Abstract
Poor oral bioavailability has been a major limitation for the successful use of dietary flavonoids as cancer chemopreventive agents. In this study, we examined fully methylated flavones as promising improved agents. In the humanoral SCC-9 cancer cells, 5,7-dimethoxyflavone and 5,7,4’-trimethoxyflavone were both ten times more potent inhibitors of cell proliferation (IC50 values 5-8 μM) than the corresponding unmethylated analogs chrysin and apigenin. Flow cytometry indicated that both methylated flavones arrested the SCC-9 cells in the G1 phase with a concomitant decrease in the S phase, dramatically different from the unmethylated analogs, which promoted G2/M phase arrest. Both methylated compounds inhibited the proliferation of two other cancer cell lines with very little effect on two immortalized normal cell lines. Examination of additional flavone structures indicated that methylated flavones in general have antiproliferative properties. Finally, we demonstrated that 5,7-dimethoxyflavone, in contrast to its unmethylated analog chrysin, was well absorbed and had high oral bioavailability as well as tissue accumulation in vivo in the rat. Thus, fully methylated flavones appear to have great potential as cancer chemopreventive/chemotherapeutic agents, in particular in oral cancer.
Keywords: Flavonoids, Methylated flavones, Cancer prevention, Proliferation, Bioavailability
1. Introduction
Dietary flavonoids and other polyphenols have over many years been demonstrated to have cancer chemopreventive properties in various biological systems [1-3]. Novel biochemical mechanisms are still being revealed at an accelerating rate in cell culture experiments [4-7]. However, extending such studies to the in vivo situation in animals or even more so in humans, using modest clinically tolerable doses, in most cases have been unsuccessful. This clearly can be explained by very poor oral bioavailability of the polyphenols, as has been shown directly in humans for chrysin [8], quercetin [9,10] the tea flavonoids [11, 12] and resveratrol [13, 14]. Mechanistically, this is related to the free hydroxyl groups of the polyphenols, giving rise to rapid intestinal/hepatic conjugation by glucuronidation and/or sulfation and excretion [15].
In contrast, we have recently described high metabolic stability as well as high intestinal transport of fully methylated flavones [16, 17]. Although many of these compounds are present in plants, they have attracted only a modest interest as potential chemopreventive agents. One reason may be their lack of antioxidant properties, which in general has been associated with free hydroxyl groups [18]. However, some of these methylated flavones show remarkable inhibitory effects on carcinogen activating enzymes, thus, making them potentially useful as inhibitors of carcinogenes is at the initiation stage[19-21]. Although inhibition of cancer cell proliferation has been reported for methylated flavones [22, 23], some of which appear highly potent [23], these studies were not pursued further. In contrast, methylation even reduces the biological activities of the isoflavones [24]. Thus, the effect of methylation on the cancer chemopreventive activities of flavonoids is not clearly understood. Our hypothesis was that methylation does not diminish the ability of the flavones to inhibit the proliferation of oral cancer cells while increasing their oral bioavailability.
In this study, we therefore compared the effects of two methylated flavones, i.e. 5,7-dimethoxyflavone (5,7-DMF) and 5,7,4’-trimethoxyflavone (5,7,4’-TMF), with their unmethylated analogs chrysin and apigenin (structures in Fig. 1) on cell proliferation. Both chrysin and in particular apigenin have been extensively studied in the past [5, 25-27]. Several other flavones were investigated as well to get a preliminary feel for chemical structure - activity relationships. Our cell models contained cancer as well as noncancer cells and cell proliferation was measured using incorporation of BrdU into new lysynthesized DNA as the assay. Effects on cell cycle progression were also investigated. In addition, we determined the cellular uptake as well as the oral bioavailability of 5,7-DMF compared to chrysin in the rat in vivo.
Fig. 1.
Structures of flavones used in this study.
2. Materials and methods
2.1. Materials
Chrysin, apigenin, flavone, propidium iodide and Cremophor EL®were obtained from Sigma Chemical Co. (St. Louis, MO). 5,7-Dimethoxyflavone (5,7-DMF), 5,7,4’-trimethoxyflavone (5,7,4’-TMF),7,4’-dimethoxyflavone (7,4’-DMF), 7-methoxyflavone (7-MF) and 5,4’-dimethoxyflavone (5,4’-DMF) were obtained from Indofine Chemical Co., Inc. (Hillsborough, NJ).5,6,7,8,4’-Pentamethoxyflavone (tangeretin) was purchased from ChromaDex (Santa Ana, CA). All other chemicals were purchased from Sigma or Fisher.
2.2. Cell culture and treatments
The human oral (tongue) squamous cell carcinoma SCC-9 cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA) and cultured in DMEM/Ham’s F12 medium (Fisher, Pittsburgh, PA), supplemented with 10% fetal bovine serum (FBS) (Atlas Biologicals, Fort Collins, CO), 1% penicillin/streptomycin and 0.4 μg/ml hydrocortisone in a 5% CO2 atmosphere at 37°C. Vehicle dimethyl sulfoxide (DMSO, 0.1% final volume) was used as a control in all experiments. The cells were used at passage 18-30. The human pharynx SCC FaDu cells (ATCC) were cultured in minimum essential medium (Eagle) with Earle’s salts, nonessential amino acids, sodium pyruvate and 10% FBS. Human mammary MCF-7 cancer cells (ATCC) were grown in minimum essential medium (Eagle) with Earle’s salts, 20 mM HEPES, insulin (10 μg/ml), sodium pyruvate and 10% FBS. Virus-transformed normal human esophageal epithelial HET-1A cells [28] were cultured in DMEM with 2% FBS, sodium pyruvate, insulin, transferrin, hydrocortisone and cholera toxin, as previously described[29]. Virus-transformed normal human lung epithelial BEAS-2B cells [30] were cultured in serum-free bronchial epithelial growth medium (Cambrex, Walkersville, MD).
2.3. Cell Proliferation Assay
The rate of proliferation of cells was determined as the rate of incorporation of bromodeoxyuridine (BrdU) into cellular nucleic acids [31], using a kit from Calbiochem/EMD Biosciences (La Jolla, CA). Briefly, cells seeded in 96-wells at 104 cells/well were allowed to attach overnight prior to incubation with flavones (0-100 μM) in serum-free medium with 0.1 % bovine serum albumin for 24 h. The cells were then pulsed with BrdU in the presence of the flavone for 4 h. The cells were fixed, denatured and incubated with anti-BrdU primary antibody, secondary antibody and substrate according to the manufacturer’s protocol. The absorbance was read at 450 nm with 590 nm background subtraction with appropriate blanks on a FLUOStar Optima platereader (BMG Labtech Inc., Durham, NC).
2.4. Cell uptake Assay
Confluent SCC-9 cells cultured in 6-well plates were incubated at 37° with 25 μM chrysin or 5,7-DMF for 0.5, 2, 6 or 24 hr in complete cell culture medium. The uptake was terminated by rinsing the cell layers twice with ice-cold saline. The flavones were extracted twice from the cells with 1 ml of methanol for 10 min on an orbital shaker. The combined methanol extracts were taken to dryness under nitrogen, reconstituted in 500 μl mobile phase and spun down. An aliquot (100 μl) of the supernatant was analyzed by HPLC as described below. The peak areas were compared to standard curves and the uptake was normalized for cellular protein [32].
2.5. Cell toxicity assay
SCC-9 cells seeded in 96-well plates (2500 cells/well) were allowed to attach overnight. The cells were then treated with 0-100 μM 5,7-DMF or chrysin for 24 h as above. After removal of the medium, the cells were fixed with glutaraldehyde, washed, and stained with 0.1% crystal violet, as previously . described [33]. The washed and dried plates were shaken with 10% acetic acid and the absorbance was read at 570 nm.
2.6. Cell cycle analysis
Asynchronized cells were plated in 60 mm2 plates at 0.1×106 cells/ml, grown until 75-80% confluency then treated with 0, 5, 10, 25 and 50 μM flavonoid for 24, 48 and 72 h [4]. The cells were collected by trypsinization, washed twice with cold phosphate-buffered saline (PBS), and centrifuged. The pellets were resuspended in 100 μl cold PBS and 900 μl cold 70% ethanol and incubated overnight at 4°C. After centrifugation, the cell pellets were washed with cold PBS and resuspended in PBS containing 1mg/ml RNase and 100 μg/ml propidium iodide. Following incubation in the dark for 30 min at 4°C, the cells were analyzed by flow cytometry. Data were acquired on a BD FACSCalibur using Cell Quest software and were analyzed using Modfit LT software.
2.7. In vivo bioavailability study
2.7.1.Animals
Male F344 rats, 4 weeks old, were obtained from Charles River Laboratories (Wilmington, MA) and quarantined for 10 days. They were kept under controlled conditions with 12-h light/dark cycle and constant temperature and humidity in the Dept. of Laboratory Animal Resources and had free access to water and standard rat chow (Harlan Teklad). The protocol was approved by the Institutional Animal Care and Use Committee. The rats were killed by an overdose of gas anesthetic.
2.7.2.Experimental procedure
A suspension of chrysin and 5,7-DMF (2 mg/ml of each) was prepared in Cremophor EL®/ethanol/saline (1/1/8,v/v/v). After 24 h of fasting, the rats were administered 0.2-0.3 ml of the suspension by gavage (for a dose of 5 mg/kg). Control animals received the same volume of vehicle. At 0.5, 1, 2 or 3 h after the drug dosing, the rats were sacrificed. Five animals were used at each time-point. Blood was obtained from the inferior vena cava and centrifuged . after the addition of EDTA. The plasma was frozen and stored at -20°C. Lungs, liver, kidneys and colon were excised and immediately frozen in liquid nitrogen. The tissues were stored at -20°C until analyzed.
2.7.3.Analysis
Plasma was mixed with an equal volume of methanol, vortex-mixed and centrifuged at 14,000 × g for 5 min. Aliquots of the supernatant (100 μl) were analyzed by HPLC, using a Symmetry C18 column (3.9 mm × 150 mm, 5 μm particle size) from Waters Corporation (Milford, MA). The mobile phase was methanol/trifluoroacetic acid/water (55/0.3/45, v/v/v) and detection was with a Model 996 photodiode array detector (Waters) at 268 nm. Quantitation was based on a comparison of peak areas with standard curves (0.05 - 25 μM) for 5,7-DMF and chrysin. The minimum detectable concentration (two times background noise) was 0.05 μM. Aliquots of lung, liver and kidney tissue (100-400 mg) were homogenized on ice in 1 ml of phosphate-buffered saline, using a Polytron homogenizer. To 0.5 ml of the homogenate was added 0.5 ml of methanol. After 1 min of vortex-mixing, the samples were centrifuged as above and the supernatant analyzed by HPLC. With the colon, the top 2 cm with the first fecal pellet were homogenized separately as above.
2.8. Statistical analysis
Data were expressed as means ± SEM. Statistical significance of differences between samples were calculated by Student’s two-tailed t-test or ANOVA with Dunnett multiple comparison post-test. P <0.05 was considered significant. The IC50 values were calculated using Prism (GraphPad Software, San Diego, CA).
3. Results
3.1. Effects on cell proliferation and toxicity
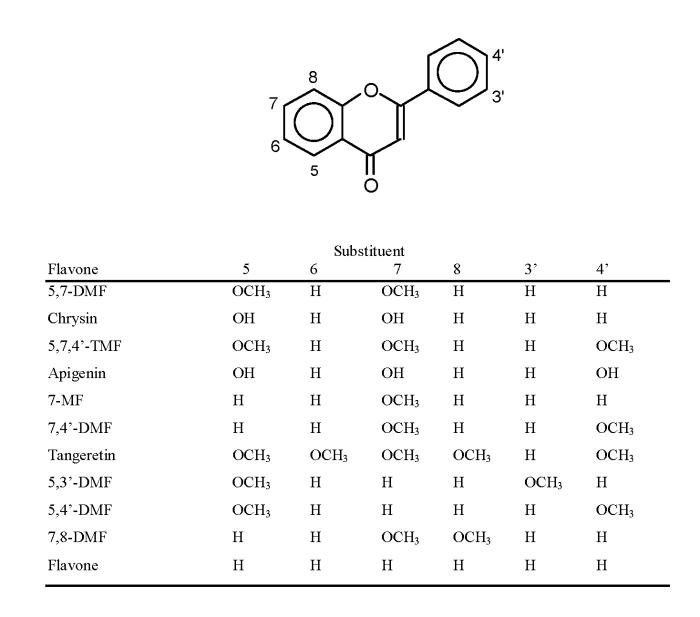
Two pairs of compounds were selected for these studies, i.e. the simple flavones 5,7-dimethoxyflavone (5,7-DMF) and its unmethylated analog chrysin and 5,7,4’-trimethoxyflavone (5,7,4’-TMF) and its unmethylated analog apigenin (structures, see Fig. 1). Chrysin and apigenin are both well studied flavonoids. Our cancer model was the human oral squamous carcinoma SCC-9 cell, a rather aggressively growing cell line [34], and the effect on cell proliferation was measured as de novo DNA synthesis, using the BrdU incorporation assay [31]. 5,7-DMF inhibited cell proliferation with an IC50 value of 8 μM (Fig. 2A), which was about 10 times more potent than chrysin. With chrysin, but not with 5,7-DMF, there was a slight activation at the 10 μM concentration. 5,7,4’-TMF had a similar potency as 5,7-DMF (Fig. 2B) with an IC50 value of 5 μM, 8 times more potent than apigenin. Also with apigenin there was a slight activation at the 5 μM concentration.
Fig. 2.
Effect of the methylated flavones 5,7-DMF and 5,7,4’-TMF compared to the unmethylated analogs chrysin and apigenin on SCC-9 cell proliferation. Cell proliferation, expressed as percent of control (DMSO-treatment), was measured as BrdU incorporation into cellular DNA after a 24-h exposure of the cells to the flavones. Mean values ± SEM are shown (n = 10). The numbers shown in the figure are the calculated IC50 values.
* significantly lower than control, P < 0.05.
# significantly higher than control, P < 0.05.
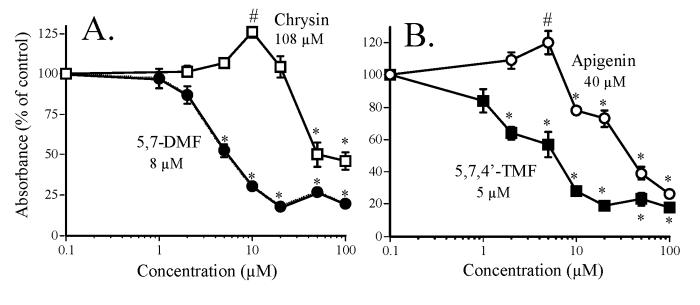
The greater potency of the methylated versus the unmethylated flavones in Fig. 2A and B could conceivably be due to greater cell uptake of the methylated flavones. This was examined with 5,7-DMF compared to chrysin. After incubation of the SCC-9 cells for up to 24 hr with 25 μM flavones, the uptake was rapid and virtually identical for the two compounds except for the somewhat higher 24 hr uptake of chrysin (Fig. 3).
Fig. 3.
SCC-9 cell uptake of chrysin and 5,7-DMF. The cell monolayers were incubated with 25 μM flavones in complete medium for various time.
* significantly higher than 0.5, 2 and 6 hr uptake, P < 0.05; (n = 6).
A small number of additional methylated flavones were investigated for antiproliferative effects compared to 5,7,4’-TMF and 5,7-DMF in the SCC-9 cells. The calculated IC50 values were 36.5 μM (7-MF), 24.2 μM (7,4’-DMF), and 19.3 μM (tangeretin). In addition, 5,4’-DMF, 5,3’-DMF and 7,8-DMF showed weaker effects. The unsubstituted flavone had an IC50 value of 8.3 μM.
As evidence of cell toxicity, we used both crystal violet staining [33] and the MTT assay [35]. Results from the crystal violet assay for 5,7-DMF and chrysin in the SCC-9 cells showed an IC50 value of about 50 to 100 μM for both compounds (n = 12). Virtually identical results were obtained with the MTT assay (data not shown).
3.2. Effects on the proliferation of cancer versus noncancer cells
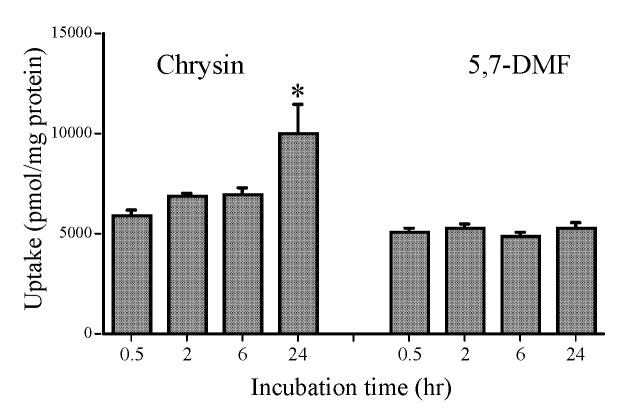
To determine if the potent antiproliferative effects observed by the methylated flavones on the SCC-9 cells in Fig. 2 were selective for cancer cells vs. noncancerous cells, we compared the effects of 5,7-DMF and chrysin in two additional cancer cell lines with those in two noncancer cell lines. In the FaDu human larynx SCC cells (Fig. 4A), both 5,7-DMF and chrysin showed similar potency as 5,7-DMF in the SCC-9 cells (IC50 8-10 μM). In the MCF-7 human breast cancer cells, both compounds again had similar but slightly lower potency (Fig. 4B) with IC50 values of 10-20 μM. 5,7-DMF seemed to exert a bimodal effect with stimulation of cell growth at higher concentrations. In contrast, two normal but transformed human cell lines, i.e. the HET-1A esophageal cells [28] and the BEAS-2B bronchial epithelial cells [30], were much less sensitive to both 5,7-DMF and chrysin. Both of these cell lines retain epithelial morphology and are non-tumorigenic in nude mice [28, 30]. Thus, both flavones had estimated IC50 values towards cell proliferation of as high as 115-150 μM in these cells (Figs. 4C and 4D).
Fig. 4.
Effect of 5,7-DMF (●) compared to chrysin (□) on the proliferation of cancer cells (A and B) and noncancer cells (C and D). A, FaDu cells;B, MCF-7 cells;C, HET-1A cells and D, BEAS-2B cells. Cell proliferation, expressed as percent of control (DMSO-treatment) was measured as BrdU incorporation into cellular DNA after a 24-h exposure of the cells to the flavones. Mean values ± SEM are shown (n = 10). The numbers shown in the figure are the calculated IC50 values.
3.3. Effects on cell cycle regulation
To determine whether the growth inhibitory effect of the two pairs of flavones was accompanied by cell cycle arrest, SCC-9 cells were treated with increasing concentrations, i.e. 0, 5, 10, 25 and 50 μM, of the compounds for 72 h. Asynchronized cells were harvested, fixed in ethanol, stained with propidium iodide and analyzed for cell cycle distribution by flow cytometry. Fig. 5A shows that 5,7-DMF caused a dose-dependent increase in the G1 phase with a concomitant decrease in the S phase and no change in the G2/M population. This was distinctly different from the more modest effects of chrysin on the cell cycle distribution (Fig. 5B) with an increase in the G2/M phase but at the highest concentration (50 μM) only.
Fig. 5.
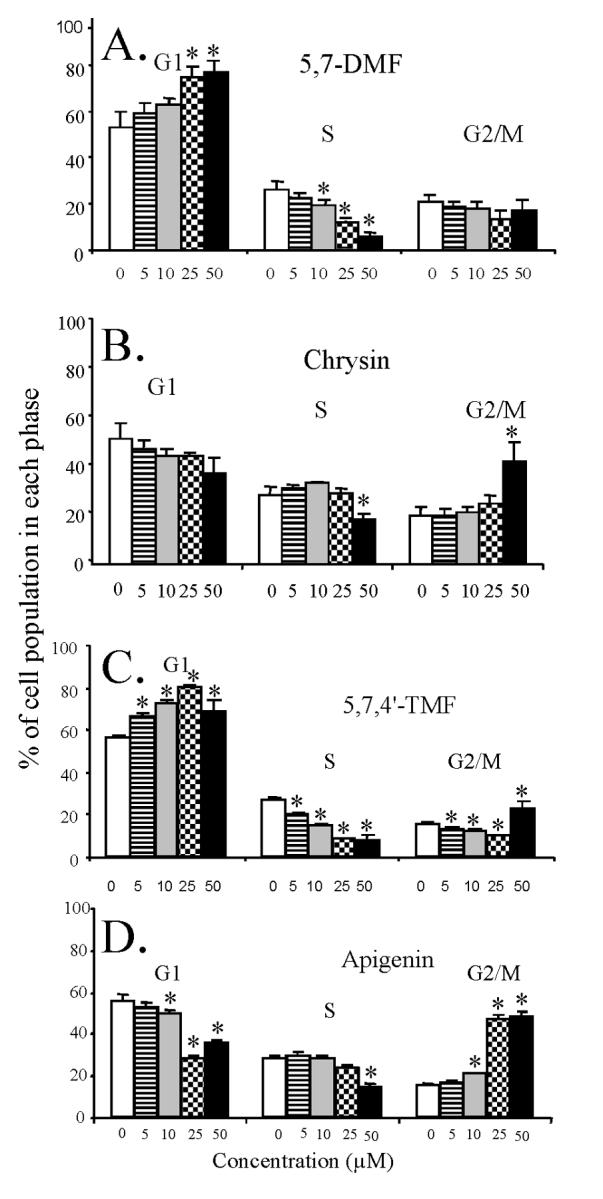
Effect of 5,7-DMF (A) compared to chrysin (B) and 5,7,4’-TMF (C) compared to apigenin (D) on SCC-9 cell cycle progression. The cells were exposed to varying concentrations of the flavones for 48 h. The percentage of cells in G1, S and G2/M phase was measured by flow cytometry after propidium iodide staining. Mean values of 3 experiments with duplicate samples are shown.
* significantly different from control, P < 0.05 or better.
Fig. 5C shows the corresponding effects of 5,7,4’-TMF with a dose-dependent increase in the G1 phase, as with 5,7-DMF, and a decrease in the S phase and not much change in the G2/M phase population. Fig. 5D shows the effects of apigenin, which qualitatively is similar to the effects of chrysin with a distinct increase in the G2/M phase population, very different from the effects of 5,7,4’-TMF. When examining the quantitative changes in the G1 phase caused by 5,7-DMF and 5,7,4’-TMF, they appear very similar, with effects already at the lowest concentration investigated (5 μM). This was statistically significant for 5,7,4’-TMF. The results seen by flow cytometry were thus very similar to those seen in the BrdU incorporation assay (Fig. 2).
3.4. Oral bioavailability in the rat
In previous in vitro studies, we showed that the methylated flavones had increased hepatic metabolic stability as well as intestinal transport in comparison with their unmethylated analogs [16,17]. In the present investigation, these observations were extended to the examination of the oral absorption and bioavailability of one of these compounds, i.e., 5,7-DMF, compared to chrysin in the rat in vivo. This test was considered particularly stringent as the rat is well known to be a species with very high activity of the xenobioticoxidizing enzymes. Both compounds were administered simultaneously by gavage (5 mg/kg each) and plasma and tissue concentrations were measured by HPLC/UV using a mobile phase that permitted separation of the two compounds. 5,7-DMF was clearly detected in plasma (Fig. 6A) with a peak concentration of 2.5 ± 0.8 μM (mean ± SEM) at 1 h after the dose. The area under the plasma concentration-time curve was 58.8 μg • ml-1 • min. Chrysin was not detectable in plasma at any time-point. 5,7-DMF was also clearly detected in liver, lung and kidney tissues with concentrations in the liver exceeding those in the plasma by as much as 7-fold, i.e. 16.5 ± 5 μM at 1 h after the dose (Fig. 6B). The half-life for 5,7-DMF in plasma was short,i.e. 16 min, most likely reflecting the high oxidative capacity of the rat. Interestingly, the half-life for 5,7-DMF calculated from the tissue measurements was considerably longer, ranging from 29 to 68 min, the latter in the lungs, indicating this tissue as a favorable accumulation site. The liver, which showed the highest accumulation of 5,7-DMF, should be predicted to be a site where 5,7-DMF might exert its greatest activity.
Fig. 6.
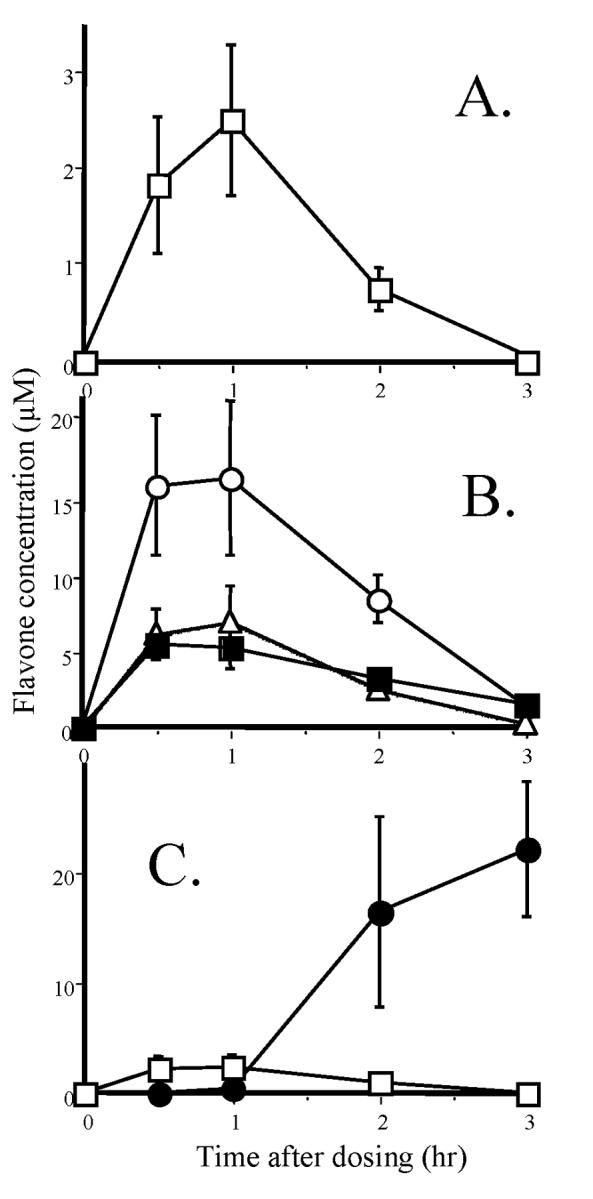
Plasma and tissue levels of 5,7-DMF and chrysin after oral administration of 5 mg/kg in rats. A. Plasma 5,7-DMF (no chrysin could be detected at any time-point);B. 5,7-DMF in post-absorption tissues, liver (○), lung (■), and kidney (Δ) (chrysin was barely detectable in a few animals and tissues);C. 5,7-DMF (□) and chrysin (●) in the proximal 2 cm of the colon with associated fecal pellet. The data represent the mean ± SEM of 5 animals at each time-point.
The colon presented a different problem, as the tissue was contaminated with fecal matter. The flavone content in the first 2 cm of the colon and the associated fecal pellet is shown in Fig. 6C. For 5,7-DMF, the low levels were almost exclusively from the colon tissue. These levels were about one third of those in the kidneys and lungs, but following the same time-course as in plasma and the other tissues. For chrysin, there were no levels detectable in the tissues. However, in the upper colonic fecal matter, there were considerable amounts of chrysin, but only at 2 and 3 h after administration of the flavone mixture (Fig. 6C).
There were no clearly detectable metabolites of the two flavones in either plasma or tissues. Conjugated metabolites of chrysin, as seen in human plasma [8], might have been present but likely were obscured by other highly polar materials present in the samples.
4. Discussion
The fact that the two methylated flavones selected for this study, i.e. 5,7-DMF and 5,7,4’-TMF, both were about 10 times more potent than their unmethylated analogs in inhibiting DNA synthesis in the SCC-9 cells (Fig. 2) was surprising, as many studies in the past have assumed that the free hydroxyl groups of the flavonoids, or polyphenols more generally, are necessary for biological effects. This certainly appears to be the case for antioxidant effects [18], long believed to be the main mechanism of action of these naturally-occurring compounds. However, recent findings point to effects on signal transduction pathways as the main sites of action for the flavonoids [36]. Free hydroxyl groups, in particular in the 4’-position of the B-ring, are critically important for the isoflavones [24].
The results of our study suggest a different mechanism of action for the methylated flavones than for the unmethylated analogs. This becomes evident most clearly in their differential effects on cell cycle progression. Apigenin has been studied extensively as a potential dietary chemopreventive agent and several studies [5, 25-27], including this one, have shown apigenin to induce cell cycle arrest in the G2/M phase. However, the methylated analog 5,7,4’-TMF as well as 5,7-DMF clearly induced arrest of most cells in the G1 phase (Fig. 4). These differential effects should be worthy of further investigation. It should be pointed out that the 50 μM flavonoid concentration appeared to produce somewhat different effects on the cell cycle progression than the lower concentrations. This may reflect a different, i.e. toxic, response of the cells. This is supported by the data obtained by crystal violet staining and the MTT assay, demonstrating IC50 values of 50-100 μM for effects on cell numbers by both 5,7-DMF and chrysin.
Of particular interest was the wide variation in effects on cell proliferation between different cell types. Thus, although 5,7-DMF showed similar potency in the FaDu laryngeal SCC cells as in the tongue SCC-9 cells, chrysin was much more potent in the FaDu cells. These two compounds also had similar effects on the MCF-7 cell proliferation with somewhat diminished potency, in particular for 5,7-DMF, which had a bimodal effect. However, most important, both 5,7-DMF and chrysin had greatly diminished effects on the noncancer transformed esophageal HET-1A cells and lung BEAS-2B cells, emphasizing high selectivity for cancer cells, in particular the tongue cancer SCC-9 cells.
The importance of the chemical structure of the methylated flavones on SCC-9 cell proliferation is not entirely clear from this study. All of the methylated compounds studied so far appear to have some inhibitory effect on SCC-9 cell proliferation, with 5,7-DMF and 5,7,4’-TMF appearing to be two of the most effective agents. These structure-activity relationships will need further investigation with additional methylated flavones. In a survey of various other methoxylated flavones in other types of human cancer cells, using the thymidine incorporation assay, a wide variety of responses were likewise observed [23], some of which indicated high potency.
Although flavonoids commonly are known to inhibit cancer cell proliferation, there are also reports suggesting that some of them may stimulate growth, presumably due to an estrogen-like effect, i.e., to have bimodal effects such as with quercetin [37]. Following this complex pattern of effects the promising polyphenolic flavonoid mixture silymarin was recently unexpectedly demonstrated to stimulate the growth of MCF-7 breast cancer cells [38]. Whereas both chrysin and apigenin in our study had a tendency toward a biphasic response, with a statistically significant stimulation at low concentrations, this was most certainly not the case with the two methylated analogs (Fig. 2). This may be related to stimulation of the estrogen receptor, such as with quercetin [37].
Although the methylated flavones investigated here were synthetic compounds, some of them have been identified in plants. For example, 5,7,4’-TMF is a citrus flavonoid [39], also present in other plants used in folk medicine [40, 41]. 7,4’-DMF has been identified in fruits and leaves from neotropical nutmeg species [42, 43] as well as from propolis [44]. Also, 5,7-DMF is a natural product [45, 46] highly abundant in pepper vine leaves. In view of the present findings, the search for methylated flavones in plants may be accelerated. The presence of some of these methylated flavones in citrus fruits is of interest in regard to a very recent epidemiological study demonstrating citrus fruits and juices to be protective against oral premalignant lesions [47].
Finally, our previous in vitro studies have demonstrated that the methylated flavones have dramatically increased metabolic stability as well as intestinal transport [16, 17]. In the present study,. we showed in vivo in the rat that one of these flavones, i.e., 5,7-DMF, indeed had high oral absorption as well as bioavailability (Fig. 6). In contrast, its unmethylated analog, chrysin, could not be detected except in the stool samples, reflecting negligible systemic availability. Of particular interest was the finding that 5,7-DMF accumulated highly in tissues, in particular in the liver. This is in contrast to observations with unmethylated flavonoids like quercetin, for which tissue accumulation, measured either directly [48] or through volume of distribution measurements [49, 50], has been very limited.
In summary, this study demonstrates that fully methylated flavones can be more potent inhibitors of cancer cell proliferation than their corresponding nonmethylated analogs. These effects appear to be selective for cancer versus noncancer cells. In addition, their mode of inhibition appears to be distinctly different. Moreover, we showed that one of these methylated flavones in vivo in the rat had high oral absorption, bioavailability and tissue distribution. Together with our previous findings on inhibition of carcinogen bioactivation [19-21], this study suggests that these compounds should be highly attractive as cancer chemopreventive/chemotherapeutic agents deserving further attention. In vivo chemopreventive studies are now in progress.
Acknowledgements
This study was supported by the National Institutes of Health grant GM55561and the Department of Defense/Hollings Cancer Center grant N6311602MD200. The HET-1A cells were kindly provided by Dr. Gary Stoner of Ohio State University. We would like to acknowledge the MUSC Center for Oral Health Research for use of the plate reader and the Hollings Cancer Center Flow Cytometry Facility for analyzing the cell cycle samples.
Abbreviations
- SCC
squamous cell carcinoma
- DMF
dimethoxyflavone
- TMF
trimethoxyflavone
- DHF
dihydroxyflavone
- MF
methoxyflavone
- HF
hydroxyflavone
- PMF
pentamethoxyflavone
- BrdU
bromodeoxyuridine
- FBS
fetal bovine serum
- DMSO
dimethyl sulfoxide
- PBS
phosphate-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Middleton EJ, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- [2].Yang CS, Prabhu S, Landau J. Prevention of carcinogenesis by tea polyphenols. Drug Metab Rev. 2001;33:237–53. doi: 10.1081/dmr-120000651. [DOI] [PubMed] [Google Scholar]
- [3].Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–85. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- [4].Haghiac M, Walle T. Quercetin induces necrosis and apoptosis in the SCC-9 oral cancer cells. Nutr Cancer. 2005;53:220–31. doi: 10.1207/s15327914nc5302_11. [DOI] [PubMed] [Google Scholar]
- [5].Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang B-H. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–53. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- [6].Hou Z, Sang S, You H, Lee M-J, Hong J, Chin K-V, et al. Mechanism of action of (-)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–56. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- [7].Lu J, Papp LV, Fang J, Rodriguez-Nieto S, Zhivotovsky B, Holmgren A. Inhibition of mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Res. 2006;66:4410–8. doi: 10.1158/0008-5472.CAN-05-3310. [DOI] [PubMed] [Google Scholar]
- [8].Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51:143–6. doi: 10.1111/j.1365-2125.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Rad Res. 2004;38:771–85. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- [10].Walle T. Absorption and metabolism of flavonoids. Free Rad Biol Med. 2004;36:829–37. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [11].Chow HHS, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of teapolyphenols following single-dose administration of epigallocatechin gallate and polyphenol E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- [12].Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA. Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutr. 2001;131:1731–7. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- [13].Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols inthree different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- [14].Walle T, Hsieh F, DeLegge MH, Oatis JEJ, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- [15].Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos. 2002;30:576–81. doi: 10.1124/dmd.30.5.576. [DOI] [PubMed] [Google Scholar]
- [16].Wen X, Walle T. Methylation protects dietary flavonoids from rapid hepatic metabolism. Xenobiotica. 2006;36:387–97. doi: 10.1080/00498250600630636. [DOI] [PubMed] [Google Scholar]
- [17].Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos. 2006;34:1786–92. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- [18].Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- [19].Wen X, Walle UK, Walle T. 5,7-Dimethoxyflavone down-regulates CYP1A1 expression and benzo[a]pyrene-induced DNA binding in Hep G2 cells. Carcinogenesis. 2005;26:803–9. doi: 10.1093/carcin/bgi015. [DOI] [PubMed] [Google Scholar]
- [20].Wen X, Walle T. Preferential induction of CYP1B1 by benzo[a]pyrene in human oral epithelial cells: Impacton DNA adduct formation and prevention by polyphenols. Carcinogenesis. 2005;26:1774–81. doi: 10.1093/carcin/bgi127. [DOI] [PubMed] [Google Scholar]
- [21].Tsuji PA, Walle T. Inhibition of benzo[a]pyrene-activating enzymes and DNA-binding in human bronchial epithelial BEAS-2B cells by methoxylated flavonoids. Carcinogenesis. 2006;27:1579–85. doi: 10.1093/carcin/bgi358. [DOI] [PubMed] [Google Scholar]
- [22].Pouget C, Lauthier F, Simon A, Fagnere C, Basly J-P, Delage C, et al. Flavonoids: structural requirements for antiproliferative activity on breastcancer cells. Bioorg Med Chem Lett. 2001;11:3095–7. doi: 10.1016/s0960-894x(01)00617-5. [DOI] [PubMed] [Google Scholar]
- [23].Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Agric Food Chem. 2002;50:5837–43. doi: 10.1021/jf020121d. [DOI] [PubMed] [Google Scholar]
- [24].Setchell KDR, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, et al. Bioavailability of pure is of lavonesinhealthyhumans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- [25].McVean M, Weinberg WC, Pelling JC. Ap21(waf1)-independent pathway for inhibitory phosphorylation of cyclin-dependent kinase p34 (cdc2) and concomitant G(2)/M arrest by the chemopreventive flavonoid apigenin. Mol Carcinogenesis. 2002;33:36–43. doi: 10.1002/mc.10016. [DOI] [PubMed] [Google Scholar]
- [26].Takagaki N, Sowa Y, Oki T, Nakanishi R, Yogosawa S, Sakai T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int J Oncol. 2005;26:185–9. [PubMed] [Google Scholar]
- [27].Trochon V, Blot E, Cymbalista F, Engelmann C, Tang RP, Thomaidis A, et al. Apigenin inhibits endothelial-cell proliferation in G(2)/M phase whereas it stimulates smooth-muscle cells by inhibiting P21 and P27 expression. Int J Cancer. 2000;85:691–6. doi: 10.1002/(sici)1097-0215(20000301)85:5<691::aid-ijc15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [28].Stoner G, Kaighn M, Reddel R, Resau J, Bowman D, Naito Z, et al. Establishment and characterization of SV40 T-antigen immortalized human esophageal epithelial cells. Cancer Res. 1991;51:365–71. [PubMed] [Google Scholar]
- [29].Orlando G, Tobey N, Wang P, Abdulnour-Nakhoul S, Orlando R. Regulatory volume decrease in human esophageal epithelial cells. Am J Physiol. 2002;283:G932–G7. doi: 10.1152/ajpgi.00455.2001. [DOI] [PubMed] [Google Scholar]
- [30].Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, et al. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–9. [PubMed] [Google Scholar]
- [31].Porstmann T, Ternynck T, Avrameas S. Quantitation of 5-bromo-2-deoxyuridine incorporation into DNA: an enzyme immunoassay for the assessment of the lymphoid cell proliferative response. J Immun Methods. 1985;82:169–79. doi: 10.1016/0022-1759(85)90236-4. [DOI] [PubMed] [Google Scholar]
- [32].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- [33].Kueng W, Silber E, Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989;182:16–9. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- [34].Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981;41:1657–63. [PubMed] [Google Scholar]
- [35].van de Loosdrecht AA, Beelen RHJ, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MMAC. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods. 1994;174:311–20. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]
- [36].Williams RJ, Spencer JPE, Rice-Evans C. Flavonoids: antioxidants and signalling molecules. Free Radic Biol Med. 2004;36:838–49. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- [37].Dihal AA, Woutersen RA, van Ommen B, Rietjens IMCM, Stierum RH. Modulatory effects of quercetin on proliferation and differentiation of the human colorectal cell line Caco-2. Cancer Lett. 2006;238:248–59. doi: 10.1016/j.canlet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- [38].Malewicz B, Wang Z, Jiang C, Guo J, Cleary MP, Grande JP, et al. Enhancement of mammary carcinogenesis intworodent models by silymarindietary supplements. Carcinogenesis. 2006;27:1739–47. doi: 10.1093/carcin/bgl032. [DOI] [PubMed] [Google Scholar]
- [39].Mizuno M, Iinuma M, Ohara M, Tanaka T, Iwamasa M. Chemotaxonomy of the genus Citrus based on polymethoxyflavones. Chem Pharm Bull. 1991;39:945–9. [Google Scholar]
- [40].Jaipetch T, Reutrakul V, Tuntiwachwuttikul P, Santisuk T. Flavonoids in the black rhizomes of Boesenbergia pandurata. Phytochemistry. 1983;22:625–6. [Google Scholar]
- [41].Yenjai C, Prasanphen K, Daodee S, Wongpanich V, Kittakoop P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia. 2004;75:89–92. doi: 10.1016/j.fitote.2003.08.017. [DOI] [PubMed] [Google Scholar]
- [42].Cavalcante SH, Fernandes D, Paulino Fo HF, Yoshida M, Gottlieb OR. Lignoids from the fruit of three Virola species. Phytochemistry. 1985;24:1865–6. [Google Scholar]
- [43].Santos LS, Corréa MJC, Campos LMO, Andrade MA. Constituents from the leaves of Virola michelli. Fitoterapia. 1996;67:555–6. [Google Scholar]
- [44].Popravko SA, Gurevich AI, Kolosov MN. Flavonoid components of propolis. Khimiya Prirodnykh Soedinenii. 1969;5:476–82. [Google Scholar]
- [45].Ahmad F, Bakar SA, Ibrahim AZ, Read RW. Constituents of the leaves of Piper caninum. Planta Med. 1997;63:193–4. doi: 10.1055/s-2006-957648. [DOI] [PubMed] [Google Scholar]
- [46].Ahmad F, Poongavanam N, Taher M, Arbain D, Jamal R, Read RW. Flavones from Piper ungaromense C. DC. ACGC Chem Commun. 2002;14:64–52. [Google Scholar]
- [47].Maserejian NN, Giovannucci E, Rosner B, Zavras A, Joshipura K. Prospective study of fruits and vegetables and risk of oral premalignant lesions in men. Am J Epidemiol. 2006 doi: 10.1093/aje/kwj233. Advance Access July 17. [DOI] [PubMed] [Google Scholar]
- [48].Ueno I, Nakano N, Hirono I. Metabolic fate of [14C]quercetin in the ACI rat. Jap J Exp Med. 1983;53:41–50. [PubMed] [Google Scholar]
- [49].Gugler R, Leschik M, Dengler HJ. Disposition of quercetin in man after single oral and intravenous doses. Eur J Clin Pharmacol. 1975;9:229–34. doi: 10.1007/BF00614022. [DOI] [PubMed] [Google Scholar]
- [50].Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, et al. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2:659–68. [PubMed] [Google Scholar]