Abstract
Long-lasting increases in REM sleep are induced in the rat following injection of small amounts of muscarinic receptor agonists into the caudal oral pontine reticular formation. By injecting carbachol at the beginning of the light period or beginning of the dark period, we sought to determine whether the muscarinic, REM sleep induction is influenced by the time of day it is initiated. We found that carbachol is more effective at increasing REM sleep when administered at the beginning of the dark in 87% of the cases. Of these cases, 43% showed evidence of a decreased potency of carbachol by a shift in the dose-response curve to the right. The lack of agreement in efficacy and potency to increase REM sleep supports a conclusion that alterations in local muscarinic receptors are not mediating the effect of time of day. REM sleep control mechanisms down stream of the muscarinic receptors may be the responsible factors.
Keywords: pontine reticular formation, muscarinic, homeostatic control, circadian control
1. INTRODUCTION
Microinjection at lights-on of the cholinergic agonist, carbachol, into the caudal oral pontine reticular formation (PnOc) of the rat is capable of producing an increase in REM sleep lasting at least eight hours2,5,6. This response is mediated by muscarinic cholinergic receptors inasmuch as REM sleep increases produced by carbachol are blocked by preinjecting the muscarinic receptor antagonist atropine at doses in which atropine alone does not reduce REM sleep2,5. The REM sleep induction also is dose-dependent expressing a dose-response relationship of carbachol to increase REM sleep in the form of an inverted “U” with a narrow range of effective doses2,6.
REM sleep expression in the rat appears to be determined by a combination of homeostatic and circadian mechanisms. Experiments comparing intact and suprachiasmatic nucleus lesioned rats support a circadian influence promoting REM sleep in the light, rest, period of the day with little effect in the dark, active, period10,15. Compensatory REM rebound from sleep deprivation or selective REM sleep deprivation in lesioned and intact rats support a homeostatic influence operating independently of time of day10,15.
In this study, we sought to determine whether carbachol induction of REM sleep in the rat is dependent upon time of day. We asked the question: does the cholinergic REM sleep induction operate like the homeostatic process, independent of time of day or like the circadian process with time of day dependence? The results may provide insight into the role of the pontine reticular formation, as well as local interactions with cholinergic mechanisms, in the control of REM sleep.
2. RESULTS
In the eight pontine sites analyzed, at least one of the four microinjections of carbachol resulted in a significant increase in REM sleep beyond the 95% confidence interval for the control distribution in the aggregate of the 8 hrs following the injection. All these injection sites were found to be located in the oral pontine reticular formation (see Fig. 1).
Fig. 1.
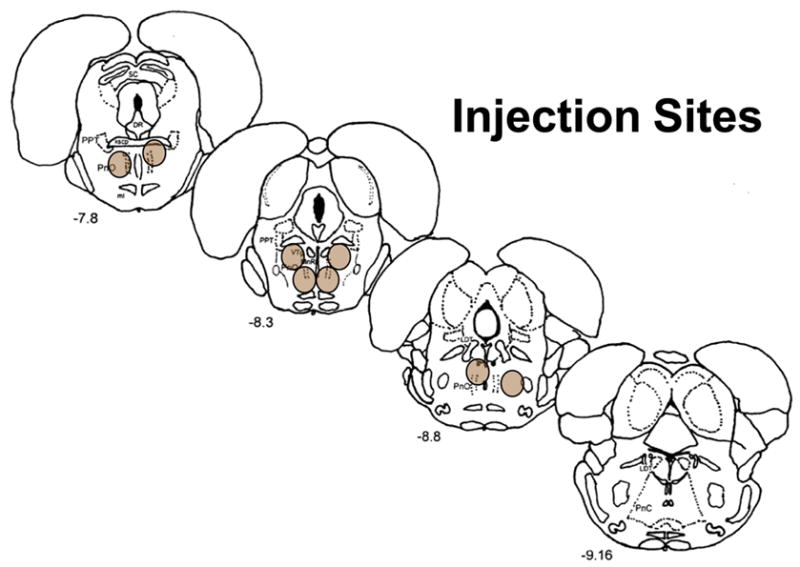
Schematic illustration in coronal section of the eight injection sites in the oral pontine reticular formation. Each site is represented by a shaded oval. Numbers in the lower left indicate distance from the bregma suture in mm.
REM sleep amounts in the 8 hrs following control injections were significantly greater for L-on compared to L-out. No difference was found between the two control injections given at each time of day (p=0.675). The mean of each pair of injections was used to compute drug effects as a percentage of control. When REM sleep amounts as a percentage of control in the 8 hrs following carbachol injections were grouped by the order in which the four injections were given, no difference in the means were found (p=0.214). This indicates that the effect of multiple cannula insertions and injections did not account for a significant amount of variance in the drug effect on REM sleep.
Independent of dose, the group mean percentage increase over control in REM sleep amount and REM bout frequency, but not REM bout duration, for the 8 hrs following carbachol injections, were significantly greater when administered at L-out compared to L-on. These differences were not significant when 24 hrs post-injection was considered (see Fig. 2). The entire data-set for 8 hr-REM sleep increases appears in Fig. 3 and shows that the greatest increases occurred with injections at L-out in seven out of the eight cases. Also shown in Fig. 3, is the lack of a consistent relationship across sites of the two doses on effect to increase the percentage of REM sleep. Each dose produced the greater effect at an equal number of sites injected with carbachol.
Fig. 2.
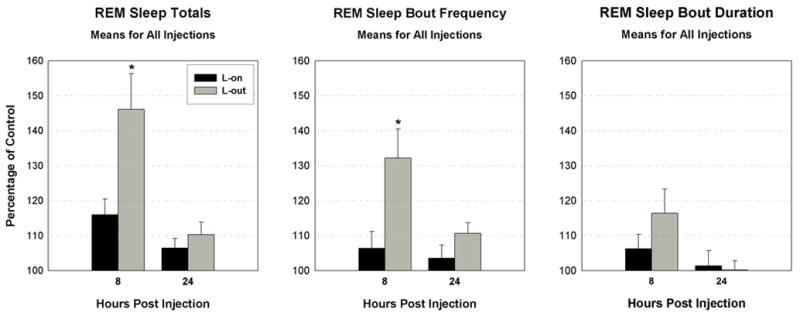
Bar graphs of the means as a percentage of control of all injections of carbachol for three measures of REM sleep in the 8 and 24 hr period following injection. Black bars indicate means for injections given at lights-on (L-on) and grey bars indicate means for injections given at lights-out (L-out). * at 8 hrs for REM sleep totals and bout frequency indicates means for L-on and L-out are significantly different (p<.05). Error bars are ± SEM.
Fig. 3.
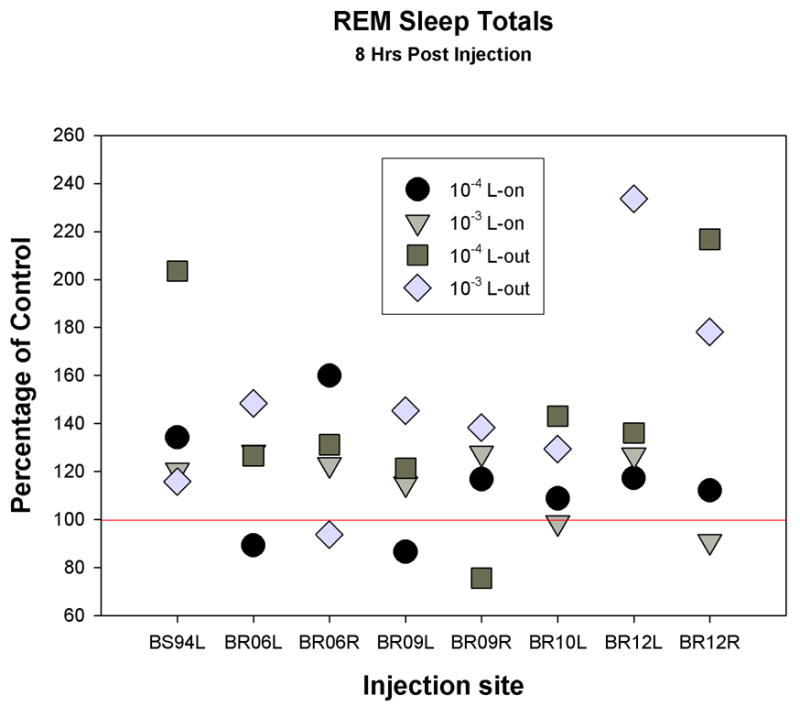
Representation of the entire data set, 32 carbachol injections at 8 pontine sites as a percentage of control in the 8 hrs following injection. Circles are 10−4 M dose given at lights-on (L-on); inverted triangles are 10−3 M dose given at L-on; squares are 10−4 M dose given at lights-out (L-out); and diamonds are 10−3 M dose given at L-out.
Owing to the site-associated variability in the most effective dose, the temporal distribution of carbachol’s effects were plotted for two-hour intervals using group means, as a percentage of control, for the single dose-level at each site that produced the greatest effect (Fig. 4). Based on the intervals with significant increases over control, the duration of the drug effect to increase REM sleep is about 8 hrs for administration at both times of day. Increases in REM sleep are not consistently elevated for each block over the 8 hrs. Differences among animals in what times were elevated resulted in high variability in the grouped data (see error bars, ±SEM, Fig. 4). The trend for means to remain elevated until lights-on for L-out injections and to be increased during the dark-period following L-on injections, reflects appearance of this temporal pattern in a few of the individual cases. A large, though not significant, peak during the 19th–20th hr interval following L-on injection owes to a single case with consistently very low amounts of REM sleep in the 19th hr on control recordings.
Fig. 4.
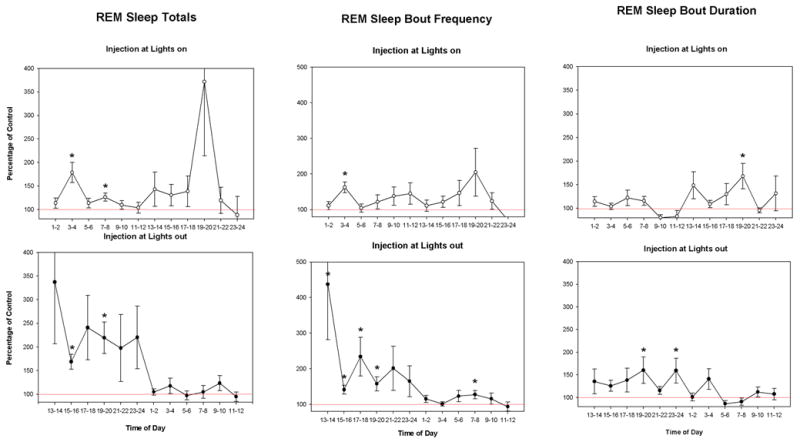
Line graphs of REM sleep measures for the means of two hour blocks as a percentage of control for the dose of carbachol producing the greatest effect at each site. All panels start at the injection time. The time of day on the abscissa is referenced to lights-on (e.g. 1–2 is the first 2 hr block after lights-on). Open circles represent the means for lights-on injections and filled circles the means of injections at lights-out. * indicates means that are significantly different from 100% (p<0.05). Error bars are ± SEM.
The distribution for SW sleep and wake totals are shown in Fig. 5. Here the 19th–20th hr interval for L-on injections indicates a more generalized influence where there are both a significant decrease for wake and increase for SW sleep in the group. The opposite effect was observed in the 5th–6th hr following L-on injection. No two-hour blocks for SW sleep or wake were observed to differ statistically from the same time in the control condition for injections made at L-out.
Fig. 5.
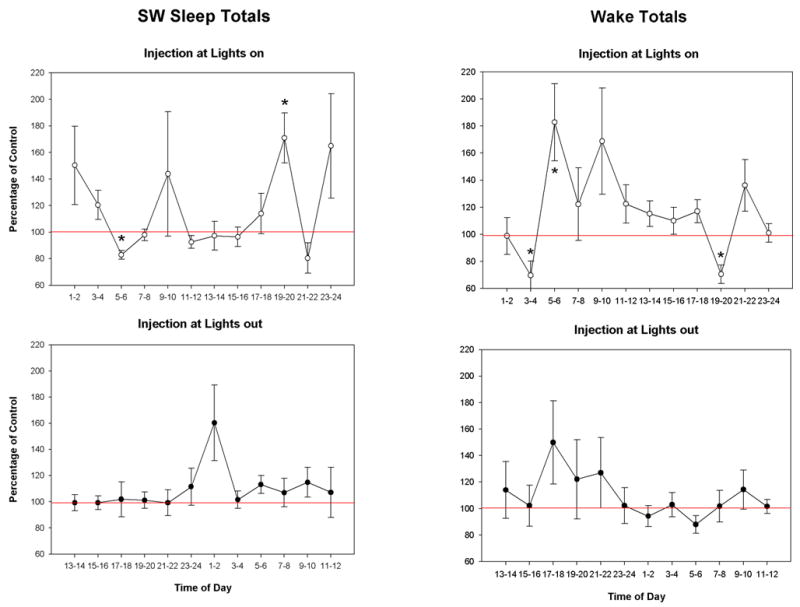
Line graphs of Slow wave sleep (SW) and wake (AW) amounts for the means of two hour blocks as a percentage of control for the dose of carbachol producing the greatest effect at each site. Graph features are the same as Fig. 4.
The finding that drug induced increases were accompanied by increases in REM bout frequency and not REM bout duration confirms previous observations that carbachol increases REM sleep in the rat predominantly by augmenting the occurrence of REM periods5,6. In the control conditions during the 8 hr period post injection, the mean hourly bout frequency of REM sleep expressed the well known circadian distribution with significantly greater frequency in the light-condition compared to the dark-condition (4.5±0.6 vs 3.1±0.5, mean±SEM respectively). Given the lesser frequency in the dark and the current finding of greater carbachol-increases of REM sleep in the dark, a significant negative correlation was found between the variables of REM bout frequency in the control condition and the percentage increase in REM bout frequency following the drug injection of most effective dose (r=−.72). The high amount of variance accounted for, over 50%, indicated that there may be more to this relationship than the light/dark differences in drug effect. We then computed the strength of relationship at a single time of day, during the dark when drug effects were greater. The correlation was still significant (r=−.70) indicating that cases with lower frequencies of REM bouts in control conditions had greater increases in frequency following drug administration. The time spent in REM sleep in control recordings was more poorly related to the drug induced increase in REM time (r=−.34) and increase in REM bout frequency (r=−.20).
The most effective doses of carbachol had only modest effects on latency when administered at either time of day (see Fig. 6). The reductions in latency to REM sleep onset following the injections were statistically significant and similar, 81.65±4.3% and 77.42±8.6% of control for L-on and L-out, respectively. Considering the mean REM sleep latency in the control conditions were 76 min for L-on and 96 min for L-out, latency to REM onset is long even after drug injection. This is a consistent finding for carbachol induction of REM sleep in the rat and differs from the short latency onset typically produced in cat1,5,6. SW sleep latency was reduced for the L-on injections only, 71.47±7.3% of control. Inasmuch as SW sleep latency was not reduced for the Lout injections, the comparable reductions in REM sleep latency at both times of day resulted in a significant reduction in the latency for REM sleep onset following SW sleep onset for the L-out injections, 52.43±10.2% of control. This latter finding may be an indication of increased REM sleep pressure following carbachol injections at L-out.
Fig. 6.
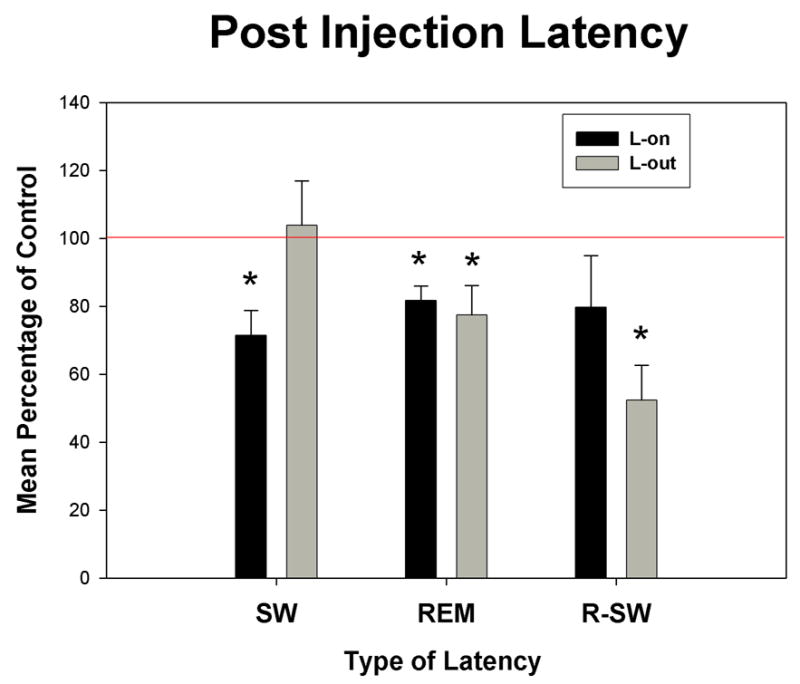
Bar graphs of three latency measures of the means as a percentage of control for the dose of carbachol producing the greatest effect at each site. Black bars indicate means for injections given at lights-on (L-on) and grey bars indicate means for injections given at lights-out (L-out). SW, time to slow wave sleep onset; REM, time to REM sleep onset; and R-SW, time to REM sleep onset following SW onset. * indicate significant differences from 100% (p<0.05). Error bars are ± SEM.
Lack of consistent relationship in the order of effectiveness of the two doses is related to the inverted “U” dose-response relationship and the narrow range of effective dose. Small shifts in the sensitivity from one site to another determine the slope of the relationship between the doses of 10−3 and 10−4 M carbachol depending on whether they are on the rising (left) or declining (right) side of the apex of the inverted “U” curve. An increase in potency, shift in dose-response curve to the left, would increase the response to a dose on the rising side of the curve as long as the shift did not pass the apex, but a dose formerly on the falling side of the curve would produce a decreased response with increased drug potency. If it is assumed that when the slope relating the response of the two doses is of the same sign when administered at L-on and L-out, which is so for all cases here, that responses for both times of day are on the same side of the dose-response curve, then an inference can be drawn as to the change in potency of carbachol between the two conditions.
In half the sites for the 8 hrs following the injection, REM sleep increases produced by 10−3 M were greater than 10−4 M indicating the doses are on the rising side of the dose-response curve (illustrated case in Fig. 7B). An increase in potency on this side of the curve is indicated by a greater response. All these cases showed greater responses in the dark and therefore also leftward shifts or greater potency of carbachol injected at L-out. In the other half of sites the dose relationship was reversed, 10−4 >10−3, indicating the doses are on the declining side of the dose-response curve. An increase in potency on this side of the curve is indicated by a reduced response. This group contained the one case in which the larger response was produced at L-on (illustrated in Fig. 7A). The diminished response observed at L-out indicates a greater potency of carbachol at the L-out time of day. The remaining three cases in this group showed greater responses in the dark and therefore lowered potency of carbachol to increase REM sleep when injected at this time (illustrated case in Fig. 7C).
Fig. 7.
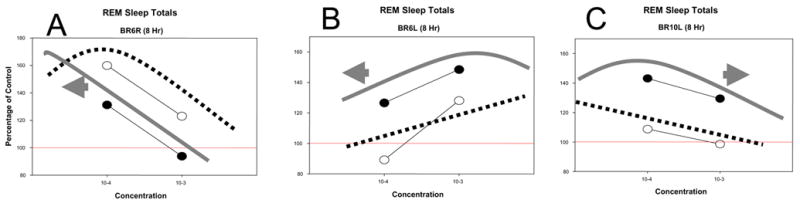
Representative examples (A-C) of the three types of changes observed in dose-response relationships for carbachol to increase REM sleep in the eight hours following injections at different times of day. Data points are REM sleep amounts as a percentage of control, open circles for injections of carbachol given at lights-on and closed circles when given at lights-out. A fine line connects the two doses, 10−3 and 10−4 M, at each time. Hypothetical dose-response curves are drawn on each graph: dotted line, lights-on injections; solid line, lights-out injections. The arrow indicates the direction of displacement of the lights-out curve with respect to the lights-on curve. (See text for additional discussion)
In summary, responses to carbachol injected in the PnO to increase REM sleep were observed to be greater when injected at L-out compared to L-on in 7 of 8 cases. In the exceptional case in which injection at L-on produced the larger response, potency of carbachol was increased for the L-out injection condition. Decreased potency of carbachol at L-out was observed in 3 of 8 cases total or 3 of 7 cases in which responses were greater at L-out.
3. DISCUSSION
The basic finding in this study was that a cholinergic agonist injected into the oral pontine reticular formation of the rat has a greater effect to increase REM sleep over control values when administered at the beginning of the dark, active, period than when administered at the beginning of the light, rest, period (7/8 cases). This drug-induced increase in REM sleep differs from REM sleep increases resulting from recovery following selective REM sleep deprivation. REM sleep-rebound as a percentage of the corresponding baseline period is not altered when allowed to occur at different times of day10. To the extent that the REM sleep-rebound phenomenon is mediated by homeostatic control mechanisms, the pontine area injected in this study may be a locus more associated with the circadian control of REM sleep.
The specific mechanism of carbachol’s time of day dependence to increase REM sleep may be subserved by the circadian rhythms in neurotransmitter receptors, which include muscarinic receptors16. Rats bred for hyper-cholinergic responses express increased numbers of pontine muscarinic receptors and high amounts of REM sleep14 consistent with muscarinic receptors as one control mechanism of REM sleep. The number of high affinity binding sites available for muscarinic receptors in the brainstem measured at different times of day is highest in the light period when REM sleep amounts are high and lowest in the dark period when REM sleep amounts are low7. Non-circadian alterations of brainstem muscarinic receptors appear differently related to specific changes in REM sleep. Muscarinic receptor binding at a single time of day in the brainstem, including the pons, indicates that receptor numbers are high in REM sleep, but also in wake as compared to SW sleep12. There are indications for other than a positive relationship between pontine muscarinic receptor numbers and the propensity to express REM sleep. Receptor binding with AF-DX 384, relatively selective for m2/m4 receptor subtypes, is reduced following REM sleep deprivation8,13 as is m2 mRNA levels in the pons4. Thus a time when REM sleep propensity is high can be associated with reductions in receptor number. Muscarinic receptors in the brainstem could mediate circadian control of REM sleep but not homeostatic control.
As a mechanism of REM sleep control, muscarinic receptors require binding acetylcholine. The local abundance of the natural ligand exerts a high degree of control over receptor number available for binding3. The reduction in antagonist binding of brainstem muscarinic receptors in the dark period has been shown to be due to a high level of endogenous acetylcholine-receptor complexes7, indicating increased acetylcholine-release in the low-REM sleep active period. In addition, fewer muscarinic receptor binding sites in the PnOc is not consistent with muscarinic receptors mediating an increased response to injected carbachol observed in the dark. The finding that 43% of the cases of carbachol’s increased effects in the dark period were accompanied by a reduction in potency, shift of the dose-response curve to the right, may indicate that altered muscarinic receptor numbers is not the mechanism underlying the differential increases in REM sleep at different times of day.
It is highly unlikely that receptor occupancy by carbachol directly controls the REM sleep-induction through its duration. Even though carbachol is resistant to hydrolysis, it is most probable that the drug diffuses away from the injection site rendering concentrations too low to be effective, long before the 8 hrs of observed elevated REM sleep. Therefore, the cholinergic agonist only initiates the response in the PnOc that is maintained by other neural mechanisms. We believe that it is a mechanism other than muscarinic receptors in the PnOc that is responsible for the influence of time of day on the REM sleep-inducing response to carbachol.
One clue to this mechanism is the negative relationship of REM bout frequency in the control condition to the drug-induced increase in REM sleep. A homeostatic control mechanism of REM sleep has been shown to operate on a time scale of the inter-episode interval9. In other words, the expression of REM sleep exerts a time-dependent suppression of the next REM episode. Such a mechanism would act to resist actions to increase the frequency of REM bouts. The current finding of increased REM sleep by carbachol in the dark may be due to the lower REM bout frequency in the dark where the longer intervals between episodes meets lower resistance to increase than in the light when REM bout frequency is higher and episodes occur closer in time.
The current finding of a time of day effect may be the product of the circadian influence regulating bout frequency. The greater facility to increase REM sleep when bout frequency is low, however, may be a product of the homeostatic regulatory mechanism. Therefore, both circadian and homeostatic processes may contribute to carbachol induction of REM sleep in the rat being more effective at L-out than L-on.
4. Experimental procedure
All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by our local Institutional Animal Care and Use Committee. Male, Long-Evans Hooded rats (Harlan) weighing between 255 and 446 gms were instrumented for chronic sleep-recording under ketamine/acepromazine anesthesia (80/2.5 mg/kg). As previously described5, this consisted of electrodes for recording: cortical electroencephalogram (EEG), two screws tapped ipsilaterally into skull respectively over somatosensory and motor cortex; hippocampal EEG, a stainless steel wire (200 μm dia.), insulated except at the tip, implanted in the dorsal hippocampus referenced to a cortical screw over the cerebellum; and the nuchal electromyogram (EMG), two spring electrodes sutured under the trapezious muscle. In addition, guide cannulas with stylettes inserted (Plastics One, Inc.) were stereotaxicaly, bilaterally aimed at sites in the PnOc previously shown to support REM sleep increases in rat after carbachol infusion2,5,6. Lateral to medial angles of approach were used (14°). See figure 1 for injection-site localization.
After one week recovery from surgery, animals were placed individually in sound-proof, video-monitored, light- (12 h light/12 h dark) and temperature- (22±1°C) controlled running rooms. A cable connected to a swing-arm and commutator tethered each rat to the recording equipment while allowing unrestricted movement in the cage. Animals remained in the recording environment except when removed for adaptation to handling (each day for the first week) or during the injection procedures starting after the first week. Injections were performed unilaterally by inserting a cannula (28-gauge), back-filled with drug solution, connected to a 1.0-μl syringe (Hamilton) through, oil-filled, polyethylene tubing (Plastics One, Inc.). The volume injected was held constant at 60 nl, of which 20 nl were injected every 45 s followed by a 3-min wait before cannula was removed and stylette reinserted. The rat was then returned to his cage. Each pontine site received eight injections over a period of months, with a minimum of seven days between injections.
Eight pontine sites were investigated in five rats. Two additional sites were out of the PnOc, produced no response to carbachol injections and are not included in the analysis. In three rats, both left and right placements were used. Two doses of carbachol (carbamylcholine chloride, RBI) were injected at concentrations of 10−4 and 10−3 M. Injections of both doses were administered at two different times of day. One time within one hour after lights-on (L-on) and the other time within 30 min before lights-out (L-out) under the 12/12 light/dark schedule. The mean of an additional two saline-vehicle injections at each time of day was used as a control for each respective pair of drug injections. Across animals, the sequence of injections was randomized within each injection-time of day as was the order of which injection-time came first.
Polygraphic recordings were obtained for 24 hrs following each injection. Electrical potentials were digitized (256 S/s) and stored on optical disks. The recordings were visually scored (Rodent sleep stager, Grass) utilizing standard criteria without knowledge of the experimental condition. Each 15-s epoch was assigned a score of wake, slow wave (SW) sleep or REM sleep. From these data, stage totals, bout frequency and mean bout duration were determined for each hour. Bout frequency was a count of the number of times an epoch was scored a particular stage that was preceded by an epoch scored a different stage. Mean bout duration was computed by dividing the time in stage by the bout frequency. REM sleep and SW sleep latencies were computed based on the interval from stylette reinsertion after injection to the first continuous 30-s (2 epochs) episode of each respective state.
All statistical tests conducted were two-tail, α<0.05. Individual drug responses were tested to determine if they fell outside the 95% confidence interval of the control recordings. Analysis of grouped data included the 8 pontine sites. The t test was used to determine differences from 100% in grouped data as a percentage of control for blocks of 8hr and 24 hr means. For the determination of the distribution of state in time, measures were divided into 2 hr blocks. The 2 hr blocks taken from different times of day had unequal variances in every measure tested. The violation of the homogeneity of variance assumption precluded the use of ANOVA in this analysis. Instead, each block was treated as an independent experiment and t tests were used to determine differences in group means. One way ANOVA with repeated measures was used to test for effects of injection order on group means for the 8 hr blocks following carbachol injections. Two way ANOVA with repeated measures was used to test for order effects in the control injections. Correlations were computed as Pearson product moment coefficients and significance determined as different from zero.
After completion of the injection experiments, rats were killed by decapitation under an overdose of pentobarbital and injection sites were determined in coronal sections (50 μm) stained with cresyl violet and mapped with the aid of the atlas of Paxinos and Watson11.
Acknowledgments
Supported by a grant from the National Institute of Mental Health, Grant number: RO1 MH57434.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- 2.Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. Neuroreport. 1995;6:532–536. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Costa LG, Kaylor G, Murphy SD. In vitro and in vivo modulation of cholinergic muscarinic receptors in rat lymphocytes and brain by cholinergic agents. Int J Immunopharmacol. 1990;12(1):67–75. doi: 10.1016/0192-0561(90)90069-y. [DOI] [PubMed] [Google Scholar]
- 4.Kushida CA, Zoltoski RK, Gillin JC. The expression of m1-m3 muscarinic receptor mRNAs in rat brain following REM sleep deprivation. Neuroreport. 1995;6(12):1705–1708. doi: 10.1097/00001756-199508000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Marks GA, Birabil CG. Enhancement of rapid eye movement sleep in the rat by cholinergic and adenosinergic agonists infused into the pontine reticular formation. Neuroscience. 1998;86:29–37. doi: 10.1016/s0306-4522(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 6.Marks GA, Birabil CG. Comparison of three muscarinic agonists injected into the medial pontine reticular formation of rats to enhance REM sleep. Sleep Res Online. 2001;4:17–24. [Google Scholar]
- 7.Mash DC, Flynn DD, Kalinoski L, Potter LT. Circadian variations in radioligand binding to muscarinic receptors in rat brain dependent upon endogenous agonist occupation. Brain Res. 1985;331:35–38. doi: 10.1016/0006-8993(85)90712-7. [DOI] [PubMed] [Google Scholar]
- 8.Nunes-Junior GP, Tufik S, Nobrega JN. Decreased muscarinic receptor binding in rat brain after paradoxical sleep deprivation: an autoradiographic study. Brain Res. 1994;645(1–2):247–252. doi: 10.1016/0006-8993(94)91658-6. [DOI] [PubMed] [Google Scholar]
- 9.Ocampo-Garces AVivaldi EA. Short-term homeostasis of REM sleep assessed in an intermittent REM sleep deprivation protocol in the rat. J Sleep Res. 2002;11:81–89. doi: 10.1046/j.1365-2869.2002.00281.x. [DOI] [PubMed] [Google Scholar]
- 10.Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci. 2000;20(11):4300–4310. doi: 10.1523/JNEUROSCI.20-11-04300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando: 1986. [Google Scholar]
- 12.Pompeiano M, Tononi G. Changes in pontine muscarinic receptor binding during sleep-waking states in the rat. Neurosci lett. 1990;109:347–352. doi: 10.1016/0304-3940(90)90020-a. [DOI] [PubMed] [Google Scholar]
- 13.Salin-Pascual RJ, Diaz-Munoz M, Rivera-Valerdi L, Ortiz-Lopez L, Blanco-Centurion C. Decrease in muscarinic M2 receptors from synaptosomes in the pons and hippocampus after REM sleep deprivation in rats Sleep Res. Online. 1998;1(1):19–23. [PubMed] [Google Scholar]
- 14.Shiromani PJ, Velazquez-Moctezuma J, Overstreet D, Shaauta M, Lucero S, Floyd C. Effects of sleep deprivation on sleepiness and increased REM sleep in rats selectively bred for cholinergic hyperactivity. Sleep. 1991;14(2):116–120. [PubMed] [Google Scholar]
- 15.Tobler I, Borbely AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neurosci lett. 1983;42:49–54. doi: 10.1016/0304-3940(83)90420-2. [DOI] [PubMed] [Google Scholar]
- 16.Wirz-Justice A. Circadian rhythms in mammalian neurotransmitter receptors, Prog. Neurobiol. 1987;29:219–259. doi: 10.1016/0301-0082(87)90022-0. [DOI] [PubMed] [Google Scholar]


