Abstract
Objective
The mechanisms responsible for maintaining the differentiated phenotype of adult vascular smooth muscle cells (VSMCs) are incompletely understood. Reactive oxygen species (ROS) have been implicated in VSMC differentiation, but the responsible sources are unknown. In this study, we investigated the role of Nox1 and Nox4-derived ROS in this process.
Methods and Results
Primary VSMCs were used to study the relationship between Nox homologues and differentiation markers such as smooth muscle α-actin (SM α-actin), smooth muscle myosin heavy chain (SM-MHC), heavy caldesmon, and calponin. We found that Nox4 and differentiation marker genes were downregulated from passage 1 to passage 6 to 12, whereas Nox1 was gradually upregulated. Nox4 co-localized with SM α-actin–based stress fibers in differentiated VSMC, and moved into focal adhesions in de-differentiated cells. siRNA against nox4 reduced NADPH-driven superoxide production in serum-deprived VSMCs and downregulated SM-α actin, SM-MHC, and calponin, as well as SM-α actin stress fibers. Nox1 depletion did not decrease these parameters.
Conclusion
Nox4-derived ROS are critical to the maintenance of the differentiated phenotype of VSMCs. These findings highlight the importance of identifying the specific source of ROS involved in particular cellular functions when designing therapeutic interventions.
Keywords: reactive oxygen species, vascular smooth muscle, differentiation, gene expression
Smooth muscle cells (SMCs) from the media of adult blood vessels exhibit a highly specialized, differentiated phenotype whose function is contraction and regulation of blood vessel diameter.1 They express a unique repertoire of contractile proteins to support this function, such as smooth muscle myosin heavy chain (SM-MHC), smooth muscle α-actin (SM α-actin), heavy-caldesmon (H-caldesmon), and calponin.1 In contrast, SMCs from neointima of diseased blood vessels are less differentiated, and express low levels of these marker proteins as well as different isoforms of myosin or actin as their function changes toward a more synthetic, proliferative state.2 The switch from the differentiated phenotype to the less differentiated, proliferative state is triggered by changes in local environmental cues, such as an increase in mitogenic cytokines, but the factors involved in the maintenance of the differentiated state are less understood.
Reactive oxygen species (ROS) are involved in promoting pathophysiological events, such as proliferation and migration of SMCs,3 as well as physiological processes, such as contraction and differentiation.4,5 A major source of ROS is NAD(P)H oxidases, of which 2 forms are present in rodent vascular SMCs (VSMCs). The Nox1-based oxidase consists of 2 membrane-bound components, Nox1 and p22phox, and regulatory cytosolic components, p47phox, NoxA1, and Rac1.6 The Nox4-based oxidase consists of Nox4 and p22phox, but it appears not to require known cytosolic subunits.7 Nox1 has been shown to promote proliferation,8,9 whereas the role of Nox4 in SMCs is not yet elucidated. It has been suggested that Nox4 is responsible for baseline ROS production,10,11 and previous studies found a correlation between Nox4 and some differentiation markers of VSMCs.12,13 Moreover, a recent study performed in fibro-blasts showed that Nox4 mediates transforming growth factor (TGF)-β1–induced differentiation of fibroblasts into contractile myofibroblasts.14 These lines of evidence suggest that Nox4-derived ROS may be required for the maintenance of the differentiated phenotype of VSMCs. To test this hypothesis, we isolated primary VSMCs from rat aorta and studied the relationship between Nox1, Nox4 and differentiation markers. We found that Nox4 correlates with smooth muscle differentiation markers in vivo and in vitro, and that it is necessary for differentiation marker gene expression.
Materials and Methods
An expanded materials and methods section is available online (please see http://atvb.ahajournals.org).
Materials
Rabbit polyclonal antibodies anti-nox4 and anti-nox1 were kind gifts from Dr David Lambeth (Emory University) and Dr H.H.H. Schmidt (University of Melbourne), respectively, and were characterized previously.15,16
Cell Culture
VSMCs were isolated from male Sprague-Dawley rat (Harlan Sprague-Dawley, Prattville, Ala) thoracic aorta by enzymatic digestion and grown in Dulbecco's modified Eagle's medium (DMEM) with 25 mmol/L HEPES and 4.5 g/L glucose as described previously.17 Cells at passages 1 and 2 (early passage) were used as a model of the differentiated phenotype, whereas late passage cells (passages 6 to 13), were used as a model of the proliferative phenotype as described previously by others.18,19
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was purified from cells using the RNeasy kit, as recommended by the manufacturer. Quantitation of nox1, nox4, 18S rRNA, SM α-actin, SM-MHC, H-caldesmon, and calponin was performed by amplification of rat VSMC cDNA using the LightCycler (Roche) real-time thermocycler. Copy number was calculated by the instrument software from standard curves of genuine templates.
Immunoblotting
VSMCs were lysed in the presence of 1% Triton X-100 as described previously,15 and lysates were separated into Triton-soluble and insoluble fractions by centrifugation. Fractions were separated using SDS-PAGE and transferred to nitrocellulose membranes, blocked, and incubated with primary antibodies. Proteins were detected by ECL. Band intensity was quantified by densitometry of immunoblots using Image J.
siRNA Design, Synthesis, and Transfection
Rat aortic SMCs were trypsinized and plated at 30% confluence 24 hours before transfection. siRNA-oligofectamine complexes were then incubated with the cells for 24 hours. Subsequently, cells were washed and placed in serum free OPTI-MEM for an additional 6 to 7 days to achieve an efficient downregulation of the protein. To control for possible nonspecific effects of siRNAs, multiple control siRNAs were used: sequence specific Nox4 scrambled, siLuc (siRNA against luciferase) and the universal negative control siRNA#3 (Ambion). A second siNox4 sequence was used in some experiments and gave similar results.
Preparation of Recombinant Adenoviruses and Infection of VSMCs
Antisense Nox1 adenovirus (AdASNox1) and antisense Nox4 (AdASNox4) were prepared as described previously.9 GFP-tagged Nox4 adenovirus (AdNox4) was prepared using the same protocol after excision from a plasmid kindly provided by P. Hordijk (University of Amsterdam). VSMCs were infected for 2 hours with recombinant adenovirus in serum free culture medium, and incubated for another 4 days in the same medium without virus before harvesting for protein extraction.
Immunofluorescent Histochemistry
Carotid arteries from rats were harvested at different time points after injury and prepared for histochemistry as described previously.13
Immunofluorescent Cytochemistry
VSMCs on glass coverslips were processed as previously described.15 Cells were incubated with anti-Nox4 antibody as before,15 antibodies for differentiation marker proteins, or phalloidin (for total filamentous actin) for 1 hour at room temperature, and then incubated with either fluorescein isothiocyanate-conjugated, Rhodamine Red X-conjugated, or Cy5-conjugated secondary antibodies for 1 hour at room temperature. Fluorescence was visualized with a Zeiss LSM 510 confocal microscope. Controls with no primary antibody showed no fluorescence labeling and single label controls were performed in double labeling experiments.
NAD(P)H Oxidase Activity Assay Using Electron Spin Resonance
VSMCs were harvested and resuspended in phosphate buffer containing protease inhibitors. Cells were sonicated and the membrane pellet was resuspended in electron spin resonance buffer. Electron spin resonance spectroscopy using 1-hydroxy-3-carboxy-pyrrolidine (a nitrone spin trap) and 200 μmol/L NADPH was used for quantitative measurements of O2− production as described previously.14 Superoxide dismutase (50 U/mL) added directly to the sample inhibited 95% to 98% of CP· production.
Detection of Intracellular Superoxide Using High-Performance Liquid Chromatography
To evaluate intracellular production of superoxide, the formation of oxyethidium from dihydroethidium was measured using high-performance liquid chromatography analysis as recently reported.14 Hydroxyethidium was expressed per mg protein. In some studies polyethylene glycol–SOD (50 U/mL) was added overnight. Polyethylene glycol–SOD inhibited the dihydroethidium signal by 60% to 70%.
Statistical Analysis
Results are expressed as mean± standard error of mean (SEM). Statistical significance was assessed by using 1-way ANOVA using Origin software version 4.1. A value of P<0.05 was considered to be statistically significant.
Results
Correlation of Nox4 and Differentiation Markers In Vivo
Previous work by Szöcs et al12 suggested a correlation between Nox4 and the differentiation marker calponin in rat carotid artery injury. The expression of both Nox4 and calponin increased only in the late stage of neointimal formation, when re-endothelization and re-differentiation of VSMCs occur.20 This system thus provides a model for monitoring the process of SMC differentiation in vivo. To examine the correlation between Nox4 and re-expression of one of the most specific markers of differentiation for VSMCs, SM-MHC, we performed double label immunohistochemistry for Nox4 and SM-MHC on frozen sections of rat carotid arteries harvested at 7, 10, and 15 days after injury. Similar to calponin, the SM-MHC protein starts to appear in the neointimal cells at 10 days, with a prominent expression at 15 days (Figure 1), whereas Nox4 expression in neointima is detectable at 7 days, gradually increases at 10 days, and reaches a maximum by 15 days (Figure 1). The increase in Nox4 precedes the appearance of both calponin and SM-MHC, suggesting that Nox4 may be involved in the re-differentiation process of neointimal VSMCs.
Figure 1.
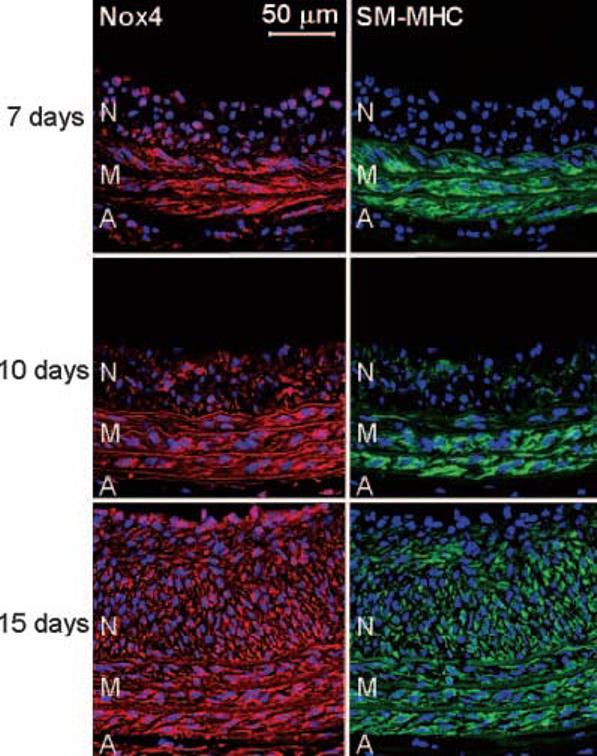
Nox4 correlates with SM-MHC differentiation markers in vivo. Immunofluorescence labeling using antibodies to Nox4 (red) and SM-MHC (green) of rat carotid arteries sections harvested at indicated time points after balloon injury. Cell nuclei are stained blue with DAPI. Lumen is to the top of each panel. N=neointima; M=media; A=adventitia. Elastic laminae appear light red in the left panels or green in the right panels. Bar=50 μm.
Characterization of VSMC Differentiation State In Vitro
To investigate a causal relationship between Nox4 and VSMC differentiation, we used an in vitro system in which we compared differentiation marker expression in early and late passage VSMCs.18,21 As previously noted, even short-term culture of VSMCs reduces smooth muscle differentiation markers compared with aorta (ref. 2 and Figure 2). As shown in Figure 2, however, early passage (1–2) VSMCs still express significantly more SM-MHC, H-caldesmon, calponin, and SM α-actin mRNA and protein than late passage (6–13) VSMCs. Therefore, we used these 2 culture conditions as representative of differentiated and dedifferentiated VSMC phenotypes.
Figure 2.
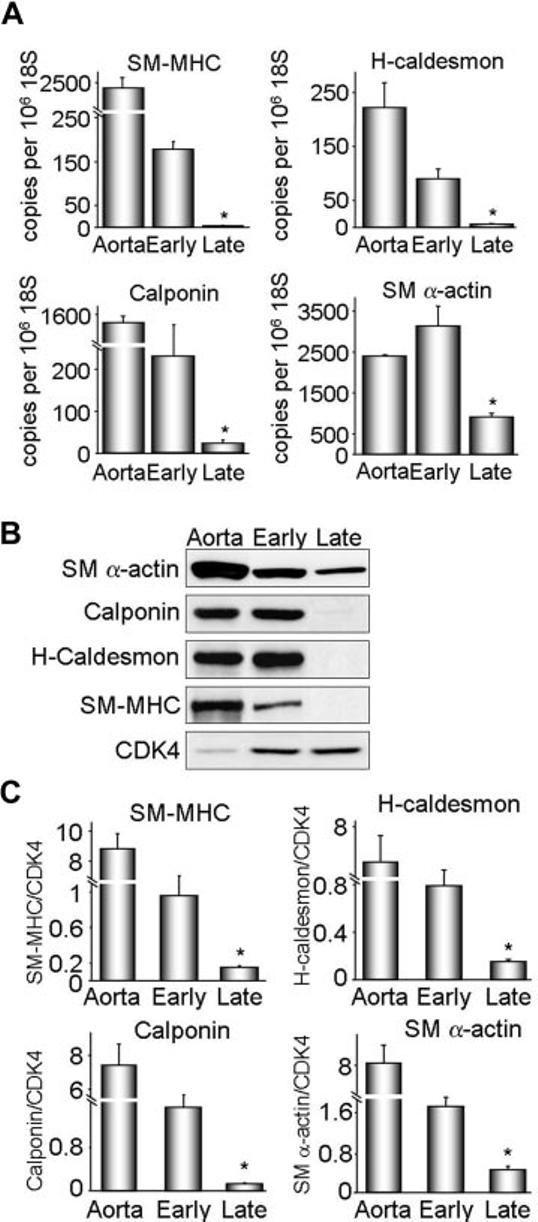
Characterization of VSMC differentiation state in vitro. Protein and RNA were extracted from early (1–2) and late (6–13) passage VSMCs. A, SM-MHC, H-caldesmon, calponin, and SM α-actin cDNA levels were measured by quantitative real-time polymerase chain reaction. Data were normalized to 106 copies 18S cDNA. Whole aorta cDNA was used as a positive control. Results are expressed as mean± SEM (n=4 to 6). *P<0.01 compared with early passage. B, Western blot analysis of differentiation marker protein expression. Cyclin-dependent kinase 4 (CDK4) was used as a loading control. Each differentiation marker gene was normalized to CDK4. C, Quantification of protein expression for 4 to 6 experiments. Results are expressed as mean± SEM. *P=0.01 compared with early passage.
Nox4, but not Nox1, Correlates With Differentiation In Vitro
To gain insight into the relationship between nox homologues and cell phenotype, we analyzed nox mRNA expression by real-time polymerase chain reaction in both differentiated and dedifferentiated VSMCs. Nox4 and Nox1 had opposite patterns of expression: Nox4 was high in early passage cells and decreased as the cells dedifferentiated, whereas Nox1 was expressed at low levels in early and increased in high-passage VSMCs (Figure 3A). Western blot analysis of VSMC lysates confirmed these findings, showing a 50% decrease in Nox4 protein expression in late compared with early passage cells (Figure 3B). Of importance, Nox4 protein expression correlated strongly with that of the differentiation markers SM α-actin (r=0.999, P<0.0001), calponin (r=0.998, P=0.0001), H-caldesmon (r=0.995, P=0.0003), and SM-MHC (r=0.989, P=0.0013) over multiple passages. As further confirmation of the correlation between Nox4 and differentiation marker genes, we also examined Nox4 and calponin expression in passaged VSMCs deprived of serum for 6 to 7 days, another model of VSMC differentiation.4 As shown in supplemental Figure I, serum deprivation increased both Nox4 and calponin expression, supporting this correlation.
Figure 3.
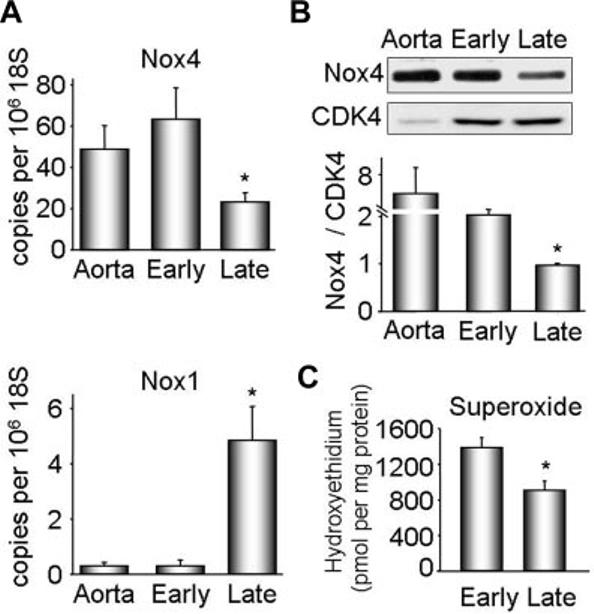
Nox1 and Nox4 expression and superoxide production in early and late passage VSMCs. Protein and RNA were extracted from early (1–2) and late (6 –13) passage VSMCs. A. Nox4 and Nox1 cDNAs were measured by quantitative real-time polymerase chain reaction. Data are normalized to 106 copies 18S cDNA. Whole aorta cDNA was used as a positive control. Results are expressed as mean±SEM (n=6). *P<0.01 compared with early passage. B, Representative Western blot analysis of Nox4 protein expression (upper panel). CDK4 was used as a loading control. Lower panel, Quantification of protein expression for 6 experiments. Nox4 was normalized to CDK4. Results are expressed as mean± SEM. *P<0.01 compared with early passage. C, Superoxide production in quiescent early and late passage VSMCs, measured with dihydroethidium–high-performance liquid chromatography. Results are expressed as mean±SEM (n=3) and are normalized to protein.
Nox4 is the Nox homologue responsible for basal ROS production in these cells.11 Therefore, we analyzed superoxide production in serum-deprived early and late passage cells using dihydroethidium–high-performance liquid chromatography. Figure 3C shows that differentiated VSMCs produce more basal superoxide than dedifferentiated cells. Together with Figure 2, these data show that Nox4, but not Nox1, correlates with differentiation marker expression and constitutive superoxide production in differentiated VSMCs.
Localization of Nox4 on SM α-actin–Based Stress Fibers and in Focal Adhesions
Because previous work demonstrated that subcellular localization of oxidases is important in determining their function,15 we investigated the localization of Nox4 in both phenotypes by immunofluorescent cytochemistry, using a specific antibody for Nox4, which we characterized previously.15 Nox4 localizes to focal adhesions and nuclei in dedifferentiated VSMCs (Figure 4A, lower panels). In contrast, differentiated VSMCs show a prominent distribution of Nox4 along SM α-actin– based stress fibers, as shown in yellow in the merged panel of Figure 4A. This localization pattern disappears early in the dedifferentiation process that normally occurs in culture, being undetectable by passage 3 (data not shown). Therefore, we compared Nox4 localization with that of more specific differentiation markers, such as SM-MHC, H-caldesmon, and calponin. Figure 4B illustrates that Nox4 appears along stress fibers only in those cells expressing these differentiation markers for SMC lineage at the highest levels, suggesting that this localization is a distinctive characteristic of the most differentiated phenotype of VSMCs.
Figure 4.
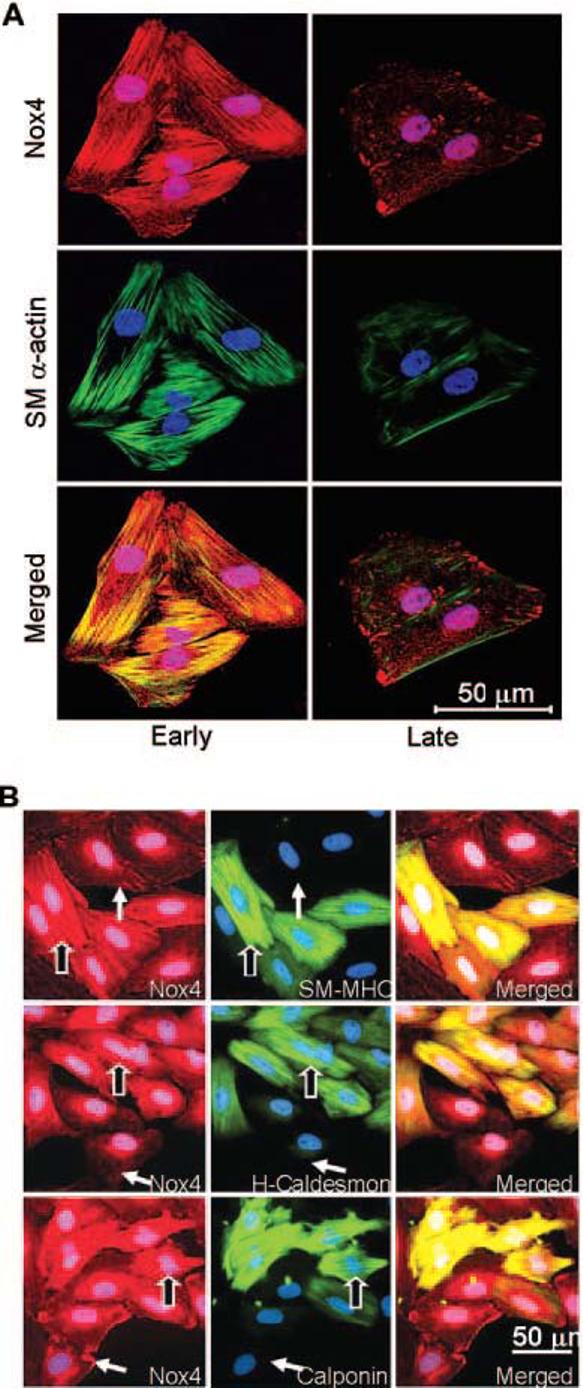
Differential localization of Nox4 in early and late passage VSMCs. A, Immunofluorescence labeling using specific antibodies to Nox4 (red) and SM α -actin (green). Nuclei are stained with DAPI (blue). Optical sectioning of the same cells shows a stress fiber distribution of Nox4 in early passage cells, and a focal adhesion pattern in late passage cells. Nox4 appears to colocalize with SM μ-actin in early passage (yellow color in bottom left panel), whereas there is no colocalization in the late passage cells (bottom right panel). Images are representative of at least four separate experiments. Bar=50 μm. B, Immunofluorescent cytochemistry for Nox4 (red), differentiation markers (green): SM-MHC, H-caldesmon, and calponin, and nuclei (blue) in early passage VSMCs. Nox4 appears along stress fibers only in the most differentiated cells (outlined arrows), whereas in cells expressing lower levels of differentiation markers, Nox4 moves to focal adhesions (plain arrows). Note: all cells express SM α-actin (not shown).
Nox4 Is Required for the Expression of Differentiation Markers
After establishing a correlation between Nox4 and differentiation markers, we next investigated whether Nox4 is required for differentiation marker gene expression. We down-regulated Nox4 using siRNA (siNox4) and antisense adenovirus (AdASNox4) (supplemental Figures II to IV), and analyzed the expression of differentiation markers. First, we showed that both constructs decreased Nox4 expression (supplemental Figures II and III). Electron spin resonance measurement of NADPH oxidase activity in serum-deprived VSMCs showed a significant decrease of ROS in both siNox4 and AdASNox4 cells, compared with control cells (supplemental Figures II and III). A second siRNA sequence against Nox4 gave similar results (data not shown). This confirms that the siNox4 and AdASNox4 are functional, and that indeed, Nox4 is responsible for baseline ROS production in these cells, as previously shown.11
siNox4 decreased SM-MHC, SM α-actin, and calponin mRNA and protein (Figure 5), whereas the expression of β-actin (the nonmuscle isoform) was unchanged, suggesting that Nox4 is required for the expression of differentiation markers, but not structural proteins in general. Similar results were obtained with AdASNox4 (supplemental Figure IV). Conversely, overexpression of GFP-tagged Nox4 increased expression of SM α-actin (supplemental Figure V). In contrast, downregulation of Nox1 with an adenovirus expressing antisense Nox1 (AdASNox1), which we have previously shown to decrease Nox1 protein expression and activity,9 did not reduce the expression of SM α-actin or calponin (supplemental Figure VI). These data support a specific requirement of a Nox4-based oxidase for differentiation marker expression in VSMCs.
Figure 5.
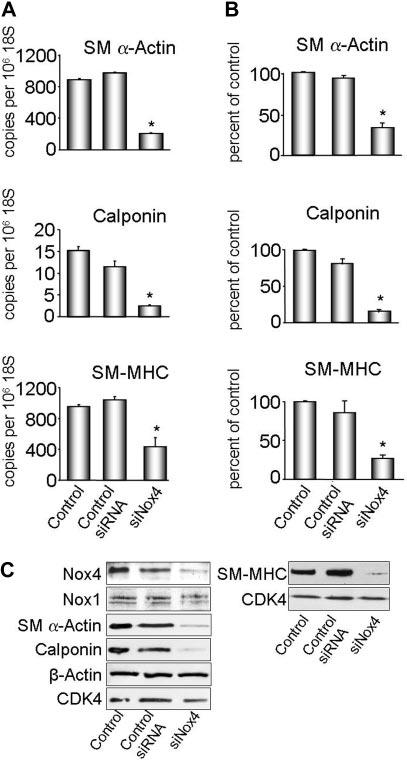
Nox4 is necessary for expression of differentiation markers. VSMCs were transfected with siNox4 or control siRNA, and harvested for RNA or protein. A, SM α-actin, calponin and SM-MHC cDNA levels were measured by quantitative real-time polymerase chain reaction. Data are normalized to 106 copies 18S cDNA. Results are expressed as mean±SEM (n=3, n=2 for SM-MHC). *P<0.01 for SM α-actin and calponin and P<0.05 for SM-MHC compared with control siRNA. B, Quantification of protein expression (n=3). Results are normalized to CDK4 and are expressed as mean±SEM. *P<0.05 compared with control siRNA. C, Western blot analysis of Nox1, Nox4, and differentiation marker protein expression. CDK4 was used as a loading control, and β-actin as a nondifferentiation marker protein.
Nox4 Is Required for the Maintenance of the SM α-Actin–Based Stress Fibers
As VSMCs dedifferentiate, they gradually lose their contractile-type stress fibers (ie, SM α-actin), and nonmuscle type isoforms (ie, β-actin) constitute the majority of the remaining stress fibers.22 To investigate whether SM α-actin–based stress fibers require the presence of Nox4, we transfected early passage VSMC (in which these fibers are prominent) with siNox4 and examined SM α-actin and F-actin stress fiber distribution using immunofluorescent cytochemistry. F-actin identifies the total filamentous actin fibers in cells, including both α-actin and β-actin. As shown in supplemental Figure VII, siNox4 induces a dramatic decrease in intensity and distribution of SM α-actin along fibers, whereas the F-actin staining reveals that the remaining stress fibers (presumably those containing β-actin) are not affected by siNox4. These findings suggest that Nox4 may be specifically required for the maintenance of the contractile-type stress fibers in VSMCs.
Mechanism of Nox4-Mediated Regulation of Differentiation Gene Expression
Because siNox4 reduces differentiation marker mRNA expression, Nox4 may control this subset of genes by regulating a common transcription factor. Smooth muscle specific differentiation marker genes are uniformly regulated by the binding of serum response factor (SRF) to 1 or more CArG boxes in their promoters. SRF expression is thus an absolute prerequisite for SMC-CArG–dependent gene expression.23 To determine whether Nox4 regulates SRF expression, we analyzed SRF levels after treatment of cells with siNox4. As shown in Figure 6, suppression of Nox4 resulted in a clear reduction in SRF expression, suggesting that this is one mechanism by which Nox4 regulates expression of differentiation marker genes.
Figure 6.
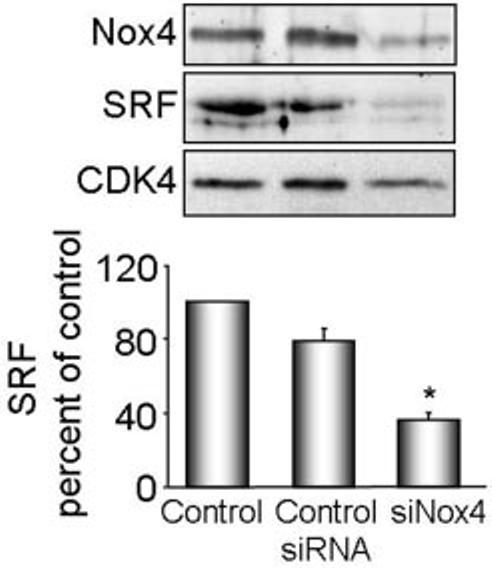
Nox4 regulates SRF expression. VSMCs were transfected with siNox4 or control siRNA, and harvested for protein. Nox4 and SRF were measured by Western analysis. Protein expression was quantified by densitometry (n=4). Results are normalized to CDK4 and are expressed as mean± SEM. *P<0.001 compared with control siRNA.
Discussion
ROS mediate multiple cellular functions, including proliferation, apoptosis, migration, and differentiation. Exactly how these molecules regulate such diverse functions is an active area of investigation. While Nox1 is localized in caveolae, Nox4 is found in focal adhesions and the nucleus in actively growing cells, suggesting that they mediate distinct functions.15 We previously showed that Nox1 mediates serum-induced proliferation and angiotensin II-stimulated hypertrophy in VSMCs.8,9 The data presented here suggest that in contrast to Nox1, Nox4 sustains VSMC differentiation, indicating that the source and location of ROS production is of paramount importance in dictating the cellular response.
Regulated cell differentiation is critical to embryogenesis and wound healing in the adult. Previous work established a relationship between ROS and differentiation in both situations. Studies in embryoid bodies showed that in the early stages of cardiac cell differentiation, ROS inhibit MEF2C and block expression of cardiac-specific genes, whereas in the later stages, they facilitate differentiation via the phosphoinositide-3 kinase (PI-3K) pathway.24 ROS also mediate skeletal muscle differentiation. Depletion of Nox2 in skeletal myoblasts prevents induction of the differentiated phenotype and decreases expression of myosin heavy chain and myogenin.25 In VSMCs, catalase and N-acetylcysteine block the induction of calponin, SM α-actin and myosin heavy chain in response to serum withdrawal,4 suggesting that H2O2 mediates differentiation in these cells. However, the endogenous source of ROS was not identified. The current data suggest that Nox4-derived ROS are required for differentiation marker gene expression in VSMCs. Depletion of Nox4 downregulates calponin, SM-MHC, and SM α-actin mRNA and protein without affecting expression of unrelated proteins, and over-expression of Nox4 induces SM α-actin expression. Of interest, inhibition of Nox1 did not decrease these genes, indicating that Nox4-derived ROS specifically mediates differentiation marker gene expression in these cells. Although we cannot fully exclude the possibility that Nox4 could function in the nucleus in a ROS-independent fashion, we believe this is unlikely given the previous data showing that H2O2 mediates differentiation in VSMCs.4 These findings highlight the importance of identifying the specific source of ROS involved in particular cellular functions.
VSMCs have a unique characteristic known as phenotype plasticity; that is, not only can they switch from a fully differentiated state (media of blood vessels) to a proliferative state (early stage of neointima formation) but also they may return to the differentiated state (late stage of neointima formation) once they are exposed to the appropriate environmental conditions.1 In this study, we used 2 different models to assess the relationship between Nox4 and SMC differentiation. The first system was an in vivo re-differentiation of intimal SMCs after balloon injury of rat carotid artery. We previously noted that Nox4 mRNA and protein expression increased only at the late stages of the response to injury when differentiation markers were re-expressed.12 This was confirmed in the present study, in which we found that Nox4 protein expression in the neointima began to appear at 7 days, and preceded the reappearance of SM-MHC (Figure 1), suggesting that Nox4 may be involved in the differentiation process. The second model used in this study was de-differentiation of primary VSMCs in culture. Even though early passage cells do not represent fully differentiated VSMCs, they retain abundant expression of contractile proteins compared with late passage cells, as shown in Figures 2 and 4. Of importance, primary VSMCs co-expressed Nox4 and contractile markers in a myofibrillar pattern, which is specific for the differentiated phenotype (Figure 4). This myofibrillar pattern of contractile proteins was lost when Nox4 was deleted, suggesting that Nox4 is required for the expression of fully differentiated phenotype (supplemental Figure VII). The change in localization of Nox4 from stress fibers in differentiated cells to focal adhesions in proliferating cells underlines the importance of signaling compartmentalization and its correlation with function. It suggests that not only is the source of ROS critical (that is, Nox1 versus Nox4), but also that the localized production of ROS within the cells dictates the ultimate function. For example, the stress fiber-associated Nox4 emerges as a positive regulator of differentiation, whereas the focal adhesion-located Nox4 is associated with a proliferative phenotype. However, the loss of Nox4 and of differentiation markers is reproduced spontaneously with passage in cultured SMCs. Even in the most proliferative SMC phenotype, where only SM α-actin and calponin are present, deletion of Nox4 potently diminishes their expression (Figure 5). Taken together, our data from these 2 models strongly support an important role for Nox4 in the early induction of differentiation (in vivo model), and in the maintenance of the fully differentiated phenotype (in vitro model) (Figure 5 and supplemental Figure VII).
Additional data concerning the correlation between Nox4 and differentiation comes from several studies. In human coronary arteries, Nox4 expression correlates with SM α-actin.13 In osteoclasts, overexpression of Nox4 upregulates osteoclast markers, whereas antisense treatment reduces their expression.26 More relevant to the current study, Spin et al27 showed by microarray analysis of differentiating VSMCs that Nox4 is upregulated early in the differentiation process, and remains elevated throughout. Of interest, the increase in Nox4 precedes significant upregulation of many of the differentiation markers. Moreover, Sturrock et al28 showed that Nox4, ROS, and SM α-actin decrease with passage, and that TGF-β–induced expression of differentiation marker genes is ROS-sensitive. However, in their study, 24-hour treatment with siNox4 did not block TGF-β induction of these genes. In our experience, 6 to 7 days of siRNA treatment are required to downregulate Nox4 protein, which may explain these disparate results. In support of this interpretation, long-term treatment with siNox4 abrogates the upregulation of SM α-actin during TGF-β–induced conversion of cardiac fibroblasts to myofibroblasts.14 Similar to these results, our data clearly show that Nox4 is required for SM α-actin, SM-MHC and calponin expression.
A number of physiological and pathophysiologic roles for Nox4 have been proposed. Nox4 was originally described as a pro-senescent, anti-proliferative factor in NIH3T3 cells.29,30 Subsequently, Nox4 was proposed to mediate insulin signaling by inhibiting the tyrosine phosphatase PTB1B.31 In mouse aortic VSMCs, Nox4 was shown to be responsible for basal superoxide production, but its physiological role was not investigated.11 A recent study on urokinase plasminogen activator-induced VSMC growth found that both Nox1 and Nox4 are necessary for a full proliferative response.32 As summarized, Nox4 is clearly important in differentiation of multiple cell types,4,24-26 but how Nox4 mediates such diverse functions remains unclear. It is possible that the function of Nox4 is dictated by a specific combination of cofactors and environmental stimuli that temporally coexist. Alternatively, Nox4 may regulate metabolic pathways that are necessary for many cellular functions. Finally, the intracellular localization of Nox4 may influence its downstream targets. When Nox4 is localized in the nucleus,15,33 it may regulate gene expression, whereas Nox4 association with stress fibers may influence contractility or cytoskeletal rearrangement. One mechanism by which Nox4 regulates gene expression appears to be through modulation of SRF levels (Figure 6). Further studies are required to elucidate the molecular mechanisms by which Nox4 controls specific cell functions.
Acknowledgments
We thank Dr David Lambeth for providing the Nox4 antibody.
Footnotes
Sources of Funding
This work was supported by NIH grants HL38206, HL075209, and HL058000, to K.K.G. and an American Heart Association Scientist Development Award (0335244N) to D.S.
Disclosures
None.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Rovner AS, Murphy RA, Owens GK. Expression of smooth muscle and nonmuscle myosin heavy chains in cultured vascular smooth muscle cells. J Biol Chem. 1986;261:14740–14745. [PubMed] [Google Scholar]
- 3.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 4.Su B, Mitra S, Gregg H, Flavahan S, Chotani MA, Clark KR, Goldschmidt-Clermont PJ, Flavahan NA. Redox regulation of vascular smooth muscle cell differentiation. Circ Res. 2001;89:39–46. doi: 10.1161/hh1301.093615. [DOI] [PubMed] [Google Scholar]
- 5.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 6.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Suh Y, Arnold RS, Lassègue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 9.Lassègue B, Sorescu D, Szöcs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91phox homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 10.Sorescu D, Somers MJ, Lassegue B, Grant S, Harrison DG, Griendling KK. Electron spin resonance characterization of the NAD(P)H oxidase in vascular smooth muscle cells. Free Radic Biol Med. 2001;30:603–612. doi: 10.1016/s0891-5849(00)00507-4. [DOI] [PubMed] [Google Scholar]
- 11.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Szöcs K, Lassègue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 13.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 14.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 15.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 16.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 17.Griendling KK, Taubman MB, Akers M, Mendlowitz M, Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem. 1991;266:15498–15504. [PubMed] [Google Scholar]
- 18.Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int Rev Cytol. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- 19.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Christen T, Verin V, Bochaton-Piallat M, Popowski Y, Ramaekers F, Debruyne P, Camenzind E, van Eys G, Gabbiani G. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation. 2001;103:882–888. doi: 10.1161/01.cir.103.6.882. [DOI] [PubMed] [Google Scholar]
- 21.Campbell JC, Campbell GR, Ross R. The smooth muscle cell in culture. Am J Physiol. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Worth NF, Rolfe BE, Song J, Campbell GR. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell Motil Cytoskeleton. 2001;49:130–145. doi: 10.1002/cm.1027. [DOI] [PubMed] [Google Scholar]
- 23.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 24.Puceat M. Role of Rac-GTPase and reactive oxygen species in cardiac differentiation of stem cells. Antioxid Redox Signal. 2005;7:1435–1439. doi: 10.1089/ars.2005.7.1435. [DOI] [PubMed] [Google Scholar]
- 25.Piao YJ, Seo YH, Hong F, Kim JH, Kim YJ, Kang MH, Kim BS, Jo SA, Jo I, Jue DM, Kang I, Ha J, Kim SS. Nox 2 stimulates muscle differentiation via NF-kappaB/iNOS pathway. Free Radic Biol Med. 2005;38:989–1001. doi: 10.1016/j.freeradbiomed.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, Zhang Y, Ries W, Key L. Expression of Nox4 in osteoclasts. J Cell Biochem. 2004;92:238–248. doi: 10.1002/jcb.20048. [DOI] [PubMed] [Google Scholar]
- 27.Spin JM, Nallamshetty S, Tabibiazar R, Ashley EA, King JY, Chen M, Tsao PS, Quertermous T. Transcriptional profiling of in vitro smooth muscle cell differentiation identifies specific patterns of gene and pathway activation. Physiol Genomics. 2004;19:292–302. doi: 10.1152/physiolgenomics.00148.2004. [DOI] [PubMed] [Google Scholar]
- 28.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming Growth Factor {beta}1 Induces Nox 4 NAD(P)H Oxidase and Reactive Oxygen Species-Dependent Proliferation in Human Pulmonary Artery Smooth Muscle Cells. Am J Physiol Lung Cell Mol Physiol. 2005;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 29.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 31.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol Mar. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menshikov M, Plekhanova O, Cai H, Chalupsky K, Parfyonova Y, Bash-trikov P, Tkachuk V, Berk BC. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler Thromb Vasc Biol. 2006 Apr;26:801–807. doi: 10.1161/01.ATV.0000207277.27432.15. [DOI] [PubMed] [Google Scholar]
- 33.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]


