increased number of infused cells after G-CSF mobilization positively influenced marrow recovery and reduced the time to neutrophil and platelet production.3,4 However, G-CSF mobilization demonstrates broad inter-individual variations in circulating progenitor cell numbers.5,6 At suboptimal HPC doses hematopoietic recovery becomes significantly delayed or incomplete, and may result in prolonged neutropenia, bleeding, increased infectious complications, increased hospital stay, and even graft failure.7–10 As a consequence, alternative mobilization strategies have been sought to safely increase CD34+ cell isolation, particularly in patients heavily pretreated with chemotherapeutic agents, who fail HPC mobilization using G-CSF mobilization.11,12
Interactions between the chemokine receptor CXCR4 and its cognate ligand, CXCL12 or stromal derived factor-1α (SDF-1), regulate the survival,13–15 directed chemotaxis,16–19 and engraftment of CD34+ HPC.20,21 CXCR4/SDF-1 interaction is also involved in the retention of HSC within the BM.22,23 Thus, pharmacological interference in the axis between marrow-derived SDF-1 and CXCR4 expressed on CD34+ human HSC or HPC has been investigated as a mobilization strategy.13,20,24,25 Subsequently, AMD3100 has been shown to reversibly bind CXCR4 and inhibit the interaction between SDF-1 and CXCR4 within the BM microenvironment resulting in the mobilization of CD34+ cells into the circulation in healthy volunteers and in cancer patients who have been treated with chemotherapeutic agents.11,26 In these studies, AMD3100 was well tolerated without significant toxicity. The collection of total CD34+ cells was comparable between individuals mobilized 4 hours after a single injection of AMD3100 (240μg/kg) and individuals mobilized by a 5 day treatment regimen with G-CSF.27 In a randomized trial, the combination of G-CSF treatment with AMD3100 administration resulted in superior CD34+ cell mobilization when compared to G-CSF alone, and patients receiving AMD3100 + G-CSF autologous transplants for non-Hodgkins lymphoma and multiple myeloma demonstrated strong early engraftment with recovery of neutrophil production between days 10 and 13.12 Collectively, these studies demonstrated that AMD3100 is a safe and effective agent for the rapid mobilization of CD34+ cells in healthy donors and in patients who had received prior chemotherapy. However, these clinical studies did not address the repopulating capacity of mobilized CD34+ cells for engraftment and reconstitution potential after AMD3100 treatment alone, without G-CSF administration, and that was the goal of the current studies.
Preliminary preclinical evaluation of the engrafting capacity of AMD3100-mobilized cells has been reported recently in murine, canine, and primate transplantation models.28–30 Both autologous and allogeneic AMD3100-mobilized HPC led to immediate, long-term engraftment in dogs and rhesus macaques after lethal irradiation.28,30 Broxmeyer and colleagues showed that AMD3100 induced rapid mobilization of murine HPC and synergistically augmented G-CSF mobilization.29 AMD3100 was capable of mobilizing long-term repopulating murine stem cells that engrafted primary and secondary recipients. In studies performed with human cells, the greatest number of NOD/SCID repopulating cells (SRC) were found in donors mobilized by a combination of 5 days G-CSF followed by one injection of AMD3100, as compared to the traditional regimen of 5 days G-CSF alone.29 However, these studies were normalized for the number of SRC per kilogram donor body weight and did not include human cell chimerism information for donors mobilized with AMD3100 alone, which we assessed in the current studies. In rhesus macaques, CD34+ cells mobilized by AMD3100 expressed higher CXCR4 and VLA-4 expression, and demonstrated increased levels of gene marking than GCSF mobilized samples. These data demonstrate that AMD3100 mobilized HPC with intrinsically different characteristics than HPC mobilized with G-CSF.24
In the current studies, we directly compared the NOD/SCID repopulating function of cells from the same normal donors who were rapidly mobilized (4 hours) by AMD3100 versus cells mobilized after 5 days of G-CSF treatment. The SRC values for both CD34+ cells and total MNC from the mobilized products were analyzed for each donor using limiting dilution and Poisson statistics. A total of 6 donors were leukapheresed after a single injection of 240ug/kg AMD3100. After 10 days of drug clearance, the same donor was mobilized with G-CSF, allowing a paired comparison of the repopulating function of cells mobilized by the two agents. The studies demonstrated that human AMD3100 purified MNC possess enhanced repopulating frequency, on a cell-per-cell basis, as compared to G-CSF mobilized cells from the same donor. However, purified CD34+ cells from AMD3100-mobilized peripheral blood demonstrated a more modest increase in repopulating function. Thus, AMD3100-mobilized peripheral blood represents a rapidly obtained and highly functional source of repopulating hematopoietic stem cells for clinical transplantation procedures.
Materials and Methods
Donor selection and leukapheresis
Healthy donors for allogeneic transplantation were accrued as part of a clinical trial evaluating the utility of AMD3100 for the mobilization and transplantation of HPC in patients with advanced malignancies. Clinical samples were obtained at the Washington University School of Medicine, St Louis, MO. according to protocols approved by the Human Subjects Committee. Donors received one subcutaneous injection of 240μg/kg AMD3100 (AnorMED, Langley, BC), followed by leukapheresis beginning 4–6 hours after drug treatment. 10–15 mL of the total leukapheresis product (representing <2–3% of the total leukapheresis product) was reserved for cellular processing and transplantation experiments. After 10 days of drug clearance, the same donors were mobilized with 5 days subcutaneous injection of 10μg/kg/day G-CSF, and leukapheresed on day 5.
Human cell purification
Blood and leukapheresis aliquots for experiments were processed immediately after harvest. Erythrocyte contamination was depleted by washing in human red cell lysis buffer (0.8% ammonium chloride solution, Stem Cell Technologies, Vancouver, BC, Canada), then cells were counted and divided for MNC transplantation or further purification of CD34+ progenitors. Human CD34+ cells were purified by positive selection with a Magnetic Affinity Cell Selection (MACS) CD34 isolation kit (Miltenyi Biotec, Auburn, CA). The purity of isolated cells was quantified by FACS analysis (>86% for all experiments).
Flow cytometry analysis
Before and after CD34 cell selection cells were stained with anti-human monoclonal antibodies for flow cytometric analysis of cell surface markers expressed on primitive human hematopoietic stem cell subsets, as well as T-lymphocyte, B-lymphocyte and myeloid subsets. 2–4 parameter flow cytometric analysis was performed on a Coulter FC-500 flow cytometer (Beckman-Coulter, Miami, FL) and analyzed using Cytomics RXP analysis software. To prepare cells for flow cytometric analysis, harvested MNC or freshly purified CD34+ cells were blocked using CD16/32w FcIII-receptor blocker (Pharmingen). Relevant isotype controls against human IgG1/2a (X0969, DakoCytomation, Glostrup, Denmark) were performed on all samples. The following anti-human monoclonal antibodies were used for phenotype analysis of human progenitor cells (CD34-FITC, CD38-PE, and CD133-APC (Miltenyi Biotechnology), human T-lymphocytes (CD4-FITC, and CD8-PE, CD3-APC,), human B-lymphocytes (CD20-FITC and CD19-PE), and human myeloid cells (CD14-FITC, CD33-PE), (all antibodies were from Beckton Dickinson, San Jose, CA unless otherwise indicated).
Transplantation and analysis of human cell engraftment
NOD/SCID mice were obtained from Jackson Laboratories, Bar Harbor, ME, and were bred at the animal facilities of the Washington University School of Medicine. The Animal Studies Committee approved all animal experiments. Human mobilized peripheral blood MNC and purified CD34+ cells were transplanted by tail vein injection into 8–10 week, sublethally irradiated (300cGy) NOD/SCID mice. A range of human MNC (106 – 40×106 cells) or purified CD34+ cells (2×104 – 1×106 cells) were injected into quadruplicate mice at a minimum of 3 different doses per donor to allow direct statistical comparison of human cell engraftment between AMD3100 and G-CSF mobilization at limiting dilution. 7–8 weeks post-transplantation, BM, spleen, and peripheral blood were harvested and labeled using monoclonal antibodies for the human pan-leukocyte marker CD45-APC, in combination with CD38-PE or isotype controls (BD) as previously described.31,32 Cells were analyzed on a Coulter FC-500 flow cytometer (Beckman-Coulter). An engrafted mouse for SRC frequency quantification was defined as 0.2% human CD45+/CD38+ co-expressing cells and >10,000 events were recorded per mouse. Low levels of human engraftment (0.2%) were confirmed by P17H8 PCR.32 Engrafted mouse BM (>2% CD45+) was further analyzed for the frequency of B-lymphoid cells (CD20-FITC, CD19-PE), myeloid cells (CD14-FITC, CD33-PE), T-lymphoid cells (CD4-FITC, CD8-PE), and primitive cells (CD34-FITC, CD38-PE) (all antibodies, Beckton Dickinson).
Statistics
Levels of human engraftment were reported as the mean ± SEM for mice grouped according to transplanted cell numbers and compared using a student’s t test. SRC analysis was performed using the single hit model and Poisson statistics at limiting dilution with 95% confidence intervals. Statistical analysis software was provided by Stem Cell Technologies, Vancouver, BC, Canada.
Results
Mobilization of human leukocytes and CD34+ cells by AMD3100 vs. G-CSF mobilization in paired healthy volunteers
Liles et al,26 followed by Broxmeyer et al,29 were the first to report that functional human hematopoietic progenitor cells (HPC) were effectively mobilized in healthy volunteers within 4–6 hours after injection of AMD3100. These investigators and others have augmented mobilization and collection of CD34+ cells when G-CSF treatment was combined with a single injection of AMD3100.12,27We directly assessed the engraftment capacity of AMD3100 and G-CSF leukapheresis products obtained following sequential mobilization by both agents in the same healthy individual. The mobilization protocol is illustrated in Figure 1.
Figure 1. Study design for the treatment of healthy human volunteers with AMD3100 followed by G-CSF.

Normal donors (n=6) were recruited for allogeneic BM transplantation and HPC were mobilized using both AMD3100 and G-CSF administration in the same individual. Baseline peripheral blood samples were drawn from each healthy donor prior to AMD3100 injection for CBC counts and differential. Each donor was administered 240mg/kg AMD3100 at the beginning of the study protocol and leukapheresis was collected 4 – 6 hours after injection. After a 10 day washout period, the same donors were mobilized with 5 days administration of G-CSF at 10mg/kg/day. Leukapheresis products were then collected on the 5th day of rhG-CSF administration. Leukapheresis products from both leukapheresis protocols were analyzed for the mobilization of total leukocytes and total CD34 cells. An aliquot from each leukapheresis product (10–15mL) from each donor were collected for phenotypic analysis of HPC markers, CD34+ cell purification and for the quantification of SRC frequency after transplantation into sublethally irradiated NOD/SCID mice. This protocol design allowed for a direct comparison of HPC mobilized by each agent in the same individual.
AMD3100 and G-CSF administration both induced a significant increase in blood leukocyte counts (WBC), in comparison to baseline controls (*p<0.001) (Table 1). Total blood leukocyte counts were increased 3-fold from baseline in peripheral blood within 4 hours of AMD3100 administration (6.5±0.3×103 versus 19.1±0.6×103 WBC/mL), whereas 5 days of G-CSF administration induced a greater than 5-fold increase from baseline (7.0±0.5×103 versus 39.0±3.0×103 WBC/mL) (*p<0.001). Overall, G-CSF mobilized 2-fold higher numbers of leukocytes, as compared to AMD3100 administration (p<0.001). Red blood cell, platelet and hemoglobin concentrations were not altered from baseline by AMD3100 or G-CSF administration (data not shown). As expected, CBC differentials also indicated an increased frequency of neutrophils (p<0.05) in G-CSF leukapheresis, with a concomitant decrease in lymphocyte frequencies (data not shown). However, AMD3100 administration did not result in an increased frequency of granulocyte or neutrophil mobilization. The rapid mobilization demonstrated by AMD3100 is advantageous and cost effective in a clinical setting.
Table 1.
Blood cell counts, total CD34 cell enumeration, and SRC frequencies from individual donors after administration of AMD3100 (240μg/kg) or G-CSF (10μg/kg/day).
| Donor # | Mobilizing agent | WBC, ×103 / mL | Total CD34+, ×106 cells/kg | Total CD34+, cells/μL | SRC frequency, ×106 MNC | 95% confidence, ×106 MNC | SRC frequency, ×103 CD34+ | 95%confidence, ×103 CD34+ | |
|---|---|---|---|---|---|---|---|---|---|
| 0 hours | At Leuk. | ||||||||
| 1 |
AMD3100
G-CSF |
7.6
9.2 |
17.8
40.1 |
1.05
1.72 |
260
350 |
1 in 3.5
1 in 7.3 |
1.4–9.1
3.4-15.8 |
1 in 79
1 in 101 |
30 – 211
44 – 229 |
| 2 |
AMD3100
G-CSF |
5.5
5.7 |
21.1
45.2 |
0.40
2.50 |
320
1210 |
1 in 34.0
*0% eng. |
10.5–110.6
*0% eng. |
1 in 55
*0% eng. |
18 – 174
*0% eng. |
| 3 |
AMD3100
G-CSF |
5.6
6.3 |
18.9
30.0 |
1.49
3.29 |
470
970 |
1 in 7.9
1 in 69.0 |
3.0–20.6
16.6–286.2 |
1 in 40
1 in 234 |
9 – 178
93– 590 |
| 4 |
AMD3100
G-CSF |
7.1
7.8 |
19.7
43.3 |
4.56
ND |
1290
ND |
**100% eng.
***ND |
**100% eng.
***ND |
1 in 35
***ND |
12 – 101
***ND |
| 5 |
AMD3100
G-CSF |
7.0
6.5 |
19.5
45.8 |
6.25
18.7 |
2510
4690 |
1 in 7.2
1 in 19.8 |
2.9–17.6
8.0–48.7 |
1 in 158
1 in 97 |
67 – 378
39 – 242 |
| 6 |
AMD3100
G-CSF |
6.1
6.6 |
17.4
29.4 |
1.97
5.01 |
720
1140 |
1 in 11.0
****ND |
6.9–17.6
****ND |
1 in 104
1 in 109 |
64 – 172
61 – 179 |
| Mean |
AMD3100
G-CSF |
6.5±0.3
7.0±0.5 |
*19.1±0.6
*39.0±3.0 |
2.9±1.0
6.2±3.2 |
928±351
1672±768 |
*1 in 8.7
1 in 29.0 |
6.0–12.7
17.5–47.9 |
1 in 117
1 in 177 |
75 – 181
113–275 |
ND = not determined, Eng. = engrafted
0% eng. G-CSF MPB MNC (n=10) and purified CD34+ cells (n=10) from donor 2 produced no engraftment in transplanted animals. Thus, calculation of SRC frequency could not be performed.
100% eng. AMD3100 MPB MNC from donor 4 showed significant human cell engraftment (>1.5% CD45+ and CD38+) in 100% of transplanted mice (dose range = 5×106–40×106 MNC, n=9). Thus, calculation of SRC frequency by Poisson statistics could not be performed.
ND. After G-CSF administration, donor 4 required a catheter for leukapheresis and G-CSF MPB was not used for animal experiments.
ND. After G-CSF administration, donor 6 leukapheresis was used for CD34+ cell selection only.
Blood for CBC counts was drawn from each donor before drug administration and just prior to leukapheresis (4 hours after injection of AMD3100 and on Day 5 of G-CSF treatment). CD34+ cell enumeration was performed on collected leukapheresis product. AMD3100 and G-CSF administration induced a significant increase in blood leukocyte counts (WBC) as compared to baseline controls (*p<0.001). Red blood cells (RBC), hemoglobin, platelets, and hematocrits were not altered by AMD3100 or G-CSF administration. Total leukocyte and total CD34+ cell mobilization was increased approximately 2-fold in G-CSF versus AMD3100 leukapheresis. For each paired donor, sublethally irradiated (300cGy) NOD/SCID mice were transplanted using a minimum of 3 cell concentrations for MNC (doses= 1×106–40×106 cells) and purified CD34+ cells (doses = 0.2×105–106 cells) at limiting dilution using 3–5 mice per dose. SRC frequencies and 95% confidence intervals were calculated by Poisson statistics using the single hit model. Mice were considered engrafted if the level of human cell engraftment in the murine BM was >0.2% CD45+ and CD38+. Human cell engraftment was confirmed by PCR for human DNA sequences (P17H8) for mice demonstrating 0.1% human cells by flow cytometry.
Determination of total CD34+ cell number and function allows prediction of the rate of hematopoietic recovery in transplanted individuals. Table 1 compares the mobilization of total CD34+ cells harvested from paired healthy donors, allowing for a direct comparison of mobilization efficiency for both agents in the same individual. Total CD34+ cell mobilization was 2-fold higher in the G-CSF leukapheresis product (6.2±3.2×106 CD34+ cells/kg or 1672±768 CD34+ cells/μL), as compared to the AMD3100 leukapheresis product (2.9±1.0×106 CD34+ cells/kg or 928±351 CD34+ cells/μL). Although these data were not statistically significant in our study design, the modest increase in CD34+ cell mobilization by G-CSF may reduce the requirement for secondary leukapheresis procedures by assuring optimal accrual of target HPC (>5×106 CD34+ cells/kg body weight) previously associated with rapid neutrophil recovery in transplanted patients.5,6 However, CD34+ cells from alternate sources (BM or UCB), as well as CD34+ cells mobilized by different agents may demonstrate widely variable repopulating functions in vivo.33,34 Thus we performed a detailed comparison of the NOD/SCID repopulating function of total MNC and purified CD34+ cells collected from the same donors by both mobilization regimens.
Expression of primitive HPC cell surface markers is similar in AMD3100 and G-CSF leukapheresis
Prior to the transplantation of MPB into immune deficient NOD/SCID mice, we performed a detailed cell surface marker analysis of HPC collected by AMD3100 or G-CSF treatment, to directly compare the frequencies of primitive hematopoietic cells contained within injected cohorts. Figure 2 shows the flow cytometric analysis of lysed leukapheresis product MNC (Fig. 2A-D), or purified CD34+ cells (Fig. 2E-H) for the cell surface expression of primitive hematopoietic markers CD34 with CD133, or CD34 with CD38. These markers represent the gold standard for predicting NOD/SCID repopulating phenotype in vivo.32,35–38 CD34 was expressed on approximately 0.5–1.0% of total leukocytes after AMD3100 or G-CSF administration. There was no difference in the frequency of CD34 expression between paired AMD3100 and G-CSF mobilized samples. Likewise, the percentages of co-expression of CD34 with or without CD133, or CD34 with or without CD38 were similar after comparison of pooled data from n=5 paired individual donors. However, G-CSF leukapheresis contained a significantly increased (p<0.05) frequency of primarily differentiated CD34−CD38+ cells,32 indicating that the increased total yield of CD34+ cells after G-CSF administration was due to the maintenance of CD34+ cell frequency combined with increased overall sample cellularity (Table 1).
Figure 2. Comparison of primitive hematopoietic cell surface marker expression on AMD3100 and G-CSF mobilized cells.
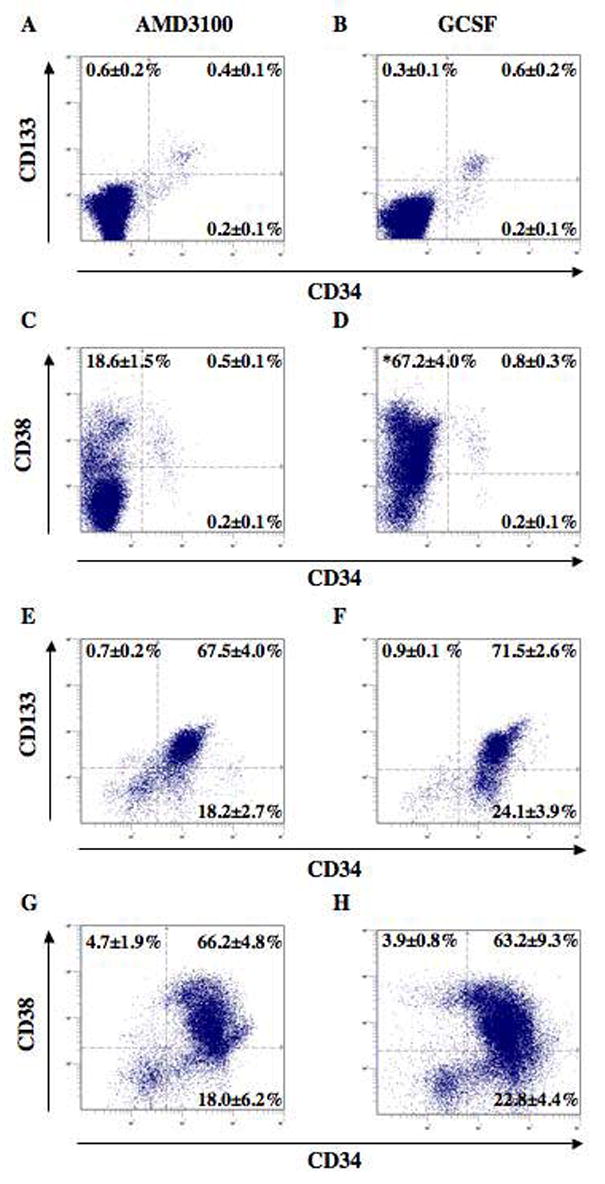
Paired human MPB MNC or purified CD34+ cell populations were analyzed for the co-expression of CD34 with CD133, or CD34 with CD38 prior to functional analysis. (A–D) Unpurified leukapheresis MNC demonstrated a low frequency of CD34+ cells (<1%). Comparison of AMD3100 versus G-CSF mobilized cells demonstrated no differences in the frequencies of cells expressing primitive phenotypic markers. However, G-CSF leukapheresis products (n=5) contained a significantly greater (p<0.01) frequency of mature CD34−CD38+ cells, as compared to AMD3100 leukapheresis products (n=5). (E–H) After CD34 positive selection, the purified cells were >86% CD34+. CD34-purified cells showed a high proportion of CD38+ progenitors and cells with primitive (CD34+CD38− or CD34+CD133+) phenotypes. Comparison of AMD3100 versus G-CSF mobilized CD34+ cells demonstrated no significant differences in the frequencies of cells expressing primitive phenotypic markers. Phenotypic analysis of purified CD34+ cells was performed after AMD3100 and G-CSF mobilization in paired individuals (n=3).
Positive selection of cells expressing the CD34 surface antigen resulted in greater than 86% purity, confirmed by flow cytometry for processed samples from each paired donor (range 86–96% CD34+ cells, n=5). In addition, there were no comparative differences in the frequency of cells expressing CD34, CD133, or CD38 after CD34+ cell selection in paired AMD3100 and G-CSF mobilized samples (Figure 2, E–H). Thus, transplanted purified CD34+ cells mobilized by AMD3100 or G-CSF administration displayed equivalent phenotypes at the time of injection into NOD/SCID mice. This allowed a direct functional comparison of the repopulating capacity of MNC and CD34+ cells harvested after AMD3100 or G-CSF mobilization in each donor.
NOD/SCID repopulating cells are present at a significantly higher frequency in AMD3100 mobilized, as compared to G-CSF mobilized peripheral blood mononuclear fractions from the same donor
Transplantation of human stem cell populations at limiting dilution into immune deficient mice is used as a quantitative assay to calculate the relative frequency of primitive NOD/SCID repopulating cells (SRC) using Poisson statistics with the single hit model.32,33,36,37,39 Wang et al. used these statistical methods to calculate the relative frequencies of stem cells isolated from human UCB (1 SRC in 9.3×105 cells), bone marrow (1 SRC in 3.0×106 cells), and from G-CSF mobilized peripheral blood (1 SRC in 6.0×106 cells) after intravenous transplantation.33 Likewise, this standardized assay has been used to compare stem and progenitor cell repopulating frequencies from various stem cell subpopulations using a variety of purification methods exploiting cell surface markers and functional activities.32,34,39 Here, we employed the quantitative SRC assay to compare the frequency of repopulating cells from AMD3100 and G-CSF mobilized samples for unfractionated leukapheresis MNC and purified CD34+ cells from 6 paired individuals receiving each mobilizing agent as described in Figure 1. In order to optimize analysis using Poisson statistics, sublethally irradiated (300cGy) NOD/SCID mice were transplanted by intravenous injection at a minimum of 3 cell concentrations for MNC (doses = 1×106 – 40×106 cells) and CD34 purified cells (doses = 0.2×105 – 1×106 cells) using 3–5 mice per dose. This strategy achieved successful human engraftment in multiple mice, without a situation where 0% or 100% of the mice were engrafted, a circumstance where Poisson statistics cannot be performed.
Figure 3 demonstrates the flow cytometric detection of human cell engraftment in the BM of mice injected with 2×107 MNC from AMD3100 and G-CSF MPB from donor 1. Injected mouse BM, spleen and peripheral blood were analyzed for human cell engraftment 7–8 weeks after transplantation. Murine samples were analyzed for the expression of the human pan leukocyte marker CD45 (Fig. 3A, R1) and for the expression of the human leukocyte differentiation marker CD38 (Figure 3B, R2). R1 and R2 frequencies were <0.1% in non-engrafted controls (Fig. 3, insets). The overall level of human cell engraftment was quantified by flow cytometry for the co-expression of CD45 and CD38. An engrafted mouse was defined as 0.2% human CD45+/CD38+ cells. BM repopulation was significantly increased (*p<0.05) in mice transplanted with 2×107 MNC from AMD3100 MPB (1.8±0.5%, n=5) (Fig. 3A–C), in comparison to an equivalent dose from G-CSF MPB (0.2±0.1%, n=5) (Fig. 3, D–F). The increased level of engraftment of transplanted AMD3100 cells corresponds to the increased in vitro progenitor capacity of cells mobilized by AMD3100 in comparison to G-CSF that has been previously reported.26,29
Figure 3. Detection of human cell engraftment in the BM of NOD/SCID mice transplanted with MNC from AMD3100 or G-CSF MPB.

Representative flow cytometric analysis of NOD/SCID mice transplanted with 2×107 MNC after AMD3100 (A–C) or G-CSF mobilization (D–F) from donor 1. Mouse BM, spleen and peripheral blood samples were harvested 7–8 weeks after transplantation. Human cell engraftment in BM was detected by flow cytometry using the co-expression of the pan-leukocyte marker CD45 (R1) and human CD38 (R2). R1 and R2 frequencies were <0.1% in non-engrafted controls (insets). BM engraftment was increased (*p<0.05) in mice transplanted with 2×107 MNC from AMD3100 MPB (1.8±0.5%, n=5) as compared to an equivalent dose from G-CSF MPB (0.2±0.1%, n=5). (G) Comparison of human engraftment in the BM of NOD/SCID mice transplanted with AMD3100 MPB MNC (n=15,
 ) or G-CSF MPB MNC (n=15,
) or G-CSF MPB MNC (n=15,
 ) isolated from the same donor. Mice were considered engrafted if the frequency of human cells was ≥0.2% CD45+ and CD38+. Poisson statistics were performed on mouse engraftment data to calculate the SRC frequency at limiting dilution for cells derived from AMD3100 versus G/CSF mobilization in each donor.
) isolated from the same donor. Mice were considered engrafted if the frequency of human cells was ≥0.2% CD45+ and CD38+. Poisson statistics were performed on mouse engraftment data to calculate the SRC frequency at limiting dilution for cells derived from AMD3100 versus G/CSF mobilization in each donor.
A total of 30 mice were transplanted with MNC from donor 1, with cell doses ranging from 1×106 to 20×106 cells isolated after AMD3100 (n=15) and G-CSF (n=15) mobilization (Fig. 1G). Mice were considered engrafted if the frequency of human cells was >0.2% CD45+ and CD38+. Transplantation of 106, 107 or 2×107 AMD3100-mobilized MNC produced engraftment in 3 of 5 mice, 4 of 5 mice, and 5 of 5 mice respectively, corresponding to a NOD/SCID repopulating cell (SRC) frequency of 1 SRC in 3.5×106 cells by Poisson statistics (Table 1). Mice that did not receive a full dose by tail vein injection were excluded from the analysis. Similar to previously reported SRC frequency,27 equivalent doses from G-CSF MPB produced an SRC frequency of 1 SRC in 7.3×106 cells. Although the calculated SRC frequencies were not statistically different using 95% confidence intervals for this donor (Table 1), these analyses provided a representative example for the in vivo functional comparison of HPC isolated after mobilization with AMD3100 and G-CSF in the same individual. Similar analysis was performed using the MNC from 6 individual donors, and pooled engraftment data from paired donors provided greater statistical power and an overall SRC frequency (Table 1). Although SRC frequencies by Poisson statistics could not be performed for donor 4 and 6, AMD3100-mobilized MNC consistently repopulated NOD/SCID mice with high efficiency (Table 1).
Figure 4 shows a similar analysis performed on purified CD34+ cells isolated from the samples obtained from donor 1. CD34+ cells were isolated from each donor using magnetic adherence cell sorting (MACS) and were >86% CD34+ (range 86 – 96% CD34+ for each donor analyzed). Similar to the engraftment results obtained with donor 1 MNC, BM repopulation was significantly enhanced (*p<0.05) in mice transplanted with 5×105 CD34+ cells from AMD3100 MPB (3.7±0.4%, n=5) (Fig. 4A–C) as compared to an equivalent dose from G-CSF MPB (0.8±0.5%, n=5) (Fig. 4, D–F). Overall engraftment results for CD34+ cell transplantation into NOD/SCID mice (dose range = 0.5×105 – 5×105 CD34+ cells) were similar for AMD3100 (1 SRC in 7.9×104 CD34+ cells, n=12) and G-CSF (1 SRC in 105 CD34+ cells, n=15) mobilized MPB from donor 1 (Table 1). Purified CD34+ cell transplantation from each donor produced more variable SRC frequencies (Table 1). These results highlight the degree of donor-to-donor variability in the overall repopulating function of human CD34+ cells mobilized by AMD3100 or by G-CSF.
Figure 4. Detection of human cell engraftment in the BM of NOD/SCID mice transplanted with CD34+ cells from AMD3100 or G-CSF MPB.
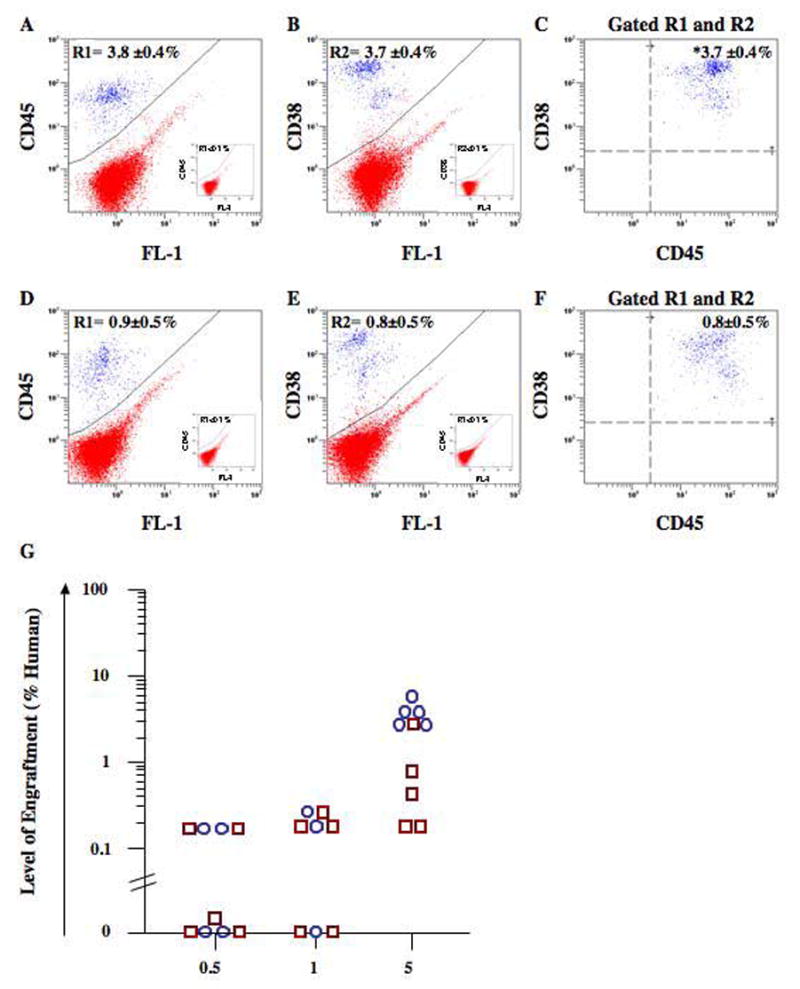
Representative flow cytometric analysis of NOD/SCID mice transplanted with 5×105 purified CD34+ cells after AMD3100 (A–C) or G-CSF mobilization (D–F) from donor 1. Mouse BM, spleen and peripheral blood were harvested 7–8 weeks after transplantation and human cells were detected by flow cytometry using the co-expression of the pan-leukocyte marker CD45 (R1) and human CD38 (R2). R1 and R2 frequencies were <0.1% in non-engrafted controls (insets). BM engraftment was increased (**p<0.01) in mice transplanted with 5×105 CD34+ cells isolated from AMD3100 MPB (3.7±0.4%, n=5) as compared to G-CSF MPB (0.8±0.5%, n=5). (G) Comparison of human engraftment in the BM of NOD/SCID mice transplanted with AMD3100 MPB CD34+ cells (n=12,
 ) or G-CSF MPB CD34+ cells (n=15,
) or G-CSF MPB CD34+ cells (n=15,
 ) isolated from the same donor. Mice were considered engrafted if the frequency of human cells was ≥0.2% CD45+ and CD38+. Poisson statistics were performed on mouse engraftment data to calculate the SRC frequency at limiting dilution for cells derived from AMD3100 versus G-CSF mobilization in each donor.
) isolated from the same donor. Mice were considered engrafted if the frequency of human cells was ≥0.2% CD45+ and CD38+. Poisson statistics were performed on mouse engraftment data to calculate the SRC frequency at limiting dilution for cells derived from AMD3100 versus G-CSF mobilization in each donor.
AMD3100 MPB CD34+ cells demonstrate multilineage differentiation in vivo
Chimeric murine/human BM resulting from the transplantation of CD34+ cells from AMD3100 and G-CSF mobilized MPB was analyzed for the expression of human-specific, lineage-restricted cell surface markers (Fig. 5). Transplantation of 5×105 purified CD34+ cells produced human engraftment averaging 14.7±5.5% CD45+ (n=5) in a subset of mice, indicating that these cells demonstrated extensive proliferation in vivo. Gated human CD45+ cells (R1, Fig. 5A) demonstrated surface markers for cells of the B-lymphoid (Fig. 5C) and myeloid (Fig. 5D) lineages, a typical development pattern in the NOD/SCID strain. Cells with co-expression of CD14 with CD33 (10.2±1.0%) and CD20 with CD19 (15.2±4.1%) confirmed the presence of maturing human monocyte/macrophages and B-lymphocytes, respectively. Although human cells expressing CD4 were detected (14.5±2.0%, Fig. 5E), CD8 expression was absent, demonstrating the well documented lack of mature T lymphocyte development in NOD/SCID mice.36,39,40 This pattern of predominantly B-lymphoid ( 70%) and myeloid ( 30%) cell differentiation in NOD/SCID mice was similar for transplanted G-CSF MPB cells in previously reported studies,33,34 suggesting that AMD3100-mobilized HPC demonstrate normal hematopoietic differentiation in vivo.
Figure 5. Multilineage differentiation of transplanted human CD34+ cells mobilized with AMD3100.
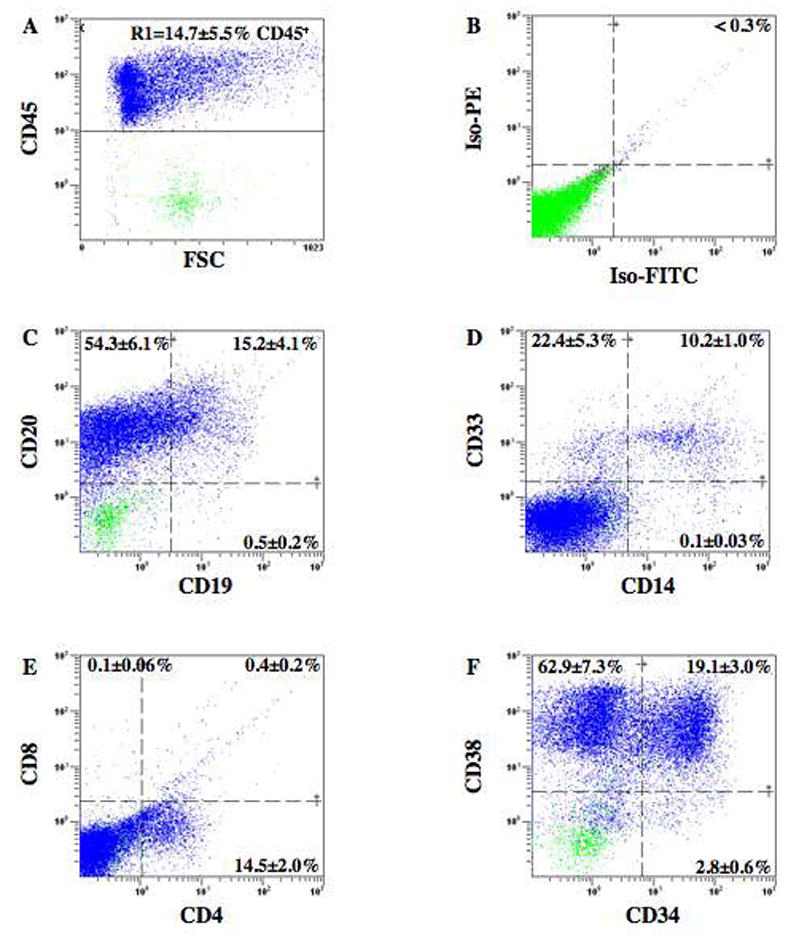
BM from a mouse transplanted with 5×105 AMD3100 mobilized CD34+ cells was stained with antibodies for markers expressed on mature human hematopoietic and primitive progenitor cells. (A) Human hematopoietic cells were selected by the expression of CD45 (R1) and analyzed for isotype controls (B), B-cell markers CD19 and CD20 (C), myeloid cell markers CD33 and CD14 (D), T-cell markers CD4 and CD8 (E), maintenance of human progenitors by CD34 and CD38 expression (F). Data represents the mean±SEM expression on human cells derived from murine BM (n=5).
To assess whether the AMD3100 and G-CSF-mobilized CD34+ cells maintained primitive cell phenotypes after transplantation, we compared engrafted human cells from the bone marrow of the mice for the co-expression of the primitive cell surface marker CD34 with CD38 (Fig. 5). Representative analyses of CD45+ human cells expressing these primitive cell surface markers are shown in Figure 5F. As is typical in the NOD/SCID mouse strain transplanted with primitive progenitors from any source, transplantation of AMD3100-mobilized CD34+ cells resulted primarily in the generation of maturing, CD34−CD38+ cells (62.9±7.3%) and committed CD34+CD38+ progenitors (19.1±3.0%). Primitive, undifferentiated hematopoietic cells were also represented phenotypically by CD34+CD38− (2.8±0.6%) expression. Thus, transplanted AMD3100 MPB CD34+ cells maintained primitive marker expression in transplanted NOD/SCID mice. These data demonstrate that AMD3100 rapidly and effectively mobilized a heterogeneous mixture of proliferative progenitors as well as more primitive SRC. Presumably, from the nature of the mobilization, the mobilized cells had expressed the SDF-1 receptor CXCR4. Cells with the phenotype CD34+/CXCR4+ have been shown to demonstrate efficient BM homing after irradiation and to correlate well with efficient neutrophil recovery and long-term graft survival in transplanted patients.1,5,20,23,41
AMD3100-mobilized MNC possess enhanced SRC capacity as compared to G-CSF-mobilized cells
Mouse engraftment data from all transplanted donors (donors 1–6) were compiled in order to allow a statistical comparison of overall SRC function between AMD3100 and G-CSF MPB. Figure 6 demonstrates human engraftment in the BM of NOD/SCID mice transplanted with MNC (closed symbols) from AMD3100 MPB (n=61) and G-CSF MPB (n=46)(Fig. 6A), or transplanted with purified CD34+ cells (open symbols) from AMD3100 MPB (n=58) and G-CSF MPB (n=56)(Fig. 6B). For the transplantation dose of 107 human MNC, AMD3100 MPB MNC showed higher levels of human repopulation (3.8±1.6%, n=15) compared to G-CSF MPB MNC (0.3±0.1%, n=7)(Figure 6A). In addition, AMD3100 MPB MNC (1 SRC in 8.7×106 MNC) demonstrated a 3-fold increase in SRC frequency when directly compared to G-CSF MPB MNC (1 SRC in 29.0×106 MNC), and represents a statistically significant increase in repopulating ability for AMD3100 MPB MNC using 95% confidence intervals (Table 1). The enhanced engraftment characteristics were obtained despite a decrease in peripheral blood total leukocyte and CD34+ cell counts when compared to G-CSF mobilization.
Figure 6. Summary of human cell repopulation in the BM of NOD/SCID mice transplanted with MNC after AMD3100 or G-CSF mobilization.

(A) Summary of the level of human engraftment in the BM of NOD/SCID mice transplanted with MNC after AMD3100 (n=61,
 ) or G-CSF (n=46,
) or G-CSF (n=46,
 ) mobilization from donors 1–6. (B) Summary of the level of human engraftment in the BM of NOD/SCID mice transplanted with purified CD34+ cells from AMD3100 (n=58,
) mobilization from donors 1–6. (B) Summary of the level of human engraftment in the BM of NOD/SCID mice transplanted with purified CD34+ cells from AMD3100 (n=58,
 ) or G-CSF (n=56,
) or G-CSF (n=56,
 ) MPB from donors 1 – 6. G-CSF leukapheresis from donor 4 was not transplanted into NOD/SCID mice. Mouse BM was extracted 7–8 weeks after transplantation and human cell engraftment was detected by co-expression of human CD45 and human CD38. Mice were considered engrafted if the frequency of human cells was ≥0.2% CD45+ and CD38+. Poisson statistics were performed on pooled mouse engraftment data to calculate SRC frequencies at limiting dilution for cells derived from AMD3100 versus G/CSF mobilization.
) MPB from donors 1 – 6. G-CSF leukapheresis from donor 4 was not transplanted into NOD/SCID mice. Mouse BM was extracted 7–8 weeks after transplantation and human cell engraftment was detected by co-expression of human CD45 and human CD38. Mice were considered engrafted if the frequency of human cells was ≥0.2% CD45+ and CD38+. Poisson statistics were performed on pooled mouse engraftment data to calculate SRC frequencies at limiting dilution for cells derived from AMD3100 versus G/CSF mobilization.
For transplanted purified CD34+ cells, AMD3100 MPB demonstrated at least equivalent repopulating levels and frequency when compared to G-CSF MPB. The calculated SRC frequency using pooled engraftment data from all donors was 1 SRC in 1.17×105 CD34+ cells for AMD3100 MPB, compared with 1 SRC in 1.77×105 CD34+ cells for G-CSF MPB. Although there was considerable overlap in the 95% confidence intervals for the overall repopulating function of purified CD34+ cells mobilized by these two agents, CD34+ cells isolated after AMD3100 mobilization possessed strong repopulating capacity that was at least equivalent to CD34+ cells mobilized by G-CSF.
Discussion
The development of AMD3100 as a rapid and efficient mobilization agent used in allogeneic and/or autologous transplantation procedures for hematopoietic malignancies has generated considerable clinical interest. The beneficial feature of this agent is the rapid egress of HPC from the BM into the periphery.26,42 In contrast to growth factor (G-CSF) based mobilization, which requires up to 4 days of repeated therapy before significant increases in circulating CD34+ cells are observed, AMD3100 significantly mobilized CD34+ cells and HPC maximally within 6 hours of single administration.26 AMD3100 was well tolerated in preliminary clinical trials, with minimal drug toxicities and donor side effects, and can be successfully used in patients with malignant disease that fail to achieve significant mobilization after G-CSF administration.11 Although data generated recently in murine, canine, and primate models suggests that there is no deleterious effects of prior treatment of AMD3100 on engraftment.28,29,30 HPC mobilization by AMD3100 exploits inhibition of the SDF-1/CXCR4 axis, a key pathway involved in the BM homing and migratory capacity of transplanted HPC.2,21,23 Therefore, determining whether CD34+ HPC mobilized by AMD3100 will possess potent engraftment following transplantation warrants further investigation with human cells in preclinical models and clinical trials.
In this study we exploited a unique clinical design where healthy donors were first leukapheresed 4–6 hours after AMD3100 administration, and were subsequently mobilized with 5 days of treatment with G-CSF two weeks after the initiation of the trial (Figure 1). G-CSF mobilized leukapheresis was acquired as an additional source of HPC for patients undergoing transplantation with AMD3100 MPB isolated from healthy matched sibling donors for allogeneic transplantation. A similar trial design has recently been used to compare endothelial progenitor function from the same individual mobilized with AMD3100 or G-CSF treatment.43 Additive or synergistic effects incurred by the use of two mobilization strategies in the same donor were minimized by the 10 day washout period before G-CSF treatment.27 Although we did not measure peripheral blood CD34+ cell counts at later time points following AMD3100 injection, Liles et al showed that circulating CD34+ progenitors as well as AMD3100 plasma concentration returned to baseline within 24 hours.26,44 Although SRC function was compared on cells mobilized by AMD3100 first and GCSF treatment second, mobilization of CD34+ cells by the secondary G-CSF treatment (documented in table 1) did not differ from routine G-CSF mobilization.34 In a similar trial, patients administered G-CSF for 4 cycles in rapid succession (10 day washout period between cycles), showed consistent mobilization of WBC and CD34+ cells after each cycle, suggesting that prior conditioning with a mobilizing agents did not affect subsequent mobilization.45 The major advantage of our study design was the ability to directly compare the NOD/SCID repopulating function of AMD3100 MPB to that of G-CSF MPB in a paired manner highlighting variation by cells isolated from the same donor.
We selected the NOD/SCID xenotransplantation model exclusively for the comparison of repopulating cell capacity after AMD3100 or G-CSF mobilization. NOD/SCID mice represent the most highly studied immune deficient animal for quantitative comparison of HPC, and stringently engraft only primitive human hematopoietic stem cells (SRC) while not engrafting with more committed progenitors that contribute to engraftment in the NOD/SCID/B2M null strain.33,36 Total leukocyte and CD34+ cells/μl leukapheresed were two-fold lower than the levels obtained by 5 days administration of G-CSF in the same individual. At the same time AMD3100 mobilized MNC demonstrated an overall 3-fold increase in repopulating frequency after transplantation into immune-deficient NOD/SCID mice. Therefore, AMD3100-mobilized leukapheresis MNC collected 4 – 6 hours after drug administration contained a higher frequency of SRC on a cell-per cell basis, and at least equivalent total SRC numbers, as compared to G-CSF-mobilized leukapheresis products. In addition, drug inhibition of CXCR4 appeared short-lived, and did not significantly interfere with the overall engraftment of human cells in the murine BM.
For transplanted purified CD34+ cells, AMD3100 MPB demonstrated at least equivalent repopulating frequency as compared to G-CSF MPB. The calculated SRC frequency using pooled engraftment data from all donors was only modestly increased, with considerable overlap in the 95% confidence intervals (Table I). Our transplantation data produced 1 SRC in 1.17×105 CD34+ cells for AMD3100 MPB, compared with 1 SRC in 1.77×105 CD34+ cells for G-CSF MPB. These frequencies correspond with a similar comparison performed by Broxmeyer et al. using non-paired subjects. The Broxmeyer group achieved an overall repopulating frequency of 12.68± 6.36 SRC per 106 CD34+ cells (approximately 1 SRC in 0.8×105 cells) for AMD3100 MPB versus 3.35±2.04 SRC per 106 CD34+ cells (approximately 1 SRC in 3×105 cells) for G-CSF MPB.29 This group also reported an overall 4.5±3.4 fold increase in SRC frequency from AMD3100 leukapheresis products, as compared to G-CSF MPB.29 In paired subjects, we observed a modest (<2-fold) increase in SRC frequency comparing AMD3100 and G-CSF mobilized CD34+ cells. The discrepancy observed within these studies may be explained by inherent differences in trial design including donor characteristics and variability in AMD3100 and G-CSF dose, or by variance in NOD/SCID colony housing, and detection methods for human engraftment.. Furthermore, the enhanced SRC capacity elicited by AMD3100 MPB MNC in combination with the similar SRC frequency observed for CD34+ cells may be partially explained by the increased number of circulating myeloid cells mobilized by G-CSF. AMD3100 mobilized CD34+ cells may also possess repopulating characteristics different than CD34+ cells mobilized with G-CSF.29 AMD3100 may also mobilize repopulating cells with alternate phenotypes that do not express CD34. Further characterization of repopulating cells mobilized by AMD3100 using non-CD34-expressing stem cells or alternate methods of HPC purification, such as the use of aldehyde dehydrogenase or ABCG2 transporter activity, are clearly warranted.31,46
It is also possible that non-CD34+ engraftment-enhancing populations such as mesenchymal stem cells or T lymphoid facilitator cells could be mobilized at higher levels by AMD3100, causing the more dramatic increase in reconstitution capacity from the AMD3100 MPB MNC in comparison to the G-CSF MPB MNC. Future studies should employ AMD3100 as a valuable source for the isolation of alternate cell types putatively released into the circulation after mobilization. CXCR4+ cells with endothelial progenitor or mesenchymal functions may be rapidly mobilized by AMD3100, and these cells may be exploited in future the development of cell therapies for a wide variety of tissue injury or disease.
In summary, AMD3100 MPB represents a rapidly obtained and highly repopulating source of hematopoietic progenitors suitable for clinical transplantation procedures. In allogeneic transplantation protocols, AMD3100 administration is non-invasive and effective for the mobilization of peripheral blood stem cells from healthy donors in a same day procedure, without the associated bone pain or pleiotropic effects on neutrophils that are evident after G-CSF treatment. Further clinical evaluation should establish AMD3100, alone or in combination with G-CSF, as a primary method for the mobilization of HPC for transplantation.
Footnotes
Research Support:
Supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK #R01DK61848 (JN)), National Heart, Lung and Blood Institute (NHLBI #1RO1HL073256 (JN)), D.A.H. was supported by a fellowship award from the Canadian Institutes of Health Research (CIHR).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 2.Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–1585. doi: 10.1182/blood-2003-05-1595. [DOI] [PubMed] [Google Scholar]
- 3.Bensinger W, Appelbaum F, Rowley S, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–2555. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 4.Bensinger WI, Weaver CH, Appelbaum FR, et al. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony-stimulating factor. Blood. 1995;85:1655–1658. [PubMed] [Google Scholar]
- 5.Roberts AW, DeLuca E, Begley CG, Basser R, Grigg AP, Metcalf D. Broad inter-individual variations in circulating progenitor cell numbers induced by granulocyte colony-stimulating factor therapy. Stem Cells. 1995;13:512–516. doi: 10.1002/stem.5530130508. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AW, Foote S, Alexander WS, Scott C, Robb L, Metcalf D. Genetic influences determining progenitor cell mobilization and leukocytosis induced by granulocyte colony-stimulating factor. Blood. 1997;89:2736–2744. [PubMed] [Google Scholar]
- 7.Shpall EJ. The utilization of cytokines in stem cell mobilization strategies. Bone Marrow Transplant. 1999;23 (Suppl 2):S13–19. doi: 10.1038/sj.bmt.1701669. [DOI] [PubMed] [Google Scholar]
- 8.Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–3969. [PubMed] [Google Scholar]
- 9.Weaver CH, Potz J, Redmond J, et al. Engraftment and outcomes of patients receiving myeloablative therapy followed by autologous peripheral blood stem cells with a low CD34+ cell content. Bone Marrow Transplant. 1997;19:1103–1110. doi: 10.1038/sj.bmt.1700808. [DOI] [PubMed] [Google Scholar]
- 10.Kiss JE, Rybka WB, Winkelstein A, et al. Relationship of CD34+ cell dose to early and late hematopoiesis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1997;19:303–310. doi: 10.1038/sj.bmt.1700671. [DOI] [PubMed] [Google Scholar]
- 11.Devine SM, Flomenberg N, Vesole DH, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 12.Flomenberg N, Devine SM, Dipersio JF, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 13.Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broxmeyer HE, Kohli L, Kim CH, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol. 2003;73:630–638. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer HE, Cooper S, Kohli L, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol. 2003;170:421–429. doi: 10.4049/jimmunol.170.1.421. [DOI] [PubMed] [Google Scholar]
- 16.Lane WJ, Dias S, Hattori K, et al. Stromal-derived factor 1-induced megakaryocyte migration and platelet production is dependent on matrix metalloproteinases. Blood. 2000;96:4152–4159. [PubMed] [Google Scholar]
- 17.Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000;105:101–111. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naiyer AJ, Jo DY, Ahn J, et al. Stromal derived factor-1-induced chemokinesis of cord blood CD34(+) cells (long-term culture-initiating cells) through endothelial cells is mediated by E-selectin. Blood. 1999;94:4011–4019. [PubMed] [Google Scholar]
- 19.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- 20.Kollet O, Spiegel A, Peled A, et al. Rapid and efficient homing of human CD34(+)CD38(−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 21.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 22.Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 23.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 24.Peled A, Grabovsky V, Habler L, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 26.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 27.Liles WC, Rodger E, Broxmeyer HE, et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 28.Burroughs L, Mielcarek M, Little MT, et al. Durable engraftment of AMD3100-mobilized autologous and allogeneic peripheral blood mononuclear cells in a canine transplantation model. Blood. 2005 doi: 10.1182/blood-2005-05-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larochelle A, Krouse A, Metzger M, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 32.Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2005 doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- 34.Hess DA, Levac KD, Karanu FN, et al. Functional analysis of human hematopoietic repopulating cells mobilized with granulocyte colony-stimulating factor alone versus granulocyte colony-stimulating factor in combination with stem cell factor. Blood. 2002;100:869–878. doi: 10.1182/blood.v100.3.869. [DOI] [PubMed] [Google Scholar]
- 35.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 36.Dick JE, Bhatia M, Gan O, Kapp U, Wang JC. Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells. 1997;15(Suppl 1):197–199. 203–204. doi: 10.1002/stem.5530150826. [DOI] [PubMed] [Google Scholar]
- 37.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 39.Bhatia M, Bonnet D, Kapp U, Wang JC, Murdoch B, Dick JE. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997;186:619–624. doi: 10.1084/jem.186.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larochelle A, Vormoor J, Hanenberg H, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 41.Kahn J, Byk T, Jansson-Sjostrand L, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 42.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd RM, Capoccia BJ, Devine SM, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006 doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lack NA, Green B, Dale DC, et al. A pharmacokinetic-pharmacodynamic model for the mobilization of CD34+ hematopoietic progenitor cells by AMD3100. Clin Pharmacol Ther. 2005;77:427–436. doi: 10.1016/j.clpt.2004.12.268. [DOI] [PubMed] [Google Scholar]
- 45.Huttmann A, Gutersohn A, Noppeney R, Neumann T, Erbel R, Duhrsen U. Rapid succession of peripheral blood progenitor cell mobilization cycles in patients with chronic heart failure: effects on the hematopoietic system. Transfusion. 2006;46:1424–1431. doi: 10.1111/j.1537-2995.2006.00912.x. [DOI] [PubMed] [Google Scholar]
- 46.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]


