Abstract
CD34 is a heavily glycosylated transmembrane protein of ∼110 kd whose function is essentially uncharacterized. First identified in a myeloid leukemia cell line, immunohistological reactivity with anti-CD34 antibodies is also encountered in a histologically diverse subset of nonhematolymphoid neoplasms including angiosarcoma, solitary fibrous tumors, epithelioid sarcomas, spindle cell lipomas, dermatofibrosarcoma protuberans, and myofibroblastomas. Immunohistological reactivity for CD34 in hematopoietic stem cells and endothelial cells has been shown to correspond to the expression of the CD34 protein. With the exception of gastrointestinal stromal tumors, CD34 protein expression has not been investigated in other CD34 immunohistologically reactive nonhematolymphoid neoplasms. We undertook this study to examine whether the observed reactivity for anti-CD34 antibodies in apparently unrelated tumors is due to the expression of the same protein or whether shared epitopes elaborated by other proteins could account for this reactivity. Immunoblot analyses with anti-CD34 antibodies of six different CD34 immunohistologically reactive lesions show the same ∼110-kd molecular weight protein. In addition, two cases of dermatofibrosarcoma protuberans show double bands at ∼110 kd. Laser-capture microdissection of CD34 immunohistologically reactive epithelioid sarcoma and nonreactive epidermal cells illustrates that this reactivity is specific to tumor cells. These results show that the observed immunohistological reactivity with anti-CD34 antibodies is due to the expression of the CD34 protein and not to shared epitopes on unrelated proteins.
The human CD34 molecule was originally identified in a myeloid leukemia cell line (KG1a) and was initially characterized as a marker for hematopoietic progenitor cells and endothelial cells. 1-6 Subsequent investigations have found that in addition to immature leukemias 7-10 and vascular tumors, 11-15 anti-CD34 antibodies react with a specific subset of histologically diverse nonhematolymphoid neoplasms. These neoplasms include solitary fibrous tumors (SFT), 16,17 gastrointestinal stromal tumors (GIST), 18 spindle cell lipomas, 19,20 dermatofibrosarcoma protuberans (DFSP), 11,13,21,22 epithelioid sarcomas, 14,23,24 myofibroblastomas, 25,26 and neural tumors. 17 Although a variety of neoplasms display immunohistological reactivity with anti-CD34, this immunophenotype is restricted and can aid in distinguishing specific tumors from histological mimics in their differential diagnoses. Most significantly, very few carcinomas (1%) and melanomas (0.5%), and no Hodgkin’s or non-Hodgkin’s lymphomas, with the exception of lymphoblastic lymphomas, have been reported to express CD34. 11,13-15,17,27,28
CD34 is a type I integral membrane protein of ∼110 kd molecular weight whose DNA sequence has no known homologue and whose postulated function in cytoadhesive signaling is largely uncharacterized. 5,29-32 Two types of murine CD34 mRNA that differ at the cytoplasmic portion of the molecule generated by alternative splicing have been described. 33 The protein backbone, based on its unique DNA sequence, is estimated to be 45 kd. A significant portion of the molecular weight of CD34 is contributed by posttranslational modifications leading to embellishment of the protein core by carbohydrate moieties. These modifications are determined by the protein sequence and include several O-linked and N-linked glycosylation sites located especially in the extracellular domain. 5,6,30,34 Monoclonal anti-CD34 antibodies such as MY10 and QBEND10 that are commonly used in the practice of diagnostic surgical pathology for detection of CD34 reactivity are known to recognize oligosaccharide side chains borne on this protein. 34 Thus, posttranslational modifications are important modulators of antigen recognition in CD34 reactivity.
The specificity of antigen-antibody recognition relies on complex and precise protein-protein interactions. The breakdown of this specificity can lead to severe consequences that impact on autoimmunity and neoplasia. Multiple binding capabilities (also known as binding promiscuity, cross-reactivity, polyspecificity, and molecular mimicry) of polyclonal as well as high-affinity monoclonal antibodies have been well documented. 35-39 Conservation of important residues or consensus motifs within different polypeptides and the utilization of structural similarities are thought to facilitate these interactions. It is therefore unclear whether the observed immunohistological reactivity for anti-CD34 with a variety of different tumors is due to the expression of the same protein or whether cross-reactivity of epitopes present on unrelated proteins could explain this reactivity on unrelated neoplasms. In addition, because many anti-CD34 antibodies react with antigenic sites on glycosylated side chains, proteins elaborating similar glycosylation motifs may exhibit reactivity with anti-CD34 antibodies. In hematopoietic stem cells and in endothelial cells the expression of the CD34 gene correlates with protein expression. 5,6 Among soft tissue tumors correlation of CD34 immunohistological reactivity with protein expression has been demonstrated in GIST 18 ; however, this has not been tested in other CD34 immunohistologically reactive tumors. The importance of CD34 as a marker for hematopoietic stem cells and its diagnostic utility in a variety of nonhematolymphoid neoplasms prompted us to investigate whether CD34 immunohistological reactivity corresponds to the expression of a distinct protein.
Materials and Methods
Case Selection
Fresh frozen tissues of SFTs, GISTs, DFSPs, epithelioid sarcomas, myofibroblastomas, and spindle cell lipomas from specimens submitted to the pathology departments of Stanford University Medical Center (Stanford, CA), University of Pennsylvania Medical Center (Philadelphia, PA), and The Royal Marsden National Health Service Trust (London, UK) comprise this study. All cases had histological and immunohistochemical features characteristic of the lesion in question.
Immunohistochemistry
The prototype antibody directed against CD34 (anti-HPCA-1, clone MY10; Becton-Dickinson, Mountain View, CA) was used as the primary antibody for assessment of CD34 reactivity by immunohistochemistry. Four-micron paraffin-embedded tissue sections were hydrated in a graded series of alcohol and incubated with 1:10 dilution of anti-CD34 antibody. Detection was performed on an automated staining machine (Ventana Medical Systems, Tucson, AZ).
Immunoblotting
Lysates were prepared from fresh frozen tissue samples and analyzed by 7.5% acrylamide sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Coomassie staining was used to quantitate amounts of protein and equal quantities from each sample were run on SDS-PAGE and transferred to nitrocellulose by electrophoresis. The CD34 molecule was detected by anti-CD34 (clone MY10) alkaline phosphatase-conjugate driven NBT/BCIP or with DAB-peroxidase staining. A lysate from KG1a, the CD34 immunohistologically reactive myeloid leukemia cell line known to express the ∼110-kd CD34 protein, 3 was used as a positive control. Tumor cell lysates in which the anti-CD34 antibody detected a product identical in migratory characteristics to that seen in the KG1a lysate were considered to express CD34 protein. A lysate from a CD34 immunohistologically nonreactive GIST sample (in which CD34-immunoreactive normal endothelial cells were present) was used as a negative control.
Microdissection
Separation of tumor cells from surrounding nontumor cells was accomplished by microscopic dissection using a laser-capture microdissection device (Arcturus PXL-200), which allows for isolation of small groups of cells in the areas of interest from frozen tissue sections onto a transfer film. 40-42 After selection of cells, the film was transferred to a microcentrifuge tube and protein isolation was performed. The laser-capture microdissection technique was applied to a sample of epithelioid sarcoma as unselected material from this tumor yielded a broad smear on CD34 immunoblots, presumably due to the high fat content of this sample. Microdissected material from the CD34 immunohistologically nonreactive GIST and microdissected epidermis overlying lesional cells was used as negative controls while a KG1a lysate was used as a positive control.
Results
Histology and Immunohistochemistry
All cases in this study were typical examples of the rendered diagnoses as summarized in Table 1 ▶ . Both malignant GISTs showed interlacing bundles of dense spindled cells with brisk mitotic activity and mild pleomorphism. The two SFTs displayed spindled cells that lacked significant pleomorphism and mitotic activity and were variably associated with dense collagen. The cases of DFSP showed bland spindled cells arranged in fascicles as well as in a storiform pattern. The case of myofibroblastoma consisted of a monotonous spindled cell proliferation arising in the background of benign breast parenchyma. The spindle cell lipomas displayed clusters of adipocytes with intervening spindled cells, minimal atypia, and focal myxoid changes. The epithelioid sarcoma showed a proliferation of epithelioid cells with abundant cytoplasm and moderate nuclear pleomorphism. In contrast to the other tumors, the epithelioid sarcoma was surrounded by a significant amount of subcutaneous fat and was also associated with marked tumor cell necrosis.
Table 1.
Summary of Clinical, Immunohistological, and Immunoblot Findings
| Case | Age/Sex | Diagnosis | Site | CD34 IHC | Immunoblot |
|---|---|---|---|---|---|
| 1 | 41 F | Epithelioid sarcoma | Thigh | ++ | broad smear* |
| 2 | 73 M | Malignant gastrointestinal stromal tumor | Stomach | +++ | broad band |
| 3 | 31 M | Malignant gastrointestinal stromal tumor | Peritoneum | − | no band |
| 4 | 75 F | Solitary fibrous tumor | Mediastinum | +++ | broad band |
| 5 | 45 M | Solitary fibrous tumor | Parapharyngeal space | +++ | broad band |
| 6 | 40 M | Dermatofibrosarcoma protuberans | Scalp | +++ | double bands |
| 7 | 18 F | Dermatofibrosarcoma protuberans | Breast | ++ | double bands |
| 8 | 76 F | Dermatofibrosarcoma protuberans | Leg | + | broad band |
| 9 | 77 M | Dermatofibrosarcoma protuberans | Chest | − | no band |
| 10 | 62 M | Myofibroblastoma | Breast | ++ | broad band |
| 11 | 65 M | Spindle cell lipoma | Soft tissue | ++ | broad band |
| 12 | 55 M | Spindle cell lipoma | Neck | ++ | broad band |
IHC, immunohistochemistry.
*Non-microdissected lysate.
The intensity of CD34 staining was scored as follows: +++ = strong, ++ = moderate, + = weak.
Immunohistochemistry for CD34 showed strong reactivity in one of two malignant GISTs and in both SFTs. The CD34-negative GIST (case 3) was histologically compatible with a diagnosis of GIST and stained for the muscle marker, smooth muscle actin, and for CD117 (c-kit). This lesion was used as a negative control in the immunoblot experiments described below. In the CD34 immunohistologically reactive neoplasms a majority of the neoplastic cells stained positive. The myofibroblastoma and spindle cell lipomas showed moderate CD34 reactivity. The epithelioid sarcoma displayed moderate membrane staining. Among the four DFSPs, two showed moderate to strong CD34 reactivity, the DFSP from the leg showed weak staining, and the DFSP from the chest wall showed no immunoreactivity. The epithelioid sarcoma was the only lesion in which predominantly membrane staining was seen; all other lesions showed cytoplasmic staining. This tumor was also positive for low-molecular-weight keratin, which is typical of epithelioid sarcoma. Typical lesional areas of epithelioid sarcoma, malignant GIST, DFSP, and myofibroblastoma, and their immunohistological reactivity with anti-CD34, antibody are shown in Figure 1 ▶ .
Figure 1.
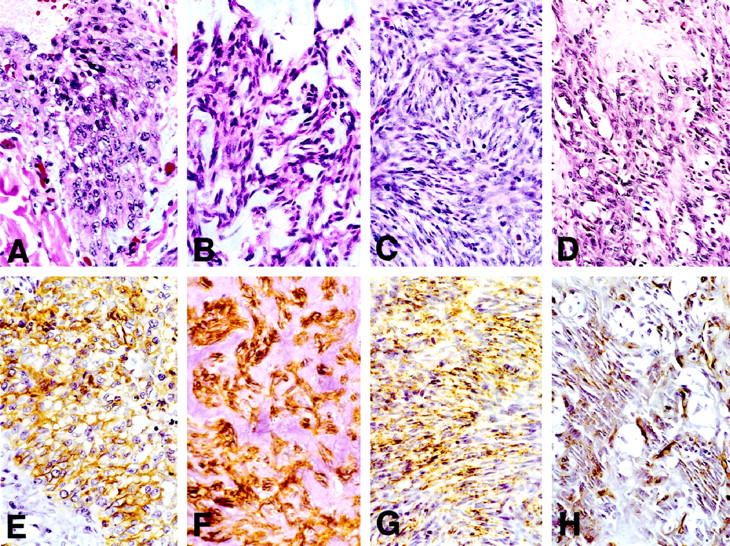
Typical lesional areas of epithelioid sarcoma (A), malignant GIST (case 2; B), DFSP (case 7; C), and myofibroblastoma (D). E-H: Immunoreactivity with anti-CD34 antibody exhibited by each of these tumors, respectively.
Immunoblotting
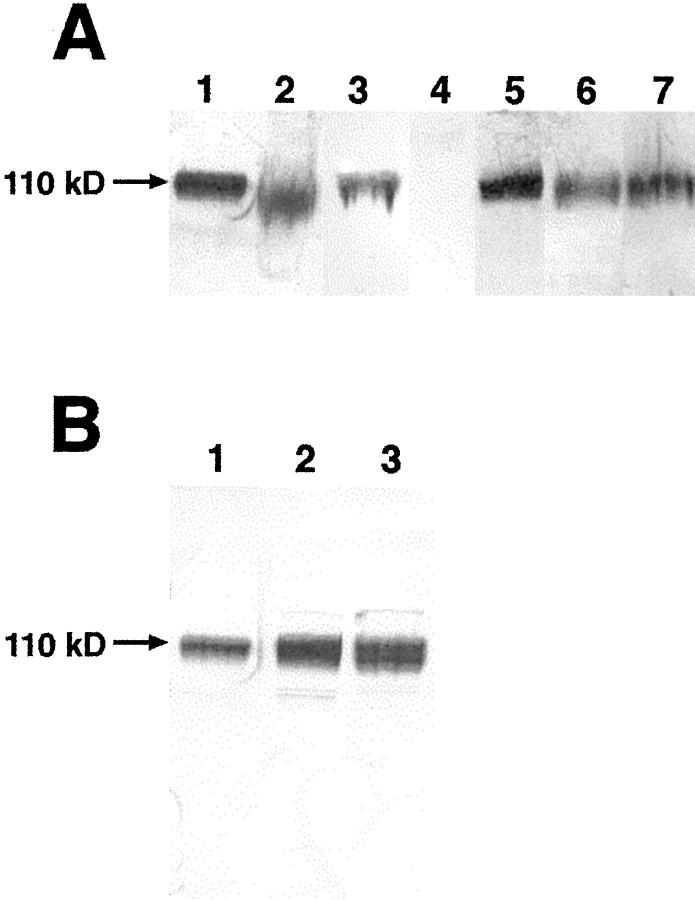
A broad band migrating at approximately 110 kd, comparable to that seen in the lysate from the KG1a cell line, was detected in the two cases of SFTs, one malignant GIST, one myofibroblastoma, and the two spindle cell lipomas (Figure 2A) ▶ . Among the four DFSPs, three cases showed detectable bands. One case showed one band at ∼110 kd, which was comparable to that seen in the KG1a lysate and other tumors shown in Figure 2 ▶ . Two other cases of DFSP exhibited identical double bands at approximately 110 kd (Figure 2B) ▶ . The lysate from nonmicrodissected epithelioid sarcoma demonstrated a broader smear that appeared to run at a slightly lower molecular weight (Figure 2A) ▶ . Only the band from the nonmicrodissected epithelioid sarcoma extended beyond the confines of the band from the KG1a lysate. The ∼110-kd band was absent from the GIST and the DFSP that were nonreactive for CD34 by immunohistochemistry. None of the lysates exhibiting the ∼110-kd band, including the KG1a cell line, displayed a single sharp band.
Figure 2.
A: Immunoblot showing a single ∼110-kd band in the KG1a control lysate (lane 1), solitary fibrous tumor (case 4; lane 3), malignant GIST (case 2; lane 5), myofibroblastoma (lane 6), and spindle cell lipoma (case 12; lane 7), a broad smear at somewhat lower molecular weight in epithelioid sarcoma (nonmicrodissected, see text; lane 2), and lack of a band of similar mobility in CD34 immunohistologically nonreactive malignant GIST (case 3; lane 4). Lanes 1 and 2 are from the same immunoblot; lanes 3–7 are taken from separate experiments. All SDS-PAGE analyses were performed with standard protein molecular weight markers and the KG1a control lysate to facilitate comparison of electromobility of the proteins in separate experiments. B: Immunoblot showing a single ∼110-kd band in KG1a control lysate (lane 1) and in the DFSP from the leg (case 8; lane 2). The DFSP from the breast (case 7) shows double bands that span the thickness of the single bands (lane 3).
Microdissection
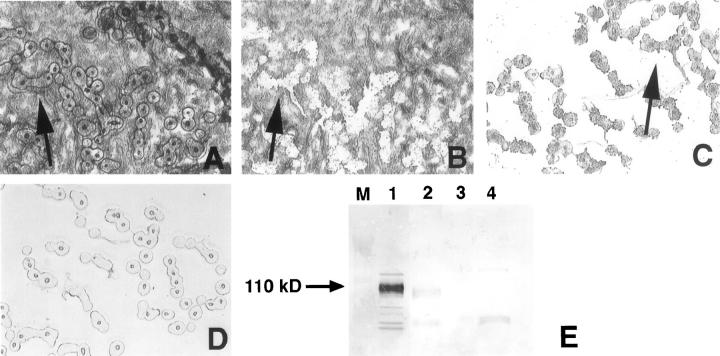
Laser-capture microdissection performed on the case of epithelioid sarcoma allowed the separation of the tumor cells from surrounding adipose tissue and skin (Figure 3, A ▶ -D). Lysates prepared from microdissected tissue were analyzed by immunoblotting and showed a band at ∼110 kd in the lysate from tumor cells, but not in the lysate containing only epidermis devoid of tumor cells (Figure 3E) ▶ . This band was not a broad smear as seen in the lysate from the undissected tumor, and migrated within the confines of the band from the KG1a lysate. An area of tissue similar in size was microdissected from the CD34 immunohistologically nonreactive GIST and used as a negative control.
Figure 3.
Laser capture microdissection of epithelioid sarcoma showing frozen tissue section after laser pulsation (A), residual tissue after cap is lifted off from the section (B), transfer film containing the selected tumor cells (C), and transfer film after elution of sample in SDS-containing sample buffer (D). Although the fields of view are shifted, arrows indicate identical sites on panels A-C. E: Immunoblot of KG1a control lysate (lane 1), lysate of epithelioid sarcoma cells from cap after microdissection (lane 2), lysate containing only overlying epidermis from the same frozen section devoid of tumor cells (lane 3), and lysate from an area of similar size microdissected from CD34 immunohistologically nonreactive malignant GIST (case 3; lane 4). Lane M indicates protein molecular weight markers.
Discussion
Anti-CD34 antibodies are remarkable in that they recognize specific but histologically divergent cell types and neoplasms ranging from hematopoietic and endothelial neoplasms to a variety of soft tissue tumors. In addition to these known divergent lineages, the various soft tissue tumors exhibit a broad range of histological appearances that do not suggest a common cell of origin or type of differentiation. Although some CD34 immunohistologically reactive soft tissue tumors exhibit a bland spindled cell composition, others are cytologically malignant or display an epithelioid morphology. However, other spindled cell lesions that show histological overlap, such as synovial sarcomas and fibrous histiocytomas, display no CD34 immunohistological reactivity. 28 Importantly, the vast majority of carcinomas, melanomas, and lymphomas are negative for CD34. 11,13-15,17,27,28 Despite the differences in appearance, CD34 immunohistological reactivity has led to postulation of a common cell of origin, especially for soft tissue tumors. Although the dendritic interstitial cell could be considered a candidate from which these tumors derive, because these cells are CD34-positive and widely present in organ parenchyma and soft tissues, 28 there is no direct link between this cell type and CD34 immunohistologically reactive tumors. Recent investigations suggest that the interstitial cells of Cajal, which regulate peristalsis in the gut and are immunoreactive for CD34 and CD117, give rise to GISTs, 43-45 although CD34 expression by these cells has been called into question. 46 It is unclear whether this cell type or a counterpart exists outside the gastrointestinal tract. In addition, CD34 immunohistologically reactive hematopoietic, vascular, endothelial, and neural tumors are unlikely to share a common lineage with soft tissue tumors. Thus, the observed CD34 immunohistological reactivity in disparate neoplasms raises the questions whether the CD34 protein is expressed in diverse cell types and whether recognition of shared epitopes among different proteins by anti-CD34 antibodies can explain this finding.
The function of the CD34 gene is unknown, although its localization with other known adhesion molecules on chromosome 1q32 suggests a cytoadhesive role for CD34. 31-33,47 In hematopoietic cells direct phosphorylation of CD34 antigen by protein kinase C has been demonstrated, suggesting a further role for CD34 in regulating cell signaling. 47 Engagement of specific epitopes on the CD34 molecule elicits enhanced cytoadhesiveness. 31 However, no effect on cell proliferation is detected. 32 Thus, the function of CD34 and the molecular basis for CD34-reactivity in hematopoiesis and in tumorigenesis remains unresolved.
Molecular mimicry among viral and host proteins are thought to trigger a number of autoimmune disorders including ankylosing spondylitis, Reiter’s syndrome, celiac disease, multiple sclerosis, myocarditis, and glomerulonephritis. 35,37-39,48 An elegant example of binding promiscuity is that of HIV anti-p24 monoclonal antibody, which is capable of binding five unrelated polypeptides. 37,38 Another example of antibody recognition of unrelated targets is that of monoclonal A103, an antibody that recognizes Melan-A/MART-1, a molecule initially characterized as a melanoma-associated antigen for the development of immunotherapy. 49,50 Although mRNA analysis reveals that Melan-A/MART-1 is not expressed in normal adrenal, A103 reacts with normal and neoplastic adrenal on immunohistochemistry. 51-53 Shared epitopes between unrelated proteins are thought to be responsible for the observed immunohistological reactivity to Melan-A/MART-1 in adrenal and steroid cells. 52,53 A similar mechanism could have been responsible for CD34 immunohistological reactivity in unrelated tumors. Anti-CD34 antibodies have been classified into three groups based on the epitopes of the protein with which they react. Class I antibodies are sensitive to cleavage by neuraminidase and glycoprotease, whereas class II antibodies are removed only by glycoprotease. CD34 reactivity by class I and II anti-CD34 antibodies can be abolished in KG1a cells using a specific protease that recognizes O-linked glycosylation sites, indicating that these antibodies are dependent on carbohydrate moieties for immunoreactivity. 34 These carbohydrate side chains may not be unique to the CD34 protein and could function as cross-reacting antigenic determinants in unrelated tumors. CD34 immunohistological reactivity in soft tissue tumors has been determined mostly using antibodies such as MY10 (class I) and QBEND10 (class II), which are directed against glycosidase sensitive epitopes, 13-16,18,20,22-24,54-56 Anti-CD34 antibodies directed against the protein backbone (class III) have not been applied to soft tissue tumors, with the exception of DFSP. 21
In the current study we tested the reactivity of the anti-CD34 clone MY10 using immunohistochemistry and immunoblotting in a variety of nonhematolymphoid neoplasms. We found that all cases in which CD34-reactivity was demonstrable by immunohistochemistry showed a ∼110-kd band on immunoblots, with the exception of two cases of DFSP, which showed double bands at ∼110 kd. These tumors include one epithelioid sarcoma, one of two malignant GISTs, two SFTs, one of four DFSPs, one myofibroblastoma, and two spindle cell lipomas. A malignant GIST and a DFSP that showed no CD34 reactivity on immunohistological stains failed to show the ∼110-kd band on immunoblots. These results indicate that CD34 immunohistological reactivity correlates with CD34 protein expression in these neoplasms. Northern blot or reverse transcription-polymerase chain reaction analysis of mRNA expression could provide additional support for this conclusion.
Of interest is that in two of the cases of DFSP, two bands were detected on immunoblots instead of one. Both bands were close to 110 kd as determined by the KG1a control lysate. The bands were of identical molecular weights in both cases of DFSP. The significance of these two bands is unclear. Although they may reflect two entirely different proteins recognized by anti-CD34 antibodies, it is more likely that they represent two different forms of the protein generated by alternatively spliced mRNAs as shown in a murine system, 33 or by posttranslational modifications including glycosylation 5,6,30,34 and phosphorylation. 31,47 The two bands also span the thickness of the single bands observed in the lysates for KG1a, SFTs, GIST, and spindle cell lipomas, suggesting that the electromobility pattern encompasses several species of the CD34 protein generated by posttranslational modifications. Further analysis is needed to clarify what these modifications might imply and also whether different posttranslational modifications may play a role in the expression of CD34 protein in different neoplasms.
Our analysis on a microdissected epithelioid sarcoma shows that the ∼110-kd band is present in the sample containing tumor cells, which excludes the possibility that adjacent nonneoplastic cells are responsible for the immunoblot result. In addition, microdissection of tumor cells from the epithelioid sarcoma away from a background rich in fat facilitated better characterization of the protein band on immunoblots and confirmed that the broad smear in the undissected tumor lysate was in fact identical to the ∼110-kd band from the KG1a control lysate. The two cases of spindle cell lipoma also showed broader bands compared to GIST, SFT, and DFSP, most likely due to the high content of lipid within these tumors.
In summary, we have demonstrated that the CD34 immunohistological reactivity detected by anti-CD34 antibodies in a variety of spindled cell and soft tissue tumors correlates with the expression of the ∼110-kd CD34 protein. Although we cannot formally exclude the possibility that the different tumors tested in this study express unrelated ∼110-kd proteins recognized by anti-CD34 antibodies on immunoblots, this supposition is highly unlikely.
Footnotes
Address reprint requests to Yasodha Natkunam, Department of Pathology, Stanford University Medical Center, 300 Pasteur Drive, Stanford, California 94305. E-mail: ynatkunam@yahoo.com.
References
- 1.Andrews RG, Singer JW, Bernstein ID: Monoclonal antibody 12-8 recognizes a 115-kd molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors. Blood 1986, 67:842-845 [PubMed] [Google Scholar]
- 2.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B: Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA 1992, 89:2804-2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH: Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol 1984, 133:157-165 [PubMed] [Google Scholar]
- 4.Strauss LC, Rowley SD, La Russa VF, Sharkis SJ, Stuart RK, Civin CI: Antigenic analysis of hematopoiesis. V. Characterization of My-10 antigen expression by normal lymphohematopoietic progenitor cells. Exp Hematol 1986, 14:878-886 [PubMed] [Google Scholar]
- 5.Greaves MF, Brown J, Molgaard HV, Spurr NK, Robertson D, Delia D, Sutherland DR: Molecular features of CD34: a hemopoietic progenitor cell-associated molecule. Leukemia 1992, 6:31-36 [PubMed] [Google Scholar]
- 6.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF: Expression of the CD34 gene in vascular endothelial cells. Blood 1990, 75:2417-2426 [PubMed] [Google Scholar]
- 7.Matutes E, Rodriguez B, Polli N, Tavares de Castro J, Parreira A, Andrews C, Griffin JD, Tindle RW, Catovsky D: Characterization of myeloid leukemias with monoclonal antibodies 3C5 and MY9. Hematol Oncol 1985, 3:179–186 [DOI] [PubMed]
- 8.Borowitz MJ, Shuster JJ, Civin CI, Carroll AJ, Look AT, Behm FG, Land VJ, Pullen DJ, Crist WM: Prognostic significance of CD34 expression in childhood B-precursor acute lymphocytic leukemia: a Pediatric Oncology Group study. J Clin Oncol 1990, 8:1389-1398 [DOI] [PubMed] [Google Scholar]
- 9.Pui CH, Hancock ML, Head DR, Rivera GK, Look AT, Sandlund JT, Behm FG: Clinical significance of CD34 expression in childhood acute lymphoblastic leukemia. Blood 1993, 82:889-894 [PubMed] [Google Scholar]
- 10.Tindle RW, Nichols RA, Chan L, Campana D, Catovsky D, Birnie GD: A novel monoclonal antibody BI-3C5 recognises myeloblasts and non-B non-T lymphoblasts in acute leukaemias and CGL blast crises, and reacts with immature cells in normal bone marrow. Leuk Res 1985, 9:1-9 [DOI] [PubMed] [Google Scholar]
- 11.Ramani P, Bradley NJ, Fletcher CD: QBEND/10, a new monoclonal antibody to endothelium: assessment of its diagnostic utility in paraffin sections. Histopathology 1990, 17:237-242 [DOI] [PubMed] [Google Scholar]
- 12.Nickoloff BJ: The human progenitor cell antigen (CD34) is localized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Kaposi’s sarcoma. Arch Dermatol 1991, 127:523-529 [PubMed] [Google Scholar]
- 13.Cohen PR, Rapini RP, Farhood AI: Expression of the human hematopoietic progenitor cell antigen CD34 in vascular and spindle cell tumors. J Cutan Pathol 1993, 20:15-20 [DOI] [PubMed] [Google Scholar]
- 14.Traweek ST, Kandalaft PL, Mehta P, Battifora H: The human hematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol 1991, 96:25-31 [DOI] [PubMed] [Google Scholar]
- 15.Aziza J, Mazerolles C, Selves J: Comparison of the reactivities of monoclonal antibodies QBEND10 (CD34) and BNH9 in vascular tumors. Appl Immunohistochem 1993, 1:51-57 [Google Scholar]
- 16.van de Rijn M, Lombard CM, Rouse RV: Expression of CD34 by solitary fibrous tumors of the pleura, mediastinum, and lung. Am J Surg Pathol 1994, 18:814-820 [DOI] [PubMed] [Google Scholar]
- 17.Weiss SW, Nickoloff BJ: CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am J Surg Pathol 1993, 17:1039-1045 [DOI] [PubMed] [Google Scholar]
- 18.van de Rijn M, Hendrickson MR, Rouse RV: CD34 expression by gastrointestinal tract stromal tumors. Hum Pathol 1994, 25:766-771 [DOI] [PubMed] [Google Scholar]
- 19.Suster S, Fisher C: Immunoreactivity for the human hematopoietic progenitor cell antigen (CD34) in lipomatous tumors. Am J Surg Pathol 1997, 21:195-200 [DOI] [PubMed] [Google Scholar]
- 20.Templeton SF, Solomon AR Jr.: Spindle cell lipoma is strongly CD34 positive: an immunohistochemical study. J Cutan Pathol 1996, 23:546–550 [DOI] [PubMed]
- 21.Abenoza P, Lillemoe T: CD34, and factor XIIIa in the differential diagnosis of dermatofibroma, and dermatofibrosarcoma protuberans. Am J Dermatopathol 1993, 15:429-434 [DOI] [PubMed] [Google Scholar]
- 22.Kutzner H: Expression of the human progenitor cell antigen CD34 (HPCA-1) distinguishes dermatofibrosarcoma protuberans from fibrous histiocytoma in formalin-fixed, paraffin-embedded tissue. J Am Acad Dermatol 1993, 28:613-617 [DOI] [PubMed] [Google Scholar]
- 23.Arber DA, Kandalaft PL, Mehta P, Battifora H: Vimentin-negative epithelioid sarcoma: the value of an immunohistochemical panel that includes CD34. Am J Surg Pathol 1993, 17:302-307 [PubMed] [Google Scholar]
- 24.Sirgi KE, Wick MR, Swanson PE: B72.3, and CD34 immunoreactivity in malignant epithelioid soft tissue tumors: adjuncts in the recognition of endothelial neoplasms. Am J Surg Pathol 1993, 17:179-185 [DOI] [PubMed] [Google Scholar]
- 25.Thomas TM, Myint A, Mak CK, Chan JK: Mammary myofibroblastoma with leiomyomatous differentiation. Am J Clin Pathol 1997, 107:52-55 [DOI] [PubMed] [Google Scholar]
- 26.Fukunaga M, Ushigome S: Myofibroblastoma of the breast with diverse differentiations. Arch Pathol Lab Med 1997, 121:599-603 [PubMed] [Google Scholar]
- 27.Hanson CA, Ross CW, Schnitzer B: Anti-CD34 immunoperoxidase staining in paraffin sections of acute leukemia: comparison with flow cytometric immunophenotyping. Hum Pathol 1992, 23:26-32 [DOI] [PubMed] [Google Scholar]
- 28.van de Rijn M, Hendrickson MR, Rouse RV: CD34: a review. Appl Immunohistochem 1994, 21:71-80 [Google Scholar]
- 29.He XY, Antao VP, Basila D, Marx JC, Davis BR: Isolation and molecular characterization of the human CD34 gene. Blood 1992, 79:2296-2302 [PubMed] [Google Scholar]
- 30.Simmons DL, Satterthwaite AB, Tenen DG, Seed B: Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol 1992, 148:267-271 [PubMed] [Google Scholar]
- 31.Majdic O, Stockl J, Pickl WF, Bohuslav J, Strobl H, Scheinecker C, Stockinger H, Knapp W: Signaling and induction of enhanced cytoadhesiveness via the hematopoietic progenitor cell surface molecule CD34. Blood 1994, 83:1226-1234 [PubMed] [Google Scholar]
- 32.Hu MC, Chien SL: The cytoplasmic domain of stem cell antigen CD34 is essential for cytoadhesion signaling but not sufficient for proliferation signaling. Blood 1998, 91:1152-1162 [PubMed] [Google Scholar]
- 33.Suda J, Sudo T, Ito M, Ohno N, Yamaguchi Y, Suda T: Two types of murine CD34 mRNA generated by alternative splicing. Blood 1992, 79:2288-2295 [PubMed] [Google Scholar]
- 34.Sutherland DR, Marsh JC, Davidson J, Baker MA, Keating A, Mellors A: Differential sensitivity of CD34 epitopes to cleavage by Pasteurella haemolytica glycoprotease: implications for purification of CD34- positive progenitor cells. Exp Hematol 1992, 20:590-599 [PubMed] [Google Scholar]
- 35.Wucherpfennig KW, Strominger JL: Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 1995, 80:695-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang ZX, Chen M, Wallhagen K, Trojnar J, Magnius LO, Wahren B, Sallberg M: Molecular basis for antibody cross-reactivity between the hepatitis C virus core protein and the host-derived GOR protein. Clin Exp Immunol 1994, 96:403-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer A, Keitel T, Winkler K, Stocklein W, Hohne W, Schneider-Mergener J: Molecular basis for the binding promiscuity of an anti-p24 (HIV-1) monoclonal antibody. Cell 1997, 91:799-809 [DOI] [PubMed] [Google Scholar]
- 38.Keitel T, Kramer A, Wessner H, Scholz C, Schneider-Mergener J, Hohne W: Crystallographic analysis of anti-p24 (HIV-1) monoclonal antibody cross- reactivity and polyspecificity. Cell 1997, 91:811-820 [DOI] [PubMed] [Google Scholar]
- 39.Oldstone MB: Molecular mimicry and autoimmune disease. Cell 1987, 50:819-820 [DOI] [PubMed] [Google Scholar]
- 40.Bonner RF, Emmert-Buck M, Cole K, Pohida T, Chuaqui R, Goldstein S, Liotta LA: Laser capture microdissection: molecular analysis of tissue. Science 1997, 278:1481,1483 [DOI] [PubMed]
- 41.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA: Laser capture microdissection. Science 1996, 274:998-1001 [DOI] [PubMed] [Google Scholar]
- 42.Simone NL, Bonner RF, Gillespie JW, Emmert-Buck MR, Liotta LA: Laser-capture microdissection: opening the microscopic frontier to molecular analysis. Trends Genet 1998, 14:272-276 [DOI] [PubMed] [Google Scholar]
- 43.Sakurai S, Fukasawa T, Chong JM, Tanaka A, Fukayama M: Embryonic form of smooth muscle myosin heavy chain (SMemb/MHC-B) in gastrointestinal stromal tumor and interstitial cells of Cajal. Am J Pathol 1999, 154:23-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seidal T, Edvardsson H: Expression of c-kit (CD117) and Ki67 provides information about the possible cell of origin and clinical course of gastrointestinal stromal tumours. Histopathology 1999, 34:416-424 [DOI] [PubMed] [Google Scholar]
- 45.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH: Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol 1999, 23:377-389 [DOI] [PubMed] [Google Scholar]
- 46.Vanderwinden JM, Rumessen JJ, De Laet MH, Vanderhaeghen JJ, Schiffmann SN: CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest 1999, 79:59-65 [PubMed] [Google Scholar]
- 47.Fackler MJ, Civin CI, Sutherland DR, Baker MA, May WS: Activated protein kinase C directly phosphorylates the CD34 antigen on hematopoietic cells. J Biol Chem 1990, 265:11056-11061 [PubMed] [Google Scholar]
- 48.Srinivasappa J, Saegusa J, Prabhakar BS, Gentry MK, Buchmeier MJ, Wiktor TJ, Koprowski H, Oldstone MB, Notkins AL: Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol 1986, 57:397-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fetsch PA, Cormier J, Hijazi YM: Immunocytochemical detection of MART-1 in fresh and paraffin-embedded malignant melanomas. J Immunother 1997, 20:60-64 [DOI] [PubMed] [Google Scholar]
- 50.Chen YT, Stockert E, Jungbluth A, Tsang S, Coplan KA, Scanlan MJ, Old LJ: Serological analysis of Melan-A (MART-1), a melanocyte-specific protein homogeneously expressed in human melanomas. Proc Natl Acad Sci USA 1996, 93:5915-5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busam KJ, Chen YT, Old LJ, Stockert E, Iversen K, Coplan KA, Rosai J, Barnhill RL, Jungbluth AA: Expression of melan-A (MART1) in benign melanocytic nevi and primary cutaneous malignant melanoma. Am J Surg Pathol 1998, 22:976-982 [DOI] [PubMed] [Google Scholar]
- 52.Busam KJ, Iversen K, Coplan KA, Old LJ, Stockert E, Chen YT, McGregor D, Jungbluth A: Immunoreactivity for A103, an antibody to melan-A (Mart-1), in adrenocortical and other steroid tumors. Am J Surg Pathol 1998, 22:57-63 [DOI] [PubMed] [Google Scholar]
- 53.Fetsch PA, Marincola FM, Abati A: The new melanoma markers: MART-1 and Melan-A (the NIH experience). Am J Surg Pathol 1999, 23:607-610 [DOI] [PubMed] [Google Scholar]
- 54.Monihan JM, Carr NJ, Sobin LH: CD34 immunoexpression in stromal tumours of the gastrointestinal tract, and in mesenteric fibromatoses. Histopathology 1994, 25:469-473 [DOI] [PubMed] [Google Scholar]
- 55.Westra WH, Gerald WL, Rosai J: Solitary fibrous tumor: consistent CD34 immunoreactivity and occurrence in the orbit. Am J Surg Pathol 1994, 18:992-998 [DOI] [PubMed] [Google Scholar]
- 56.Miettinen M, Virolainen M, Sarlomo-Rikala M: Gastrointestinal stromal tumors: value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol 1995, 19:207-216 [DOI] [PubMed] [Google Scholar]