Abstract
In preeclampsia, poor placental perfusion may result in maternal endothelial dysfunction, but the pathways involved are largely unknown. Candidate placental mediators include products of oxidative stress released into the maternal circulation. Xanthine oxidase has been implicated in postischemic-reperfusion injury via the generation of superoxide anion radicals (superoxide; O2.−) and hydrogen peroxide. We examined placentas and placental bed curettings and/or biopsies from preeclamptic control pregnant women to test the hypothesis that xanthine oxidase is a mediator of oxidative stress in placentas from women with preeclampsia. The expression of xanthine dehydrogenase/xanthine oxidase holoenzyme and the activity of xanthine oxidase, the isoform known to generate reactive oxygen species, were increased in a subpopulation of cytotrophoblasts of preeclamptic women. Additionally, the expression of superoxide dismutase, which would scavenge superoxide produced by xanthine oxidase, was reduced in the same cells. Furthermore, fluorescence immunostaining for nitrotyrosine, which was suggestive of superoxide-nitric oxide interactions to form peroxynitrite anion (ONOO−) in vivo, was increased in these cells and in villous vessels. Thus, our data indicate an increased capacity of placental cells to generate reactive oxygen species in preeclampsia.
Preeclampsia, a pregnancy-specific disorder, is the leading cause of maternal mortality in the Western world and increases perinatal mortality fivefold. The clinical diagnosis is based on the new onset of hypertension and the appearance of proteinuria and edema during pregnancy. It is evident, however, that these findings are a small component of a multisystemic disease that reduces perfusion to virtually all of the organs in the body. 1 The hypothesis has been advanced that much of the disease could be explained by alterations in the function of vascular endothelium. Increasing data support this hypothesis. 2 The more complete hypothesis posits that the placenta, likely in response to reduced perfusion, produces a circulating factor(s) that alters endothelial cell function. 2 Among the candidate molecules are products resulting from oxidative stress. 3 Oxidative stress, the result of free-radical generation in excess of protective mechanisms, is suggested as an important component of other disorders that affect endothelial function, including diabetes and atherosclerosis. 4,5 Evidence for oxidative stress in preeclampsia includes elevated lipid hydroperoxides or their metabolites and reduced plasma antioxidant activity. 3,6-8
Xanthine oxidase, which metabolizes xanthine and hypoxanthine to uric acid with production of superoxide and hydrogen peroxide, is an integral mediator of reactive oxygen species generation in many settings. Usually, this enzyme is present as the holoenzyme xanthine dehydrogenase/xanthine oxidase (XDH/XO). The dehydrogenase isoform (XDH) converts purines to uric acid with reducton of nicotinamide-adenine dinucleotide (NAD) to reduced NAD (NADH). With hypoxia and in response to several cytokines, XDH/XO synthesis increases, and the conversion of the enzyme to the XO form is enhanced. 9 Reduced organ perfusion and subsequent reperfusion are proposed to result in increased XO during reduced flow and in subsequent formation of reactive oxygen species with reperfusion. The inadequate placental perfusion that characterizes preeclampsia is believed to reduce oxygen delivery to the placenta. Vasospasm may result in intermittent variations of utero-placental perfusion during preeclampsia. Hence, although it is unclear whether postischemic reoxygenation occurs in the preeclampsia placenta, we proposed that XO might be a source of reactive oxygen species in the disorder.
In previous studies, we documented that XO, which was thought to be absent from the placenta, 10 was present and active. 11 In this study, we examined XDH/XO protein expression and activity in placental villi and placental bed biopsies from normal and preeclamptic women. We also studied expression (at the protein level) of enzymes that degrade superoxide (copper, zinc-superoxide dismutase (SOD); CuZn-SOD) and hydrogen peroxide (catalase). We looked for evidence of enhanced immunoreactive nitrotyrosine in placentas from women with preeclampsia, an oxidative change suggestive of interaction of O2.− with nitric oxide (NO) to form a peroxynitrite anion (ONOO−). We report that invasive cytotrophoblasts from preeclamptic women manifest increased amounts and activity of XDH/XO accompanied by reduced expression of the buffering enzyme SOD but no reduction in catalase and that the cells show increased nitration of tyrosine indicative of oxidative modification.
Materials and Methods
Study Subjects
Subjects in these studies were cared for at Moffitt Hospital and San Francisco General Hospital, University of California at San Francisco (San Francisco, CA); at Magee-Women’s Hospital, University of Pittsburgh (Pittsburgh, PA); and at the University of Tennessee Medical Center (Memphis, TN). The study was approved by the institutional review boards of the participating institutions.
Preeclampsia was diagnosed by similar criteria at all institutions. The diagnosis required gestational hypertension, proteinuria, hyperuricemia, and reversal of hypertension and proteinuria after pregnancy. 12 Hypertension was defined as an increase of 30 mmHg systolic or 15 mmHg diastolic blood pressure as compared with values obtained before 20 weeks of gestation or an absolute blood pressure >140/90 mmHg. Proteinuria was defined as >500 mg/24-hour collection or >2+ on voided or 1+ on catheterized random urine specimens. Hyperuricemia was defined as >1 SD above usual values at the time of gestation. Chronic hypertensive women or those with other medical complications were excluded. Women in the control group had normal blood pressure and no other medical complications.
Tissue Processing
Samples of floating villi and basal plate for immunofluorescence staining were obtained from elective terminations and at the time of term cesarean section and vaginal delivery (Moffitt Hospital and San Francisco General Hospital). Tissue biopsy and preparation for immunostaining were as described previously. 13,14 Placental villi (from Magee-Women’s Hospital) and placental site curettings (from University of Tennessee Medical Center) for determining XDH/XO activity were obtained after separation of the placenta, immediately frozen in liquid nitrogen, and stored at −80°C until further processing. In this case, placental samples were obtained only from women delivered by cesarean section without prior contractions. This eliminated any potential influence of uterine contraction on XDH/XO activity. The placental bed was sampled by sharp curetting of the uterine site to which the placenta was attached.
Immunohistochemical Staining
Primary Antibodies
Immunostaining used specific antibodies to XO (rabbit polyclonal immunoglobulin G (IgG), 1:3000 dilution; Rockland, Gilbertville, PA), nitrotyrosine (mouse monoclonal IgG, 1:100 dilution; from J. S. Beckman, University of Alabama, Birmingham, AL, and subsequently from Upstate Biotechnology, Inc., Lake Placid, NY), Cu,Zn-SOD (mouse monoclonal IgG, 1:500 dilution; Sigma Chemical Co., St. Louis, MO), catalase (rabbit polyclonal IgG, 1:100 dilution; from P. B. Lazarow, Mount Sinai Medical Center, New York, NY), and cytokeratin (rat monoclonal IgG, 1:200 dilution 14 ).
Specimens and Antibody Localization
Double indirect immunofluorescence was used to localize the primary antibody. Sections of floating villi, basal plate, and placental bed were exposed to an appropriate dilution of primary antibodies for 60 minutes. Negative-control incubations omitting either the primary or secondary antibodies were routinely performed. We always stained with only one antibody (either anti-cytokeratin or the experimental reagent) to eliminate red/green crossover. No nonspecific contribution to staining intensity was observed. For catalase, specificity was confirmed by using equal dilutions of rabbit pre-immune sera in place of the primary antibody as a negative control. Specificity of anti-XO and nitrotyrosine immunostaining was confirmed using equivalent dilutions of antigen-preabsorbed antibodies. Tissues were incubated for 30 minutes with fluorescein- and rhodamine-conjugated secondary antibodies. 14 Sections were viewed with a Zeiss Axiophot Epifluorescence microscope equipped with filters to selectively view the rhodamine and fluorescein images without cross contamination.
Control specimens (10) were obtained at the following gestational ages: 7 weeks (1 sample obtained at the time of pregnancy termination), 15 weeks (1 sample obtained at the time of pregnancy termination), 18 weeks (2 samples obtained at the time of pregnancy termination), 26 weeks (2 samples–one obtained from a woman who delivered due to cervical incompetence and another obtained after termination of a pregnancy due to inoperable conjoined twins). The remainder of the samples were obtained from control nulliparous women who underwent cesarean sections at the following gestational ages: 33 weeks (1 sample), 34 weeks (1 sample), and 38 weeks (2 samples). None of the control patients had evidence of preeclampsia, gestational hypertension, chorioamnionitis, or chronic hypertension. Likewise, none had a medical history that suggested they were at risk for developing preeclampsia. We also obtained 10 specimens from patients with preeclampsia as follows: 24 weeks (1 sample), 26 weeks (2 samples), 28 weeks (2 samples), 32 weeks (2 samples) 33 weeks (1 sample), 35 weeks (1 sample), and 38 weeks (1 sample). In all cases, sections from three different biopsy sites within the placental bed were examined. Clinical characteristics of patients are summarized in Table 1 ▶ . An atheromatous coronary artery specimen (provided by S. C. Watkins, Department of Cell Biology and Physiology, University of Pittsburgh) from a 73-year-old female who died of aortic aneurysm was used as a positive control tissue for anti-nitrotyrosine immunostaining. This vessel showed nitrotyrosine staining in atheroma and foam cells (but not the underlying smooth muscle).
Table 1.
Clinical Characteristics of Patients from Whom Placental Bed Curettings Were Obtained for XDH/XO Enzyme Analyses
| Clinical characteristic | Normal (n = 12) | Preeclampsia (n = 10) | P value |
|---|---|---|---|
| Maternal age (years) | 24.9 ± 6.2 | 24.3 ± 8.5 | N.S. |
| Prepregnant body mass index (kg/m2) | 28.4 ± 6.1 | 23.9 ± 8.5 | N.S. |
| Prepregnant blood pressure (mmHg) | 105/68 ± 7/8 | 110/71 ± 10/6 | N.S. |
| Blood pressure at term (mmHg) | 123/78 ± 9/6 | 155/98 ± 19/9 | <0.001 |
| Weeks gestation at delivery | 39.6 ± 1.2 | 33.0 ± 5.1 | <0.01 |
| Hematocrit (v/v %) | 36.4 ± 2.2 | 34.8 ± 3.7 | N.S. |
| Serum creatinine (mg/dl) | 0.8 ± 0.1 | 0.8 ± 0.2 | N.S. |
| Infant birthweight (grams) | 3440 ± 439 | 1822 ± 760 | <0.001 |
| Serum uric acid (mg/dl) | 5.1 ± 0.8 | 7.1 ± 1.1 | <0.001 |
Data are means ± SDs and were analyzed using the Student’s t-test. N.S., not significant (P > 0.05). Blood pressures are systolic/diastolic.
XDH/XO Enzyme Activity
Materials
[14C] Xanthine, specific activity 55 Ci/mmol, was purchased from Dupont-NEN, Boston, MA. All other materials were purchased from Sigma.
Tissue Preparation
For XDH/XO analyses, clinical characteristics of patients from whom samples were obtained are summarized in Table 2 ▶ . Approximately 0.5 g of tissue was frozen in liquid nitrogen and ground into powder. Cold buffer (50 mmol/L potassium phosphate, 10 mmol/L dithiothreitol, 1 mmol/L phenylmethyl sulfonyl fluoride, 0.1 mmol/L ethylenediaminetetraacetic acid, pH 7.8) was added to the powder (1:5 w/v). The slurry was centrifuged at 40,000 × g for 30 minutes at 4°C, and the supernatant was applied to a G-25 Sephadex column at 4°C to eliminate endogenous xanthine and low-molecular-weight inhibitors. The excluded volume was collected for assay. No more than 60 minutes elapsed from the beginning of the tissue processing to the beginning of the activity assay.
Table 2.
Summary of Placental Staining for XO, SOD, Catalase, and Nitrotyrosine in Normal Pregnancy and in Preeclampsia
| STB | Villous CTB | Villous SC | Villous EC | EV-CTB | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | PE | 1st | 2nd | 3rd | PE | 1st | 2nd | 3rd | PE | 1st | 2nd | 3rd | PE | 1st | 2nd | 3rd | PE | |
| XO | − | − | ± | + | − | − | ± | + | − | − | ± | ± | − | − | −/± | +/++ | − | − | −* | ++† |
| SOD | − | ++ | ++ | ++ | + | + | + | + | − | − | − | − | − | − | − | − | − | ± | ++ | −/±† |
| Catalase | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Nitrotyrosine | − | − | − | − | − | − | − | − | − | − | ± | + | − | − | − | ++ | − | −/± | ± | +/++ |
++, Very strong staining, ie, comparable to staining for cytokeratin; +, strong staining, but weaker than cytokeratin; ±, weak staining in some cells, whereas others were negative; −, no staining detected; Essentially the same results were obtained in the control and experimental samples. If discrepancies were noted, then both results (separated by a slash) were included. STB, syncytiotrophoblast; CTB, cytotrophoblast; SC, stroma cell; EC, endothelial cell; EV, extravillous; 1st, control first trimester; 2nd, control second trimester; 3rd, control third trimester; PE, preeclampsia.
*In 1 of 10 control samples, the EV-CTB showed ++ staining. In 1 of 10 samples obtained from patients with preeclampsia, the EV-CTB showed − staining.
†No − staining was observed in samples obtained at ≤28 weeks gestation. Weak (±) staining was observed in samples obtained at ≥28 weeks gestation.
Enzyme Assay
The XDH/XO enzyme assay was performed as previously described. 11,15 Briefly, total XDH/XO activity was determined by the conversion of [14C] xanthine to [14C] uric acid. The reaction mixture contained NAD (500 μmol/L), [14C] xanthine (34 μmol/L), and sample in a total volume of 175 μl. To eliminate inhibition by accumulation of NADH, lactate dehydrogenase (5 U/ml) and pyruvate (5 mmol/L) were added. In this system, NADH is continuously converted to NAD. The proportions of enzyme in the different isoforms were evaluated by comparing [14C] uric acid production in the presence (XDH/XO) and absence (XO) of NAD. The mixture was incubated for 90 minutes, and the reaction was terminated by adding 25 μl of 40% trichloroacetic acid and centrifuging for 7 minutes at 16,000 × g. The reaction was linear for at least 120 minutes. The supernatant (175 μl) was applied to a 1-ml Dowex 50W-X8-H column and eluted with 0.1 mol/L HCl. All results were adjusted to the same protein concentration, determined with the Bradford protein assay. 16 Enzyme activity was expressed as picomoles of uric acid production per milligram of protein per minute. [14C] Xanthine was completely adsorbed (>99.9%) when applied to the Dowex column. Adding allopurinol (100 μmol/L) completely inhibited enzyme activity.
Statistical Analysis
Data were presented as mean ± SEM. Nonparametric analysis (Mann Whitney U test) was used to compare groups. A P value of less than 0.05 was considered significant.
Results
The Effects of Preeclampsia on XDH/XO Expression by Cells of Floating and Anchoring Placental Villi
We used an antibody that specifically reacted with XDH/XO (anti-XO) to localize expression of this holoenzyme in tissue sections taken from the major compartments of the human placenta. These findings are summarized in Table 3, together with the results of additional immunolocalization experiments described below. First, we examined XO expression by cells found in the two populations of chorionic villi: floating villi, which float in maternal blood, and anchoring villi, which anchor the placenta to the uterine wall. Both villus structures have a trophoblast covering that surrounds a stromal core, which is composed primarily of fibroblasts and fetal vessels. No staining of control samples with anti-XO was detected until the third trimester, when relatively weak immunoreactivity was found in association with syncytiotrophoblasts (Figure 1, B and D) ▶ . In addition, weak staining of the other cell types resident in the villi was usually observed. When the pregnancy was complicated by preeclampsia, antibody reactivity dramatically increased. In addition to strong staining of the trophoblast layers, blood vessels of the stromal cores were clearly demarcated, a particularly striking feature of this staining pattern (Figure 1F) ▶ .
Figure 1.
XDH/XO expression is increased in floating and anchoring villi from placentas of patients with preeclampsia. A–D: Tissue sections obtained from control placentas at the gestational ages indicated in weeks (wk). E and F: Tissue sections obtained from the placenta of a patient diagnosed at 32 weeks of gestation with preeclampsia (PE). Panels show staining with anti-cytokeratin (CK; A, C, and E) and anti-XO (B, D, and F). In general, control first- (data not shown) and second-trimester (B) villi did not express XO. By the third trimester, the cellular components of the villi stained weakly (D), but never as intensely as when the pregnancy was complicated by preeclampsia (F). In the latter situation immunoreactive cells were detected in the villous stromal core, particularly evident in association with blood vessels (arrowheads), and the trophoblast syncytium. Essentially the same results were obtained in analyses of 10 of 10 control samples and 10 of 10 samples obtained from women with preeclampsia.
Second, we examined XDH/XO expression by invasive cytotrophoblasts that emanate from anchoring villi and ultimately reside within the uterine wall. In this location no staining of either the fetal or the maternal cells was observed in control samples (Figure 2, B, D, and F) ▶ . In sharp contrast, when the pregnancy was complicated by preeclampsia, invasive cytotrophoblasts were strongly immunoreactive with anti-XO (Figure 2H) ▶ .
Figure 2.

XDH/XO expression by invasive cytotrophoblasts within the uterine wall is increased when the pregnancy is complicated by preeclampsia. A–F: Tissue sections of basal plate or placental bed biopsies obtained from control patients at the gestational ages indicated in weeks (wk). G and H: Tissue section of a placental bed biopsy obtained from a patient diagnosed at 26 weeks of gestation with preeclampsia (PE). Panels show staining with anti-cytokeratin (CK; A, C, E, and G) and anti-XO (B, D, F, and H). In control samples, no XO expression was detected in association with the subpopulation of cytotrophoblasts that invaded the uterine wall (B, D, and F). In sharp contrast, the invasive cytotrophoblast subpopulation stained intensely with anti-XO when the pregnancy was complicated by preeclampsia (H). Essentially the same results were obtained in analysis of 9 of 10 control samples and 9 of 10 samples obtained from women with preeclampsia.
XDH/XO Enzyme Activity
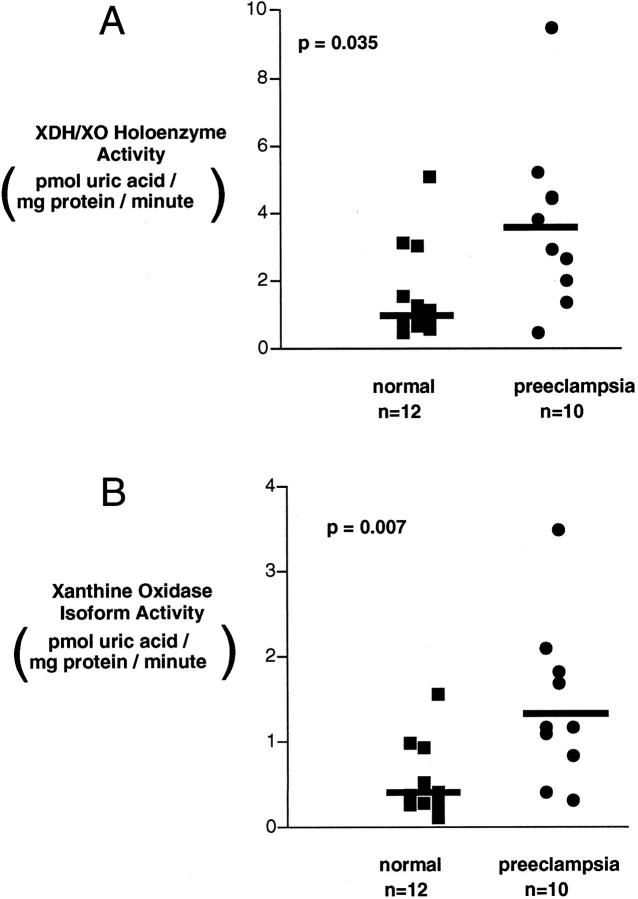
We compared XDH/XO holoenzyme and specific XO enzyme activity in placental villi and placental bed curettings from preeclamptic and control pregnancies. No difference in XDH/XO activity was detected between the villous samples from the preeclamptic (n = 16) and normal (n = 17) groups (5.7 ± 1.4 versus 8.5 ± 1.4 pmol/mg protein per minute; P = 0.12). Likewise, XO isoform activity was also not significantly different (3.1 ± 1.0 versus 4.2 ± 0.7 pmol/mg protein per minute; P = 0.14). As shown in Figure 3 ▶ , however, the XDH/XO activity in placental bed curettings was significantly higher in biopsies from 10 preeclamptic samples compared with control samples obtained from 12 control pregnant women (3.6 ± 0.8 versus 1.6 ± 0.4 pmol/mg protein per minute; P < 0.035). XO activity was similarly increased (1.4 ± 0.5 versus 0.5 ± 0.1 pmol/mg protein per minute; P < 0.007; Figure 3 ▶ ). The activity ratio, XO/(XDH + XO), was not different in the two groups (42% ± 5% versus 35% ± 3%; P = 0.35). Although the gestational age of the preeclamptic women sampled (33.0 ± 5 weeks) was significantly less (P < 0.01) than that of controls (39.6 ± 1.2 weeks), there was no evident effect of gestational age on XDH/XO or XO activity in the preeclamptic or control women (P = 0.15; r 2 = 0.1).
Figure 3.
A: XDH/XO activity in placental bed currettings from normal and preeclamptic women. B: XO activity in placental bed currettings from normal and preeclamptic women: The conversion of [14C] xanthine to [14C] uric acid was determined in cytosol prepared from placental site currettings as described in Materials and Methods, in the presence of total XDH/XO (A, NADH) or its absence (B, XO activity). Samples from preeclamptic women manifest greater activity for both total activity (P < 0.04) and XO activity (P < 0.01). All samples were obtained from women delivered by cesarean section without labor. Data are displayed as individual patients (10 preeclamptic and 12 control women). Analysis was by Mann-Whitney U test.
The Effects of Preeclampsia on SOD and Catalase Expression by Cells of Floating and Anchoring Placental Villi
We also investigated which cells among those of the placenta proper or found at the maternal-fetal interface expressed two major intracellular antioxidant enzymes—the cytosolic form of SOD (Cu,Zn-SOD), which inactivates superoxide, and catalase, which inactivates hydrogen peroxide. The results are summarized in Table 3. Immunostaining for SOD was detected exclusively in association with the various trophoblast subpopulations. In first-trimester samples, only cytotrophoblasts of floating villi were stained (data not shown). From the second trimester onward, all of the trophoblast populations expressed SOD to various degrees. By the end of pregnancy, staining of the syncytiotrophoblasts (data not shown) and invasive cytotrophoblasts within the uterine wall was the strongest (Figure 4 ▶ ; cf B, D, and F). When the pregnancy was complicated by preeclampsia, SOD expression by the trophoblast components of floating and anchoring villi was unaffected (data not shown), but invasive cytotrophoblasts reacted only weakly, if at all, with anti-SOD (Figure 4H) ▶ .
Figure 4.
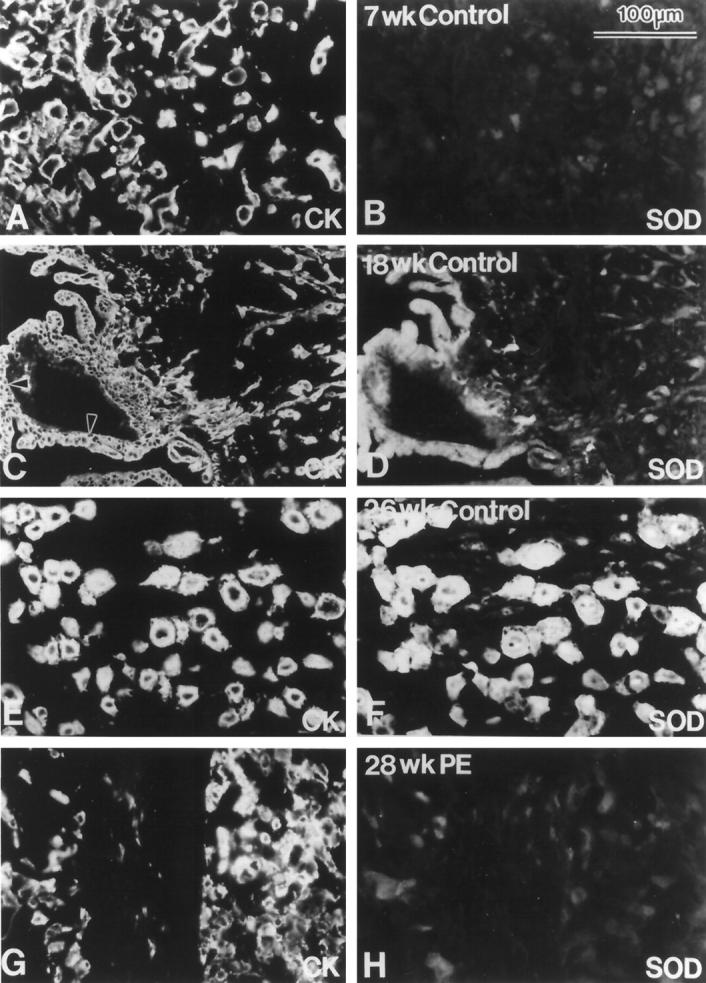
A–F: SOD expression by invasive cytotrophoblasts markedly decreased when the pregnancy was complicated by preeclampsia. Shown are sections of basal plate or placental bed biopsies obtained from control patients at the gestational ages indicated in weeks (wk). G and H: Tissue section of a placental bed biopsy obtained from a patient diagnosed at 28 weeks of gestation with preeclampsia (PE). Panels show staining with anti-cytokeratin (CK; A, C, E, and G) and anti-SOD (B, D, F, and H). Within the placenta and placental bed, SOD expression is confined to the various trophoblast subpopulations. In general, anti-SOD reactivity increased with advancing gestational age (cf. B, D, and F). When the pregnancy was complicated by preeclampsia, this increase was markedly blunted (H). Essentially the same results were obtained in analyses of 10 of 10 control samples and 10 of 10 samples obtained from women with preeclampsia.
We also studied catalase expression in the same tissue compartments (Table 3). Essentially, all of the trophoblast, stromal, and vascular cells of floating villi expressed this enzyme, and the staining pattern did not change when the pregnancy was complicated by preeclampsia (data not shown).
The Effects of Preeclampsia on Nitrotyrosine Generation by Cells of Floating and Anchoring Placental Villi
The placenta is capable of generating NOs, 17 which can in turn react with superoxide to form peroxynitrite anions (ONOO−) that covalently modify proteins by nitration of tyrosine residues. 18 Accordingly, we also used immunolocalization techniques to determine whether the apparent preeclampsia-associated changes in invasive cytotrophoblast XDH/XO staining and XO activity were accompanied by changes in the oxidative status of the tissue. The results are summarized in Table 3. For control floating villi, nitrotyrosine expression was not observed in the first (data not shown) or second trimester of pregnancy (Figure 5B) ▶ . At term, weak staining was detected in association with stromal cells of the villus core (data not shown). For control invasive cytotrophoblasts, no staining was observed until the second trimester, when a few cells reacted weakly with anti-nitrotyrosine (Figure 5D) ▶ . By term, faint immunoreactivity was observed in association with most cytotrophoblasts within the uterine wall (data not shown).
Figure 5.

Trophoblast nitrotyrosine (NT) expression is increased when the pregnancy is complicated by preeclampsia. A and B: Tissue section of floating villi obtained from the placenta of a control patient at 26 weeks of gestation. C and D: Tissue section of a placental bed biopsy obtained from the same control patient. E and F: Tissue section of floating villi obtained from the placenta of a patient diagnosed at 26 weeks of gestation with preeclampsia (PE). G and H: Tissue section of a placental bed biopsy obtained from the same preeclampsia patient. Panels show staining with anti-cytokeratin (CK; A, C, E, and G) and anti-NT (B, D, F, and H). In general, NT expression was not detected in either the first (data not shown) or the second trimester (B and D) of pregnancy. By the third trimester, the stromal components of the villous cores and invasive cytotrophoblasts stained weakly (data not shown). When the pregnancy was complicated by preeclampsia, particularly intense immunoreactivity was detected in association with the fetal blood vessels of floating villi (compare arrowheads in B and F) and in association with invasive cytotrophoblasts (H). Essentially the same results were obtained in analyses of 10 of 10 control samples and 4 of 4 samples obtained from patients with preeclampsia.
When the pregnancy was complicated by preeclampsia, several changes in nitrotyrosine expression were noted. For floating villi, the fetal blood vessels found within the villus cores stained very intensely (Figure 5F) ▶ . Likewise, the staining intensity of invasive cytotrophoblasts also increased (Figure 5H) ▶ . The latter two aspects of the expression pattern resembled the effects of preeclampsia on staining to detect XDH/XO expression.
Discussion
Oxidative stress has been proposed as an important component of the pathophysiology of preeclampsia. It is posited that reactive oxygen species alter endothelial cell function. 3,6 In support of this proposal, lipid peroxidation products are increased in the blood and tissues of preeclamptic women. 3,6,7 XO, a component of the XO holoenzyme XDH/XO, produces superoxide and hydrogen peroxide and is a mediator of posthypoxic-reperfusion injury. 19 Abundant evidence indicates reduced placental perfusion in preeclampsia. 1 Implantation is superficial in preeclampsia. In particular, cytotrophoblasts fail to invade the spiral arterioles. As a result, these vessels do not enlarge, severely compromising their ability to deliver maternal blood to the intervillous space. Predisposing medical conditions (such as hypertension, diabetes, and collagen vascular disease) share the common pathophysiological feature of microvascular involvement, whereas obstetrical predisposition includes disorders with excessively large placentas that are at risk for relative hypoperfusion. 1 Variations in intervillous blood flow with alterations in oxygenation may occur during preeclampsia. Therefore, we considered increased XO as a candidate source for oxidative stress from the poorly perfused placentas of preeclamptic women. It is interesting that the other product of xanthine breakdown by XO is uric acid, known to be consistently increased in the circulation of preeclamptic women and also known to possess antioxidant properties. 20
Our data demonstrate XDH/XO immunostaining in syncytiotrophoblasts of normal pregnant women. We previously reported that XDH/XO enzyme activity and messenger RNA (mRNA) are present in the placenta (although at low levels), 11 contradicting older findings. 10 We now find that in normal pregnancy much of this activity is localized to the syncytiotrophoblasts. Although immunostaining intensity was greater in syncytiotrophoblasts from preeclamptic, compared with normally pregnant, women, XDH/XO enzyme activity levels were not different. There was markedly greater holoenzyme immunostaining in the invasive cytotrophoblasts from preeclamptic women as compared with controls. Additionally, placental site curettings from preeclamptic women (which contain this cytotrophoblast population) had increased holoenzyme and increased XO isoform activity as compared with samples from normal women. We did not demonstrate an increased proportion of the enzyme present as XO (ratio change), which has been reported in response to hypoxia and cytokines in other systems. Although mean XO/(XDH + XO) activity ratios were greater in samples from women with preeclampsia, the differences did not achieve statistical significance perhaps due to lack of power with a small number of samples. Increased XO activity was associated with an increase in staining for nitrotyrosine associated with these cells, suggesting in vivo interaction of superoxide and NO. We have no data regarding the dynamic range of activity levels that give rise to immunofluorescence signals in the tissue localization studies. However, it should be noted that the two assays point to the same conclusion, namely, that XO expression/activity, which is normally very low, is substantially elevated at the maternal-fetal interface in preeclampsia.
We sought to determine whether the increase in superoxide indicated by these findings was associated with modifications of degradatory enzymes for superoxide. SOD converts superoxide to hydrogen peroxide, whereas catalase converts the product of that reaction, hydrogen peroxide, to water and molecular oxygen. We found no difference in catalase immunostaining within the placenta. CuZn-SOD immunostaining, however, was reduced in trophoblast cells from preeclamptic women. This is consistent with previous data measuring placental and erythrocyte CuZn-SOD activity in these patients. 21-25
We note that there are several other potential enzymatic sources of superoxide in addition to XO and other isoforms of SOD (manganese SOD and extracellular SOD). These have different tissue and cellular localizations that could potentially explain our overall findings. Nevertheless, a scenario in which shallow implantation results in reduced oxygen delivery with subsequent down-regulation of SOD and up-regulation of XDH/XO would be compatible with our findings. In other settings, hypoxia is a potent stimulus that increases XDH/XO expression. Alternatively, the effect of hypoxia might not be direct, but mediated by cytokines such as tumor necrosis factor-α (TNF-α). TNF-α is known to increase XDH/XO 26 and is increased in conditioned media from cytotrophoblast and villous explants cultured under hypoxic conditions in vitro. 27 There is also a report of increased TNF-α production by preeclamptic placentas. 28 If hypoxia is a stimulus, then it is perhaps not surprising that invasive cytotrophoblast, with oxygenation determined by nonanastamosing basal arteries and diffusion through several cell layers of decidua, would be affected whereas villous cytotrophoblast exposed to extensively comingled intervillous blood flow would not. Our findings of changes in the basal plate/placental bed suggest that oxidative stress is more pronounced at sites where maternal and fetal tissues intermingle, as distinct from changes in purely fetal tissue (the villous placenta). Studies of villous explants and isolated cytotrophoblast maintained under standard and hypoxic culture conditions should prove useful in determining the influence of hypoxia on XDH/XO and SOD expression.
Under appropriate stoichiometric conditions, NO will interact with superoxide to form the reactive oxygen species peroxynitrite. 18 Peroxynitrite causes nitration of tyrosine residues on proteins to form nitrotyrosine. 29 This has been used as a marker of in vivo activity of peroxynitrite and, for example, is found in the intima of vessels with atherosclerotic lesions. 30 Myatt and coworkers have reported increased nitrotyrosine immunostaining, particularly in villous vascular endothelium, in preeclampsia. 31 It is known that trophoblast can produce NO. 32 Thus, the finding of nitrotyrosine residues in the invasive cytotrophoblast may reflect increased production of superoxide in this setting. Recently, however, pathways independent of superoxide have been reported for the nitration of tyrosine, such as interaction of nitrogen dioxide (NO2) with tyrosyl radicals generated by myeloperoxidase during oxidative stress. 33 Thus, nitrotyrosine detection more broadly provides evidence for damage by reactive nitrogen species and, although explainable by the interaction of superoxide with NO as the pathway for formation, does not necessarily imply an increase in this interaction. There are obvious limitations of immunostaining as a quantitative technique. However, the results we obtained for XDH/XO and SOD in invasive cytotrophoblasts and for nitrotyrosine in fetal blood vessels within the villous core fall into the category of dramatic differences that were largely “all or none” and devoid of nonspecific staining.
The relationship of available concentrations of superoxide and NO with SOD is critical to determining whether NO will out-compete SOD for superoxide and thus form peroxynitrite. 18 In this regard, we did not find evidence of CuZn-SOD immunostaining in fetal vessels. Our data are also compatible with altered stoichiometry of superoxide, NO, and SOD in fetal placental vessels compared with vessels in similar sites from normal infants. There are reports that the NO metabolites, nitrite and nitrate, are increased in the cord blood of infants of preeclamptic women. 34 In addition, we reported that exposure of endothelial cells to plasma from cord blood of infants of preeclamptic mothers increases NO production. 35
These data provide overall support for a local increase in reactive oxygen species generation in the placental beds of preeclamptic women. Whether this could lead to oxidative stress systemically is uncertain. The activity of XO in placental site curettings was greater, but the activity of XO in villous trophoblast was similar in samples from both preeclamptic and control women. The activity of the enzyme was, in general, greater in villous trophoblast than in placental site curettings, and there is much more villous than invasive trophoblast tissue. However, despite similar XO activity of villous trophoblast, the fetal vessels inside the villi of preeclampsia placentas manifest increased nitrotyrosine labeling, which might arise from increased exposure to superoxide. Could there be systemic effects from the elevated placental XO in the invasive cytotrophoblast of preeclamptic women? It is possible that the relatively small amount of enzyme in invasive cytotrophoblast could result in greater production of superoxide (and peroxynitrite) than in the villous trophoblast, if the stoichiometry of NO, superoxide, and SOD was appropriate. It could also be possible that, in the decidual environment, a relative paucity of antioxidants or presence of other seeding-reactive oxygen species might favor propagation of oxidative damage. Further support for linkage between XO, superoxide production, and oxidative damage will ultimately require measurement of control and preeclamptic placental tissue superoxide production and the impact of inhibitors of XO. In this regard, it is noteworthy that a recent reappraisal of XO in human tissues has suggested that both XDH and XO isoforms can generate reactive oxygen species during posthypoxic reperfusion. 36
In summary, we have demonstrated increased activity of XO, a free-radical–generating enzyme, in invasive cytotrophoblast from preeclamptic women. This activity is accompanied by a reduction of SOD expression and increased nitrotyrosine labeling, suggestive of local peroxynitrite generation. The mechanisms explaining the XO increase, and the implications of this local increase to generalized oxidative stress remain to be determined.
Acknowledgments
We thank Dr. S. A. Friedman, University of Tennessee Medical Center, for providing the placental bed specimens. We thank the Clinical Data Core and nursing staffs for their assistance, which made this study possible.
Footnotes
Address reprint requests to James M. Roberts, M.D., Magee-Women’s Research Institute, 204 Craft Avenue, Pittsburgh, PA 15213. E-mail: jimrob+@pitt.edu.
Supported by National Institutes of Health grant PO1 HD30367 and the Irene McClenahan Young Investigator Award Fund of Magee Women’s Health Foundation.
References
- 1.Friedman SA, Taylor RN, Roberts JM: Pathophysiology of preeclampsia. Clin Perinatol 1991, 18:661-682 [PubMed] [Google Scholar]
- 2.Roberts JM, Redman CWG: Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 1993, 341:1447-1451 [DOI] [PubMed] [Google Scholar]
- 3.Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rodgers GM, McLaughlin MK: Lipid peroxidation in pregnancy: new perspectives on preeclampsia. Am J Obstet Gynecol 1989, 161:1025-1034 [DOI] [PubMed] [Google Scholar]
- 4.Parthasarathy S, Santanam N: Mechanisms of oxidation, antioxidants, and atherosclerosis. Curr Opin Lipidol 1994, 5:371-375 [DOI] [PubMed] [Google Scholar]
- 5.Nakao-Hayashi J, Ito H, Kawashima S: An oxidative mechanism is involved in high glucose-induced serum protein modification causing inhibition of endothelial cell proliferation. Atherosclerosis 1992, 97:89-95 [DOI] [PubMed] [Google Scholar]
- 6.Walsh SC: Lipid peroxidation in pregnancy. Hypertens Pregnancy 1994, 13:1-25 [Google Scholar]
- 7.Hubel CA: Dyslipidemia, iron, and oxidative stress in preeclampsia: assessment of maternal and feto-placental interactions. Semin Perinatol 1998, 16:75-92 [DOI] [PubMed] [Google Scholar]
- 8.Davidge ST, Hubel CA, Brayden RD, Capeless EC, McLaughlin MK: Sera antioxidant activity in uncomplicated and preeclamptic pregnancies. Obstet Gynecol 1992, 79:897-901 [PubMed] [Google Scholar]
- 9.Terada LS, Leff JA, Repine JE: Measurement of Xanthine Oxidase in Biological Tissues. 1990. Academic Press, New York [DOI] [PubMed]
- 10.Hayashi T, Baldridge RC, Olmsted PS, Kimmel DL: Purine nucleotide catabolism in placenta. Am J Obstet Gynecol 1964, 88:470-478 [DOI] [PubMed] [Google Scholar]
- 11.Many A, Westerhausen-Larson A, Kanbour-Shakir A, Roberts JM: Xanthine oxidase/dehydrogenase is present in human placenta. Placenta 1996, 17:361-365 [DOI] [PubMed] [Google Scholar]
- 12.Chesley LC: Diagnosis of preeclampsia. Obstet Gynecol 1985, 65:423-425 [PubMed] [Google Scholar]
- 13.Khong TY, De Wolf F, Robertson WB, Brosens I: Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986, 93:1049-1059 [DOI] [PubMed] [Google Scholar]
- 14.Damsky CH, Fitzgerald ML, Fisher SJ: Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 1992, 89:210-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty TM: A sensitive assay for xanthine oxidase using commercially available [14C] xanthine. Anal Biochem 1976, 74:604-608989261 [Google Scholar]
- 16.Bradford M: A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 17.Conrad KP, Vill M, McGuire PG, Dail WG, Davis AK: Expression of nitric oxide synthase by syncytiotrophoblast in human placental villi. FASEB J 1993, 7:1269-1276 [DOI] [PubMed] [Google Scholar]
- 18.Beckman JS, Chen J, Ischiropoulos H, Crow JP: Oxidative chemistry of peroxynitrite. 1994. Academic Press, New York [DOI] [PubMed]
- 19.Parks DA, Williams TK, Beckman JS: Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol 1988, 254:G768-G774 [DOI] [PubMed] [Google Scholar]
- 20.Many A, Hubel CA, Roberts JM: Hyperuricemia and xanthine oxidase in preeclampsia revisited. Am J Obstet Gynecol 1996, 174:288-291 [DOI] [PubMed] [Google Scholar]
- 21.Poranen AK, Ekblad U, Uotila P, Ahotupa M: Lipid peroxidation and antioxidants in normal and pre-eclamptic pregnancies. Placenta 1996, 17:401-405 [DOI] [PubMed] [Google Scholar]
- 22.Pandey S, Gujrati VR, Chandravati, Sanger KCS, Shanker K: Status of human placental lipid peroxidation, superoxide dismutase and catalase during pregnancy-induced hypertension (PIH). Asia Pacific J Pharmacol 1995, 10:41–44
- 23.Wang Y, Walsh SW: Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Invest 1996, 3:179-184 [PubMed] [Google Scholar]
- 24.Wisdom SJ, Wilson R, McKillop JH, Walker JJ: Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obstet Gynecol 1991, 165:1701-1704 [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Wilson R, Boyd P, McKillip J, Leitch C, Walker JJ, Burdon R: Normal superoxide dismutase (SOD) gene in pregnancy-induced hypertension: is the decreased SOD activity a secondary phenomenon? Free Radic Res 1994, 21:59-66 [DOI] [PubMed] [Google Scholar]
- 26.Faggioni R, Gatti S, Demitri MT, Delgado R, Echtenache R, Gnocchi P, Heremans H, Ghezzi P: Role of xanthine oxidase and reactive oxygen intermediates in LPS and TNF induced pulmonary edema. J Lab Clin Med 1994, 123:394-399 [PubMed] [Google Scholar]
- 27.Benyo DF, Miles TM, Conrad KP: Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab 1997, 82:1582-1588 [DOI] [PubMed] [Google Scholar]
- 28.Wang YP, Walsh SW: TNF α concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol 1996, 32:157-169 [DOI] [PubMed] [Google Scholar]
- 29.Ohshima H, Friesen M, Brouet I, HB, Bartsch H: Nitrotyrosine as a new marker for endogenous nitrosation and nitration of proteins. Food Chem Toxicol 1990, 28:647–652 [DOI] [PubMed]
- 30.Beckmann JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MA, White CR: Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler 1994, 375:81–88 [DOI] [PubMed]
- 31.Myatt L, Rosenfield R, Eis A, Brockman D, Greer I, Lyall F: Nitrotyrosine residues in placenta: evidence of peroxynitrite formation and action. Hypertension 1996, 28:488-493 [DOI] [PubMed] [Google Scholar]
- 32.Eis AL, Brockman DE, Pollock JS, Myatt L: Immunohistochemical localization of endothelial nitric oxide synthase in human villous and extravillous trophoblast populations and expression during syncytiotrophoblast formation in vitro. Placenta 1995, 16:113-126 [DOI] [PubMed] [Google Scholar]
- 33.Halliwell B: What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett 1997, 411:157–160 [DOI] [PubMed]
- 34.Lyall F, Young A, Greer IA: Nitric oxide concentrations are increased in the fetoplacental circulation in preeclampsia. Am J Obstet Gynecol 1995, 173:714-718 [DOI] [PubMed] [Google Scholar]
- 35.Davidge S, Signorella A, Lykins D, Gilmour C, Roberts J: Evidence of endothelial activation and endothelial activators in cord blood of infants of preeclamptic women. Am J Obstet Gynecol 1996, 175:1301-1306 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Blake DR, Stevens CR, Kanczler JM, Winyard PG, Symons MCR, Benboubetra M, Harrison R: A reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of NADH as an electron donor. Free Radic Res 1998, 28:151-164 [DOI] [PubMed] [Google Scholar]