Abstract
To investigate the distribution of acyl-coenzyme A:cholesterol acyltransferase-1 (ACAT-1) in various human tissues, we examined tissues of autopsy cases immunohistochemically. ACAT-1 was demonstrated in macrophages, antigen-presenting cells, steroid hormone-producing cells, neurons, cardiomyocytes, smooth muscle cells, mesothelial cells, epithelial cells of the urinary tracts, thyroid follicles, renal tubules, pituitary, prostatic, and bronchial glands, alveolar and intestinal epithelial cells, pancreatic acinar cells, and hepatocytes. These findings showed that ACAT-1 is present in a variety of human tissues examined. The immunoreactivities are particularly prominent in the macrophages, steroid hormone-producing cells, followed by hepatocytes, and intestinal epithelia. In cultured human macrophages, immunoelectron microscopy revealed that ACAT-1 was located mainly in the tubular rough endoplasmic reticulum; immunoblot analysis showed that the ACAT-1 protein content did not change with or without cholesterol loading; however, on cholesterol loading, about 30 to 40% of the total immunoreactivity appeared in small-sized vesicles. These vesicles were also enriched in 78-kd glucose-regulated protein (GRP 78), a specific marker for the endoplasmic reticulum. Immunofluorescent microscopy demonstrated extensive colocalization of ACAT-1 and GRP 78 signals in both the tubular and vesicular endoplasmic reticulum before and after cholesterol loading. These results raise the possibility that foam cell formation may activate an endoplasmic reticulum vesiculation process, producing vesicles enriched in the ACAT-1 protein.
Acyl-coenzyme A:cholesterol acyltransferase (ACAT) is a key enzyme involved in cellular cholesterol metabolism. It catalyzes the formation of cholesteryl esters from cholesterol and long-chain fatty acyl-coenzyme A. 1 ACAT activities are present in various tissues such as liver, intestines, adrenal glands, and aorta and are involved in intracellular cholesterol storage, lipoprotein assembly, steroid hormone production, and dietary cholesterol absorption. 2 Previous studies showed that ACAT is a membrane-bound enzyme; its activity is found only in the membrane fractions of intracellular organelles, especially in the rough endoplasmic reticulum. 3,4 More recently, molecular probes of ACAT have become available. In 1993, the first cDNA of ACAT, designated as ACAT-1, was cloned from a human THP-1 cell cDNA library by using a somatic cell and molecular genetic approach. 5 The sequence of human ACAT-1 cDNA led to the clonings of its homologues from various other species, including mice. 6 ACAT-1 gene knockout mice were produced. 7,8 Analyses of these mice showed that ACAT activities were significantly decreased in selected tissues examined but not in the livers, strongly suggesting that one or more additional ACAT genes, distinct from the ACAT-1 gene, probably exist in mice. More recently, a different ACAT cDNA, designated ACAT-2, was cloned. 9-11 The sequence of ACAT-2 is homologous but different from that of ACAT-1. Earlier, two different ACAT-like genes were found in the simple eukaryote Sachromycetes saravesea. 12,13 The exact roles of ACAT-1 and ACAT-2 in different species remain unknown. Their physiological functions are currently under intensive investigation in several laboratories.
Recently, specific polyclonal antibodies against the ACAT-1 protein from humans or mice have been produced. 14-16 In human cells and tissues, these antibodies recognize the ACAT-1 protein as a single 50-kd protein in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. 14 Using an antibody against the human ACAT-1 protein, DM10, we performed immunoblot analysis and ACAT enzyme activity assay and found that human monocyte-derived macrophages expressed high levels of ACAT-1 protein and high ACAT enzyme activity during the early stage of monocyte/macrophage differentiation. 17 We also demonstrated that the ACAT-1 protein was amply present in macrophages, but not in smooth muscle cells, within the atherosclerotic lesions of human aorta. 17 Other than the atherosclerotic lesions, little is known about the distribution of ACAT-1 protein in normal human organs at the cellular level, particularly in those that play important roles in cholesterol homeostasis. At the single cell level, using the specific anti-ACAT-1 antibody (DM10) for immunofluorescent microscopy, Chang et al reported in human melanoma cells that ACAT-1 is mainly located in endoplasmic reticulum. 14 Using a similar method, Khelef et al reported in murine macrophages that a minor portion of ACAT-1 protein resides in membranes other than the endoplasmic reticulum 16 ; the non-rough endoplasmic reticulum localization of ACAT-1 may be the trans-Golgi network. 18 The distribution of ACAT-1 at the ultrastructural level in any cell type has not been reported yet.
In the present study, we examined by immunohistochemistry the distribution and localization of ACAT-1 in various normal human tissues. In addition, to investigate the intracellular localization of ACAT-1 at both the protein and cellular levels, we performed immunoblot analysis, immunoelectron microscopy, and immunofluorescent microscopy of cultured human macrophages before and after cholesterol loading by treating cells with acetylated low density lipoprotein (AcLDL).
Materials and Methods
Tissue Preparation
For immunohistochemistry, tissue specimens were obtained from various organs and tissues of 8 autopsy cases (6 males and 2 females, 41 to 75 years old) within 4 hours postmortem. These specimens were fixed in an ice-cold 2% periodate-lysine-paraformaldehyde fixative for 6 hours and washed with phosphate-buffered saline, pH 7.2, containing a graded series of sucrose (10, 15, and 20%). To prevent ice crystal formation, the specimens were immersed in 0.01 mol/L phosphate-buffered saline containing 20% sucrose and 10% glycerol for 30 minutes and embedded in OCT compound (Miles, Elkhart, IN). These embedded materials were frozen and cut sequentially into 5-μm-thick sections with a cryostat (HM 500 M; MICROM, Waldorf, Germany).
Preparation of AcLDL
Low density lipoprotein (LDL, d = 1.091 to 1.063) was isolated by sequential ultracentrifugation from normolipidemic human plasma, dialyzed in 0.15 mol/L NaCl and 1 mmol/L ethylenediamine tetraacetic dihydrate, pH 7.4 (Nacalai Tesque, Kyoto, Japan), and treated with acetic anhydride to prepare AcLDL as described previously. 19
Cell Culture
Monocytes were collected from peripheral blood of healthy volunteers according to the method described elsewhere, with a minor modification. 20 Briefly, mononuclear leukocytes were segregated from peripheral blood using the Ficoll/Hypaque gradient centrifugation method, resuspended in RPMI1640 (Nissui Pharmaceutical Co., Tokyo, Japan) containing 10% autologous serum or 10% fetal calf serum, and plated in culture chambers (Lab-Tek Chamber Slide, Nalge Nunc International, Naperville, IL) for 2 hours. After nonadherent cells were removed by gently washing with culture medium, adherent monocytes were cultured for 7 days to induce differentiation and maturation into macrophages. Cultured macrophages were further incubated for 3 more days with a culture medium containing 100 μg/ml of AcLDL. After AcLDL treatment, foam cell transformation was confirmed by observing the accumulation of lipid droplets under phase-contrast microscopy.
Antibodies
Specific polyclonal rabbit antibody for human ACAT-1, DM10, was generated and affinity-purified as described elsewhere. 14 The antibody recognizes the first 131 amino acids residues in N-terminal region of the human ACAT-1. To confirm the formation of mature macrophages derived from cultured peripheral monocytes, we used a mouse anti-human macrophage monoclonal antibody, AM-3K, 21 generated in our laboratory. To detect rough endoplasmic reticulum by immunofluorescent and immunoelectron microscopy, we used a goat polyclonal antibody N-20 raised against a human 78-kd glucose-regulated protein, GRP 78 (Santa Cruz Biotechnology, Santa Cruz, CA).
Immunohistochemistry
To detect the expression of ACAT-1 in the normal human tissues, we performed the indirect immunoperoxidase method with a minor modification as described previously. 17 Briefly, after inhibition of endogenous peroxidase activity according to the method of Li et al, 22 the frozen sections were incubated with 5% normal donkey serum for 20 minutes and sequentially reacted with DM10 diluted 1:200 as primary antibody at room temperature for 1 hour. The sections were rinsed 5 times with ice-cold 0.01 mol/L phosphate-buffered saline, pH 7.2, and incubated with peroxidase-labeled anti-rabbit immunoglobulin F(ab′)2 (Amersham, Little Chalfont, UK) diluted 1:100 as second antibody. After washing, the peroxidase activity was visualized as black color with a solution containing Ni, Co, and 3,3′-diaminobenzidine (Dojin Chemical Co., Kumamoto, Japan), 23 and the sections were stained with Methylgreen and mounted with Malinol (Mutoh Chemical Co., Tokyo, Japan). To assure the immunoreactive specificity of DM10 in each tissue, control stainings were done in the same manner, omitting the primary antibody. In each case, the control stainings provided only a very weak positivity on background.
Immunoblotting
Immunoblot analysis for cultured macrophages with or without AcLDL treatment was performed as described elsewhere. 17 Briefly, the 1 × 10 7 cells seeded in 10-cm dishes were washed several times with phosphate-buffered saline, stored at −80°C, and dried in monolayers for up to 7 days. The frozen cell monolayers were thawed and extracted with 0.1 ml of 10% sodium dodecyl sulfate per dish. Cells were scraped and sheared, using syringes with 25-gauge needles. Protein concentrations of cellular extracts were determined by the method of Lowry et al. 24 Samples were run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to immunoblotting. The primary antibody (DM10) was used at a final concentration of 0.5 μg/ml.
Immunoelectron Microscopy
Immunoelectron microscopy was performed according to the same method as described previously. 21 Briefly, cultured macrophages with or without AcLDL treatment were fixed with 4% periodate-lysine-paraformaldehyde and 0.1% glutaraldehyde (Nacalai Tesque, Kyoto, Japan) in phosphate-buffered saline, pH 7.2, at 4°C for 20 minutes. After washing with phosphate-buffered saline and treating with 0.005% saponin containing phosphate-buffered saline for 10 minutes, the cells were stained by the immunoperoxidase method, using DM10 or N-20 as primary antibody. After visualization with 3,3′-diaminobenzidine for 5 minutes, the cells were postfixed with 1% osmium tetroxide at 4°C for 30 minutes. After rinsing, the cells were dehydrated with a graded series of ethanol and embedded in Epok 812. Ultrathin sections were made by an ultramicrotome MT7000 ULTRA (RMC Inc., Tucson, AZ) and observed under an electron microscope H-7500 (Hitachi, Tokyo, Japan).
Immunofluorescent Double Staining
Immunofluorescent double staining with DM10 and N-20 was used to confirm the localization of ACAT-1 in rough endoplasmic reticulum. Briefly, cultured human macrophages with or without AcLDL treatment were fixed in 2% periodate-lysine-paraformaldehyde solution at 4°C for 20 minutes and rinsed with phosphate-buffered saline containing 0.005% saponin. The cells were incubated with 5% normal donkey serum and 0.1% Triton X-100 for 20 minutes and reacted to primary antibodies containing 200-fold-diluted DM10 with 20-fold-diluted N-20 for 1 hour at room temperature. After rinse, the cells were incubated with secondary antibody sequentially, FluoroLink Cy3-labeled donkey anti-goat IgG(H+L) (Amersham, Little Chalfont, UK) diluted 1:1000 was reacted to the cells, and FluoroLink Cy2-labeled goat anti-rabbit IgG(H+L) (Amersham) were further applied to the cells for 1 hour at room temperature after washing out the former fluorescent labeled antibody. The specimens were mounted using Dako fluorescent mounting medium (Dako, Carpinteria, CA) after washing out nonreacted secondary antibody and observed by a confocal laser scanning microscope (FLUOVIEW, Olympus, Tokyo, Japan).
Results
Immunohistochemical Detection of ACAT-1 in Human Tissues
Table 1 ▶ summarizes the distribution of ACAT-1 in various organs and tissues of autopsy cases as revealed by immunohistochemistry, using the specific anti-ACAT-1 antibodies DM10. Immunoreactivity was demonstrated in various cells and tissues. The specificity of immunoreactivity using DM10 was demonstrated previously by immunoblot analysis: Lee et al reported that a single specific signal at 50 kd was detected in human liver, adrenal glands, and kidneys as well as in macrophages. 25 In the digestive tract, immunoreactivity was expressed in the epithelia of the small intestines and gastric fundic glands, mesothelial cells, and smooth muscle cells. In the small intestine, absorptive epithelia expressed ACAT-1, whereas the goblet cells and Paneth cells showed a very weak immunoreactivity (Figure 1, A and B) ▶ . Negative control staining (without incubation with the first antibodies) in specimens obtained from small intestine, liver, adrenal glands, and neurons confirmed that the immunosignals were specific for ACAT-1 (Figure 1C) ▶ . Myenteric ganglion cells also showed a weak reactivity (data not shown). A very weak immunoreactivity was found in the squamous epithelium of esophagus and in the absorptive epithelial cells of the large intestine (Figure 1D) ▶ , though these regions are not involved in cholesterol absorption. Kupffer cells in the liver expressed a marked immunoreactivity for ACAT-1; hepatocytes also stained positive with DM10, whereas the epithelial cells of bile ducts showed a much weaker immunoreactivity (Figure 1, E and F) ▶ . No immunoreactivity was found in endothelial cells of the hepatic sinusoids (Figure 1F) ▶ . Marked immunoreactivity for ACAT-1 was found in steroid hormone-producing cells including the adrenal cortical cells, Leydig cells in the testis, and granulosa cells in the ovary (Figure 2, A and B) ▶ . In contrast, other types of endocrine cells that are not involved in steroid hormone production showed only an extremely weak immunoreactivity.
Table 1.
Distribution of ACAT-1 in Normal Human Tissues
| Tissues and cells | Immunoreactivity |
|---|---|
| Liver | |
| Hepatocytes | ++ |
| Kupffer cells | +++ |
| Sinusoidal endothelia | − |
| Intrahepatic bile duct | + |
| Adrenal glands | |
| Adrenal cortex | +++ |
| Adrenal medulla | − |
| Gonads | |
| Leidig/granulosa cells | ++ |
| Stromal cells | − |
| Digestive tract | |
| Esophagus | |
| Squamous epithelia | − |
| Esophageal glands | ++ |
| Stomach | |
| Foveolar epithelium | − |
| Fundic glands | ++ |
| Small intestine | |
| Mucosal epithelial cells | ++ |
| Large intestine | |
| Mucosal epithelial cells | − |
| Respiratory system | |
| Alveolar epithelia | ++ |
| Bronchial epithelia | ++ |
| Alveolar macrophages | +++ |
| Cardiovascular system | |
| Cardiomyocytes | + |
| Endothelial cells | − |
| Smooth muscle cells | + |
| Endocrine system | |
| Pituitary gland | + |
| Thyroid gland | |
| Follicular epithelial cells | ++ |
| Nervous system | |
| Neurons | ++ |
| Glial cells | − |
| Myentric ganglia | ++ |
| Kidney | |
| Epithelial cells of proximal and distal tubules | ++ |
| Glomeruli | − |
| Pancreas | |
| Exocrine glands | ++ |
| Langerhans islet cells | − |
| Duct epithelia | + |
| Urinary tract | |
| Transitional epithelial cells | ++ |
| Immune system | |
| Lymphocytes (lymph nodes, spleen, Peyer’s patches) | − |
| Macrophages (liver, lungs, kidneys) | +++ |
+++, strongly positive; ++, positive; +, slightly positive; −, not detected.
Figure 1.
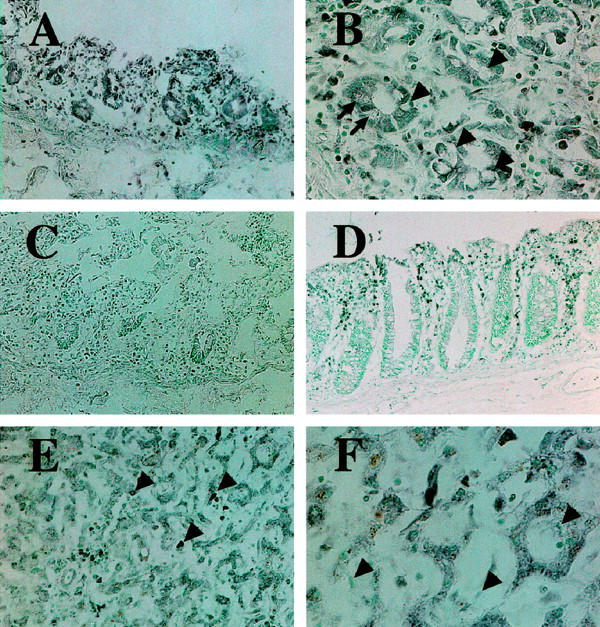
Immunohistochemical demonstration of ACAT-1 in the digestive system. A: The small intestinal absorptive epithelial cells are stained positively by ACAT-1 specific antibodies DM10 (black). B: Absorptive epithelia in small intestine express ACAT-1 strongly, but goblet cells (arrowheads) and Paneth cells (arrows) show less immunoreactivity. Infiltrated monocytes/macrophages also express ACAT-1. C: Negative control; no immunoreactivity is seen in mucosa of small intestine, when the staining procedure omits the step of incubation with the first antibody. D: Epithelial cells of the colonic mucosa are not stained, whereas infiltrated mononuclear phagocytes in the colonic mucosa highly express ACAT-1. E: In the liver, ACAT-1 is most prominent in Kupffer cells (arrowheads) and in hepatocytes. F: Higher magnification view revealed much stronger immunosignals are detected in Kupffer cells than hepatocytes, and no positive signal is detected in hepatic sinusoidal endothelial cells (arrowheads). Original magnifications: A, C, and E, ×120; B and F, ×300; D, ×75.
Figure 2.
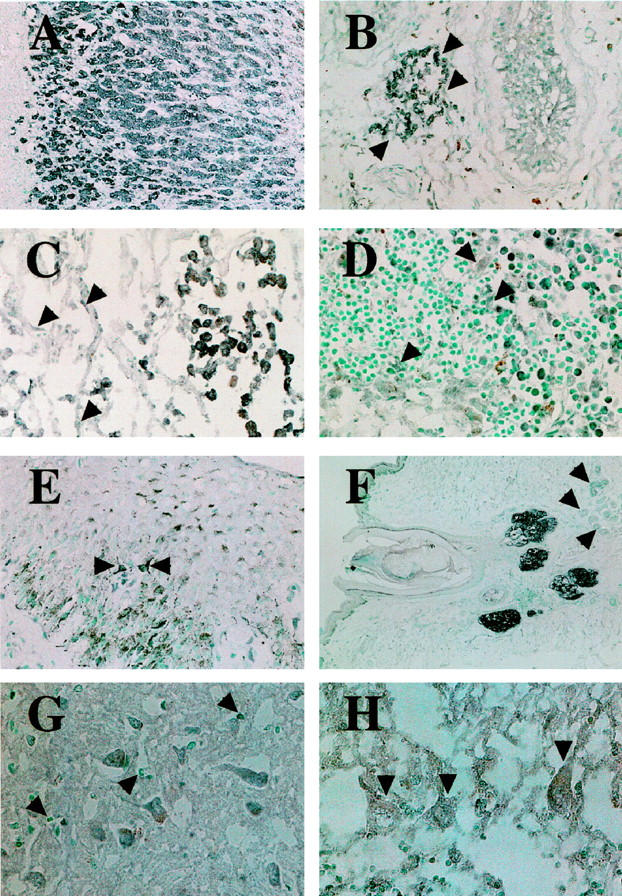
Immunohistochemical demonstration of ACAT-1 in normal human tissues. A: Strong ACAT-1 signals are found in the parenchymal cells of adrenal cortex. B: Arrowheads indicate the presence of ACAT-1 in interstitial Leydig cells of the testis. C: Alveolar macrophages express an intense signal for ACAT-1, whereas alveolar epithelial cells (arrowheads) express a much weaker signal. D: Numerous macrophages in lymphatic sinuses of a lymph node show marked immunoreactivity for ACAT-1, whereas sinusal endothelial cells and dendritic cells (arrowheads) are only weakly positive for ACAT-1. E: Arrowheads indicate a positive ACAT-1 signal in epidermal Langerhans cells. F: Prominent immunosignals are shown in sebaceous glands in the skin, whereas other skin appendages such as hair follicles and eccrine sweat glands (arrowheads) stained negative. G: Neurons in cerebral cortex express ACAT-1, whereas no immunoreactivity was observed in glia (arrowheads). H: Purkinje cells (arrowheads) and their dendrites in cerebellar cortex also stained positive for ACAT-1. Original magnifications: A, ×120; B and C, ×150: D, ×230; E, ×190; F, ×40; G and H, ×300.
Macrophages in the alveolar spaces of lungs (Figure 2C) ▶ , in the lymphatic sinuses of the lymph nodes, and in the red pulp of spleen, expressed an intense immunoreactivity to the ACAT-1 antibodies. Dendritic cells and endothelial cells of lymphatic sinuses in lymph nodes indicated immunoreactivity to DM10 (Figure 2D) ▶ and epidermal Langerhans cells were also positively stained by the antibodies (Figure 2E) ▶ . Control experiments showed that macrophages in these tissues were positively stained with the anti-macrophage antibody AM-3K, while dendritic cells were not stained by AM-3K (data not shown). In the skin, the most prominent signals were observed in the sebaceous glands, while the eccrine sweat glands did not show immunoreactivity (Figure 2F) ▶ . Neurons and their dendrites express ACAT-1 in the cerebral cortex, basal ganglia, cerebellar cortex, pontine nucleus, and spinal cord (Figure 2, G and H) ▶ , but neural glia showed no immunoreactivities. In data not shown, DM10-positive immunoreactivities were also found in the tubular epithelial cells of kidneys, transitional epithelia of urinary tracts, cardiomyocytes, alveolar or bronchial epithelium, and bronchial glands in the respiratory tract, epithelial cells of the prostate and thyroid follicles, and pancreatic acinar and ductal epithelial cells.
Immunoblot Analysis of ACAT-1 in Cultured Human Macrophages with or without Cholesterol Loading
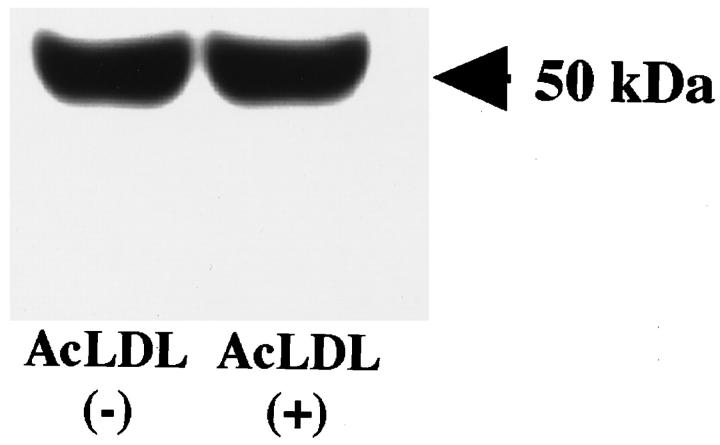
Monocytes collected from peripheral blood of normal healthy human volunteers were incubated in culture for 7 days. Immunostaining using the macrophage-specific antibody AM-3K confirmed that at this stage, all of the cells were differentiated into mature macrophages (data not shown). Modified LDL, such as AcLDL, are known to induce cholesteryl ester accumulation in macrophages and cause subsequent foam cell formation. 20 To investigate whether AcLDL induces any changes in the ACAT protein content in human macrophages, we used DM10 as the primary antibody to perform immunoblot analysis. The results showed that a single 50-kd protein, corresponding to the human ACAT-1 protein, 25 was detected in sodium dodecyl sulfate-polyacrylamide gel electrophoresis; the intensity of the 50-kd protein signal remained the same in cells with or without AcLDL for 3 days (Figure 3) ▶ .
Figure 3.
Immunoblot analysis of ACAT-1 in cultured human macrophages with or without AcLDL treatment. Human monocytes (1 × 10 7 cells) were seeded to 10-cm dishes and cultured in 10 ml per dish of RPMI1640 containing 10% fetal calf serum with or without 100 μg/ml AcLDL. The experiment was conducted as described in Materials and Methods. Samples of cell lysates freshly prepared in sodium dodecyl sulfate were run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (50 μg protein/lane) with 25 mmol/L dithiothreitol, followed by transblotting. The membrane was incubated with DM10 (0.5 μg/ml) as the primary antibody. The relative ACAT-1 protein content in each lane was evaluated from the images of the Western blot by quantitative densitometry analysis.
Localization of ACAT-1 by Immunoelectron Microscopy and Immunofluorescent Microscopy in Cultured Human Macrophages with or without Cholesterol Loading
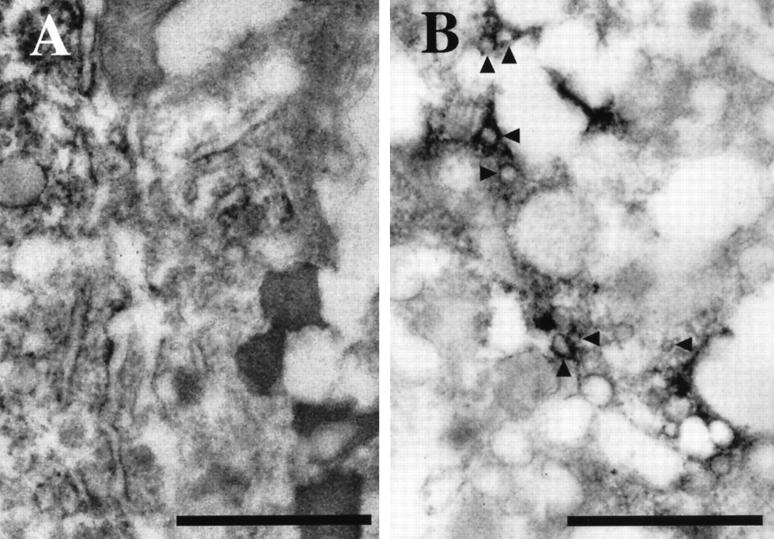
Immunoelectron microscopy was used to study the subcellular localization of ACAT-1 in macrophages. Without cholesterol loading, immunoreactivities against DM10 were found in the membranes of intracellular organelles, especially in rough endoplasmic reticulum and the nuclear membrane (Figure 4A) ▶ ; however, no immunoreactivity was detectable in the Golgi complexes (Figure 4B) ▶ . After AcLDL treatment, immunoreactivity could be found in small vesicles with diameter of 80 to 150 nm, in addition to the tubular endoplasmic reticulum structure (Figure 4, C and D) ▶ . Based on the results of examining 50 or more individual electron micrographs, we estimated that approximately 30 to 40% of the total immunoreactivity appeared in these small vesicles after the transformation of cultured macrophages into foam cells. Under this condition, despite careful examinations, we have still failed to detect any immunoreactivity in the Golgi complexes (data not shown).
Figure 4.
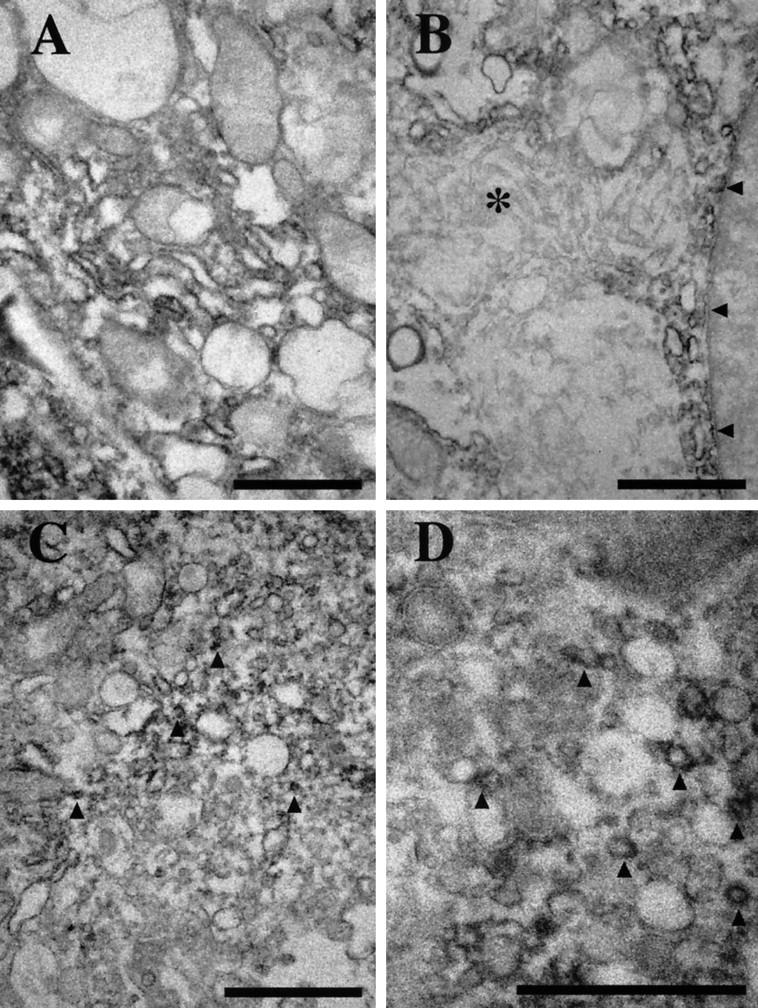
Immunoelectron microscopy of cultured human macrophages, using anti-ACAT-1 antibodies. A: Without AcLDL treatment, the signals are localized mainly in the rough endoplasmic reticulum. B: In addition to the rough endoplasmic reticulum, a few positive signals are detected in the nuclear membrane (arrowheads), but no signal is observed in the Golgi complexes (*). C: With AcLDL, in addition to the endoplasmic reticulum, immunoreactive signals are also found in small vesicles (arrowheads) present throughout the cytoplasm. D: A higher magnification view of C; small vesicles (approximately 80–150 nm in diameter) enriched in ACAT-1 signals (arrowheads) are demonstrated. Scale bars, 1 μm.
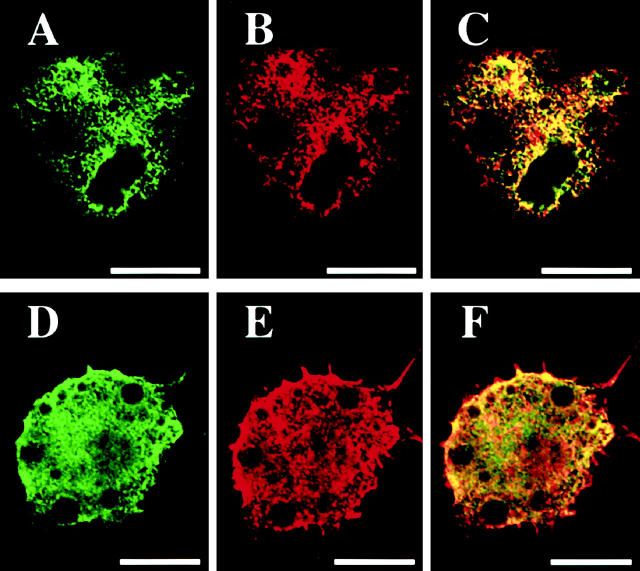
In cultured human macrophages, immunofluorescent staining for ACAT-1 revealed that the enzyme was distributed in a dense reticular network throughout the cytoplasm (Figure 5A) ▶ , a result consistent with the earlier findings implying that ACAT-1 is mainly distributed in the endoplasmic reticulum. 16 When anti-GRP 78 antibody, a specific marker for endoplasmic reticulum, was used, similar patterns were demonstrated (Figure 5B) ▶ . 26,27 By double immunofluorescent staining, the extensive colocalization of ACAT-1 and GRP 78 was demonstrated (Figure 5C) ▶ . After cholesterol loading, the extent of colocalization of ACAT-1 and GRP 78 remained extensive and essentially unaltered (Figure 5, D ▶ -F).
Figure 5.
Double immunofluorescent staining of cultured human macrophages using anti-ACAT-1 and anti-GRP 78 antibodies. A: The signals for ACAT-1 (green) are detected in the cytoplasm as reticular network pattern. B: The signals for GRP 78 (red); the patterns are very similar to those of ACAT-1. C: The overlay view of both ACAT-1 and GRP 78 signals; most of the signals are yellow in color, suggesting extensive colocalization of the green and red signals. D–F: In macrophages with AcLDL treatment, most of the green (ACAT-1) and red signals (GRP 78) overlap with each other, producing yellow signals suggesting essentially identical cellular distribution of ACAT-1 and GRP 78. Scale bars, 20 μm.
To further investigate the possibility that AcLDL treatment may cause extensive vesicular transformation of the endoplasmic reticulum, which is mainly tubular in structure, we performed additional immunoelectron microscopy using a specific antibody for GRP 78. In macrophages without AcLDL treatment, electron-dense, immunopositive deposits were observed at the tubular endoplasmic reticula (Figure 6A) ▶ . After cholesterol loading, numerous immunoreactive small vesicles appeared throughout the cytoplasm of the cells (Figure 6B) ▶ .
Figure 6.
Immunoelectron microscopy of cultured human macrophages using the anti-GRP 78 antibody. A: Without AcLDL treatment, positive signals are localized mainly in the endoplasmic reticulum. B: With AcLDL, positive signals are present in small vesicles with diameter of approximately 100 nm throughout the cytoplasm (arrowheads). Scale bars, 1 μm.
Discussion
In mammals, two ACAT cDNAs with homologous but distinct nucleotide sequences, designated ACAT-1 5,25 and ACAT-2, 9-11 have been reported in the literature. The physiological functions of these two proteins are under investigation in several laboratories. Studies show that, in mice deficient in the ACAT-1 gene, a significant decrease in cellular cholesteryl ester formation was observed in selected tissues or cells that include the adrenal glands, skin, and macrophages, but not in the liver. 7,8 This result raised the possibility that the ACAT-1 gene product may play a functional role only in certain selected tissues, but not in the liver. In human tissues, using a biochemical approach, immunodepletion experiments suggested that the ACAT-1 protein constitutes a majority of the ACAT enzyme activities displayed in extracts prepared from various human tissues or cells, including livers (hepatocytes), adrenal glands, kidneys, and macrophages; human ACAT-1 is also present in the small intestines, but it may not be the major cholesterol esterifying enzyme in that tissue. 25 Using the immunohistochemical staining in various human tissues, our current results show that the ACAT-1-expressing cells in normal human tissues can be classified into three major groups: 1) cells involved in cholesterol absorption and lipoprotein assembly, ie, the epithelia in the digestive tract and the hepatocytes in the liver; 2) steroid hormone-producing cells, such as parenchymal cells of the adrenal cortex, Leydig cells in the testis, and granulosa cells in the ovary; and 3) tissue macrophages and their related cells, such as dendritic cells. Our additional studies show that various other cell types, such as neuronal cells in central nervous system and myenteric plexus, cardiomyocytes, smooth muscle cells, or epithelial cells, also express ACAT-1. In these ACAT-1-positive tissues/cells, prominent immunoreactivities are observed in macrophages and its related cells, steroid hormone-producing cells, and sebaceous glands. Other types of cells such as intestinal enterocytes, hepatocytes, and neurons also express ACAT-1, but at a relatively weaker level. We thus conclude that the ACAT-1 protein is present in a wide variety of human tissues.
In macrophages, our current result indicated that there is no change in the ACAT-1 protein content when the cells are transformed into foam cells (by loading cells with cholesterol via AcLDL treatment). This result is consistent with previous studies in various cells and tissues supporting the notion that the main mechanism for increase in ACAT enzyme activity by cholesterol influx is probably due to the allosteric property of the ACAT-1 enzyme in combination with an increase in intracellular cholesterol trafficking toward the endoplasmic reticulum. 1,28,29 The electron microscopic studies in macrophages described in this study report ACAT-1 localization at the ultrastructural level for the first time, demonstrating that the ACAT-1 protein is located mainly in the rough endoplasmic reticulum. This result is consistent with previous biochemical studies using other cell types, suggesting that ACAT-1 is an integral membrane protein that functions in the rough endoplasmic reticulum. 1,30-32 In an earlier study using immunofluorescence microscopy, Khelef et al demonstrated that a minor portion of ACAT-1 in mouse macrophages is distributed in Golgi complexes in addition to its main location in the endoplasmic reticulum. 16 In human macrophages, however, we have examined numerous electron micrographs (more than 50) but have failed to detect any positive signals in the Golgi complexes. This discrepancy may result from species difference used in our current study and the studies by Khelef et al. 16 When human macrophages were loaded with cholesterol by treating with AcLDL, we still could not detect any ACAT-1-positive immunoreactivity in the Golgi complexes. Instead, we found that the ACAT-1 signals were present in tubular endoplasmic reticulum as well as in the small-sized vesicles, 80 to 150 nm in diameter; these small vesicles also stained positive for GRP 78, known as a specific marker for endoplasmic reticulum. In addition, our double immunofluorescent staining revealed that the ACAT-1 signals colocalized extensively with the GRP 78 signals; the extent of colocalization between ACAT-1 and GRP 78 did not change before and after cholesterol loading. Overall, our findings raise the possibility that, at least in human macrophages, cholesterol loading may induce an accelerated endoplasmic reticulum vesiculation process to produce certain novel small vesicles enriched in the ACAT-1 protein from rough endoplasmic reticulum. The vesiculation process, if it exists, may increase the accessibility of ACAT-1 protein to intracellular free cholesterol.
Acknowledgments
We thank Ms. Makiko Tanaka and Mr. Osamu Nakamura, Second Department of Pathology, Kumamoto University School of Medicine, for skillful technical assistance.
Footnotes
Address reprint requests to Dr. Kiyoshi Takahashi, Second Department of Pathology, Kumamoto University School of Medicine, 2–2-1 Honjo, Kumamoto 860-0811, Japan. E-mail: naomi@kaiju.medic.kumamoto-u.ac.jp.
References
- 1.Chang TY, Chang CCY, Cheng D: Acyl-coenzyme A: cholesterol acyltransferase. Annu Rev Biochem 1997, 66:613-638 [DOI] [PubMed] [Google Scholar]
- 2.Suckling KE, Stange EF: Role of acyl-CoA: cholesterol acyltransferase in cellular cholesterol metabolism. J Lipid Res 1985, 26:647-671 [PubMed] [Google Scholar]
- 3.Balasubramaniam S, Venkatesan S, Mitropoulos KA, Peters TJ: The submicrosomal localization of acyl-coenzyme A-cholesterol acyltransferase and its substrate, and of cholesteryl esters in rat liver. Biochem J 1978, 174:863-872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto S, Fogelman AM: Smooth microsomes: a trap for cholesteryl ester formed in hepatic microsomes. J Biol Chem 1980, 255:8678-8684 [PubMed] [Google Scholar]
- 5.Chang CCY, Huh HY, Cadigan KM, Chang TY: Molecular cloning and functional expression of human acyl-coenzyme A: cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem 1993, 268:20747-20755 [PubMed] [Google Scholar]
- 6.Uelmen PJ, Oka K, Sullivan M, Chang CCY, Chang TY, Chan L: Tissue-specific expression and cholesterol regulation of acylcoenzyme A: cholesterol acyltransferase (ACAT) in mice: molecular cloning of mouse ACAT cDNA, chromosomal localization, and regulation of ACAT in vitro and in vivo. J Biol Chem 1995, 270:26192-26201 [DOI] [PubMed] [Google Scholar]
- 7.Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M, Pitas RE, McGuire J, Herz J, Farese RV, Jr: Disruption of the acyl-CoA: cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci USA 1996, 93:14041-14046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meiner V, Tam C, Gunn MD, Dong L-M, Weisgraber KH, Novak S, Myers HM, Erickson SK, Farese RV, Jr: Tissue expression studies on the mouse acyl-CoA: cholesterol acyltransferase gene (Acact): findings supporting the existence of multiple cholesterol esterification enzymes in mice. J Lipid Res 1997, 38:1928-1933 [PubMed] [Google Scholar]
- 9.Oelkers P, Behari A, Cromley D, Billheimer JT, Sturley SL: Characterization of two human genes encoding acyl coenzyme A: cholesterol acyltransferase-related enzymes. J Biol Chem 1998, 273:26765-26771 [DOI] [PubMed] [Google Scholar]
- 10.Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RV, Jr: ACAT-2, a second mammalian acyl-CoA: cholesterol acyltransferase: its cloning, expression, and characterization. J Biol Chem 1998, 273:26755-26764 [DOI] [PubMed] [Google Scholar]
- 11.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL: Identification of a form of acyl-CoA: cholesterol acyltransferase specific to liver and intestine in nonhuman primate. J Biol Chem 1998, 273:26747-26754 [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Bard M, Bruner DA, Gleeson A, Deckelbaum RJ, Aljinovic G, Pohl TM, Rothstein R, Sturley SL: Sterol esterification in yeast: a two-gene process. Science 1996, 272:1353-1356 [DOI] [PubMed] [Google Scholar]
- 13.Yu C, Kennedy NJ, Chang CCY, Rothblatt JA: Molecular cloning and characterization of two isoforms of Saccharomyces cerevisiae acyl-CoA: sterol acyltransferase. J Biol Chem 1996, 271:24157-24163 [DOI] [PubMed] [Google Scholar]
- 14.Chang CCY, Chen J, Thomas MA, Cheng D, Priore VAD, Newton RS, Pape ME, Chang TY: Regulation and immunolocalization of acyl-coenzyme A: cholesterol acyltransferase in mammalian cells as studied with specific antibodies. J Biol Chem 1995, 270:29532-29540 [DOI] [PubMed] [Google Scholar]
- 15.Meiner V, Tam C, Gunn MD, Dong LM, Weisgraber KH, Novak S, Myers HM, Erickson SK, Farese RV, Jr: Tissue expression studies on the mouse acyl-CoA: cholesterol acyltransferase gene (Acact): findings supporting the existence of multiple cholesterol esterification enzymes in mice. J Lipid Res 1997, 38:1928-1933 [PubMed] [Google Scholar]
- 16.Khelef N, Buton X, Beatini N, Wang H, Meiner V, Chang TY, Farese RV, Jr, Maxfield FR, Tabas I: Immunolocalization of acyl-coenzyme A: cholesterol O-acyltransferase in macrophages. J Biol Chem 1998, 273:11218-11224 [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki A, Sakashita N, Lee O, Takahashi K, Horiuchi S, Hakamata H, Morganelli PM, Chang CCY, Chang TY: Expression of ACAT-1 protein in human atherosclerotic lesions and cultured human monocytes/macrophages. Arterioscler Thromb Vasc Biol 1998, 18:1568-1574 [DOI] [PubMed] [Google Scholar]
- 18.Tabas I: A portion of acyl-CoA:cholesterol acyltransferase (ACAT) colocalizes with the trans-Golgi network (TGN) in macrophages (Mφs). Circulation 1998, 97(suppl I):311 (abstr)
- 19.Horiuchi S, Takata K, Maeda H, Morino Y: Scavenger function of sinusoidal liver cells: acetylated low density lipoprotein is endocytosed via a route distinct from formaldehyde-treated serum albumin. J Biol Chem 1985, 260:53-56 [PubMed] [Google Scholar]
- 20.Sakai M, Miyazaki A, Hakamata H, Sato Y, Matsumura T, Kobori S, Shichiri M, Horiuchi S: Lysophosphatidylcholine potentiates the mitogenic activity of modified LDL for human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol 1996, 16:600-605 [DOI] [PubMed] [Google Scholar]
- 21.Zeng L, Takeya M, Takahashi K: AM-3K, a novel monoclonal antibody specific for tissue macrophages and its application to pathological investigation. J Pathol 1996, 178:207-214 [DOI] [PubMed] [Google Scholar]
- 22.Li C-Y, Ziesmer SC, Lazcano-Villareal O: Use of azide and hydrogen peroxide as an inhibitor for endogenous peroxidase in the immunoperoxidase method. J Histochem Cytochem 1987, 35:1457-1460 [DOI] [PubMed] [Google Scholar]
- 23.Adams JC: Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem 1981, 29:775. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagents. J Biol Chem 1951, 193:265-270 [PubMed] [Google Scholar]
- 25.Lee O, Chang CCY, Lee W, Chang TY: Immunodepletion experiments suggest that acyl-coenzyme A: cholesterol acyltransferase-1 (ACAT-1) protein plays a major catalytic role in adult human liver, adrenal gland, macrophages, and kidney, but not intestines. J Lipid Res 1998, 39:1722-1727 [PubMed] [Google Scholar]
- 26.Martin J, Horwich AL, Hartl FU: Prevention of protein denaturation under heat stress by the chaperonin Hsp60. Science 1992, 258:995-998 [DOI] [PubMed] [Google Scholar]
- 27.Csermely P, Miyata Y, Schnaider T, Yahara I: Autophosphorylation of grp94 (endoplasmin). J Biol Chem 1995, 270:6381-6388 [DOI] [PubMed] [Google Scholar]
- 28.Okwu AK, Xu XX, Shiratori Y, Tabas I: Regulation of the threshold for lipoprotein-induced acyl-CoA cholesterol O-acyltransferase stimulation in macrophages by cellular sphingomyelin content. J Lipid Res 1994, 35:644-655 [PubMed] [Google Scholar]
- 29.Chang CCY, Lee CYG, Chang ET, Cruz JC, Levesque M, Chang TY: Recombinant acyl-CoA: cholesterol acyltransferase-1(ACAT-1) purified to essential homogeneity utilizes cholesterol in mixed micelles or vesicles in a highly cooperative manner. J Biol Chem 1998, 273:35132-35141 [DOI] [PubMed] [Google Scholar]
- 30.Reinhart MP, Billheimer JT, Faust JR, Gaylor JL: Subcellular localization of the enzymes of cholesterol biosynthesis and metabolism in rat liver. J Biol Chem 1987, 262:9649-9655 [PubMed] [Google Scholar]
- 31.Doolittle GM, Chang TY: Solubilization, partial purification, and reconstitution in phosphatidylcholine-cholesterol liposomes of acyl-CoA: cholesterol acyltransferase. Biochemistry 1982, 21:674-679 [DOI] [PubMed] [Google Scholar]
- 32.Lange Y, Strebel F, Steck TL: Role of the plasma membrane in cholesterol esterification in rat hepatoma cells. J Biol Chem 1993, 268:13838-13843 [PubMed] [Google Scholar]