Abstract
Mucosa-associated lymphoid tissue (MALT) may accumulate within gastric mucosa as a result of long standing Helicobacter pylori infection, and this acquired MALT may eventually develop into low-grade B-cell MALT lymphoma. To determine the possible association of cell cycle regulatory proteins and apoptotic cell death in the transformation of H. pylori gastritis to MALT lymphoma, the extent of cell proliferation, cell viability, expression of Cdc2/Cdk1 and cyclin B in gastric mucosal from patients with H. pylori-positive chronic gastritis (n = 7), MALT (n = 12), or MALT lymphoma (n = 12) were undertaken. Control tissue was obtained from H. pylori- negative patients (n = 5). Proliferating cell nuclear antigen (PCNA), Cdc2, and cyclin B1 were examined in paraffin embedded tissue by immunohistochemistry, while the apoptotic index (AI) was determined using the TUNEL assay. H&E staining for histology and modified Giemsa staining for the detection of H. pylori was conducted simultaneously. When compared to chronic gastritis tissue, those with MALT or MALT lymphoma had an increase in PCNA labeling index of 3.3- and 2.7-fold, while that for Cdc2/Cdk1 increased 2.3- and 3.1-fold, respectively. cyclin B1 labeling was 1.9 and 3.0 fold, while the AI was 3.4- and 1.4-fold higher in MALT and MALT lymphoma tissue, respectively, in the same comparison. On the other hand, the AI index of MALT lymphoma was 2.5-fold lower than that for MALT tissues. The labeling scores for Cdc2/Cdk1 and cyclin B1 were significantly higher in the germinal center when compared to the mantle and marginal zones of MALT tissues. Using χ2 and Pearson/Spearman’s rho correlation coefficient with regression analyses, there was an inverse correlation between the AI and Cdc2/Cdk1 or cyclin B1 in MALT and MALT lymphoma tissues. There was no correlation between AI and PCNA labeling in any of the tissues. These results suggest that Cdc2/Cdk1 and cyclin B1 expression may be actively associated in the modulation of cellular death by apoptosis, as well as cellular proliferation and transformation during the evolution of H. pylori-associated gastritis to MALT lymphoma. Subclassification of high labeling score (≥40) for Cdc2/Cdk1 and cyclin B1 and low labeling index (<0.6) for apoptotic cells in H. pylori-associated MALT may help in identifying a population of patients with an increased risk of developing MALT lymphoma.
Normal human gastric mucosa is devoid of organized mucosa-associated lymphoid tissue (MALT). 1 MALT accumulates within gastric mucosa as a result of long-standing Helicobacter pylori infection in a subset of infected patients, and from this acquired MALT, low-grade B cell MALT lymphoma may eventually develop. 2-4 Several chromosomal and/or subchromosomal abnormalities have been detected in gastric MALT lymphomas. 5-11 However, the precise molecular mechanism of the evolution of MALT to MALT lymphoma remains uncertain.
Self-immolation or programmed cell death, apoptosis, is crucial for the overall health of an organism and essential for both the development and function of an effective immune repertoire. 12-16 The development of neoplastic growth can be considered a perturbation in the balance of cellular proliferation, differentiation, and apoptosis. 14-16 Cells that have been exposed to toxic agents or infected with harmful viruses and/or bacteria can escape apoptosis and gradually undergo transformation as a result of the malfunction of certain genes. 12,14 Recent studies on the molecular mechanism of apoptosis indicate several cell cycle regulatory genes including cyclin-dependent kinase (Cdc2/cdk1) and cyclin B1 may be directly involved in this process. 17-22 Normally the complex events of the cell cycle are regulated by cell cycle specific cyclin-dependent kinases (CDKs), which become activated as the result of phosphorylation along with interaction with cyclins. The Cdc2-cyclin B1 complex controls the G2-M phase transition in eukaryotes by promoting breakdown of nuclear membrane, chromatin condensation, and microtubule spindle formation, allowing for ordered DNA replication and repair. Unscheduled up- or down-regulation of these cell cycle regulatory genes during the cell cycle can help cells overcome the death sentence, and Cdc2-cyclin B1 complex may play a crucial role in the induction of tumorigenesis. The significance and extent of apoptosis and disturbance of cell cycle regulatory proteins in the genesis of H. pylori-associated MALT to MALT lymphoma is unknown. To determine the association between cell cycle regulatory proteins, specially Cdc2 and cyclin B1, and apoptotic cell death during the progression of MALT lymphoma and to identify protein marker(s) that may help in recognition of the population that is at increased risk of developing MALT lymphoma, we examined the extent of apoptosis, cell proliferation, and Cdc2 and cyclin B1 expression in lymphoid cells in H. pylori-associated chronic gastritis, gastric MALT, and MALT lymphoma.
Materials and Methods
Patients and Histology
After obtaining informed consent, gastric mucosal biopsies were prospectively obtained during esophagogastroduodenoscopy (EGD) with large biopsy forceps. The gastric biopsies were placed in 10% buffered formalin for routine hematoxylin and eosin (H&E), immunohistochemistry, and apoptotic studies. Determination of the presence of H. pylori was based on the results of gastric mucosal biopsies stained with H&E as well as a modified Giemsa stain. H. pylori colonization was considered to be present if one or more of the Giemsa-stained gastric biopsy specimens demonstrated typical H. pylori structures. The density of H. pylori was determined in Giemsa-stained sections according to the upgraded Sydney grading system. 23 Criteria for normal control patients included a completely normal endoscopic appearance of the stomach, the absence of H. pylori on at least six Giemsa-stained gastric surveillance biopsies, and normal histology.
H. pylori-positive patients were defined in this study as having chronic H. pylori gastritis if they were found to have a mild to moderate mononuclear cell infiltrate in the lamina propria according to the updated Sydney grading system. 23 Lymphoid aggregates could be present, but not lymphoid follicles or lympho-epithelial lesions. If a lymphoid aggregate was noted, all available serial sections were closely scrutinized to exclude the presence of a germinal center.
The diagnosis of MALT required a confidence of diagnosis of lymphoma score of 3 to 4 according to Wotherspoon et al. 24 Features of MALT included the presence of a moderate to severe dense lymphocytic infiltrate in the lamina propria, one or more germinal centers, none to rare lympho-epithelial lesions, no Dutcher bodies, and the absence of monoclonality on Southern blot gene rearrangement testing in conjunction with a benign clinical, endoscopic, radiological, and laboratory investigation.
The diagnosis of low-grade MALT lymphoma was made on the basis of histology, requiring a confidence of diagnosis of lymphoma score of 5 according to Wotherspoon et al. 24 Histological components of gastric MALT lymphoma included 1) clusters of centrocyte-like cells, both intraepithelially and intralumenally, invading mucosal epithelium and forming lympho-epithelial lesions; 2) reactive lymphoid follicles within the mucosa and submucosa exhibiting variable obliteration of the mantle zone and infiltration of germinal center by centrocyte-like cells; and 3) a dense subepithelial plasma cell infiltrate. 25-29 Low- and high-grade MALT B cell lymphomas were distinguished based on criteria described by Isaacson. 25,26 Confirmation of malignancy required demonstration of B cell immunoglobulin heavy chain monoclonality. 25 The institution’s Human Subjects Committee approved the study.
Chemicals
PCNA, Cdc2, and cyclin B1 antibodies were purchased from NeoMarkers, Inc. (Fremont, CA) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). DNA fragmentation detection kit (TdT FragEL) was purchased from Oncogene Research Products (Cambridge, MA). Immunohistochemical kits were purchased from Zymed Laboratories, Inc. (San Francisco, CA). All other materials were of the highest commercially available grade.
Immunohistochemistry
The immunohistochemical staining was carried out according to manufacturer’s recommendations (Zymed) and our previous modified method. 30 Briefly, paraffin-embedded 10-μm tissue sections were dewaxed in xylene and rehydrated in 1× PBS through different concentrations of ethanol. To block endogenous peroxidase activity, slides were incubated in 3% hydrogen peroxide (3% v/v) in methanol for 5 minutes at room temperature. The slides were then incubated in citrate buffer (Zymed) in a microwave oven at high power for 5 minutes and allowed to cool for 15 minutes. After washing the slides in water followed by 1× PBS for 5 minutes, sections were incubated in ready to use tissue blocker for 15 minutes at room temperature. Tissue blocker was replaced with primary antibodies or preimmune IgG as negative controls and incubated overnight at 4°C. Antibodies used included a mouse monoclonal antibody at a 1:300 dilution for Cdc2 (NeoMarkers); a mouse monoclonal antibody at a 1:200 dilution for cyclin B1 purchased from, and a ready-to-use monoclonal antibody for PCNA (Zymed). After incubation with primary antibodies, slides were rinsed with 1× PBS (3× 5 minutes) and incubated for 10 minutes at room temperature in biotinylated rabbit-anti-goat IgG (Zymed). After rinsing with PBS (3× 5 minutes), sections were incubated in peroxidase-conjugated linker for 10 minutes at room temperature. Antibodies were detected by incubation with 3,3′-diaminobenzidine tetrahydrochloride solution, provided by Zymed with their kit, for a period of time sufficient to yield dark brown color, usually 5 minutes. Sections were counterstained with hematoxylin for microscopic examination. Lymphoid cells were identified by CD20 immunohistochemical analysis.
Determination of PCNA Labeling Index (LI) and the Expression of Cdc2 and Cyclin B1
The LI of PCNA, Cdc2, and cyclin B1 were determined in the different portions (eg, superficial and deep regions of lamina propria, intraepithelial lymphocytes, and germinal center, mantle zone, and marginal zone of MALT) of paraffin-embedded gastric biopsy tissue sections of H. pylori-negative normal and H. pylori-positive chronic gastritis, MALT, and MALT lymphoma patients as depicted in Figure 1 ▶ . To determine the LI, the immunostained sections were first scanned under low magnification (100×) field to locate the hot spots (areas with maximal Cdc2- or cyclin B1-immunopositive lymphoid cells) in different portions of immunostained sections. The LI of PCNA, Cdc2, and cyclin B1 in a 400× field was then scored by determining the average count of the number of lymphoid cells with positively staining nuclei and/or cytoplasm at five hot spots in lamina propria, organized mucosal lymphoid tissue (ie, germinal center, mantle zone, marginal zone), and intraepithelial lymphocytes of the immunostained sections. A minimum of five sections from each patient was examined to accurately determine the LI of these proteins. All slides were scored blindly three times by observers without knowledge of the patient’s clinical and/or histological findings.
Figure 1.
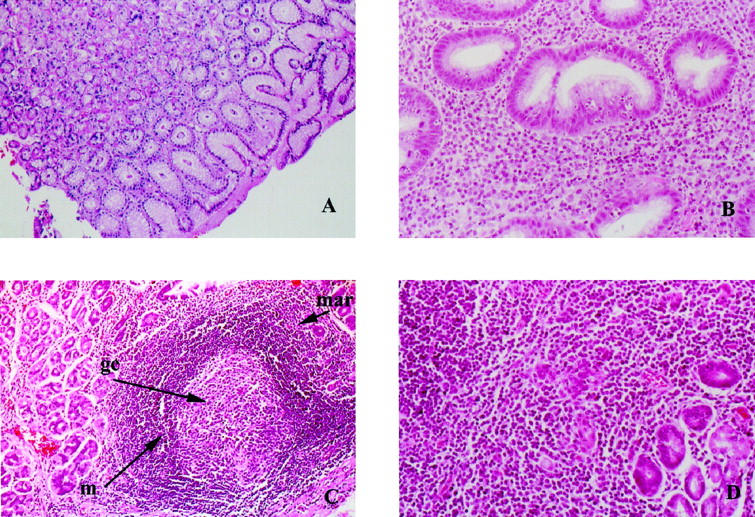
Histopathology of H&E-stained human gastric biopsy samples. A: Normal gastric mucosa; original magnification, ×100. B: Chronic active H. pylori-associated gastritis; original magnification, ×400. C: Lymphoid follicle (MALT) from a case of H. pylori-associated gastritis showing germinal center (gc), mantle zone (m) and marginal zone (mar); original magnification, ×100. D: Low grade lymphoma of MALT type. Note lymphoepithelial lesion. Original magnification, ×200.
Histochemical Detection of Apoptotic Cells and Bodies
Apoptosis was visualized using TdT FragEL DNA fragmentation detection kit (Oncogene Research Products). The staining procedures were modified based on the manufacturer’s recommendations. Briefly, after routine deparaffinization, rehydration, and washing in 1× PBS, pH 7.4, tissues were digested with proteinase K (20 mg/ml in 1× PBS) for 20 minutes at room temperature and washed. After incubation in equilibration buffer for 10 minutes, sections were treated with terminal deoxynucleotidyltransferase (TdT) enzyme at 37°C for 1 hour. After the TdT treatment and histochemical staining, slides were counterstained with methyl green to identify the normal and apoptotic cells. A specimen known to be positive for apoptotic cells was used as a positive control. Distilled water was substituted for TdT for use as a negative control.
Determination of Apoptotic Index (AI)
All slides were scored blindly three times without knowledge of the patient’s clinical and/or histological findings. The AI was determined in the paraffin-embedded gastric biopsy tissue sections of H. pylori-negative normal and H. pylori-positive chronic gastritis, MALT and MALT lymphoma patients (Figure 1) ▶ . To determine AI in normal and diseased sections, TUNEL-immunostained sections were first scanned under low power magnification (100×) to locate the apoptotic hot spots (areas with maximal TUNEL-positive lymphoid cells) within lamina propria, organized mucosal lymphoid tissue (ie, germinal center, mantle zone, marginal zone), and intraepithelial lymphocytes. The AI at 400× field was then scored by counting the number of TUNEL-positive cells. At least five hot spots in a section were selected to determine the average count. A minimum of 500 cell nuclei from each slide was counted. Positively staining cells with the morphological characteristics of apoptosis were identified using standard criteria, including chromatin condensation, nucleolar disintegration, and formation of crescentic caps of condensed chromatin at the nuclear periphery. Data were expressed as a mean percentage of total cell numbers. Photographs of cells were taken using a Zeiss photomicroscope.
Colocalization of PCNA, Cdc2, Cyclin B1, and Apoptosis in Serial Sections
To define associations, PCNA, Cdc2, cyclin B1, and apoptotic cells were immunodetected using immunohistochemistry and TUNEL assay in step-serial sections of each biopsy sample of normal, chronic gastritis, MALT, and MALT lymphoma patients. The LI of PCNA, Cdc2, cyclin B1, and AI were determined in the same areas of the serial sections stained with different antibodies and/or TUNEL assay. The numbers of lymphoid cells with immunostaining nuclei and/or cytoplasm were determined at three hot spots. Data were expressed as a mean percentage of total cell numbers.
Statistical Analysis
Statistical analyses were performed using χ2, Pearson, Spearman’s rho correlation coefficient, regression analysis, and Student’s t-test. For all statistical analyses, the SPSS system and Sigma Stat for personal computer were used, with significance defined as P < 0.05.
Results
PCNA Immunohistochemistry in Normal, H. pylori-Associated Chronic Gastritis, MALT, and MALT Lymphoma
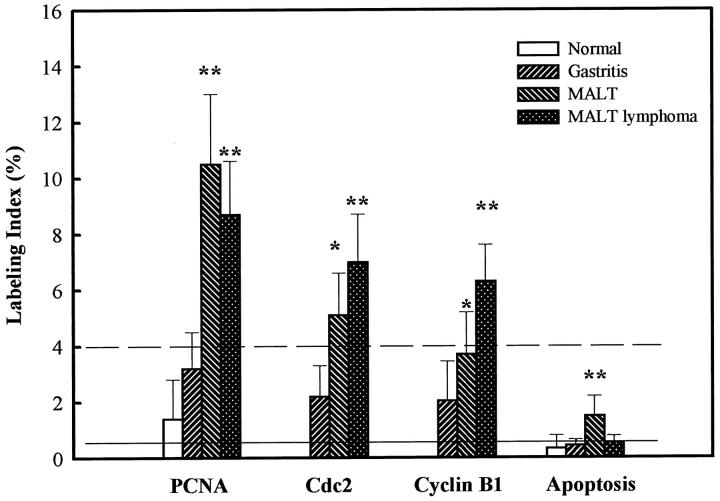
Immunohistochemistry of PCNA was performed in normal (n = 5), H. pylori-associated chronic gastritis (n = 7), gastric MALT (n = 12), and B cell low-grade gastric MALT lymphoma (n = 12). Examples of PCNA-immunostained positive lymphoid cells are shown in Figure 2A ▶ and the PCNA LI are summarized in Figure 3 ▶ and Table 1 ▶ . The LI of PCNA-positive lymphoid cells was significantly (P < 0.05, paired two-tailed Student’s t-test) elevated during the transition of chronic gastritis to MALT and MALT lymphoma. PCNA LI was increased 3.3- and 2.7-fold in MALT and MALT lymphoma, respectively, relative to chronic gastritis. In MALT, the PCNA LI was significantly (P < 0.01, paired two-tailed Student’s t-test) higher in germinal center than mantle zone and marginal zone (Table 1) ▶ . No hot spots were found in paraffin sections of normal tissue.
Figure 2.
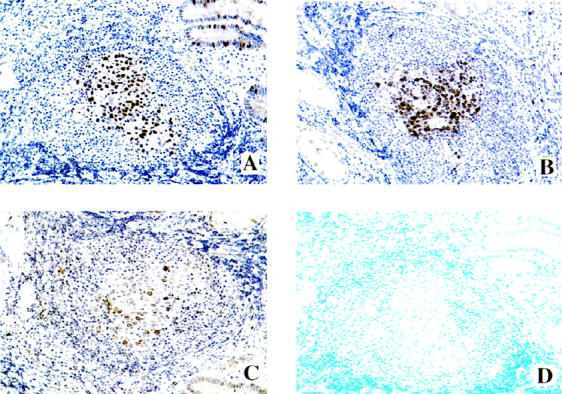
Colocalization of PCNA, Cdc2, cyclin B1, and apoptosis in paraffin-embedded tissue sections of H. pylori-associated gastric MALT using immunohistochemistry and/or TUNEL assay. A: PCNA. B: Cdc2. C: Cyclin B1. D: Apoptosis. Note that the apoptotic cells were not detected in those regions of the MALT where PCNA, Cdc2, and cyclin B1 were overexpressed. Original magnifications, ×250.
Figure 3.
Analysis of PCNA, Cdc2, and cyclin B1 labeling index and apoptotic labeling index (AI) in normal, H. pylori-positive chronic gastritis, MALT, and MALT lymphoma patients. The dashed line indicates the cutoff value for the labeling scores of Cdc2 and cyclin B1 and the straight line indicates the cutoff value for apoptosis. *P < 0.05; **P < 0.01.
Table 1.
Labeling Indices of PCNA, Cdc2, and Cyclin B1 Immunopositive Cells and Apoptotic Cells in Germinal Center, Mantle Zone, and Marginal Zone in H. pylori-Associated MALT
| Areas of MALT | PCNA labeling index (%) | Cdc2 labeling index (%) | Cyclin B1 labeling index (%) | Apoptosis (%) |
|---|---|---|---|---|
| Germinal center | 19.8 ± 6.7 | 17.1 ± 7.3 | 11.2 ± 5.6 | 1.9 ± 0.7 |
| Mantle zone | 5.7 ± 2.7* | 5.6 ± 3.3* | 3.1 ± 1.2* | 0.4 ± 0.4* |
| Marginal zone | 3.2 ± 2.3* | 3.4 ± 3.4** | 1.9 ± 1.6* | 0.3 ± 0.4* |
Results displayed are mean percentage of immunopositive cell number ± SD of five hot spot areas.
*P < 0.01; **P < 0.001.
Cdc2 and Cyclin B1 Immunohistochemistry in H. Pylori-Associated Chronic Gastritis, MALT, and MALT Lymphoma
Immunohistochemical studies demonstrated the presence of Cdc2 and cyclin B1 proteins in the lymphocytes of normal, chronic gastritis, MALT, and MALT lymphoma patients (Figures 2B, 2C, and 3) ▶ ▶ . The LI of Cdc2 and cyclin B1 were significantly higher (P < 0.05, paired two-tailed Student’s t-test) in MALT lymphoma compared to H. pylori-associated chronic gastritis and MALT tissues (Figure 3) ▶ . The mean Cdc2 LI was 2.1% (median 2.2, SD 1.13) in H. pylori-associated chronic gastritis, 5.1% (median 5.0, SD 1.5) in MALT, and 7.0% (median 7.0, SD 1.8) in MALT lymphoma. The mean cyclin B1 LI was 2.1% (median 1.5, SD 1.3), 3.7% (median 3.0, SD 1.5), and 6.3% (median 6.0, SD 1.3) in H. pylori gastritis, MALT, and MALT lymphoma, respectively.
These two proteins exhibited regional differences in the immunohistochemistry of MALT (Figure 3, B and C ▶ , and Table 1 ▶ ) and MALT lymphoma (data not shown). The comparative analysis of the labeling scores of Cdc2 and cyclin B1 indicated that the mean LI of Cdc2 was 17.1% (median 15.5, SD 7.3), 5.6% (median 1.05, SD 3.3), and 3.4% (median 2.0, SD 3.7) in germinal center, mantle, and marginal zone, respectively, and the cyclin B1 LI was 11.1% (median 12.5, SD 5.6) in germinal center, 3.1% (median 2.5, SD 1.2) in mantle zone, and 1.9% (median 1.2, SD 1.6) in marginal zone. A significantly higher labeling score (P < 0.01, paired two-tailed Student’s t-test) for these proteins was found in the germinal center relative to mantle and marginal zone of MALT (Table 1) ▶ . The overexpression of Cdc2 and cyclin B1 protein was also observed in the gastric glands surrounded by neoplastic and/or non-neoplastic lymphoid cells. No hot spots were found in paraffin sections of normal tissue.
Although mean labeling scores of PCNA, Cdc2, cyclin B1, and apoptosis exhibited significant differences in gastritis, MALT, and MALT lymphoma, several specimens of gastritis and MALT had overlapping scores of PCNA, Cdc2, and cyclin B1 LI, and apoptosis as those noted in MALT lymphoma samples. The highest common overlapping value for Cdc2 and cyclin B1 is 4.3 in both chronic gastritis and MALT specimens. Accordingly, the cutoff values for Cdc2 and cyclin B1 scores were considered as ≥4.0 (Figure 3) ▶ for subclassification of patients.
Apoptotic Index (AI) in H. pylori-Associated Chronic Gastritis, MALT, and MALT Lymphoma
Positive staining of apoptosis in lymphoid cells was observed in formalin-fixed, paraffin-embedded sections of chronic gastritis, MALT, and MALT lymphoma patients with AI ranging from 0.2 to 0.65% (median 0.4, SD 0.3), 0.8 to 3.0% (median 1.3, SD 0.9), and 0.4 to 0.85% (median 0.6, SD 0.4), respectively. The statistical analysis indicated that the apoptotic index in MALT was markedly higher (P < 0.05, paired two-tailed Student’s t-test) compared to chronic gastritis and MALT lymphoma (Figure 3) ▶ . No significant difference in AI was noted between MALT lymphoma and chronic gastritis (Figure 3) ▶ . In MALT, apoptosis was significantly higher (P < 0.01, paired two-tailed Student’s t-test) in germinal center, as compared to mantle and marginal zone (Table 1) ▶ . In several specimens of H. pylori-associated chronic gastritis and MALT, the apoptotic index had overlapping scores with those detected in MALT lymphoma. The highest overlapping value for apoptosis is 0.65% in both chronic gastritis and MALT specimens. Thus, the cutoff values for the apoptosis score was considered as <0.6% (Figure 3) ▶ for subclassification of patients.
Correlation between AI and PCNA, Cdc2, and Cyclin B1 Labeling Index
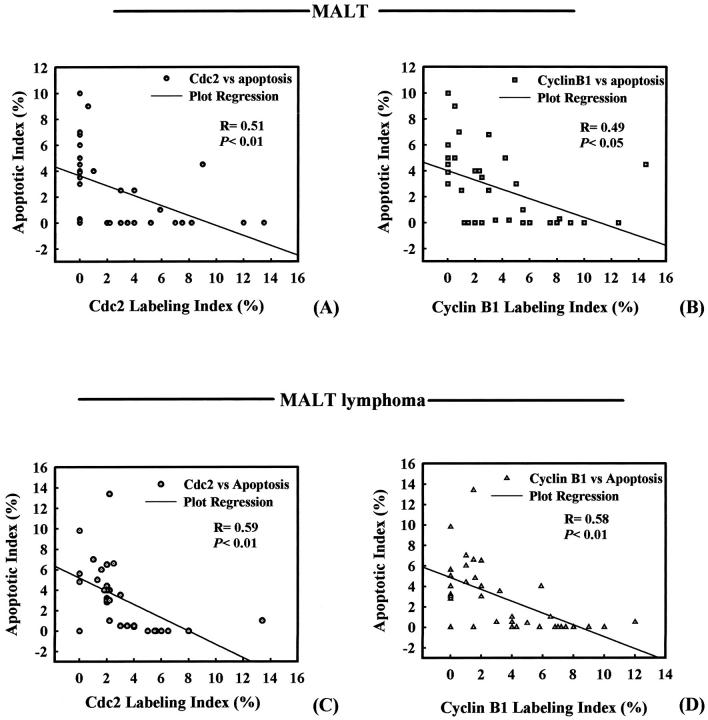
To examine the association between the induction of apoptosis and the expression of PCNA, Cdc2, and cyclin B1 in H. pylori-associated chronic gastritis, MALT, and MALT lymphoma, the labeling scores in colocalized areas of serial sections were examined using χ, 2 Pearson, and Spearman’s rho correlation coefficient with regression analysis. The immunohistochemical colocalization analysis and correlation coefficient, on plots of AI versus the Cdc2 or cyclin B1 on a per-case basis, showed a significant inverse correlation between AI and these two proteins in both MALT and MALT lymphoma patients (Figures 2, 4, and 5) ▶ ▶ ▶ . No significant correlation between AI and PCNA LI was found (data not shown).
Figure 4.
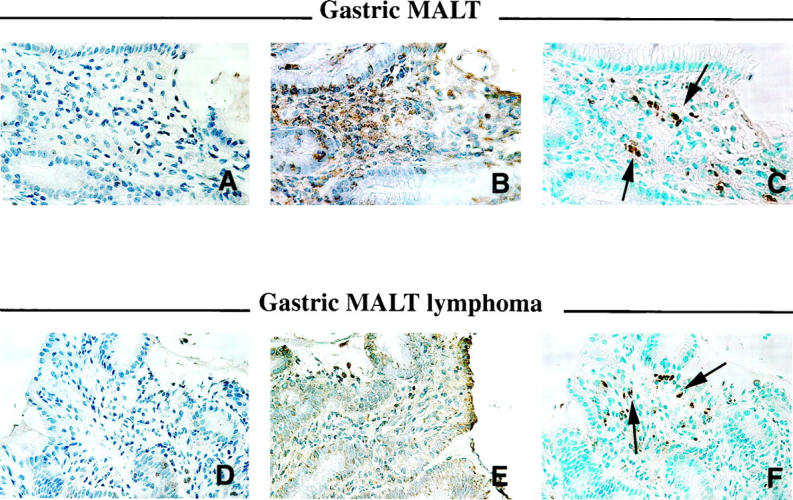
Colocalization of Cdc2, cyclin B1, and apoptosis in paraffin-embedded tissue sections of H. pylori-associated gastric MALT and MALT lymphoma using immunohistochemistry and/or TUNEL assay. A and D: Cdc2. B and E: Cyclin B1. C and F: Apoptosis. Note that the number of apoptotic cells was elevated in the MALT and MALT lymphoma where Cdc2 and cyclin B1 were underexpressed and/or not detected. Arrows indicate apoptotic cells. Original magnifications, ×250.
Figure 5.
Analysis of the χ2, Pearson, Spearman’s ρ correlation coefficient, and regression analysis for the correlation of apoptotic labeling index (AI) with Cdc2 or cyclin B1 labeling scores. A: Plot of Cdc2 labeling index against AI per MALT patient. B: Plot of cyclin B1 labeling index against apoptotic LI per MALT patient. C: Plot of Cdc2 labeling index against AI per MALT lymphoma patient. D:. Plot of cyclin B1 labeling index against apoptotic LI per MALT lymphoma patient. Note that 36 labeling spots were analyzed from either MALT or MALT lymphoma patients.
Discussion
A significant inverse correlation between apoptosis and both Cdc2 and cyclin B1 expression was found in H. pylori-associated gastric MALT and MALT lymphoma patients. This suggests that the extent of apoptosis in gastric MALT or MALT lymphoma may be associated with Cdc2 and cyclin B1 protein synthesis. To our knowledge, this is the first report of such a correlation. The number of apoptotic cells and/or bodies increased in the tissues from those patients with H. pylori-associated gastric MALT or MALT lymphoma in which the Cdc2 and/or cyclin B1 labeling indices were markedly reduced. Thus, when Cdc2 and cyclin B1 expression is increased in the lymphoid cells by chronic infection with H. pylori, apoptosis is concomitantly reduced. Our results depart from the two existing contradictory concepts regarding the role of Cdc2 in induction of apoptosis. The first concept demonstrated involvement of transcriptional activation of the Cdc2 gene in the genesis of apoptosis, 20,31-33 although other data have strongly argued against the relationship and suggest instead that Cdc2 expression is not obligatory for apoptosis. 34,35 Cdc2 kinase is a crucial protein and universally required as a master control enzyme during mitosis. 36 The appropriate signal of this kinase, in association with cyclin B1 and other cell regulatory proteins, controls the G2-M transition by promoting breakdown of nuclear membrane, chromatin condensation, and microtubule spindle formation. The contradictory data in the literature on the importance of Cdc2 in regulation of apoptosis can be reconciled based on our findings. Our results, along with those of previous studies, 31 strongly suggest that unscheduled synthesis of these proteins during a cell cycle may induce apoptotic events either by cell cycle delays from over- or underproduction of Cdc2 and cyclin B, both of which are essential for a cell to exit from mitosis, 17 or by inhibition of microtubule formation 3 as Cdc2 and/or cyclin B1 levels decreased. 37
The low labeling scores for Cdc2 and/or cyclin B1 in some areas of MALT and MALT lymphoma sections (Figure 5) ▶ lacking apoptotic cell death suggest that deficiency of these proteins may not always induce mitotic catastrophe or apoptosis in lymphoid cells. Other factors may also be important in regulation of apoptosis. High apoptotic score was always encountered in the MALT and in the deep portion where lymphoid cells are generally activated in response of specific foreign substances and/or pathogens and undergo several successive rounds of cell division over a period of several days. 38 Therefore, these studies suggest that the status of the lymphoid cell (ie, resting or activated lymphocyte) may be one crucial factor for induction of apoptosis.
While analyzing the expression of Cdc2 and cyclin B1 and apoptosis in individual patients, we observed a gradual augmentation of Cdc2- and cyclin B1-positive lymphoid cells, and inhibition of apoptosis, in H. pylori-associated MALT and MALT lymphoma. Moreover, variable distributions of cyclin B1- and Cdc2-positive lymphoid cells were observed in both MALT and MALT lymphoma in these same patients. Taken together, these studies indicate that in the initial stage of this lymphoproliferative disease, the deletion of unwanted and/or defective lymphocytes, might involve down-regulation of Cdc2 and/or cyclin B1 genes. As the disease progresses, up-regulation of these genes may ensure the survival of the defective repertoire. These defective lymphocytes may gradually acquire multiple genetic anomalies, which then eventually transform MALT into MALT lymphoma.
The diagnosis of low-grade B cell lymphoma in gastric biopsies is usually straightforward. 28 Doubtful cases are confirmed by scoring of histological appearances 24 and/or by determining B cell monoclonality using polymerase chain reaction (PCR), Southern blot, or immunohistochemical methods. 28,39 However, recent studies have raised a question about the specificity of PCR monoclonality as a method of determining the clonality of malignant cells. 40 Accordingly, identification of pure MALT and early cases of MALT lymphoma based on PCR technique is inappropriate. 40 The present immunohistochemical studies (Figure 3) ▶ suggest that the high labeling score (≥4.0) of Cdc2 and cyclin B1 in conjugation with a low labeling index (<0.6) of apoptotic cells in H. pylori-associated MALT may help identify a population that is primed to develop MALT lymphoma.
In summary, our findings indicate that Cdc2 and cyclin B1 may play an important role in the modulation of cellular death, proliferation, and transformation during the evolution of chronic gastritis to MALT lymphoma. Moreover, the labeling scores of Cdc2 and cyclin B1 may be used as one potential parameter for identifying the population at increased risk of developing MALT lymphoma.
Acknowledgments
We thank Professor K. M. Hassanein, Ph.D., Department of Biometry, for his advice regarding the choice of statistical methods, and Professor Donald C. Johnson, Ph.D., for critical reading of the manuscript and helpful suggestions.
Footnotes
Address reprint requests to Allan P. Weston, M.D. or Sushanta K. Banerjee, Ph.D., Molecular Gastroenterology & Pancreatic Cancer Research Unit, VA Medical Center, Research Division 151, 4801 Linwood Blvd., Kansas City, MO 64128.
Supported by a Department of Veterans Affairs Merit Review Grant and the Midwest Biomedical Research Foundation (Kansas City, MO).
S. K. B. and A. P. W. made equal contributions in this study.
References
- 1.Genta RM, Hamner HW, Graham DY: Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy (see comments). Hum Pathol 1993, 24:577-583 [DOI] [PubMed] [Google Scholar]
- 2.Wyatt JI, Rathbone BJ: Immune response of the gastric mucosa to Campylobacter pylori. Scand J Gastroenterol Suppl 1988, 142:44-49 [PubMed] [Google Scholar]
- 3.Stolte M, Eidt S: Lymphoid follicles in antral mucosa: immune response to Campylobacter pylori? J Clin Pathol 1989, 42:1269-1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho SB: Premalignant lesions of the stomach. Semin Gastrointest Dis 1996, 7:61-73 [PubMed] [Google Scholar]
- 5.Banerjee SK, Weston AP, Persons DL, Campbell DR: Non-random loss of chromosome 3 during transition of Helicobacter pylori-associated gastric MALT to B-cell MALT lymphoma revealed by fluorescence in situ hybridization. Cancer Lett 1997, 121:83-90 [DOI] [PubMed] [Google Scholar]
- 6.Spina D, del Vecchio MT, Leoncini L, Vindigni C, Minacci C, Valente G, Palestro G, Tosi P: Primary gastric lymphomas (MALTomas): a nuclear image analysis comparison with lymph node monocytoid B-cells and marginal zones of spleen and Peyer’s patches. Anal Cell Pathol 1995, 8:307-321 [PubMed] [Google Scholar]
- 7.Wotherspoon AC, Finn TM, Isaacson PG: Trisomy 3 in low-grade B-cell lymphomas of mucosa-associated lymphoid tissue. Blood 1995, 85:2000-2004 [PubMed] [Google Scholar]
- 8.Whang-Peng J, Knutsen T, Jaffe E, Raffeld M, Zhao WP, Duffey P, Longo DL: Cytogenetic study of two cases with lymphoma of mucosa-associated lymphoid tissue. Cancer Genet Cytogenet 1994, 77:74-80 [DOI] [PubMed] [Google Scholar]
- 9.Takahashi H, Fujita S, Okabe H, Tsuda N, Tezuka F: Estimation of silver-binding nucleolar organizer regions (AgNORs) in lymphoproliferative disorders of gastrointestinal tract. Pathol Res Pract 1994, 190:350-361 [DOI] [PubMed] [Google Scholar]
- 10.Chan WY, Wong N, Chan AB, Chow JH, Lee JC: Consistent copy number gain in chromosome 12 in primary diffuse large cell lymphomas of the stomach. Am J Pathol 1998, 152:11-16 [PMC free article] [PubMed] [Google Scholar]
- 11.Auer IA, Gascoyne RD, Connors JM, Cotter FE, Greiner TC, Sanger WG, Horsman DE: t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997, 8:979-985 [DOI] [PubMed] [Google Scholar]
- 12.Steller H: Mechanisms and genes of cellular suicide. Science 1995, 267:1445-1449 [DOI] [PubMed] [Google Scholar]
- 13.Cory S, Strasser A, Jacks T, Corcoran LM, Metz T, Harris AW, Adams JM: Enhanced cell survival and tumorigenesis. Cold Spring Harb Symp Quant Biol 1994, 59:365-375 [DOI] [PubMed] [Google Scholar]
- 14.Cory S, Adams JM: Matters of life and death: programmed cell death at Cold Spring Harbor. Biochim Biophys Acta 1998, 1377:R25-R44 [DOI] [PubMed] [Google Scholar]
- 15.McDonnell TJ: Cell division versus cell death: A functional model of multistep neoplasia. Mol Carcinog 1993, 8:209-213 [DOI] [PubMed] [Google Scholar]
- 16.Pan H, Yin C, Van Dyke T: Apoptosis and cancer mechanisms. Cancer Surv 1997, 29:305-327 [PubMed] [Google Scholar]
- 17.Hunter T, Pines J: Cyclins and cancer. Cell 1991, 66:1071-1074 [DOI] [PubMed] [Google Scholar]
- 18.Hartwell LH, Kastan MB: Cell cycle control and cancer. Science 1994, 266:1821-1828 [DOI] [PubMed] [Google Scholar]
- 19.Freeman RS, Estus S, Johnson EMJ: Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron 1994, 12:343-355 [DOI] [PubMed] [Google Scholar]
- 20.Yao SL, McKenna KA, Sharkis SJ, Bedi A: Requirement of p34cdc2 kinase for apoptosis mediated by the Fas/APO-1 receptor and interleukin 1β-converting enzyme-related proteases. Cancer Res 1996, 56:4551-4555 [PubMed] [Google Scholar]
- 21.Shen SC, Huang TS, Jee SH, Kuo ML: Taxol-induced p34cdc2 kinase activation and apoptosis inhibited by 12-O- tetradecanoylphorbol-13-acetate in human breast MCF-7 carcinoma cells. Cell Growth Differ 1998, 9:23-29 [PubMed] [Google Scholar]
- 22.Shi L, Chen G, He D, Bosc DG, Litchfield DW, Greenberg AH: Granzyme B induces apoptosis, and cyclin A-associated cyclin-dependent kinase activity in all stages of the cell cycle. J Immunol 1996, 157:2381-2385 [PubMed] [Google Scholar]
- 23.Dixon MF, Genta RM, Yardley JH, Correa P: Classification and grading of gastritis: the updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996, 20:1161-1181 [DOI] [PubMed] [Google Scholar]
- 24.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG: Regression of primary low-grade B-cell gastric lymphoma of mucosa- associated lymphoid tissue type after eradication of Helicobacter pylori (see comments). Lancet 1993, 342:575-577 [DOI] [PubMed] [Google Scholar]
- 25.Isaacson PG, Noeton AJ: Mucosa-associated lymphoid tissue (MALT) and the MALT lymphoma concept. Isaacson PG Noeton AJ eds. Extranodal Lymphoma. 1994, :pp 5-14 Churchill Livingstone, NewYork [Google Scholar]
- 26.Isaacson PG: Gastrointestinal lymphoma. Hum Pathol 1994, 25:1020-1029 [DOI] [PubMed] [Google Scholar]
- 27.Isaacson PG: Primary gastric lymphoma. Br J Biomed Sci 1995, 52:291-296 [PubMed] [Google Scholar]
- 28.Spencer J, Wotherspoon AC: Gastric MALT lymphoma and Helicobacter pylori. Cancer Surv 1997, 30:213-231 [PubMed] [Google Scholar]
- 29.Wotherspoon AC: Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu Rev Med 1998, 49:289-299 [DOI] [PubMed] [Google Scholar]
- 30.Banerjee SK, Sarkar DK, Weston AP, De A, Campbell DR: Over expression of vascular endothelial growth factor and its receptor during the development of estrogen-induced rat pituitary tumors may mediate estrogen-initiated tumor angiogenesis. Carcinogenesis 1997, 18:1155-1161 [DOI] [PubMed] [Google Scholar]
- 31.Shi L, Nishioka WK, Th’ng J, Bradbury EM, Litchfield DW, Greenberg AH: Premature p34cdc2 activation required for apoptosis (see comments). Science 1994, 263:1143-1145 [DOI] [PubMed] [Google Scholar]
- 32.Furukawa Y, Iwase S, Terui Y, Kikuchi J, Sakai T, Nakamura M, Kitagawa S, Kitagawa M: Transcriptional activation of the cdc2 gene is associated with Fas-induced apoptosis of human hematopoietic cells. J Biol Chem 1996, 271:28469-28477 [DOI] [PubMed] [Google Scholar]
- 33.Meikrantz W, Schlegel R: Suppression of apoptosis by dominant negative mutants of cyclin-dependent protein kinases. J Biol Chem 1996, 271:10205-10209 [DOI] [PubMed] [Google Scholar]
- 34.Martin SJ, McGahon AJ, Nishioka WK, La Face D, Guo X, Th’ng J, Bradbury EM, Green DR: p34cdc2 and apoptosis. Science 1995, 269:106-107 [DOI] [PubMed] [Google Scholar]
- 35.De LA, De MR, Baldi A, Trotta R, Facchiano F, Giordano A, Testi R, Condorelli G: Fas-induced changes in cdc2 and cdk2 kinase activity are not sufficient for triggering apoptosis in HUT-78 cells. J Cell Biochem 1997, 64:579–585 [DOI] [PubMed]
- 36.Nurse P: Universal control mechanism regulating onset of M-phase. Nature 1990, 344:503-508 [DOI] [PubMed] [Google Scholar]
- 37.Jackman MR, Pines J: Cyclins and G2/M transition. Cancer Surv 1997, 29:47-73 [PubMed] [Google Scholar]
- 38.Parslow TG: Lymphocytes and lymphoid tissues. Stites DP Terr AI Parslow TG eds. Basic and Clinical Immunology. 1994, :pp 22-39 Appleton & Lange, New York [Google Scholar]
- 39.Wotherspoon AC: Helicobacter pylori infection and gastric lymphoma. Br Med Bull 1998, 54:79-85 [DOI] [PubMed] [Google Scholar]
- 40.Weston AP, Banerjee SK, Horvat RT, Cherian R, Campbell DR, Zoubine MN. Specificity of polymerase chain reaction monoclonality for diagnosis of gastric mucosa-associated lymphoid tissue (MALT) lymphoma: direct comparison to Southern blot gene rearrangement. Dig Dis Sci 1998, 43:290–299 [DOI] [PubMed]