Abstract
We investigated the growth fraction and cell loss fraction in a large group of patients with Cushing’s disease subdivided according to tumor size. Fifty-one patients, 8 males and 43 females, aged 12 through 61 years (mean age 34.6 ± 1.5 years), were studied. Thirty-six patients had a microadenoma and the remaining 15 a macroadenoma. Immunohistochemical analysis was performed on paraffin-embedded material using a monoclonal antibody (MIB-1) directed against a proliferation-associated nuclear antigen, Ki-67, to measure the growth fraction. Apoptosis was assessed by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling method, using a monoclonal antibody recognizing areas of DNA fragmentation. Ki-67 labeling index and apoptosis were counted on separate slides in at least 1000 evaluable cells. Patients with a macroadenoma had a significantly higher value of Ki-67 index (9.3 ± 2.7%) than patients with microadenoma (2.8 ± 0.5%; P < 0.002), whereas the apoptotic index was not significantly different in the two groups (1.7 ± 0.8% in macroadenomas versus 0.8 ± 0.3% in microadenomas). Our study shows that ACTH-secreting macroadenomas are characterized by a higher cell growth fraction than microadenomas, whereas the cell loss fraction is not different. A high proliferation rate seems to play a major role in determining the progression from small to large pituitary tumors in Cushing’s disease.
Cushing’s disease is a rare but severe disease whose first clinical manifestations usually begin about 5 years before establishment of diagnosis. 1 Despite the delay between onset of symptoms and diagnosis, the majority of patients with Cushing’s disease have microadenomas, defined as maximum tumor diameter ≤10 mm, whereas the frequency of corticotroph macroadenomas is estimated to be 6 to 20% in large surgical series, 1-5 a value lower than that typical of other types of secreting adenomas. 6-7 Only a few studies have investigated whether corticotroph macroadenomas differ in their clinical and biochemical features from the more frequent microadenomas. 5,8
The reason for the low frequency of adrenocorticotropin (ACTH)-secreting macroadenomas has not yet been elucidated. It is likely that chronic glucocorticoid excess may play a role, as suggested by the development of aggressive tumors (Nelson’s syndrome) in about 20% of patients with Cushing’s disease treated by bilateral adrenalectomy. 9 Theoretically, the rate of growth of pituitary tumors depends on the doubling time of the adenomatous cells. Cell doubling time is slowed by many factors, among which the most important are the death of some tumor cells by apoptosis (programmed cell death), ischemic, or hemorrhagic events, and the presence of a pool of quiescent cells that do not enter the mitotic cycle. 10 Assessment of the growth fraction can be accomplished by determining the percentage of cells expressing the Ki-67 antigen, a nuclear protein of unknown function expressed only in cycling cells. 11 Apoptosis is an active process requiring protein synthesis and specific endonucleolytic digestion of cellular DNA. 12 The DNA fragmentation that ensues is one hallmark of apoptotic cell death.
Despite the interplay of both processes determines the type of neoplastic growth, only one study investigated the proliferation index of ACTH-secreting adenomas subdivided into micro- and macroadenomas, 5 whereas the relationship between tumor size and apoptosis has never been addressed previously. Only one study 13 reported the percentage of apoptotic cells in a heterogeneous group of pituitary adenomas, including 16 ACTH-secreting tumors. Therefore, we measured both the proliferation and apoptotic indices in a large series of ACTH-secreting adenomas subdivided according to tumor size.
Materials and Methods
Patients
One hundred twelve patients operated on for Cushing’s disease between January 1990 and June 1997 were eligible for the study. Diagnosis of Cushing’s disease was based on standard clinical and biochemical findings. 1 Criteria of exclusion from the study were previous pituitary surgery (11 patients), lack of pituitary adenoma at histological analysis (26 patients), and tumor size <4 mm (24 patients). The last exclusion criterion was selected because measurement of both Ki-67 antigen and apoptosis in very small tumor specimens may not be always reliable. Altogether, 51 patients, 43 female and 8 male, were included in the study. The mean age of the patients was 34.6 ± 1.5 years (range, 12–61 years). The estimated mean duration of disease was 4.6 ± 0.4 years. Maximum tumor diameter was measured on preoperative magnetic resonance imaging (MRI) or, in the smallest tumors, directly assessed by the surgeon at operation. Patients were subdivided according to tumor size in two groups: patients with microadenoma (maximum tumor diameter ≤10 mm; group A, 36 patients) and patients with macroadenoma (maximum tumor diameter >10 mm; group B, 15 patients). Eighteen patients (35%; 12 in group A, 6 in group B) had received treatment with ketoconazole before surgery; the drug was usually stopped at least 10 to 14 days before operation. In all but three patients, free urinary cortisol level was above the normal range immediately before surgery; their exclusion from analyses that included previous treatment with ketoconazole did not modify significantly any result. Successful outcome of surgery was defined according to the following criteria: clinical remission of the disease accompanied by postoperative hypocortisolism or, in the case of normal serum and urinary cortisol levels, demonstration of normal suppression of steroid levels during low-dose administration of dexamethasone. If hypercortisolism recurred within 6 months after surgery, patients were considered as treatment failures. 1
Hormone Assays
Blood samples for hormone measurements were obtained in the morning after an overnight fast from hospitalized patients. The specimens for cortisol measurement were drawn into plastic tubes and allowed to clot at room temperature before being centrifuged at 2500 rpm for 10 minutes. The samples for ACTH measurement were collected into iced plastic tubes containing sodium EDTA plus Trasylol. The tubes were then centrifuged at 4°C for 10 minutes at 2500 rpm and the plasma was transferred into polypropylene tubes and stored at −20°C until assay.
Plasma ACTH was assayed in duplicate by a commercially available solid phase two-site immunoradiometric assay (CIS Diagnostici S.p.A., Tronzano Vercellese, Italy). Serum cortisol was assayed in duplicate by a commercially available immunoradiometric assay (Biorad S.p.A., Milano, Italy). Normal ranges for ACTH and cortisol were 10 to 80 ng/L and 50 to 250 μg/L, respectively.
Histological Analysis
Surgically removed specimens were fixed immediately in 10% buffered formalin and subsequently embedded in paraffin. Standard hematoxylin-eosin sections were used for diagnosis; standard immunocytochemistry using commercially available antisera was used to characterize neoplastic areas for the presence of prolactin (PRL; Immunotech S.A., Marseille, France), growth hormone (GH; Biomeda, Foster City, CA), ACTH (Signet, Dedham, MA), thyrotropin (Immunotech), luteinizing hormone (Immunotech), and follicle stimulating hormone (Immunotech). Analysis of MIB-1 immunostaining and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL; see below) was conducted in slides adjacent to those where immunocytochemistry was performed to avoid inclusion of normal peritumoral tissue.
MIB-1 Immunostaining
Proliferation activity of the tumor was determined by calculating the percentage of cells expressing the Ki-67 antigen. To this aim we used the monoclonal antibody MIB-1, which can be also used in formalin-fixed material. MIB-1 immunostaining was performed using the avidin-biotin-peroxidase complex method 14 on 4-μm sections mounted onto glass slides treated with poly-L-lysine and dried until ready for use. Sections of human cecal appendix were used as positive controls. Tissue sections were dewaxed and endogenous peroxidase activity was blocked. 15 Then, after antigen retrieval by microwave treatment (two passages at 850 W of 5 minutes each), sections were incubated with nonimmune rabbit serum at room temperature for 20 minutes to block nonspecific links and then incubated at 37°C for 60 minutes with MIB-1 monoclonal antibody diluted 1:50 (Immunotech); no MIB-1 antibody was added on sections used as negative controls. After rinsing, slides were incubated with rabbit biotinylated anti-mouse IgG diluted 1:100 as secondary antibody for 30 minutes at room temperature and then exposed to streptavidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA) for 30 minutes. After rinsing, sections were exposed in the dark to the chromogen 3,3′-diaminobenzidine for 5 minutes, that stains Ki-67 immunopositive nuclei with a dark brown color (Figure 1) ▶ . Slides were lightly counterstained with hematoxylin, dehydrated, cleared, and mounted. Because MIB-1 positive cells may be unevenly distributed in biopsy samples, the Ki-67 antigen labeling index (Ki-67 LI) was determined by counting a total of at least 1000 neoplastic nuclei subdivided in 10 fields chosen randomly at 400× magnification.
Figure 1.
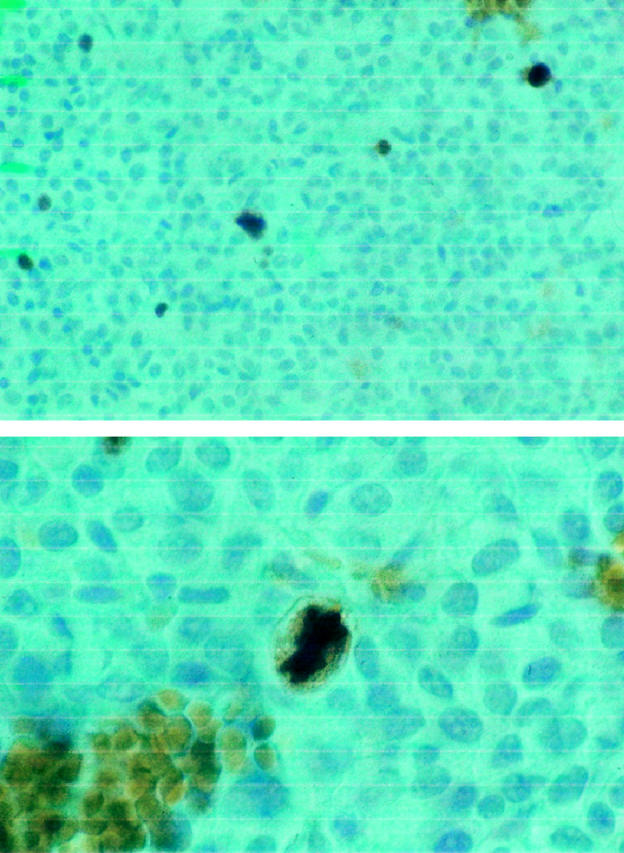
The upper panel represents anti-MIB-1 antibody-hematoxylin staining of an ACTH-secreting macroadenoma. Proliferating cells expressing the Ki-67 antigen are recognized by the intense brown coloration; original magnification, ×250. The lower panel shows a cell in mitosis from the same tumor at higher magnification (×400). The strong reaction to anti-MIB-1 is evident both at the nuclear and cytoplasmic level, whereas cells in the G1, S, or G2 phase of the mitotic cycle present a dark coloration at the nuclear level only.
Determination of Apoptosis
The method of TUNEL, originally described by Gavrieli and coworkers, 16 was used after minor modifications. Briefly, tissue sections of 4 μm were mounted onto glass slides treated with poly-L-lysine, deparaffinized, hydrated, and treated for 10 minutes at 37°C with proteinase-K (Boehringer Mannheim, Mannheim, Germany; 20 μg/ml 10 mmol/L Tris-HCl buffer, pH 7.4). Slides were rinsed twice with PBS. Then, 50 μl of TUNEL reaction mixture (450 μl nucleotide mixture containing fluoresceinated dUTP in reaction buffer plus 50 μl enzyme TdT from calf thymus) were added to samples. To ensure homogeneous spread of TUNEL reaction mixture on tissue sections and to avoid evaporative loss, slides were covered with coverslips during incubation. Slides were incubated in a humidified chamber for 60 minutes at 37°C. After rinsing, slides were incubated with anti-fluorescein antibody, Fab fragment from sheep, conjugated to alkaline phosphatase for 30 minutes at room temperature (Boehringer Mannheim). Then, on each slide, 50 μl substrate solution (blue tetrazolium) were added and slides were incubated for 10 minutes at room temperature. Positive signal was defined as the presence of a distinct blue staining on nuclei of the neoplastic cells or on apoptotic bodies as morphologically defined. The apoptotic index was determined by counting a total of at least 1000 neoplastic nuclei subdivided in 10 fields chosen randomly at 400× magnification. Apoptotic cells were identified by TUNEL in conjunction with characteristic morphological changes, such as cell shrinkage, membrane blebbing, and chromatin condensation, to distinguish apoptotic cells and apoptotic bodies from necrotic cells, which were not considered as apoptotic cells. Sections of human cecal appendix were used as positive controls.
All Ki-67 LI and apoptotic index determinations were performed by a single pathologist (F. M.) who was unaware of the clinical characteristics of the patients.
Statistical Analysis
All data are expressed as the mean ± SE. Because ACTH, Ki-67 LI, and apoptotic index showed a markedly skewed distribution, they were transformed logarithmically before statistical analysis; a value of 0% was arbitrarily assigned to be 0.05%. However, in the text and figures, ACTH, Ki-67 LI, and apoptosis index are given in the usual decimal format. Student’s t-test for unpaired data was used to compare continuous variables among groups. Correlation coefficients between continuous variables were calculated by the method of least squares. χ 2 tabulation was used to compare binomial proportions. Multivariate regression was performed to evaluate the independent effect of the proliferation index on the success rate of surgery. A P value of <0.05 was considered to indicate statistical significance. All calculations were performed using the statistical package StatView 4.0 (Abacus Concepts Inc., Berkeley, CA).
Results
Clinical Characteristics of the Patients
Thirty-six patients had a microadenoma (group A; mean tumor diameter 5.9 ± 0.2 mm; range, 4–9 mm), whereas the remaining 15 patients had a macroadenoma (group B; mean tumor diameter 19.2 ± 1.2; range, 13–36 mm). No patient in group A had MRI evidence of sinus cavernous involvement, whereas five patients in group B had involvement of one cavernous sinus by the tumor. Two additional patients in group B also showed tumor extension into the sphenoid sinus, whereas the remaining eight patients had an intrasellar or suprasellar extending macroadenoma. The clinical and hormonal characteristics of the two groups are detailed in Table 1 ▶ . No difference of age, sex distribution, or estimated duration of disease was observed. Mean plasma ACTH level was significantly higher (P < 0.01) in group B compared to group A patients and showed a highly significant correlation with the maximum tumor diameter (r = 0.67; P < 0.0001). Twelve patients in group A (33.3%) and 9 patients (60.0%) in group B had basal ACTH above the normal range. The difference of mean cortisol level was not significant, even though the P value was very close to the significance level (P = 0.06). Overall, 45 patients, 41 with postoperative hypocortisolism and 4 with normal serum and urinary cortisol levels, were considered in remission after operation. Outcome of surgery was significantly better in group A (36 of 36 patients in remission; success rate 100%) than in group B (10 out of 15 patients in remission; success rate 66.7%; P < 0.01). The mean follow-up in this group of patients was 26.5 ± 2.9 months, ranging from 2 to 71 months. Recurrence of Cushing’s disease in this period occurred in three patients, two in group A and one in group B, after 32, 55, and 71 months, respectively.
Table 1.
Clinical Characteristics of 51 Patients with Cushing’s Disease According to Tumor Size
| Tumor type | Age (years) | Female sex | Estimated duration of disease (years) | Plasma ACTH (ng/L) | Serum cortisol (μg/L) |
|---|---|---|---|---|---|
| Microadenomas (n = 36) | 34.9 ± 1.9 | 32 /36 (88.9%) | 4.8 ± 0.6 | 63.7 ± 4.8 | 207.6 ± 11.0 |
| Macroadenomas (n = 15) | 34.1 ± 2.6 | 11 /15 (73.3%) | 4.1 ± 0.7 | 185.0 ± 60.1* | 251.5 ± 22.1† |
*P < 0.01 by unpaired Student’s t-test vs. microadenomas.
†P = 0.06 by unpaired Student’s t-test vs. microadenomas.
Ki-67 LI and Apoptotic Index
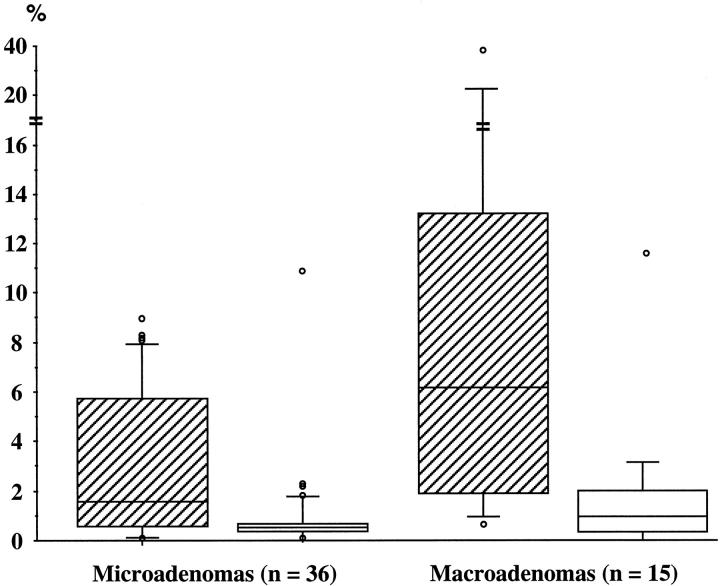
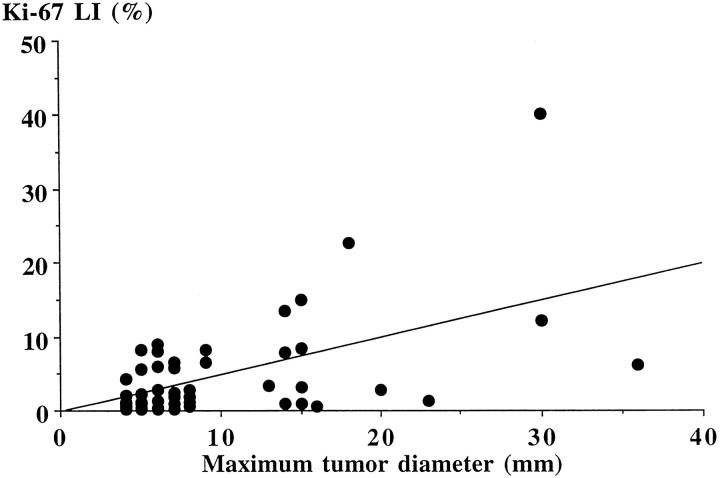
The mean Ki-67 LI in all patients was 4.7 ± 1.0%, ranging from 0.1 to 40%. The highest value of 40% was recorded in a 34-year-old male patient with an invasive tumor measuring >30 mm in maximum diameter. Although such a high value is often detected in malignant tumors, our patient did not have evidence of any metastatic lesion and experienced postoperative hypoadrenalism after apparent total removal of the tumor. Ki-67 LI was not correlated with age at operation, estimated duration of disease, female sex, previous treatment with ketoconazole, plasma ACTH, or serum cortisol levels. Group B patients had a significantly higher Ki-67 LI (9.3 ± 2.7%; range, 0.6–40.0%) than group A patients (2.8 ± 0.5%; range, 0.1–9.0%; t = 3.38; P < 0.002; Figure 2 ▶ ). Moreover, the Ki-67 LI was significantly correlated with maximum tumor diameter in the whole study group (r = 0.53; P < 0.01; Figure 3 ▶ ).
Figure 2.
Box graphs and whisker plots representing the distribution of Ki-67 LI (hatched boxes) and apoptotic index (open boxes) in 51 patients with histology-proven Cushing’s disease grouped into micro- and macroadenomas according to maximum tumor diameter. The box contains values between the upper and lower quartiles and shows the interquartile range (IQR). The line cutting the box is the median. The lines or whiskers extend from the ends of the box to either the largest values that are within the range upper quartile +1.5 × IQR or to the smallest values within the range lower quartile −1.5 × IQR. More extreme values are shown as individual points. Because of the skewed distribution, the data were logarithmically transformed before statistical analysis. The difference of Ki-67 LI between the two groups was significant by Student’s t-test for unpaired data (t = 3.38; P < 0.002), whereas no significant difference of apoptotic index was detected (t = 1.45, P > 0.10).
Figure 3.
Correlation between Ki-67 LI and maximum tumor diameter in 51 patients with histology-proven Cushing’s disease (r = 0.53; P < 0.01).
The mean apoptotic index in all patients was 1.1 ± 0.3%, ranging from 0 to 12.2%. The highest value occurred in the patient with the highest Ki-67 LI value. Apoptotic index was not correlated with age at operation, estimated duration of disease, female sex, previous treatment with ketoconazole, plasma ACTH, or serum cortisol levels. Group B patients had an apoptotic index not significantly higher than group A patients (1.7 ± 0.8% vs. 0.8 ± 0.3%, t = 1.45, P > 0.10; Figure 2 ▶ ). Moreover, maximum tumor diameter was only weakly correlated with the apoptotic index (r = 0.28, P = 0.04). There was a significant correlation between Ki-67 LI and apoptotic index in the whole group of patients (r = 0.41, P < 0.01).
Patients not in remission after surgery had a significantly higher Ki-67 LI than patients in remission (9.6 ± 2.6% vs. 4.2 ± 1.0, t = 2.15, P = 0.03), whereas the apoptotic index did not differ significantly (0.7 ± 0.3 vs. 1.1 ± 0.3%, t = −0.43, P = n.s.). However, stepwise regression analysis indicated that the apparent association between a high Ki-67 LI and a poor surgical outcome was not independent, since when all the other variables were considered together, only maximum tumor diameter and basal ACTH level remained significantly associated to a poor surgical outcome. Exclusion from the analysis of the four patients with postoperative eucortisolism rather than hypocortisolism did not modify the results.
Discussion
Our study, the first to compare both the proliferation activity and the apoptotic index with tumor size in a large group of consecutive patients with Cushing’s disease, clearly demonstrates that ACTH-secreting macroadenomas are characterized by a higher proliferation index than microadenomas, whereas the apoptotic index is not significantly different between the two groups.
Most patients with Cushing’s disease have very small tumors and consequently the distinction between microadenomas and macroadenomas, as well as the mechanism(s) underlying their different pattern of growth, has not received much attention in the literature. The frequency of ACTH-secreting macroadenomas (15 of 101, 15%), in our population is similar to that reported in other large surgical series, 1,3,4 but lower than that of smaller series. 8,17 Although arbitrary, subdivision of pituitary adenomas in micro- and macroadenomas has been widely accepted and, in the case of PRL-secreting adenomas, the two groups seem to represent entities differing in some biological characteristics. 18 In the present study we found a significantly higher proliferation index in ACTH-secreting macroadenomas compared to microadenomas. The significant correlation between Ki-67 LI and maximum tumor diameter further strengthens our finding. Moreover, it is also likely that the magnitude of the difference would have been even greater had we not excluded from the study patients with tumors smaller than 4 mm. Data on the proliferation rate of ACTH-secreting adenomas are rather scanty. In a retrospective study, Katznelson and coworkers 5 reported a higher Ki-67 LI in 16 corticotroph macroadenomas compared to 17 microadenomas, but the difference was only of borderline significance, probably because it was influenced by the presence of a single elevated value in the group of microadenomas (see Figure 3 ▶ of their paper). The five patients described by Ikeda and coworkers 17 had Ki-67 LI of 0.1, 1.7, 0.4, 0.1, and 0.2%, respectively, but they did not report for comparison the Ki-67 LI in ACTH-secreting microadenomas operated in the same period. Other studies found high Ki-67 LI values in ACTH-secreting adenomas, 19-21 but the number of patients was small and the size of the tumor was not specified. The relatively high frequency (about 30%) of ACTH-secreting tumors among the few pituitary carcinomas reported to date in the literature 22,23 supports the conclusion that the proliferative capacity of these adenomas is increased.
Which mechanism may cause the high proliferation index occurring in ACTH-secreting macroadenomas? High doses of glucocorticoids suppress the growth of the corticotrophic tumor cell line AtT20 24 in vitro. Moreover, a positive correlation between the size of the corticotroph adenoma and the degree of insensitivity to glucocorticoid feedback was demonstrated in dogs in vivo. 25 These data are consistent with studies in humans that showed a lack of cortisol suppression after administration of dexamethasone at high doses in patients with corticotroph macroadenomas only. 5,8,17,26 It has been hypothesized that resistance to high doses of glucocorticoids may be caused by the occurrence of somatic mutation(s) affecting the glucocorticoid receptor or its expression. 27,28 Adding our data to the above mentioned studies, we can speculate that, from the outset or during the course of the disease, a subset of ACTH-secreting microadenoma acquires a somatic mutation in the glucocorticoid receptor that renders the adenomatous cells insensitive to the high cortisol levels, thus unmasking the intrinsic high proliferative activity of these tumors. Further research in this area is needed to test the validity of such hypothesis.
Apoptosis was originally discovered as an important regulator of normal development and tissue homeostasis, but it has assumed increasing importance because of its perceived role in controlling the growth of neoplastic cells by counterbalancing their rate of proliferation. 10,29,30 Theoretically, then, the high frequency of small tumors among patients with Cushing’s disease could also be explained by a high apoptotic index in microadenomas. Our data do not support this possibility. The apoptotic index was not different in the two groups of patients. On the contrary, corticotroph macroadenomas showed an insignificantly higher apoptotic index compared to microadenomas, and there was a weak but significant correlation between maximum tumor diameter and apoptotic index in the entire group of patients. Interestingly, we found a significant correlation between apoptosis and proliferative activity. The latter finding has also been reported in malignant neoplasms, 31 particularly in brain tumors. 32 It has been hypothesized that an increase of the proliferation activity within a cell population leads to exhaustion of factors that promote cell multiplication and inhibit apoptosis. Apoptosis in ACTH-secreting pituitary adenomas has been investigated rarely. In a recent report Kontogeorgos and coworkers using an in situ end labeling technique 13 found a higher apoptotic index in functioning than in nonfunctioning adenomas. Corticotroph adenomas had apoptotic values lower than thyrotropic adenomas but similar to that of GH-, PRL-, and mixed GH/PRL-secreting adenomas. 13 At variance with our finding, the apoptotic index was higher in 5 ACTH-secreting microadenomas than in the remaining 10 macroadenomas; however, the difference was not significant. 13
Outcome of surgery was not influenced by either proliferation or apoptotic index when a multivariate analysis was performed. Tumor size was the strongest predictor of surgical outcome and eliminated the apparent significant association found in the univariate analysis between Ki-67 LI and surgical outcome. Our data do not support the use in routine clinical practice of proliferation and apoptotic indices in patients with Cushing’s disease. However, because of the small number of patients with recurrent Cushing’s disease and the relatively short follow-up, we cannot evaluate their importance in predicting the long term prognosis of surgically treated Cushing’s disease.
We found a significant linear correlation between maximum tumor diameter and baseline plasma ACTH level, thus reinforcing the finding reported by Selvais and coworkers 8 of a similar correlation between the tumor volume and mean plasma ACTH value. In another case control study 5 the percentage of macroadenoma patients with ACTH level above the appropriate normal range (78%) was higher than in microadenoma patients (46%; P = 0.05 by Fisher’s exact test). The relationship between tumor size and peripheral level of the secreted hormone is typical of the other types of secreting pituitary adenomas, such as prolactinomas 33 and acromegaly. 34 Basal cortisol levels were higher in macroadenoma patients, but the difference did not reach the significance level. A similar trend was observed in other reports that assessed the urinary free cortisol or 17-OHCS levels. 5,8
In conclusion, our study demonstrates that ACTH-secreting macroadenomas have a significantly higher proliferation index than microadenomas, whereas the apoptotic index is similar in the two groups. It seems reasonable to hypothesize that the difference of the proliferation activity is the principal characteristic underlying the different pattern of growth among ACTH-secreting tumors. Further studies are needed to clarify the mechanism(s) causing the growth advantage of ACTH-secreting macroadenoma.
Acknowledgments
We thank Dr. U. Pagotto, Max-Planck-Institute, Munich, Germany for critical reading and discussion of the manuscript.
Footnotes
Address reprint requests to Marco Losa, M.D., Dept. of Neurosurgery, IRCCS San Raffaele, Via Olgettina 60, 20132-Milano, Italy. E-mail: losa.marco@hsr.it.
Supported in part by grants from the Ministero della Universitǎ e della Ricerca Scientifica e Tecnologica (MURST), Rome.
References
- 1.Bochicchio D, Losa M, Buchfelder M, and the European Cushing’s Disease Survey Study Group: Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s disease survey group. J Clin Endocrinol Metab 1995, 80:3114–3120 [DOI] [PubMed]
- 2.Aron DC, Findling JW, Fitzgerald PA, Forsham PH, Wilson CB, Tyrrell JB: Cushing’s syndrome: problems in management. Endocr Rev 1982, 3:229-244 [DOI] [PubMed] [Google Scholar]
- 3.Mampalam TJ, Tyrrell JB, Wilson CB: Transsphenoidal microsurgery for Cushing’s disease. A report of 216 cases. Ann Intern Med 1988, 109:487-493 [DOI] [PubMed] [Google Scholar]
- 4.Robert F, Hardy J: Cushing’s disease: a correlation of radiological, surgical and pathological findings with therapeutic results. Pathol Res Pract 1991, 187:617-621 [DOI] [PubMed] [Google Scholar]
- 5.Katznelson L, Bogan JS, Trob JR, Schoenfeld DA, Hedley-White ET, Hsu DW, Zervas NT, Swearingen B, Sleeper M, Klibanski A: Biochemical assessment of Cushing’s disease in patients with corticotroph macroadenomas. J Clin Endocrinol Metab 1998, 83:1619-1623 [DOI] [PubMed] [Google Scholar]
- 6.Giovanelli M, Losa M, Mortini P: Acromegaly: surgical results and prognosis. Pituitary Adenomas. Edited by Landolt AM, Vance ML, Reilly PL. Edinburgh, Churchill Livingstone, 1996, pp 333–351
- 7.Losa M, Giovanelli M, Persani L, Mortini P, Faglia G, Beck-Peccoz P: Criteria of cure and follow-up of central hyperthyroidism due to thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab 1996, 81:3084-3090 [DOI] [PubMed] [Google Scholar]
- 8.Selvais P, Donckier J, Buysschaert M, Maiter D: Cushing’s disease: a comparison of pituitary corticotroph microadenomas and macroadenomas. Eur J Endocrinol 1998, 138:153-159 [DOI] [PubMed] [Google Scholar]
- 9.Miller JW, Crapo L: The medical treatment of Cushing’s syndrome. Endocr Rev 1993, 14:443-458 [DOI] [PubMed] [Google Scholar]
- 10.Landolt AM: Growth of pituitary adenomas, malignant adenomas. Pituitary Adenomas. Edited by Landolt AM, Vance ML, Reilly PL. Edinburgh, Churchill Livingstone, 1996, pp 73–82
- 11.Losa M, Franzin A, Mortini P, Terreni MR, Mangili F, Giovanelli M: Usefulness of markers of cell proliferation in the management of pituitary adenomas. Clin Sci 1998, 95:119-125 [PubMed] [Google Scholar]
- 12.Kerr JFR, Winterford CM, Harmon BV: Apoptosis: its significance in cancer and cancer therapy. Cancer 1994, 73:2013-2026 [DOI] [PubMed] [Google Scholar]
- 13.Kontogeorgos G, Sambaziotis D, Piaditis G, Karameris A: Apoptosis in human pituitary adenomas: a morphologic and in situ end-labeling study. Mod Pathol 1997, 10:921-926 [PubMed] [Google Scholar]
- 14.Hsu SM, Raine L, Fanger H: The use of antiavidin antibody and avidin-biotin peroxidase complex in immunoperoxidase technics. Am J Clin Pathol 1981, 75:816-821 [DOI] [PubMed] [Google Scholar]
- 15.Cattoretti G, Becker MHG, Key G, Duchrow M, Schluter C, Galle J, Gerdes J: Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB-1 and MIB-3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 1992, 168:357-363 [DOI] [PubMed] [Google Scholar]
- 16.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda H, Yoshimoto T, Ogawa Y, Mizoi K, Murakami O: Clinico-pathological study of Cushing’s disease with large pituitary adenoma. Clin Endocrinol (Oxf) 1997, 46:669-679 [DOI] [PubMed] [Google Scholar]
- 18.Weiss MH, Teal J, Gott P, Wycoff R, Yadley R, Apuzzo ML, Giannotta SL, Kletzky O, March C: Natural history of microprolactinomas: a six-year follow-up. Neurosurgery 1983, 12:180-183 [DOI] [PubMed] [Google Scholar]
- 19.Landolt AM, Shibata T: Growth, cell proliferation, and prognosis of pituitary adenomas: Pituitary Adenomas: New Trends in Basic and Clinical Research. Edited by Faglia G, Beck-Peccoz P, Ambrosi B, Travaglini P, Spada A. Amsterdam, Excerpta Medica, 1991, pp 169–178
- 20.Shibuya M, Saito F, Miwa T, Davis RL, Wilson CB, Hoshino T: Histochemical study of pituitary adenomas with Ki-67 and anti-DNA polymerase α monoclonal antibodies, bromodeoxyuridine labeling, and nucleolar organizer region counts. Acta Neuropathol 1992, 84:178-183 [DOI] [PubMed] [Google Scholar]
- 21.Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER, Jr: Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 1996, 38:99-107 [DOI] [PubMed] [Google Scholar]
- 22.Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs K, Horvath E, Young WF, Jr, Lloyd RV, Davis DH, Guthrie BL, Schoene WC: Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer 1996, 79:804-812 [DOI] [PubMed] [Google Scholar]
- 23.Lormeau B, Miossec P, Sibony M, Valensi P, Attali JR: Adrenocorticotropin-producing pituitary carcinoma with liver metastasis. J Endocrinol Invest 1997, 20:230-236 [DOI] [PubMed] [Google Scholar]
- 24.Van Wijk PA, van Neck JW, Rijnberk A, Croughs RJM, Mol JA: Proliferation of the murine corticotropic tumour cell line AtT20 is affected by hypophysiotrophic hormones, growth factors and glucocorticoids. Mol Cell Endocrinol 1995, 111:13-19 [DOI] [PubMed] [Google Scholar]
- 25.Kooistra HS, Voorhout G, Mol JA, Rijnberk A: Correlation between impairment of glucocorticoid feedback and the size of pituitary gland in dogs with pituitary-dependent hyperadrenocorticism. J Endocrinol 1997, 152:387-394 [DOI] [PubMed] [Google Scholar]
- 26.Gibson S, Ray DR, Crosby SR, Dornan TL, Jennings AM, Bevan JS, Davis JR, White A: Impaired processing of proopiomelanocortin in corticotroph macroadenomas. J Clin Endocrinol Metab 1996, 81:497-502 [DOI] [PubMed] [Google Scholar]
- 27.Mu Y-M, Takayanagi R, Imasaki K, Ohe K, Ikuyama S, Yanase T, Nawata H: Low level of glucocorticoid receptor messenger ribonucleic acid in pituitary adenomas manifesting Cushing’s disease with resistance to a high dose-dexamethasone suppression test. Clin Endocrinol (Oxf) 1998, 49:301-306 [DOI] [PubMed] [Google Scholar]
- 28.Karl M, von Wichert G, Kempter E, Katz DA, Reincke M, Monig H, Ali IU, Stratakis CA, Oldfield EH, Chrousos GP, Schulte HM: Nelson’s syndrome associated with a somatic frame shift mutation in the glucocorticoid receptor gene. J Clin Endocrinol Metab 1996, 81:124-129 [DOI] [PubMed] [Google Scholar]
- 29.Hoffman B, Lieberman DA: Molecular controls of apoptosis: differentiation/growth arrest primary response genes, proto-oncogenes, and tumor suppressor genes as positive and negative modulators. Oncogene 1994, 9:1807-1812 [PubMed] [Google Scholar]
- 30.Schwartzman RA, Cidlowski JA: Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev 1993, 14:133-151 [DOI] [PubMed] [Google Scholar]
- 31.Wyllie AH: The biology of cell death in tumours. Anticancer Res 1985, 5:131-136 [PubMed] [Google Scholar]
- 32.Nakagawe S, Shiraishi T, Kihara S, Tabuchi K: Detection of DNA strand breaks associated with apoptosis in human brain tumours. Virch Archiv A 1995, 427:175-179 [DOI] [PubMed] [Google Scholar]
- 33.Klijn JGM, Lamberts SWJ, De Jong FH, Docter R, van Dongen KJ, Birkenhager JC: The importance of pituitary tumour size in patients with hyperprolactinaemia in relation to hormonal variables and extrasellar extension of tumour. Clin Endocrinol (Oxf) 1980, 12:341-355 [DOI] [PubMed] [Google Scholar]
- 34.Klijn JGM, Lamberts SWJ, De Jong FH, van Dongen KJ, Birkenhager JC: 1980 Interrelationship between tumour size, age, plasma growth hormone, and incidence of extra-sellar extension in acromegalic patients. Acta Endocrinol (Copenh) 1980, 95:289-299 [DOI] [PubMed] [Google Scholar]