Abstract
Basaloid squamous cell carcinoma (BSCC) of the esophagus is a rare, poorly differentiated variant of typical esophageal squamous cell carcinoma (SCC) characterized by high proliferative activity and frequent spontaneous apoptoses. In the present study, we investigated the expression of the apoptosis-suppressing protein Bcl-2 in 23 BSCC of the esophagus and 23 stage-matched typical esophageal SCC by means of immunohistochemistry. In addition, amplification of the apoptosis- and proliferation-inducing gene c-myc was determined by means of differential polymerase chain reaction. Bcl-2 expression was found significantly more often in BSCC than in SCC (86.9% vs. 17.4%, P < 0.0001). Amplification of c-myc was nearly twice as common in BSCC as in SCC (47.8% vs. 26.1%, not significant). Bcl-2 protein expression together with c-myc amplification was detected in 43.5% of the BSCC but in none of the typical SCC (P < 0.0001). Taken together, our findings indicate that the molecular pathogenesis of esophageal BSCC differs from that of typical SCC and frequently involves coactivation of c-myc and Bcl-2.
Basaloid squamous cell carcinoma (BSCC) is an uncommon variant of squamous cell carcinoma (SCC) that arises in a variety of anatomic sites, including the upper aerodigestive tract, 1-3 the anus, 4 the thymus, 5 and the uterine cervix. 6 Histologically, BSCC is defined as an invasive carcinoma composed of closely packed cells with hyperchromatic nuclei and scant cytoplasm. The tumors display a predominantly solid growth pattern, small cystic spaces, and foci of comedo-type necrosis. Additionally, BSCC is intimately associated with dysplastic squamous epithelium, in situ SCC, invasive SCC, or foci of SCC among basaloid cells. 1 Recently, we described the histological and clinical features of a series of BSCC of the esophagus. 7 In this investigation, we were able to show that esophageal BSCC are characterized by significantly higher proliferative activity and a significantly higher rate of spontaneous apoptosis than typical SCC. In another study on the expression of the apoptosis-regulating protein Bcl-2 in esophageal SCC, we had observed that expression of Bcl-2 is more common in poorly differentiated SCC of the esophagus than in well differentiated SCC. 8 We also noted that expression of Bcl-2 was especially strong in BSCC (unpublished results). A similar observation has recently been published by Koide et al, 9 who found strong expression of Bcl-2 protein in a small series of 4 BSCC of the esophagus.
The simultaneous finding of high apoptotic rate and strong expression of Bcl-2 in BSCC is difficult to understand, given that Bcl-2 is known to function as a suppressor of apoptosis. 10 We therefore speculated that the frequent overexpression of Bcl-2 in BSCC must be counteracted by activation of one or more pro-apoptotic and/or proliferation-promoting proto-oncogenes. The proto-oncogene c-myc seemed to be a very promising candidate in this context. Thus, much in vitro and in vivo data indicate that increased expression of c-myc blocks differentiation 11-14 and enhances proliferative and apoptotic activity. 15-17 Moreover, it has been shown in experimental tumor systems that increased expression of Bcl-2 and c-myc can cooperate in tumorigenesis. 18-21
In the present study, we therefore determined the expression of Bcl-2 protein and screened for amplification of the c-myc gene in a series of 23 BSCC of the esophagus and compared the results with those obtained for 23 stage-matched typical SCC of the esophagus. Our results indicate that Bcl-2 overexpression and c-myc amplification frequently occur in BSCC but not in SCC, a finding suggesting a cooperative function of Bcl-2 and c-myc in the molecular pathogenesis of esophageal BSCC.
Materials and Methods
Specimen Selection
Twenty-three basaloid squamous cell carcinomas of the esophagus, classified according to the criteria of Wain et al, 1 were retrieved from the files of the Institutes of Pathology of the Universities of Düsseldorf and Mainz, Germany. As a control, 23 stage-matched typical squamous cell carcinomas of the esophagus were selected. All tumors had been resected between 1978 and 1998 without prior radio- or chemotherapy.
The 23 BSCC were from 19 male and 4 female patients. The median age at operation was 61 years (range, 45–72 years). The typical SCC were from 14 male and 9 female patients (median age 59 years; range, 42–67 years).
Pathological Review
The surgical specimens were fixed in 4% buffered formaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). The tumor stage was determined according to the criteria proposed by the UICC. 22 The grade of tumor differentiation of the 23 typical SCC was determined according to the criteria proposed by the World Health Organization. 23 Accordingly, 5 BSCC were in stage I, 4 were in stage IIA, 2 were in stage IIB, and 12 were in stage III. The distribution of the 23 typical SCC according to tumor stage was identical to that of the BSCC. Of the typical SCC, 2 were graded as G1, 11 as G2, and 10 as G3. No grading was performed for the BSCC because there is currently no generally accepted grading system for this tumor type.
Bcl-2 Immunohistochemistry
For each carcinoma, one representative block including central and peripheral portions of the tumor was selected. After microwave pretreatment, immunostaining was performed using the monoclonal antibody 124 (1:40; Dako, Glostrup, Denmark) as described previously. 8 Negative controls were performed by replacing the primary antibody by an irrelevant isotype-matched monoclonal mouse antibody at the same dilution as the Bcl-2 antibody. Tonsillar tissue was used as positive control. In addition, positive staining of tumor-infiltrating lymphocytes provided an internal control for Bcl-2 staining.
The percentage of Bcl-2-positive tumor cells was determined semiquantitatively by assessing the entire tumor section. Each sample was assigned to one of the following categories: 0 (0–4%), 1 (5–24%), 2 (25–49%), 3 (50–74%), or 4 (75–100%). The intensity of immunostaining was determined as 0 (negative), 1+ (weak), or 2+ (strong). Staining intensity was judged relative to lymphocytes within the sample, which were designated arbitrarily as 2+. 24 Finally, an immunoreactive score was calculated by multiplying the percentage of positive cells by the staining intensity score, as proposed by Krajewska et al. 25 In the case of heterogeneous staining intensities within one sample, each component was scored independently and the results were summed. For example, a specimen containing 25% tumor cells with strong intensity (1 × 2+ = 2), 25% tumor cells with weak intensity (1 × 1+ = 1) and 50% tumor cells without immunoreactivity received a score of 2 + 1 + 0 = 3.
Differential PCR Analysis for c-myc Amplification
For DNA preparation two 10-μm slices from paraffin blocks containing tumor tissue and adjacent normal tissue (eg, lymph-node tissue or gastric tissue from the distal resection margin) were dewaxed and lightly stained with hematoxylin. Subsequently, tumor tissue and normal tissue were dissected from the slides under light microscopic control and placed into reaction cups containing TE buffer (10 mmol/L Tris-Cl; pH 7,5; 0,1 mmol/L EDTA). After proteinase K digestion overnight (1 mg/ml; 55°C), and inactivation of proteinase K (94°C, 8 minutes), the preparations were used for PCR without further purification.
For differential PCR analysis, 26 a 96-bp fragment of the c-myc gene (target gene) located on chromosome 8 was coamplified with a 134-bp fragment of the APRT (adeninphosphoribosyl-transferase) gene (control gene) located on chromosome 16. The primer sequences were 5′ - CCT CAA CGT TAG CTT CAC CAA C-3′ and 5′-CTG CTG GTA GAA GTT CTC CTC - 3′ for c-myc, and 5′ - TGG GAA AGC TGT TTA CTG GC - 3′ and 5′ - CAG GGA ACA CAT TCC TTT GC - 3′ for APRT. Differential PCR was performed in a final volume of 50 μl with 2 μl of DNA template, PCR buffer containing 1.5 mmol/L MgCl2, 0.2 mmol/L of each dNTP, 30 pmol primer for the c-myc gene, 60 pmol primer for the APRT gene, and 2 U Taq DNA-polymerase (PCR Core Kit, Qiagen, Hilden, Germany). Initial denaturation at 94°C for 5 minutes was followed by 30 cycles on a thermocycler (UNO; Biometra, Göttingen, Germany). These included denaturation at 94°C for 1 minute, annealing at 56°C for 1 minute, and extension at 72°C for 1 minute. A final extension step at 72°C was performed for 4 minutes.
As a positive control, we used DNA from the colon carcinoma cell line COLO320DM, previously shown to have amplified c-myc 27 and DNA from a formalin-fixed, paraffin-embedded esophageal SCC with known c-myc amplification. Peripheral leukocyte DNA and normal placental DNA were used as reference templates with normal gene copy number.
PCR products were separated on 3% agarose gels, and the ethidium-bromide-stained bands were recorded by the Gel-Doc 1000 system (Bio-Rad, München, Germany). Quantitative densitometric evaluation of the target gene signal intensity relative to the control gene signal intensity was performed using Molecular Analyst software, version 2.1 (Bio-Rad, München, Germany). Only increases in the target gene/control gene quotients more than 3 times that of corresponding normal tissue were considered as evidence of gene amplification. 28,29
Tumors with evidence of c-myc amplification in the initial analysis were retested by performing a second independent PCR. In addition, differential PCR analysis using a second control gene locus was performed in all these cases. Therefore, a 82-bp fragment of the IFNG (γ-interferon) gene, located on chromosome 12, was coamplified with the 96-bp fragment of the c-myc gene. The primer sequences were 5′ - GCA GAG CCA AAT TGT CTC CT - 3′ and 5′ - GGT CTC CAC ACT CTT TTG GA - 3′ for IFNG. The PCR conditions were identical to those for the PCR with APRT as control gene, except that the quantities of primer were 60 pmol for c-myc and 30 pmol for IFNG.
Statistical Analysis
Statistical analysis was performed using the SAS software package (SAS Institute Inc., Cary, NC). Correlations between the expression of Bcl-2, amplification of c-myc, and tumor type were analyzed by means of the two-sided Fisher’s exact test; P values <0.05 were considered significant.
Results
Expression of Bcl-2 in Normal Esophageal Tissue
In normal esophageal squamous epithelium adjacent to the carcinomas, cytoplasmatic Bcl-2 immunoreactivity was found in the basal cell layer, whereas suprabasal cells showed no Bcl-2 immunoreactivity. The intensity of Bcl-2 staining was weak (1+) compared to lymphocytes within the same section. In addition, Bcl-2 expression was found in neurons of the myenteric plexus as well as in smooth muscle cells of the arterial walls, the muscularis mucosae and the muscularis propria.
Expression of Bcl-2 in BSCC and in SCC
Twenty out of 23 BSCC (Fig. 1a) ▶ showed cytoplasmatic expression of Bcl-2 (Fig. 1b) ▶ , 2 with weak (1+), and 18 with strong (2+) staining intensity, whereas 3 BSCC were completely negative. Regarding the percentage of positive tumor cells, 2 were in category 1 (5–24%), 4 in category 2 (25–49%), 7 in category 3 (50–74%), and 7 in category 4 (75–100%). The immunoreactive scores of the 20 Bcl-2-positive BSCC ranged between 2 and 8 (median, 5).
Figure 1.
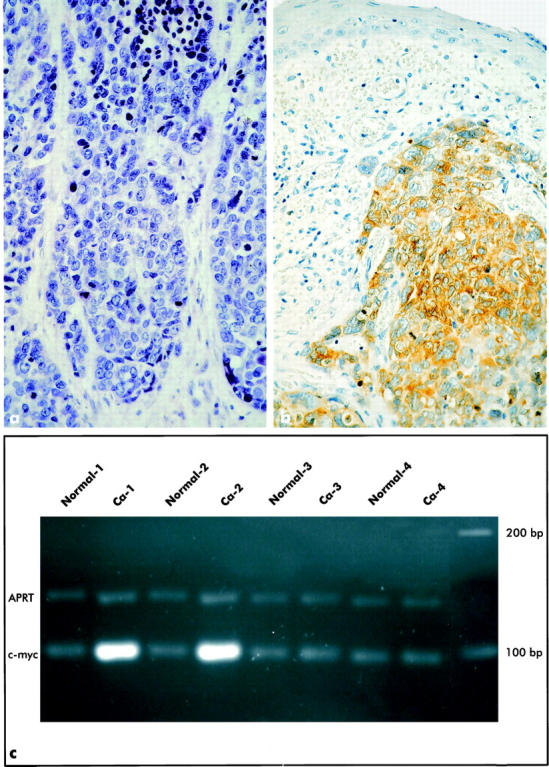
Typical light microscopic aspect of a basaloid squamous cell carcinoma (BSCC) composed of cells with hyperchromatic nuclei and scant cyctoplasm (a); H&E; original magnification, ×400. Strong expression of Bcl-2 protein (brown cytoplasmatic reaction product) in an esophageal BSCC. Note the absence of Bcl-2 expression in the neighboring nonmalignant esophageal squamous epithelium (b); original magnification, ×400. Ethidium bromide-stained agarose gel of a differential PCR for the c-myc gene and the APRT gene in carcinoma tissues (Ca) and corresponding normal tissues (Normal) from 4 cases of esophageal BSCC. Note amplification of the c-myc gene in tumors 1 and 2, no c-myc amplification in tumors 3 and 4 (c).
In contrast, only 4 SCC showed cytoplasmatic immunoreactivity for Bcl-2; 19 cases were negative. The 4 Bcl-2-positive SCC showed only weak (1+) staining intensity. With regard to the percentage of positive cells, all positive cases were in category 1 (5–24%); the immunoreactive score of each of the 4 Bcl-2-positive SCC was 1.
Upon comparison of Bcl-2 expression in BSCC and SCC (negative vs. positive), the difference between the two groups was highly significant (P < 0.0001; Fisher’s exact test).
Amplification of c-myc in BSCC and SCC
Amplification of c-myc was found in 11 out of 23 BSCC and in 6 out of 23 SCC (Fig. 1c) ▶ . Differential PCR analysis using APRT as control gene showed results identical to those for differential PCR using IFNG as control gene. None of the corresponding normal tissues under investigation showed evidence of c-myc amplification. Comparison of the frequency of c-myc amplification in BSCC and SCC revealed no significant difference (Fisher’s exact test).
Amplification of c-myc and Expression of Bcl-2 in BSCC and in SCC
Both aberrations, amplification of c-myc and expression of Bcl-2, were simultaneously detected in 10 BSCC but in none of the SCC under investigation. This difference was significant according to Fisher’s exact test (P < 0.0001).
Discussion
The present study shows that expression of Bcl-2 protein is more frequent and stronger in BSCC than in typical SCC of the esophagus. In addition, we have demonstrated that amplification of c-myc is nearly twice as common in BSCC as in typical SCC. Both aberrations, ie, expression of Bcl-2 and amplification of c-myc, were present in nearly half of the BSCC but in none of the SCC in this investigation. These data suggest that coactivation of Bcl-2 and c-myc may be important for the pathogenesis of esophageal BSCC. Prior evidence of cooperation between c-myc and Bcl-2 derives mainly from experimental data. For example, induction and progression of malignant lymphomas through cooperation of Bcl-2 and c-myc has repeatedly been shown in c-myc/Bcl-2 double-transgenic mice. 19-21 A similar effect was found for the transformation of cultured fibroblasts 30 and cultured bone marrow cells. 18 Mechanistically, this cooperation is explained by a Bcl-2-induced suppression of the c-myc-induced apoptosis without blockade of the proliferation-inducing effect of c-myc. 21,30-32 In contrast, evidence of cooperation between c-myc and Bcl-2 in clinical tumor samples is still limited. Thus, Wang et al 33 found coexpression of Bcl-2 and c-myc in the majority of pheochromocytomas. In neuroblastomas, a cooperative effect between Bcl-2 and the c-myc homologue N-myc has been suggested. 34 Except for one study on medullary thyroid cancer, 35 the possible coordinate aberration of Bcl-2 and c-myc in human carcinomas has not yet been investigated. This may be partly attributable to technical problems. Due to DNA degradation in formalin-fixed tumor samples, determination of c-myc amplification is not possible by Southern blot analysis. On the other hand, immunohistochemical analysis of c-myc protein expression has produced inconclusive results due to the short half-time of the c-myc protein 36 and the negative effects of formalin fixation on antigenicity. 37 Against this background, differential PCR analysis provides a reliable alternative method for the detection of c-myc amplification in paraffin-embedded tumor samples. 26,28,29 This can be appreciated from the fact that the frequency of c-myc amplification found in our series of typical SCC corresponds well with previously published Southern blot-based figures on the frequency of c-myc amplification in this tumor type. 38
Genetic alterations involved in the development of BSCC, especially BSCC of the esophagus, are largely unknown. Recently, Abe et al 39 have demonstrated DNA aneuploidy by means of DNA image cytometry in 100% of a small series of 7 esophageal BSCC. The main reason for the current lack of information on the molecular pathogenesis of BSCC may be that it is a relatively rare and only recently recognized variant of typical SCC. Thus, only 43 cases of esophageal BSCC, including our 23 cases, have been published to date. 7,9,39-43 Our study addresses for the first time the analysis of molecular genetic alterations in esophageal BSCC. We demonstrate that these tumors frequently show Bcl-2 overexpression and c-myc amplification. With regard to the temporal sequence of these aberrations, however, we cannot conclude from our data whether amplification of c-myc follows overexpression of Bcl-2 in the development of BSCC or vice versa. However, the first possibility seems to be more likely, because overexpression of Bcl-2 was more frequent than c-myc amplification. This hypothesis is also supported by our previously published observation that Bcl-2 overexpression may already occur in precursor lesions of esophageal cancer (ie, severe squamous dysplasias, carcinomas in situ). 8 The potential role of Bcl-2 in the development of tumors has been explained by its apoptosis-suppressing effect, which provides a growth advantage to Bcl-2-overexpressing cells and allows the accumulation of oncogenic mutations during a prolonged life span. 44 However, our data indicate that Bcl-2 overexpression may only incompletely protect malignant cells in BSCC from apoptosis because these tumors, in spite of their frequent Bcl-2 overexpression, are characterized by higher rates of spontaneous apoptoses than typical SCC. 7 Although the increased expression of c-myc due to gene amplification may at least partly account for this phenomenon, its precise molecular basis remains to be elucidated. Nevertheless, our data indicate that alterations other than concomitant Bcl-2 overexpression and c-myc amplification must be involved in the development of BSCC, insofar as only 10 of the 23 cases under investigation showed both aberrations. In this context it is possible that other mechanisms for c-myc activation than gene amplification may play a role in BSCC, eg, chromosomal translocation 21 or transcriptional up-regulation by increased expression of activating transcription factors. 17,45 Additionally, other genes may play an important role. Future investigations on mutations in the p53 gene would be especially interesting, because it well known that p53 not only plays an important role in the regulation of proliferation and apoptosis but also is frequently mutated in typical esophageal SCC. 38
In conclusion, our data provide evidence for the first time that the molecular pathogenesis of esophageal BSCC differs from that of typical SCC and frequently involves coactivation of c-myc and Bcl-2.
Footnotes
Address reprint requests to Dr. Mario Sarbia, Institute of Pathology, University of Düsseldorf, Moorenstr. 5 40225 Düsseldorf, Germany. E-mail: Sarbia@med.uni-duesseldorf.de.
References
- 1.Wain SL, Kier R, Vollmer RT, Bossen EH: Basaloid-squamous carcinoma of the tongue, hypopharynx and larynx: report of 10 cases. Hum Pathol 1986, 17:1158-1166 [DOI] [PubMed] [Google Scholar]
- 2.Luna MA, El-Naggar A, Parichatikanond P, Weber RS, Batsakis JG: Basaloid squamous carcinoma of the upper aerodigestive tract. Cancer 1990, 66:537-542 [DOI] [PubMed] [Google Scholar]
- 3.Banks ER, Frierson HF, Mills SE, George E, Zarbo RJ, Swanson PE: Basaloid squamous cell carcinoma of the head and neck: a clinicopathologic and immunohistochemical study of 40 cases. Am J Surg Pathol 1992, 16:939-946 [DOI] [PubMed] [Google Scholar]
- 4.Dougherty BG, Evans HL: Carcinoma of the anal canal: a study of 79 cases. Am J Clin Pathol 1985, 83:159-164 [DOI] [PubMed] [Google Scholar]
- 5.Walker AN, Mills SE, Fechner RE: Thymoma and thymic carcinomas. Semin Diagn Pathol 1990, 7:250-265 [PubMed] [Google Scholar]
- 6.Ferry JA, Scully RE: “Adenoid cystic” carcinoma and adenoid basal carcinoma of the uterine cervix: a study of 28 cases. Am J Surg Pathol 1988, 2:134-144 [DOI] [PubMed] [Google Scholar]
- 7.Sarbia M, Verreet P, Bittinger F, Dutkowski P, Heep H, Willers R, Gabbert HE: Basaloid squamous cell carcinoma of the esophagus: diagnosis and prognosis. Cancer 1997, 79:1871-1878 [DOI] [PubMed] [Google Scholar]
- 8.Sarbia M, Bittinger F, Porschen R, Verreet P, Dutkowski P, Willers R, Gabbert HE: Bcl-2 expression and prognosis in squamous-cell carcinoma of the esophagus. Int J Cancer 1996, 69:324-328 [DOI] [PubMed] [Google Scholar]
- 9.Koide N, Koike S, Adachi W, Amano J, Usuda N, Nagata T: Immunohistochemical expression of bcl-2 protein in squamous cell carcinoma and basaloid carcinoma of the esophagus. Surg Today 1997, 27:685-691 [DOI] [PubMed] [Google Scholar]
- 10.Strasser A, Huang DCS, Vaux DL: The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochem Biophys Acta 1997, 1333:F151-F178 [DOI] [PubMed] [Google Scholar]
- 11.Coppola JA, Cole MD: Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature 1986, 320:761-763 [DOI] [PubMed] [Google Scholar]
- 12.Freytag SO: Enforced expression of the c-myc oncogene inhibits cell differentiation by precluding entry into a distinct predifferentiation state in G0/G1. Mol Cell Biol 1988, 8:1614-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freytag SO, Dang CV, Lee WMF: Definition of the activities and properties of c-myc required to inhibit cell differentiation. Cell Growth Differ 1990, 1:339-343 [PubMed] [Google Scholar]
- 14.Henriksson M, Luscher B: Proteins of the myc network: essential regulators of cell growth and differentiation. Adv Cancer Res 1996, 68:109-182 [DOI] [PubMed] [Google Scholar]
- 15.Askew DS, Ashmun RA, Simmons BC, Cleveland JL: Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene 1991, 6:1915-1922 [PubMed] [Google Scholar]
- 16.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock: Induction of apoptosis in fibroblasts by c-myc protein. Cell 1992, 69:119-128 [DOI] [PubMed] [Google Scholar]
- 17.Bouchard C, Staller P, Eilers M: Control of cell proliferation by myc. Trends Cell Biol 1998, 8:202-206 [DOI] [PubMed] [Google Scholar]
- 18.Vaux DL, Cory S, Adama JM: Bcl-2 gene promotes haematopoetic cell survival and cooperates with c-myc to immortalize pre B cells. Nature 1988, 335:440-442 [DOI] [PubMed] [Google Scholar]
- 19.Strasser A, Harris AW, Bath ML, Cory S: Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990, 348:331-333 [DOI] [PubMed] [Google Scholar]
- 20.McDonnell TJ, Korsmeyer SJ: Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18). Nature 1991, 349:254-256 [DOI] [PubMed] [Google Scholar]
- 21.Marin MC, Hsu B, Stephens C, Brisbay S, McDonnell TJ: The functional basis of c-myc and bcl-2 complementation during multistep lymphomagenesis in vivo. Exp Cell Res 1995, 217:240-247 [DOI] [PubMed] [Google Scholar]
- 22.TNM Classification of Malignant Tumours, 5th ed. Edited by Sobin LH, Wittekind CH (eds.). New York, Wiley-Liss, 1997
- 23.World Health Organization. Histological Typing of Oesophageal and Gastric Tumours, 2nd ed. Berlin, Springer-Verlag, 1990
- 24.Sinicrope FA, Ruan SB, Cleary KR, Stephens C, Lee JJ, Levin B: bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res 1995, 55:237-241 [PubMed] [Google Scholar]
- 25.Krajewska M, Krajewski S, Epstein JL, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC: Immunohistochemical analysis of bcl-2, bax, bcl-X and mcl-1 expression in prostate cancers. Am J Pathol 1996, 148:1567-1576 [PMC free article] [PubMed] [Google Scholar]
- 26.Neubauer A, Neubauer B, He M, Iglehart D, Frye RA, Liu E: Analysis of gene amplification in archival tissue by differential polymerase chain reaction. Oncogene 1992, 7:1019-1025 [PubMed] [Google Scholar]
- 27.Schwab M, Alitalo K, Kiempnauer KH, Varmus HE, Bishop JM, Gilbert F, Brodeur G, Goldstein M, Trent J: Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumor. Nature 1983, 305:245-248 [DOI] [PubMed] [Google Scholar]
- 28.Reifenberger J, Ring GU, Gies U, Cobbers JMJL, Oberstrass J, An HX, Niederacher D, Wechsler W, Reifenberger G: Analysis of p53 mutation and epidermal growth factor amplification in recurrent gliomas with malignant progression. J Neuropath Exp Neurol 1996, 55:822-831 [DOI] [PubMed] [Google Scholar]
- 29.Cobbers JMJL, Wolter M, Reifenberger J, Ring GU, Jessen F, An HX, Niederacher D, Schmidt EE, Ichimura K, Floeth F, Kirsch L, Borchard F, Louis DN, Collins VP, Reifenberger G: Frequent inactivation of CDKN2A and rare mutation of TP53 in PCNSL. Brain Pathol 1998, 8:263-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fanidi A, Harrington EA, Evan GI: Cooperative interaction between c-myc and bcl-2 protooncogenes. Nature 1992, 359:554-556 [DOI] [PubMed] [Google Scholar]
- 31.Bissonette RP, Echeverri F, Mahboubi A, Green DR: Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature 1992, 359:552-554 [DOI] [PubMed] [Google Scholar]
- 32.Wagner AJ, Small MB, Hay N: Myc-mediated apoptosis is blocked by ectopic expression of Bcl-2. Mol Cell Biol 1993, 13:2432-2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang DG, Johnston CF, Marley JJ, Phenix KV, Atkinson AB, Russel CFJ, Buchanan KD: Expression of the apoptosis-suppressing gene Bcl-2 in pheochromocytomas is associated with the expression of c-myc. J Clin Endocrinol Metab 1997, 82:1949-1952 [DOI] [PubMed] [Google Scholar]
- 34.Castle VP, Heidelberger KP, Bromberg J, Ou X, Dole M, Nuñez G: Expression of the apoptosis-suppressing protein bcl-2 in neuroblastoma is associated with unfavorable histology and n-myc amplification. Am J Pathol 1993, 143:1543-1550 [PMC free article] [PubMed] [Google Scholar]
- 35.Wang DG, Liu WH, Johnston CF, Sloan JM, Buchanan KD: Bcl-2 and c-myc, but not Bax and p53, are expressed during human medullary thyroid tumorigenesis. Am J Pathol 1998, 152:1407-1413 [PMC free article] [PubMed] [Google Scholar]
- 36.Ong G, Gullick W, Sikora K: Oncoprotein stability after tumour resection. Br J Cancer 1990, 61:538-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loke SL, Neckers LM, Schwab G, Jaffe ES: c-myc protein in normal tissue: effects of fixation on its apparent subcellular distribution. Am J Pathol 1988, 131:29-37 [PMC free article] [PubMed] [Google Scholar]
- 38.Montesano R, Hollstein M, Hainaut P: Genetic alterations in esophageal cancer and their relevance to etiology and pathogenesis: a review. Int J Cancer 1996, 69:225-235 [DOI] [PubMed] [Google Scholar]
- 39.Abe K, Sasano H, Itakura Y, Nishira T, Mori S, Nagura H: Basaloid-squamous carcinoma of the esophagus: a clinicopathologic, DNA ploidy, and immunohistochemical study of seven cases. Am J Surg Pathol 1996, 20:453-461 [DOI] [PubMed] [Google Scholar]
- 40.Tsang WYW, Chan JKC, Lee KC, Leung AKF: Basaloid-squamous carcinoma of the upper aerodigestive tract and so-called adenoid cystic carcinoma of the oesophagus: the same tumor type? Histopathology 1991, 19:35-46 [DOI] [PubMed] [Google Scholar]
- 41.Takubo K, Mafune K, Tanaka Y, Miyama T, Fujita D: Basaloid-squamous carcinoma of the esophagus with marked deposition of basement membrane substance. Acta Pathol Jpn 1991, 41:59-64 [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa Y, Asuwa N, Ishii T, Masuda S, Kiguchi H: A case of basaloid-squamous of the esophagus: immunohistochemical and ultrastructural studies. Pathol Int 1994, 44:466-474 [DOI] [PubMed] [Google Scholar]
- 43.Cabrera E, Fernandez F, Gomez-Roman J, Val-Bernal JF: Basaloid squamous cell carcinoma of the esophagus: immunohistochemistry and flow cytometric DNA analysis in two cases. Int J Surg Pathol 1996, 3:267-274 [Google Scholar]
- 44.Bronner MP, Culin C, Reed JC, Furth EE: The bcl-2 proto-oncogene and the gastrointestinal epithelial tumor progression model. Am J Pathol 1995, 146:20-26 [PMC free article] [PubMed] [Google Scholar]
- 45.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-MYC as a target of the APC pathway. Science 1998, 281:1509-1512 [DOI] [PubMed] [Google Scholar]


