Abstract
The thresholds of compound action potentials evoked by tone pips were measured in the cochleae of anesthetized gerbils, both in adults and in neonates aged 14, 16, 18, 20 and 30 days, using round-window electrodes. Stapes vibrations were also measured, using a laser velocimeter, in many of the same ears of adults and neonates aged 14, 16, 18 and 20 days to assess cochlear sensitivity in isolation from middle ear effects and to circumvent problems associated with calibration of acoustic stimuli at high frequencies. Whether referenced to sound pressure level in the ear canal or stapes vibration velocity, thresholds in adults were roughly uniform in the entire range of tested frequencies, 1.25–38.5 kHz. In neonates, thresholds decreased systematically as a function of age, with the largest reductions occurring at the highest frequencies. Thresholds remained slightly immature at all frequencies 30 days after birth. The results for adult gerbils are consistent with the recent finding that basilar-membrane responses to characteristic frequency tones normalized to stapes vibrations are as sensitive at sites near the round window as at more apical sites. The results for neonates confirm that the extreme basal region of the cochlea is the last to approach maturity, with substantial development occurring between 20 and 30 days after birth.
Keywords: Cochlea, Basilar membrane, Development, Middle ear, Stapes, Gerbil
Introduction
The present investigation of compound action potential (CAP) thresholds in adult and neonatal gerbils was undertaken to complement studies of Overstreet and his group [Overstreet, 2000; Overstreet et al., 2002a, b; Overstreet and Ruggero, 1999, 2002] on the development of the gerbil cochlea at sites with characteristic frequencies (CFs) of 34–37 kHz. CAP thresholds or responses of auditory-nerve fibers have only exceptionally been measured at those frequencies in adult gerbils [Müller, 1996] and never in neonates. Overstreet et al. [2002a] showed that basilar-membrane vibrations in adult gerbils are as sensitive at the 34- to 37-kHz site as at sites with CFs 1 octave lower [Cooper, 2000; Ren and Nuttall, 2001]. Those basilar-membrane data are consistent with mechanical measurements in adult gerbils showing that the magnitudes of stapes vibration velocity [Overstreet and Ruggero, 2002] and pressure in the scala vestibuli [Olson, 1998] are as sensitive in the 34- to 46-kHz range as at frequencies more than 1 octave lower but contrast starkly with reports that CAP and auditory brainstem response (ABR) thresholds in adult gerbils at 30 kHz exceed those at 15 kHz by 20–40 dB (fig. 4b). We believe that the latter discrepancy is due in part to flaws in the calibration of stimulus levels at high frequencies [Khanna and Stinson, 1985; Overstreet and Ruggero, 2002; Pearce et al., 2001; Stinson and Khanna, 1994] and to noxious effects of surgical procedures, including anesthesia and/or cochlear cooling [Brown et al., 1983a, b; Ohlemiller and Siegel, 1992, 1994; Shore and Nuttall, 1985].
Fig. 4.
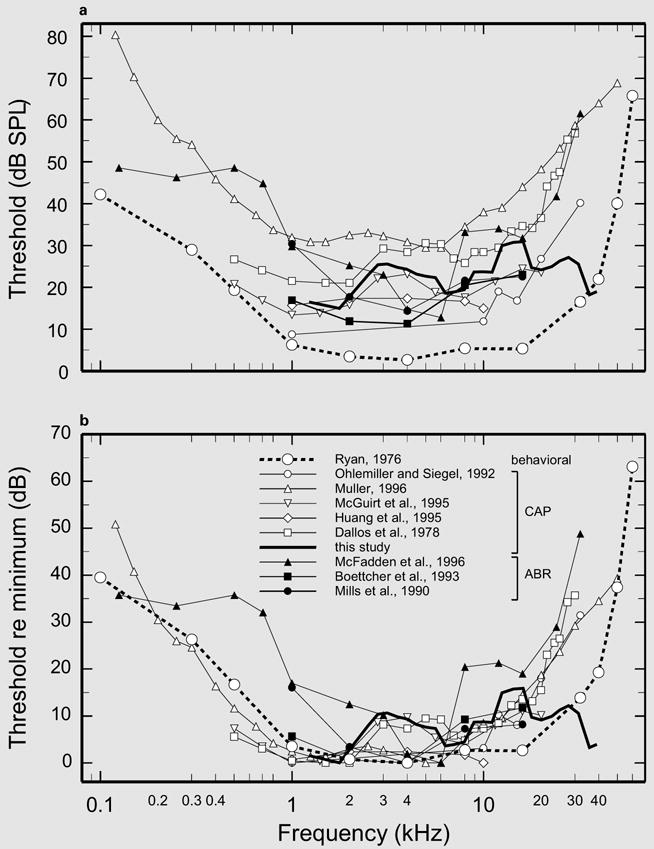
A survey of neural and behavioral thresholds in adult gerbils. a The average CAP thresholds of figure 1 (thick solid line) are compared with thresholds from five other CAP studies [Ohlemiller and Siegel, 1992; Müller, 1996; McGuirt et al., 1995; Huang et al., 1995; Dallos et al., 1978], three ABR studies [McFadden et al., 1996; Boettcher et al., 1993; Mills et al., 1990] and one behavioral study [Ryan, 1976]. b The curves of a are re-presented after normalization to the minimum threshold in each curve.
The problems posed by the calibration of high-frequency stimuli have been circumvented in the present study by referencing CAP thresholds to the velocity magnitude of stapes vibration recorded in the same ears of adult and neonatal gerbils. This procedure also simplified the study of cochlear development by isolating its age-dependent changes from the effects of middle-ear maturation [Overstreet and Ruggero, 2002]. In addition, we took elaborate precautions to minimize surgical damage and to maintain the normal temperature of the cochlea. We show here that in adult gerbils the region of the cochlea with a CF of 38 kHz is as sensitive as sites with CFs an octave lower and that the region of the cochlea near the round window is the last to approach maturity in neonatal gerbils.
Materials and Methods
Animal Preparation
All experiments were conducted in accordance with guidelines of the Northwestern University’s Animal Care and Use Committee. Subjects were anesthetized adult and neonatal Mongolian gerbils (Meriones unguiculatus). Adult gerbils in one group (A) had ages of 90–150 days after birth (DAB); those in another group (B) had ages of 40–55 DAB. Neonatal gerbils had ages of 14–30 DAB (determined with a precision of ±8 h), with day zero being the day of birth. To help insure equal maturation rates, all litters were culled to 6 pups, and only neonates weighing within 1 standard deviation of the norm for a given age [Woolf and Ryan, 1985] were used.
Neonatal and group A adult gerbils were first sedated with a 0.01-ml subcutaneous injection of ketamine HCl (100 mg/ml) and anesthetized with an intraperitoneal injection of sodium pentobarbital (48 mg/kg). Group B adult gerbils were initially anesthetized with an intraperitoneal injection of sodium pentobarbital (80 mg/kg). Immediately after the initial injection of pentobarbital, neonates and group A adult gerbils were placed in an aluminum ‘boat’ floating in hot water to prevent loss of body heat during the induction of anesthesia. Failure to carry out this procedure often resulted in elevated CAP thresholds. Additional doses of sodium pentobarbital were administered as needed to maintain deep anesthesia, as indicated by the absence of the limb withdrawal reflex (tested every 30 min).
Neonates and group A adults were kept hydrated with 0.10-ml intraperitoneal injections of Pedialyte (Abbott; each liter of this solution contains 45 mEq Na+, 20 mEq K+, 35 mEq Cl−, 30 mEq citrate and 25 g dextrose), administered at approximately 90-min intervals. All gerbils were tracheotomized to maintain a patent airway.
After the gerbil had been fully anesthetized, it was wrapped in a heating pad and placed on a metal platform, with its head firmly attached to a heated holder. The platform rested on a vibration isolation table within a sound-insulated chamber. Rectal temperature was monitored and maintained at 38°C (group B adults) or 39°C (neonates and group A adults) by means of the servo-controlled DC electrical heating pad. One or 2 small holes were opened in the ventral aspect of the bulla by scoring and cutting the bone with tiny knives. A recording electrode, fashioned from Teflon-insulated silver wire, was placed on the bone near the round window. The left pinna was removed and the tip of a speculum was positioned against the external ear canal, near the tympanic ring, without applying undue pressure to the bulla, which otherwise could become deformed and cause a conductive hearing loss. The speculum tip was then cemented to the bone surrounding the tympanic ring. For group A adults and neonatal gerbils, the speculum housed the probe tube of a miniature microphone.
Sound System and Its Calibration
Acoustic stimuli were produced by a modified tweeter [Chan et al., 1993] coupled to the ear speculum. The tweeter was driven with electrical sinusoids digitally synthesized under computer control. Preliminary experiments indicated that in situ probe calibrations introduced spurious high-frequency irregularities in the responses of the basilar membrane [Overstreet et al., 2002a] and the stapes [Overstreet and Ruggero, 2002]. Therefore, we adopted alternative calibration methods based largely on measurements of sound pressure in an artificial cavity mimicking the external ear canal and terminated with a 1/8 inch Brüel & Kjaer microphone in lieu of the tympanic membrane.
Group B Calibrations
Stimulus sound pressures as a function of frequency were estimated using an average calibration based on measurements in a tubing coupler with volume comparable to that of the external ear canal and terminated by a 1/8 inch microphone [Pearce et al., 2001].
Group A Calibrations
Stimulus sound pressures as a function of frequency were estimated for each ear using a hybrid calibration [Overstreet et al., 2002a; Overstreet and Ruggero, 2002] derived from an individual in situ calibration and an average calibration in an artificial cavity. In situ probe tube calibrations were measured in the ear canal using a miniature Knowles microphone fitted with a thin, small-diameter probe tube whose tip was placed within 2.5 mm of the tympanic membrane. The artificial-cavity calibration was an average of measurements in a closed cavity simulating the external ear canal of the gerbil with the 1/8 inch microphone as a stand-in for the tympanic membrane [Pearce et al., 2001]. At stimulus frequencies of 16.5 kHz and lower, the magnitudes of the hybrid calibrations were identical to those measured in situ; at frequencies higher than 25 kHz, they were identical to the magnitudes in the artificial-cavity calibration. Between 16.5 and 25 kHz, the magnitudes were weighted averages of the in situ and artificial-cavity calibrations, with the weight of the latter increasing linearly from zero at 16.5 kHz to 100% at 25 kHz. Further details on the hybrid calibration are given in Overstreet and Ruggero [2002].
Measurement of CAP Thresholds
CAP thresholds were measured using automated procedures which, for each stimulus frequency, determined the sound pressure level (SPL, expressed in decibels referenced to 20 μPa) required to achieve a predetermined N1/P1 CAP magnitude (7 μV for all neonates and most group A adults; 10 μV for all group B and 3 group A adults). For neonates and group A adults, stimuli were 5-ms tone pips with onset phases randomized to cancel out cochlear microphonics and ramped with a 0.75-ms rise/fall time. For group B adults, thresholds were determined with an adaptive procedure [Taylor and Creelman, 1967], using 12-ms tone pips with 1-ms rise/fall time, presented as 32 pairs in opposing phase to cancel the microphonics.
Measurement and Analysis of Stapes Vibrations
In a subset of experiments in which CAP thresholds were obtained from group A adult gerbils and neonates aged 14–20 days, the vibrations of the stapes were also measured using a Dantec laser velocimeter consisting of a He-Ne laser, a headstage and a Doppler frequency tracker [Overstreet and Ruggero, 2002; Ruggero and Rich, 1991]. The headstage was attached to an Olympus compound microscope equipped with a ×20 ultra-long working-distance objective (Mitutoyo 20SL, n.a. 0.42) which focused the laser beam on a reflective glass bead (diameter: 20–30 μm) placed on the head of the stapes near the incudostapedial joint. The voltage output of the vibrometer Doppler frequency tracker, proportional to target velocity, was digitized (16-bit resolution, sampling rate of 166 kHz) and stored on magnetic media in a computer. No cosine correction was applied to the velocity signal since the angle between the laser beam and the axis of stapes vibration was always 30° or less. MATLAB programs were used to compute the discrete Fourier transforms of the time-averaged response waveforms. The velocity-response magnitudes and phases were imported into a spreadsheet (EXCEL) for further processing.
Inspection of the responses of the stapes (and, in some cases, of the basilar membrane [Overstreet et al., 2002a]) to stimuli identical to those used for CAP threshold measurements corroborated that the acoustic stimuli closely resembled the electrical input to the earphone and, specifically, that they were free of irregularities such as onset clicks.
Results
CAP Thresholds in the Intact Cochleae of Adult Gerbils
After CAPs had been measured in all neonatal gerbils and most adult subjects in one laboratory, additional CAP data for adult gerbils were obtained independently in a second laboratory, using somewhat different methodologies (see Methods). The symbols of figure 1 indicate the medians of CAP thresholds for the two groups of adults. In one group, with 14 subjects (group A: circles), gerbils were tested at ages of 90–150 DAB, at stimulus frequencies spaced at 0.5-octave (or slightly shorter) intervals between 1.25 and 38.3 kHz.1 In the other group (group B: squares), with 5 subjects, gerbils were tested at 40–55 DAB, using stimulus frequencies spaced at approximately 1/6-octave intervals between 2.8 and 38.5 kHz. The thick solid line in figure 1 indicates a 2/3-octave running average for the entire data set from the 19 gerbils. Although the running average includes irregularities, it is roughly flat over the range of frequencies tested, 1.25–38.5 kHz, with a mean value of 24.1 dB SPL. In particular, there is no evidence that thresholds rise systematically at the highest frequencies. Most of the data points lie close to a nearly flat straight line, thresholddB SPL = 3.35 · log10 (fHz) + 10.22.
Fig. 1.

CAP thresholds in adult gerbils. The symbols and brackets indicate medians and interquartile ranges for sets of data obtained independently in two laboratories using somewhat different methods. Each group A data point (○) represents 14 subjects; each group B point (□) represents 5 subjects. The thick solid line is a weighted running average, computed at 1/6-octave intervals over 2/3-octave windows. The thin straight line depicts the function thresholddB SPL = 3.35 · log10 (fHz) + 10.22.
Vulnerability of CAP Thresholds in Adult Gerbils
The threshold data of figure 1 were obtained from gerbils tested relatively late in the two series of experiments. In earlier experiments, thresholds at frequencies >25–27 kHz were almost invariably higher, by as much as 30 dB, than those of figure 1. In group A, the improved cochlear sensitivity in the more recent experiments may have resulted from preventing a reduction of body temperature during the induction of anesthesia (see first section of the Methods). In both groups, improved sensitivity was also associated with a combination of faster performance of surgical procedures and a more restricted opening of the bulla, which may have resulted in maintenance of normal cochlear temperature. In addition, the smaller opening of the bulla may have minimized any possible acoustic trauma (see section ‘CAP thresholds normalized to stapes vibration’ of the Discussion) [Brown et al., 1983a].
The Development of CAP Thresholds in Neonatal Gerbils
CAP thresholds at 11 frequencies (1.25–28.3 kHz, at 0.5-octave intervals, and 38.3 kHz) were measured immediately after opening the bulla in 45 neonatal gerbils aged 14, 16, 18, 20 and 30 days (fig. 2).1 CAP thresholds varied systematically as a function of both age and frequency. Thresholds in neonates of all ages, 14–30 days, exceeded those in adults, with the highest thresholds corresponding to the youngest animals and the highest stimulus frequencies. Both the median thresholds and their variability (see interquartile ranges) decreased with age.
Fig. 2.
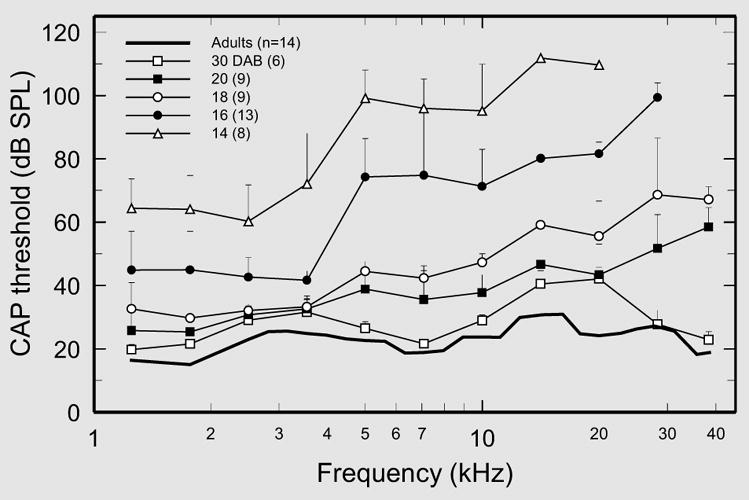
Development of CAP thresholds in neonatal gerbils. Median CAP thresholds (dB SPL) and interquartile ranges are shown as a function of frequency, with age as the parameter. The numbers of subjects used to compute the medians for each age are given in the symbol key.
CAP thresholds in neonates aged 14 and 16 days were much higher than in older animals at all frequencies >4 kHz and typically could not be measured at frequencies >20 and >28.3 kHz, respectively, at the highest pressure levels attainable with our equipment (about 100 dB at 28.3 kHz and 90 dB at 38.3 kHz). Thresholds at the highest frequencies improved considerably after 16 DAB, especially at the highest frequencies. Nevertheless, thresholds in 20-DAB neonates still exceeded adult values by 26 dB at 28.3 kHz and 40 dB at 38.3 kHz (vs. only 12 dB at 1.25 kHz). The development of thresholds at the highest frequencies caught up with those for lower frequencies during the 20- to 30-day period. However, even at 30 days, CAP thresholds for most stimulus frequencies approached, but did not quite reach, adult levels.
CAP Thresholds Normalized to Stapes Vibration
In a subset (5 group A adults and 31 neonates aged 14, 16, 18 and 20 days) of the ears included in figure 2, the responses to tones were also measured at the head of the stapes, near the incudostapedial joint (in conjunction with basilar-membrane experiments reported elsewhere [Overstreet et al., 2002a, b; Overstreet and Ruggero, 1999]). The CAP thresholds in each of those ears were normalized to the magnitude of stapes vibration velocity measured in the same animal, and the medians are plotted in figure 3. This figure, although differing in some respects from figure 2, indicates that the main features of the dependence of absolute CAP thresholds on frequency and age are reproduced in stapes-normalized thresholds. In adult gerbils, normalized CAP thresholds are scattered around a nearly flat line averaging 0.132 μm/s (SD = 0.067 μm/s), and there is no evidence of a systematic elevation as a function of increasing frequency. In contrast, stapes-normalized CAP thresholds in neonates aged 14–20 days were highest at the highest frequencies and decreased as a function of increasing age. [Only the datum for 5-kHz thresholds in 20-DAB neonates (fig. 3) departed significantly from this pattern. We view this datum as an outlier (note small sample size and large interquartile range).]
Fig. 3.
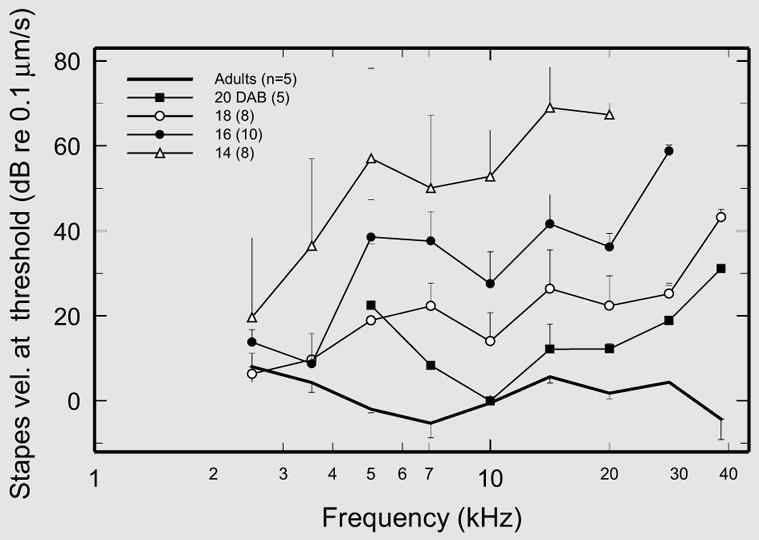
CAP thresholds in adult and neonatal gerbils normalized to stapes vibration. A subset of the threshold data in figure 2 are presented after normalization to the magnitudes of stapes velocity recorded in the same ears. Medians and interquartile ranges are indicated only for frequencies in which the data samples included 5 or more ears. (Stapes vibration was not recorded in gerbils aged 30 days.)
Discussion
Stapes-Normalized CAP Thresholds in the Cochleae of Adult Gerbils
The most important finding of the present study is that CAP thresholds in adult gerbils, expressed either relative to pressure near the tympanic membrane (fig. 1, 2) or normalized to the magnitude of stapes vibration (fig. 3), are roughly uniform in the range from 1.25 to 38.5 kHz. The finding of uniform stapes-normalized thresholds is especially significant because the normalization removes both the uncertainties of calibrating stimulus pressure at high frequencies (see Discussion on ‘Comparison of the present CAP thresholds with behavioral thresholds in adult gerbils’ below) and the signal transformations of the middle ear. Therefore, this finding establishes conclusively that intrinsic cochlear sensitivity (encompassing all stages of signal transformation within the cochlea, from the generation of pressure waves near the oval window to excitation of auditory-nerve fibers) is more or less independent of cochlear location between 1.25 and 38.5 kHz.
Stapes-Normalized CAP Thresholds in the Cochleae of Neonatal Gerbils
Two previous studies of cochlear development in gerbils attempted to separate intrinsic cochlear factors from middle-ear effects by using indirect methods based on the recording of cochlear microphonics [Harris and Dallos, 1984; Woolf and Ryan, 1988]. In the present study, normalization of CAP thresholds to stapes vibration permitted a direct assessment of the intrinsic contributions of cochlear maturation to the development of CAP thresholds, leading to this study’s second major finding. As demonstrated in figure 3, intrinsic cochlear sensitivity remains substantially immature at 20 days after birth, especially at the highest frequencies, and is not completely adult-like even 30 days after birth.
Comparison of Absolute CAP Thresholds of the Present Study with Previously Reported CAP and ABR Thresholds in Adult Gerbils
Figure 4a gathers together absolute CAP and ABR thresholds from several investigations on adult gerbils. The thresholds are widely scattered, probably due to methodological differences. Between 1 and 20 kHz, the thresholds of the present study (from fig. 1) occupy a midrange position. Much of the scatter of the curves in figure 4a disappears when the curves are individually normalized to their respective minima (fig. 4b), revealing substantial uniformity in the variation of thresholds as a function of frequency. With a single exception [McFadden et al., 1996], all normalized CAP and ABR thresholds are relatively constant up to 20 kHz [Boettcher et al., 1993; Huang et al., 1995; McGuirt et al., 1995; Müller, 1996; Ohlemiller and Siegel, 1992]. However, the thresholds of the present study stand out in that they remain relatively low even at the highest tested frequencies and are roughly similar across the entire range from 1.25 to 38.5 kHz. Thresholds in other studies exhibit large elevations at high frequencies, exceeding the thresholds of the present study by as much as 30–35 dB.
Explaining the Differences between the Present High-Frequency Thresholds and Those of Other CAP Studies
For simplicity’s sake, we confine the discussion in this section to neural studies that are directly comparable, i.e. those that measured CAP thresholds in adult gerbils and used a similar methodology. How can discrepancies among these studies be explained? Specifically, what accounts for the contrast between the thresholds of the present study, which are roughly uniform between 1.25 and 38.5 kHz, and the thresholds of other studies, which rise at frequencies higher than 10 kHz? The factors that we consider most likely to explain the discrepancies are the physiological state of the gerbils and the specification of the acoustic stimulus.
In discussing the sensory physiology of animals subjected to invasive procedures, it should be kept in mind that normal responses can be obtained only from preparations that remain relatively intact, whereas abnormalities, including threshold elevations, can be caused by any one of a myriad of possible insults. Therefore, although the ensuing discussion attempts to isolate the variables that may explain the difference between the present high-frequency thresholds and those of other CAP investigations, we submit that the task is difficult because it requires a comparison of experimental details that are rarely documented in the literature.
It is well known that high-frequency cochlear thresholds can be elevated by the effects of anesthesia, which are partly mediated by lowered cochlear temperature [Brown et al., 1983a, b; Ohlemiller and Siegel, 1992, 1994; Shore and Nuttall, 1985]. Thus, low cochlear temperature could have elevated high-frequency thresholds in most of the gerbil CAP studies, which did not monitor or control this variable. The present investigation of CAP thresholds and another by Ohlemiller and Siegel [1992] are exceptional in that they monitored cochlear temperature and took special precautions to prevent heat loss (see the first section of the Methods). Why are the high-frequency thresholds of Ohlemiller and Siegel substantially higher than those of the present study? Two possibilities come to mind. One is that by using forceps to open the bulla widely, Ohlemiller and Siegel [1992] may have generated intense high-frequency noise, causing threshold elevations [Brown et al., 1983a]. (In the present study, the bulla was opened via small holes produced by scoring and cutting the bone with a small knife; see the first section of the Methods.) The other possibility is that the acoustic calibration used by Ohlemiller and Siegel overestimated the intensity of high-frequency stimuli (see further discussion in the next section).
Comparison of the Present CAP Thresholds with Behavioral Thresholds in Adult Gerbils
Behavioral thresholds as a function of frequency were measured in adult gerbils in a single study, using a shock avoidance procedure [Ryan, 1976]. The behavioral threshold curve (large circles connected by thick-dash lines in fig. 4) exhibits a broad frequency region, from 1 to 16 kHz, which is nearly flat, a transition segment between 16 and 40 kHz in which thresholds rise at a rate of 13 dB/octave and a steep terminal slope (of about 66 dB/octave) between 40 and 60 kHz. The behavioral threshold curve has a wider pass-band than any of the threshold curves in figure 4 except for the present CAP data. Discounting the various irregularities of the present CAP thresholds as measurement errors, the main difference between CAP and behavioral threshold curves is that the former exhibit an essentially flat pass-band up to 38.5 kHz, whereas the latter rise by about 17 dB between 16 and 40 kHz (fig. 4b).
Are the Present CAP Thresholds Consistent with Behavioral Thresholds?
Given that behavioral and CAP thresholds reflect different physiological processes and are measured under disparate experimental conditions, it is unclear whether the discrepancies between the shape of the present CAP threshold curve and the behavioral threshold curve in the range of 16–38 kHz should be considered significant. Most importantly, CAP thresholds are determined solely by cochlear processes, whereas behavioral thresholds depend additionally on the immensely more complex processes in the brain. Furthermore, the behavioral thresholds were determined binaurally in nonanesthetized animals with intact pinnae and closed bullae and stimuli calibrated in the free field. CAP thresholds, on the other hand, were measured monaurally in anesthetized animals with the pinnae removed and bullae opened, and with stimuli calibrated near the tympanic membrane.
The acoustic calibration at high frequencies is the factor that we consider most likely to be responsible for differences between the present CAP thresholds and the behavioral thresholds. Even when using a probe microphone, specification of the acoustic stimulus at high frequencies is difficult because ossicular vibrations are stimulated by the weighted spatial average of the local pressures and acoustic loads distributed across the surface of the tympanic membrane [Stinson and Khanna, 1989]: ‘… above about 10 kHz … it is not possible to define the acoustical input to the ear from sound pressure level measured at any single location’ [Khanna and Stinson, 1985; see also Stinson, 1985; Stinson and Shaw, 1982]. Overstreet and Ruggero [2002] have recently noted that referencing gerbil stapes vibrations to pressure calibrations measured in situ with a probe tube introduced spurious high-frequency valleys and peaks in velocity magnitude-versus-frequency curves that had no counterpart in the effective pressure driving ossicular vibrations. In the present study, we largely circumvented these problems by adopting calibration methods that either did not use probe tubes at all (group B) or that limited their use to the measurement of frequencies <16 kHz (group A and neonates). Nevertheless, we suspect that some of the features of the CAP thresholds of figure 1 and 2 reflect calibration errors.
Most experiments of human psychoacoustics and animal auditory physiology are carried out using closed sound delivery systems, in part because specification of sound pressure is usually simpler and more accurate in closed systems than in the free field. In the case of animal psychoacoustics, the free-field acoustic stimulus can be specified with some precision by requiring the animal’s head to be in a fixed position [Heffner and Heffner, 1991]. In the shock avoidance method used by Ryan [1976], the animal moves freely in a shuttle box, leading to possible errors due to variations with position in the sound field that can reach 15 dB [Miller, 1970]. The apparent conflict between CAP and behavioral thresholds (fig. 4b) would be resolved if Ryan’s investigation overestimated behavioral thresholds at 32 and 40 kHz relative to those at 16 kHz. At least in the case of the chinchilla, behavioral procedures in which the head position is not fixed do indeed overestimate (by about 7 dB) thresholds at 32 kHz relative to those at 16 kHz [Heffner and Heffner, 1991].
Comparison of CAP and/or ABR Absolute Thresholds Recorded in Neonatal Gerbils
The absolute CAP thresholds in neonates of the present study (fig. 2) are generally consistent with, and extend to higher frequencies, the salient findings of an extensive study by McGuirt et al. [1995], which reported CAP thresholds in gerbil neonates aged 14–30 days for frequencies up to 20 kHz, and of another, limited to frequencies up to 10 kHz in neonates aged 22 and 30 days [Huang et al., 1995]. In general, thresholds in neonates are most elevated at the highest frequencies, thresholds at those frequencies are the last to reach near-adult values, and thresholds at 30 DAB remain slightly elevated at many frequencies. The results of figure 2 are also consistent with the ABR data of McFadden et al. [1996] in that thresholds improve more rapidly with age at mid-frequencies (2–8 kHz) than at higher frequencies (10–32 kHz). Müller [1996] reported CAP thresholds for frequencies up to 32 kHz in neonatal gerbils aged 14–18 days, but it is difficult to draw conclusions from his data because few animals were tested and the results were variable.
Further Evidence for Wide-Band Hearing in Adult Gerbils
The present finding of uniform CAP thresholds across a wide frequency range (1.25–38.5 kHz) in adult gerbils (fig. 1–3) is consistent with recent mechanical measurements in the same species: (1) the magnitudes of stapes vibrations, referenced to pressure at the tympanic membrane, are approximately uniform between 1 and 40 kHz [Overstreet and Ruggero, 2002]; (2) the magnitudes of pressure in the scala vestibuli near the stapes, referenced to pressure at the tympanic membrane, are also very similar across frequency up to 46 kHz [Olson, 1998], and (3) basilar-membrane vibrations are as sensitive and nonlinear at a site with CFs of 34–37 kHz [Overstreet et al., 2002a] as they are at a location 1.8 mm farther apical [Cooper, 2000; Ren and Nuttall, 2001].
The present findings are also consistent with thresholds at the auditory nerve [Müller, 1996] and the inferior colliculus [Brown, 1973b] of gerbils. Of 8 auditory-nerve fibers with CFs between 33 and 44 kHz reported by Müller, 5 had CF thresholds as low as those of fibers with CFs near 1 kHz [Müller, 1996, fig. 2]. Interestingly, Müller reported much higher CAP thresholds in the same experimental population [Müller, 1996, fig. 2]. Relatively elevated CAP thresholds vis-à-vis single-fiber CF thresholds have also been reported for the chinchilla [Dallos et al., 1978, fig. 2]. The higher CAP thresholds may reflect a decreased innervation density at the extreme base of the cochlea [Bohne et al., 1982]. Finally, recordings from the inferior colliculus of gerbils [Brown, 1973b] show that thresholds at 40 kHz are only 2–3 dB higher than at 15 kHz.
Behavioral Significance of High-Frequency Auditory Sensitivity in Gerbils
High-frequency auditory sensitivity may play an important role in social interactions among gerbils. Gerbil pups produce cries at 40–50 kHz which can be detected by hair cells in the adult gerbil cochlea, as demonstrated by recordings of cochlear microphonics [Brown, 1970, 1973a]. In addition, adult gerbils emit vocalizations at frequencies as high as 38 kHz during sexual activity [Holman and Hutchison, 1985] and upon detection of certain meaningful aromas emitted by other gerbils [Brown et al., 1988].
Acknowledgments
We thank Ed Walsh for his thorough review of a previous version of the manuscript. The work on group A adult gerbils and neonates, part of a doctoral dissertation submitted by E.H.O. to the University of Minnesota [Overstreet, 2000], was supported by grant DC-00419 to M.A.R. from the National Institute on Deafness and Other Communication Disorders (NIDCD). Data on group B adult gerbils were obtained with the support of NIDCD grant DC-00089 to Peter Dallos and National Science Foundation grant IBN-0077476 to C.-P.R.
Footnotes
The thresholds for neonates and group A adults (fig. 1, 2) differ from those originally reported in a doctoral dissertation [Overstreet, 2000]: a different method for the pressure calibrations has been adopted here, and some computational errors in the dissertation were corrected.
References
- Boettcher FA, Mills JH, Norton BL, Schmiedt RA. Age-related changes in auditory evoked potentials of gerbils. II Response latencies. Hear Res. 1993;71:146–156. doi: 10.1016/0378-5955(93)90030-5. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Kenworthy A, Carr CD. Density of myelinated nerve fibers in the chinchilla cochlea. J Acoust Soc Am. 1982;72:102–107. doi: 10.1121/1.387994. [DOI] [PubMed] [Google Scholar]
- Brown AM. Bimodal cochlear response curves in rodents. Nature. 1970;228:576–577. doi: 10.1038/228576a0. [DOI] [PubMed] [Google Scholar]
- Brown AM. High frequency peaks in the cochlear microphonic response of rodents. J Comp Physiol A. 1973a;83:377–392. [Google Scholar]
- Brown AM. High levels of responsiveness from inferior colliculus of rodents at ultrasonic frequencies. J Comp Physiol A. 1973b;83:393–406. [Google Scholar]
- Brown MC, Smith DI, Nuttall AL. Anesthesia and surgical trauma: Their influence on the guinea pig compound action potential. Hear Res. 1983a;10:345–358. doi: 10.1016/0378-5955(83)90097-7. [DOI] [PubMed] [Google Scholar]
- Brown MC, Smith DI, Nuttall AL. The temperature dependency of neural and hair cell responses evoked by high frequencies. J Acoust Soc Am. 1983b;73:1662–1670. doi: 10.1121/1.389387. [DOI] [PubMed] [Google Scholar]
- Brown RE, Hauschild M, Holman SD, Hutchison JB. Mate recognition by urine odors in the Mongolian gerbil (Meriones unguiculatus) Behav Neural Biol. 1988;49:174–183. doi: 10.1016/s0163-1047(88)90495-5. [DOI] [PubMed] [Google Scholar]
- Chan JC, Musicant AD, Hind JE. An insert earphone system for delivery of spectrally shaped signals for physiological studies. J Acoust Soc Am. 1993;93:1496–1501. doi: 10.1121/1.406807. [DOI] [PubMed] [Google Scholar]
- Cooper NP. Basilar membrane vibrations in the basal turn of the gerbil cochlea. Assoc Res Otolaryngol Mid-Winter Meet Abstr. 2000;23:205. [Google Scholar]
- Dallos P, Harris D, Özdamar Ö, Ryan A. Behavioral, compound action potential, and single unit thresholds: Relationship in normal and abnormal ears. J Acoust Soc Am. 1978;64:151–157. doi: 10.1121/1.381980. [DOI] [PubMed] [Google Scholar]
- Harris DM, Dallos P. Ontogenetic changes in frequency mapping of a mammalian ear. Science. 1984;225:741–743. doi: 10.1126/science.6463651. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Behavioral hearing range of the chinchilla. Hear Res. 1991;52:13–16. doi: 10.1016/0378-5955(91)90183-a. [DOI] [PubMed] [Google Scholar]
- Holman SD, Hutchison JB. Effects of intracranial androgen on the development of masculine ultrasonic vocalizations in the Mongolian gerbil (Meriones unguiculatus) J Endocrinol. 1985;107:355–363. doi: 10.1677/joe.0.1070355. [DOI] [PubMed] [Google Scholar]
- Huang JM, Berlin CI, Cullen JK, Jr, Wickremasinghe AR. Development of the VIIIth nerve compound action potential evoked by low-intensity tone pips in the Mongolian gerbil. Hear Res. 1995;88:14–18. doi: 10.1016/0378-5955(95)00094-k. [DOI] [PubMed] [Google Scholar]
- Khanna SM, Stinson MR. Specification of the acoustical input to the ear at high frequencies. J Acoust Soc Am. 1985;77:577–589. doi: 10.1121/1.391876. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Walsh EJ, McGee J. Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus) Hear Res. 1996;100:68–79. doi: 10.1016/0378-5955(96)00108-6. [DOI] [PubMed] [Google Scholar]
- McGuirt JP, Schmiedt RA, Schulte BA. Development of cochlear potentials in the neonatal gerbil. Hear Res. 1995;84:52–60. doi: 10.1016/0378-5955(95)00015-v. [DOI] [PubMed] [Google Scholar]
- Miller JD. Audibility curve of the chinchilla. J Acoust Soc Am. 1970;48:513–523. doi: 10.1121/1.1912166. [DOI] [PubMed] [Google Scholar]
- Mills JH, Schmiedt RA, Kulish LF. Age-related changes in auditory potentials of Mongolian gerbil. Hear Res. 1990;46:201–210. doi: 10.1016/0378-5955(90)90002-7. [DOI] [PubMed] [Google Scholar]
- Müller M. The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear Res. 1996;94:148–156. doi: 10.1016/0378-5955(95)00230-8. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Siegel JH. The effects of moderate cooling on gross cochlear potentials in the gerbil: Basal and apical differences. Hear Res. 1992;63:79–89. doi: 10.1016/0378-5955(92)90076-y. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Siegel JH. Cochlear basal and apical differences reflected in the effects of cooling on responses of single auditory nerve fibers. Hear Res. 1994;80:174–190. doi: 10.1016/0378-5955(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Olson ES. Observing middle and inner ear mechanics with novel intracochlear pressure sensors. J Acoust Soc Am. 1998;103:3445–3463. doi: 10.1121/1.423083. [DOI] [PubMed] [Google Scholar]
- Overstreet EH. The Development of Basilar Membrane Mechanics in the Mammalian Cochlea; PhD dissertation. University of Minnesota; 2000. [Google Scholar]
- Overstreet EH, Ruggero MA. The development of basilar membrane mechanics at the hook region of the Mongolian gerbil cochlea. Assoc Res Otolaryngol Mid-Winter Meet Abstr. 1999;22:135–136. [Google Scholar]
- Overstreet EH, Ruggero MA. Development of wide-band middle ear transmission in the Mongolian gerbil. J Acoust Soc Am. 2002;111:261–270. doi: 10.1121/1.1420382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet EH, Temchin AN, Ruggero MA. Basilar-membrane vibrations near the round window of the gerbil cochlea. JARO J Assoc Res Otolaryngol. 2002a doi: 10.1007/S101620023. in press. Published online 27 February 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet EH, Temchin AN, Ruggero MA. Development of passive basilar-membrane mechanics at the base of the cochlea in neonatal gerbils. J Physiol. 2002b doi: 10.1113/jphysiol.2002.025205. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce M, Richter CP, Cheatham MA. A reconsideration of sound calibration in the mouse. J Neurosci Methods. 2001;106:57–67. doi: 10.1016/s0165-0270(01)00329-6. [DOI] [PubMed] [Google Scholar]
- Ren T, Nuttall AL. Basilar membrane vibration in the basal turn of the sensitive gerbil cochlea. Hear Res. 2001;151:48–60. doi: 10.1016/s0378-5955(00)00211-2. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Application of a commercially manufactured Doppler-shift laser velocimeter to the measurement of basilar-membrane vibration. Hear Res. 1991;51:215–230. doi: 10.1016/0378-5955(91)90038-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AF. Hearing sensitivity of the Mongolian gerbil, Meriones unguiculatus. J Acoust Soc Am. 1976;59:1222–1226. doi: 10.1121/1.380961. [DOI] [PubMed] [Google Scholar]
- Shore SE, Nuttall AL. The effects of cochlear hypothermia on compound action potential tuning. J Acoust Soc Am. 1985;77:590–598. doi: 10.1121/1.391877. [DOI] [PubMed] [Google Scholar]
- Stinson MR. The spatial distribution of sound pressure within scaled replicas of the human ear canal. J Acoust Soc Am. 1985;78:1596–1602. doi: 10.1121/1.392797. [DOI] [PubMed] [Google Scholar]
- Stinson MR, Khanna SM. Sound propagation in the ear canal and coupling to the eardrum, with measurements on model systems. J Acoust Soc Am. 1989;85:2481–2491. doi: 10.1121/1.397743. [DOI] [PubMed] [Google Scholar]
- Stinson MR, Khanna SM. Spatial distribution of sound pressure and energy flow in the ear canals of cats. J Acoust Soc Am. 1994;96:170–180. doi: 10.1121/1.410461. [DOI] [PubMed] [Google Scholar]
- Stinson MR, Shaw EAG. Wave effects and pressure distribution in the ear canal near the tympanic membrane. J Acoust Soc Am. 1982;71:S88. [Google Scholar]
- Taylor MM, Creelman CD. PEST: Efficient estimates on probability functions. J Acoust Soc Am. 1967;41:782–787. [Google Scholar]
- Woolf NK, Ryan AF. Ontogeny of neural discharge patterns in the ventral cochlear nucleus of the Mongolian gerbil. Brain Res. 1985;349:131–147. doi: 10.1016/0165-3806(85)90138-5. [DOI] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF. Contributions of the middle ear to the development of function in the cochlea. Hear Res. 1988;35:131–142. doi: 10.1016/0378-5955(88)90112-8. [DOI] [PubMed] [Google Scholar]


