Abstract
Thalidomide is a teratogen with anti-angiogenic properties and causes stunted limb growth (dysmelia) during human embryogenesis. The molecular mechanisms of thalidomide action in embryopathy are currently unknown. Using the endothelial-specific antigen platelet endothelial cell adhesion molecule-1 and confocal laser scanning microscopy we have demonstrated that thalidomide exerts anti-angiogenic effects on the development of capillary structures in embryoid bodies differentiated from murine embryonic stem cells. Consequently, in thalidomide-treated embryoid bodies the diffusion properties of the tissue were deteriorated. Thalidomide raised reactive oxygen species (ROS), as revealed using 2′7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) as an indicator. A comparable ROS generation was achieved with the thalidomide hydrolysis product phthaloyl glutamic acid (PGA), but not with phthalimide (PI), the major component of thalidomide. ROS formation by thalidomide was inhibited by the hydroxyl radical scavengers mannitol and 2-mercaptoethanol. After coadministration of either 2-mercaptoethanol or mannitol with thalidomide the anti-angiogenic effects of thalidomide were abolished and the diffusion properties of the tissue were restored to the control values. In summary, our data suggest that thalidomide exerts its anti-angiogenic properties via the generation of toxic hydroxyl radicals, which impair vasculogenesis and angiogenesis during embryoid body development.
The hypnosedative drug thalidomide was introduced to the German market as Contergan in 1956 by Chemie Grunenthal, but had to be withdrawn in late 1961 after the reports of McBride 1 and Lenz, 2 who demonstrated an association between maternal thalidomide usage and infant limb defects. In recent years, however, interest has increased in the reintroduction of thalidomide, because it has been shown to exert therapeutic effects on various diseases including erythema nodosum leprosum, HIV-related wasting syndrome and esophageal ulcers, graft-versus-host disease, arthritis, and tuberculosis. In 1997 thalidomide was licensed in the United States as a treatment for a serious skin disorder that affects people with leprosy. Several South American countries had already approved the drug for this use. 3
The modes of action of thalidomide in embryopathy have been a matter of controversy for more than 30 years. 4 Recently, it has been shown that thalidomide is an inhibitor of angiogenesis induced by basic fibroblast growth factor in rabbit 5 and mouse 6 cornea micropocket assays. In further studies thalidomide has been shown to generate reactive oxygen species (ROS), which may be responsible for the teratogenic effects of this compound. 7,8 The present study was undertaken to evaluate whether ROS formation by thalidomide and the inhibition of angiogenesis are correlated. Furthermore, the anti-angiogenic properties of thalidomide have not yet been investigated in an embryonic tissue where both vasculogenesis, ie, the de novo formation of blood vessel structures from in situ differentiating endothelial progenitors (angioblasts), and angiogenesis, ie, sprouting of new capillaries from the preexisting network, occur. Previous studies on the anti-angiogenic effects of thalidomide did not offer any information on the molecular events that resulted in the impairment of blood vessel formation with its consequence of teratogenesis in the embryo.
In our experiments we used embryoid bodies, which were previously introduced by us as a novel in vitro model of anti-angiogenesis. 9 By means of this in vitro model we evaluated the mechanisms of the anti-angiogenic capacity of thalidomide. Embryoid bodies are spheroidal three-dimensional embryonic tissues grown from pluripotent murine embryonic stem (ES) cells and have been shown to differentiate vessel-like structures, 10,11 effectively improving the diffusion properties of the tissue. 9 When cultivated in spinner flask technique, embryoid bodies attain a diameter of 1 to 2 mm. 9 We investigated whether thalidomide inhibited endothelial cell growth and impaired the diffusion properties in this embryonic tissue. Because the teratogenicity of many xenobiotics, including thalidomide, is thought to depend at least in part on their bioactivation by embryonic cytochrome P-450, prostaglandin H synthase, and lipoxygenases to electrophilic and/or free radical intermediates, which in turn may oxidize DNA, proteins, and lipids, 7,8 we evaluated the induction of ROS generation by thalidomide. We demonstrate that thalidomide exerts its anti-angiogenic effects by the generation of hydroxyl radicals, in that scavengers of hydroxyl radicals abolished the inhibitory effects of thalidomide on capillary structure formation in embryoid bodies.
Materials and Methods
Spinner Flask Culture Technique for Cultivation of Embryoid Bodies
The ES cell line CCE 12 was grown on mitotically inactivated feeder layers of primary murine embryonic fibroblasts for a maximum of eight passages in Iscove’s medium (Life Technologies, Rockville, MD) supplemented with 20% heat-inactivated (56°C, 30 minutes) fetal calf serum (FCS) (Boehringer Mannheim, Mannheim, Germany), 2 mmol/L Glutamax, (Life Technologies), 100 μmol/L 2-mercaptoethanol (Sigma, Deisenhofen, Germany), 1% MEM non-essential amino acids stock solution, 100 IU/ml penicillin, and 100 μg/ml streptomycin (all from Life Technologies) in a humidified environment containing 5% CO2 at 37°C and passaged every 2 to 3 days. At day 0 of differentiation adherent cells were enzymatically dissociated using 0.2% trypsin and 0.05% EDTA in PBS (Life Technologies) and seeded at a density of 1·10 7 ml−1 cells in 250 ml siliconized spinner flasks (Live Technologies, Fernwald, Germany) containing 100 ml Iscove’s medium supplemented with the same additive as described above. After 24 hours, 150 ml medium was added to give a final volume of 250 ml. The spinner flask medium was stirred at 20 rpm using a stirrer system (Integra Biosciences, Fernwald, Germany). Every day, 125 ml of the medium was removed and replaced by fresh cell culture medium.
Treatment with Thalidomide, Thalidomide Analogues, and Hydroxyl Radical Scavengers
At day 3 of cell culture embryoid bodies were removed from spinner culture flasks. 20–30 embryoid bodies were subcultivated in liquid overlay culture for 5 days in bacteriological petri dishes (6.4 cm; Greiner, Solingen, Germany) containing 6 ml cell culture medium. Thalidomide ((±)−2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione), purchased from Biotrend (Cologne, Germany), and phthaloyl glutamic acid (PGA), purchased from Sigma, were dissolved in dimethyl formamide (DMF) and added to the cell culture medium in concentrations as indicated. Phthalimide (PI; Sigma) was dissolved in cell culture medium. Cell culture medium supplemented with the agents was completely changed every day. In experiments with hydroxyl radical scavengers either 10 mmol/L mannitol (Merck, Darmstadt, Germany) or 200 μmol/L 2-mercaptoethanol (Sigma, Deisenhofen, Germany) (final concentration 300 μmol/L) were coadministered with thalidomide. The control samples were treated with only the solvent DMF (final concentration 0.4%). Embryoid body diameters were determined every 24 hours.
Antibody Staining
The monoclonal antibody anti-mouse platelet endothelial cell adhesion molecule-1 (PECAM-1; CD31; Endogen, Woburn, MA) was used at a concentration of 5 μg/ml. Embryoid bodies were fixed in ice-cold methanol/acetone (7:3) for 60 minutes at −20°C, and washed with phosphate-buffered saline (PBS) containing 0.1% Triton X-100 (PBST; Sigma). Blocking against unspecific binding was performed for 60 minutes with 10% fat-free milk powder (Heirler, Radolfzell, Germany) dissolved in PBS. Embryoid bodies were subsequently incubated for 90 minutes at room temperature with PECAM-1 primary antibody dissolved in PBS supplemented with 10% milk powder. Embryoid bodies were then washed three times with PBST (0.01% Triton) and reincubated with a Cy5-conjugated rabbit anti-syrian hamster IgG (H + L; Dianova, Hamburg, Germany) at a concentration of 3.8 μg/ml in PBS containing 10% milk powder. After washing three times in PBST (0.01%) embryoid bodies were stored in PBS until inspection. For the excitation of the Cy5 fluorochrome the 633-nm band of a helium/neon laser of the confocal setup was used. Emission was recorded using a long-pass 655-nm filter set.
Confocal Laser Scanning Microscopy
Fluorescence recordings were performed by means of a confocal laser scanning setup (LSM 410, Zeiss, Jena, Germany) connected to an inverted microscope (Axiovert 135, Zeiss). The confocal setup was equipped with a 0.5 mW helium/neon laser, single excitation at 543 nm (excitation of doxorubicin) and a 5 mW helium/neon laser single excitation 633 nm (excitation of Cy5). Emission was recorded using long-pass filter sets LP570 and RG665, respectively. A 16× numerical aperture (N.A.) 0.5, oil immersion-corrected objective (Neofluar, Zeiss) was used.
Full frame images (512 × 512 pixels) were acquired from whole-mount embryoid bodies using the extended depth of focus algorithm of the confocal setup. In brief, 5 full frame images separated by a distance of 20 μm in the z-direction were recorded that included the information of the capillary area and spatial organization in a tissue slice 100 μm thick. From the acquired images an overlay image giving a three-dimensional projection of the vascular structures in the scanned tissue slice of 100 μm thickness was generated. The pixel intensity in optical sections of embryoid bodies treated only with the secondary antibody (background fluorescence) was separately evaluated and subtracted from the overlay image. The mean area (in mm2) of PECAM-1-positive cell structures was determined as the integrated sum of pixel values above background level in the overlay image and was calculated by the image analysis software of the confocal setup.
Diffusion Studies with Doxorubicin
For diffusion studies embryoid bodies were incubated at room temperature for 10, 30, and 60 minutes with 10 μmol/L doxorubicin (Sigma). They were subsequently washed and doxorubicin fluorescence was determined using the 543-nm line of a helium/neon laser of the confocal setup and the optical probe technique as previously described. 13,14 In brief, optical sections with a thickness of 10 μm were performed from the periphery of embryoid bodies toward the center and doxorubicin fluorescence was determined in selected regions of interest (600 μm2, 40 × 40 pixels). The depth-intensity curves were fitted using a four-parameter exponential equation and a computer-based least squares fitting routine. 13 For the determination of diffusion coefficients the doxorubicin distribution was evaluated with the optical sectioning routine of the confocal setup after 5, 20, and 30 minutes of incubation with doxorubicin. From the maximum diffusion distance x of doxorubicin after 30 minutes of incubation, diffusion coefficients were calculated according to the Einstein-Smoluchovski equation D = x2/2t where D is the diffusion coefficient, x is the maximum diffusion distance of doxorubicin from the embryoid body periphery, and t is the diffusion time of 30 minutes.
Determination of the Intracellular Redox State
The intracellular redox state was measured using the fluorescent dye 2′7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular Probes, Eugene, OR), a nonpolar compound that is converted into a nonfluorescent polar derivative by cellular esterases after incorporation into cells. H2DCF is membrane impermeable and is rapidly oxidized to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) in the presence of intracellular ROS. 15 For the experiments, embryoid bodies were incubated in E1 medium containing 135 mmol/L NaCl, 5.4 mmol/L KCl, 1.8 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L glucose, and 10 mmol/L Hepes, pH 7.4. Subsequently they were incubated in 20 μmol/L H2DCF-DA for 50 minutes at room temperature. For fluorescence excitation, the 488-nm band of the argon ion laser of the confocal setup was used. Emission was recorded using a long-pass LP515 filter set.
Statistical Analysis
Data are given as mean values ± SE with n denoting the number of experiments performed with embryoid bodies from different spinner flasks. Student’s t-test for unpaired data was applied as appropriate. A value of P < 0.05 was considered significant.
Results
Anti-Angiogenic Effects of Thalidomide in Embryoid Bodies
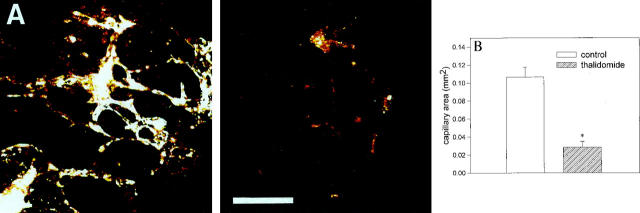
Thalidomide has been previously described as exerting anti-angiogenic properties in the corneal micropocket assay. 5,6 To evaluate whether thalidomide likewise displayed anti-angiogenic effects in the embryoid body model, embryoid bodies were treated from day 3 to day 8 of development with either 10 or 100 μg/ml thalidomide. On day 8, angiogenesis progression was evaluated by confocal laser scanning microscopy and immunohistochemistry of PECAM-1 expression in five consecutive optical sections of 20 μm thickness. This procedure yielded information on the capillary area and spatial organization of capillaries in a tissue slice of 100 μm thickness. As previously reported endothelial cell differentiation was absent in 3-day-old embryoid bodies, whereas the extension of the capillary area was maximal after 8 days of cell culture (data not shown). 9 Thalidomide significantly reduced the extension of the capillary area from 0.10 ± 0.016 mm 2 in the control to 0.026 ± 0.012 mm 2 in the treated sample (n = 10 embryoid bodies for each experimental condition in five independent experiments; Figure 1, A and B ▶ ). When embryoid bodies were treated with 10 μg/ml thalidomide, no significant effect on endothelial cell differentiation was observed (data not shown).
Figure 1.
Effect of thalidomide on the development of capillary structures in embryoid bodies. Embryoid bodies were incubated with 100 μg/ml thalidomide from day 3 to day 8 of development. They were subsequently fixed and the capillary area was evaluated by PECAM-1 immunohistochemistry and confocal laser scanning microscopy. A: Representative untreated (left panel) and thalidomide-treated (right panel) embryoid bodies. Scale bar, 200 μm. B: Quantitative analysis of the capillary areas in 8-day-old embryoid bodies which were either untreated (open bar) or treated with 100 μg/ml thalidomide (hatched bar). Shown is a representative of five independent experiments. Significance (P < 0.05) is indicated by an asterisk.
Generation of Hydroxyl Radicals by Thalidomide
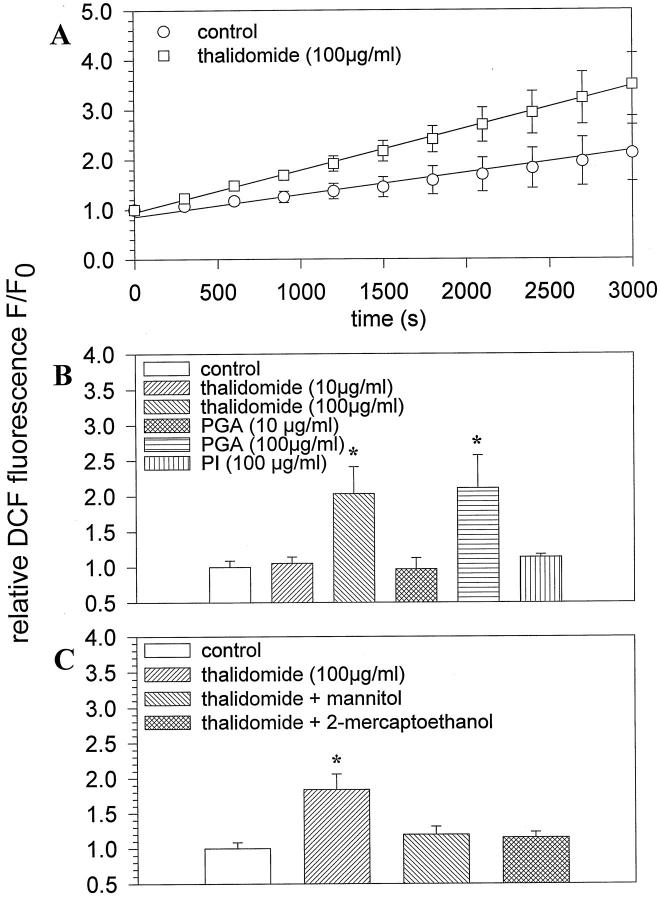
The anti-angiogenic effects of thalidomide may be due to the generation of toxic ROS, which increase during long term incubation with the compound. Moreover, ROS generation may be more efficient with hydrolysis products of thalidomide. To evaluate this issue, embryoid bodies were treated for either 4 or 24 hours with 10 and 100 μg/ml thalidomide, 10 and 100 μg/ml PGA, which is a hydrolysis product of thalidomide, and 100 μg/ml PI, which is the inactive major component of the thalidomide molecule. Subsequent ROS generation was monitored using the fluorescent ROS indicator DCF. Our data show that in control embryoid bodies ROS were continuously generated, owing to the activity of a diphenylene iodonium chloride-inhibitable NADPH-oxidase present in embryoid bodies (Sauer H, Rahimi G, Hescheler J, Wartenberg M, unpublished observations). However, the kinetics of H2DCF oxidation to fluorescent DCF was significantly increased in thalidomide treated embryoid bodies as compared to the untreated control (n = 4 embryoid bodies for each experimental condition in three independent experiments; Figure 2A ▶ ). An increase in ROS generation was observed after a 4-hour incubation with 100 μg/ml thalidomide and 100 μg/ml PGA, which elevated DCF fluorescence to 200 ± 38% (n = 3) and 211 ± 46% (n = 4), respectively, of the untreated control. Incubation with either 10 μg/ml thalidomide (n = 4), 10 μg/ml PGA (n = 3) or 100 μg/ml PI (n = 3) was without significant effect on ROS generation (Figure 2B) ▶ . An incubation for 24 hours with 100 μg/ml of either thalidomide or PGA did not result in a further increase of ROS formation. When 10 μg/ml of either thalidomide or PGA was administered for 24 hours, ROS formation was increased to 131 ± 7.5% (n = 5) and 128 ± 20% (n = 3), which was, however, not significantly different from the untreated control. The nature of the ROS generated by thalidomide was evaluated by coincubating embryoid bodies with 100 μg/ml thalidomide and either 10 mmol/L mannitol or 300 μmol/L 2-mercaptoethanol, both of which are known to be specific scavengers of hydroxyl radicals. 16,17 Figure 2C ▶ shows that after treatment of embryoid bodies for 4 hours with thalidomide the intracellular redox state as indicated by DCF fluorescence was elevated to 184 ± 23% of the control level (n = 4 embryoid bodies for each experimental condition in five independent experiments). Coadministration of either mannitol or 2-mercaptoethanol with thalidomide lowered the intracellular redox state to 120 ± 11% and 115 ± 8%, respectively, which was not statistically significant from the control (set to 100%). Comparable results were achieved when embryoid bodies were incubated with 100 μg/ml PGA in the presence of either mannitol or 2-mercaptoethanol (not shown). From these data we concluded that thalidomide specifically generated hydroxyl radicals.
Figure 2.
Generation of hydroxyl radicals by thalidomide. Embryoid bodies were preincubated with thalidomide for 4 hours. Subsequently they were stained with the ROS indicator H2DCF-DA and the generation of fluorescent DCF was monitored. A: Time course of ROS generation in control and thalidomide-treated embryoid bodies after a 4-hour incubation with the compound. Data were fitted by linear regression. B: ROS generation by different concentrations (10 μg/ml and 100 μg/ml) thalidomide, PGA, and PI (100 μg/ml). C: Effects of the hydroxyl radical scavengers mannitol (10 mmol/L) and 2-mercaptoethanol (300 μmol/L) on ROS generated by thalidomide in embryoid bodies. Control DCF fluorescence was set to 100%. Significance (P < 0.05) is indicated by an asterisk.
Reversal of the Anti-Angiogenic Effects of Thalidomide by Cotreatment with Hydroxyl Radical Scavengers
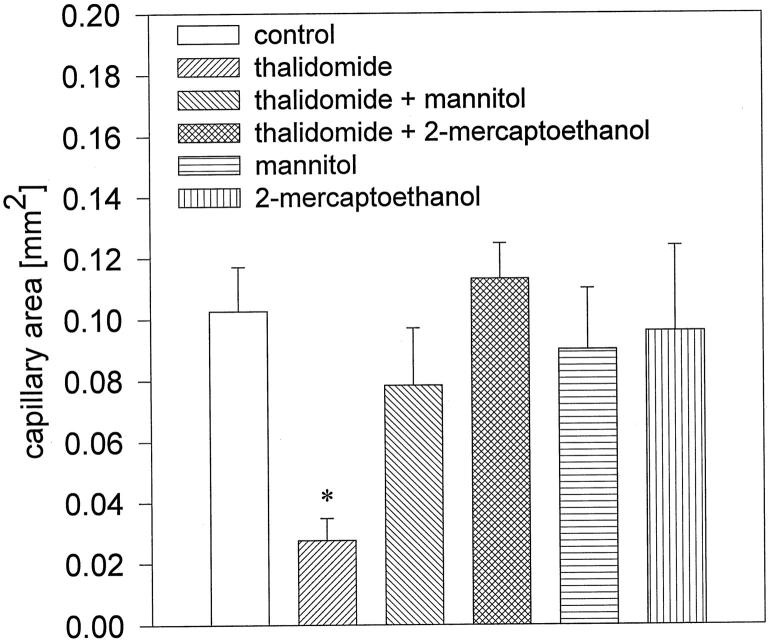
If generation of hydroxyl radicals is the cause of the anti-angiogenic effects of thalidomide, these effects should be absent when ROS formation by thalidomide is inhibited by coadministration of hydroxyl radical scavengers. We therefore incubated embryoid bodies from day 3 to day 8 with 100 μg/ml thalidomide in the presence of either 10 mmol/L mannitol or 300 μmol/L 2-mercaptoethanol, and subsequently evaluated the capillary areas in embryoid bodies. We observed that the anti-angiogenic effect of thalidomide was abolished when hydroxyl radicals were scavenged during the incubation period. The capillary area amounted to 0.08 ± 0.037 mm 2 and 0.11 ± 0.06 mm 2 in the samples treated with mannitol and 2-mercaptoethanol, respectively, which is not significantly different from control (0.10 ± 0.05 mm2; n = 10 embryoid bodies for each experimental condition in three independent experiments; Figures 3 and 4 ▶ ▶ ). Incubation with radical scavengers in the absence of thalidomide did not exert significant effects on capillary structure formation (n = 10 embryoid bodies for each experimental condition in four independent experiments). Therefore, we concluded that the anti-angiogenic effects of thalidomide were due to the generation of highly reactive hydroxyl radicals. To evaluate the specificity of thalidomide on the inhibition of angiogenesis and to exclude that the agents applied in the present study exerted cytotoxic effects, the growth kinetics of embryoid bodies was followed during the time of thalidomide treatment (n ≥ 20 embryoid bodies for each experimental condition). Neither thalidomide nor the applied hydroxyl radical scavengers, either alone or in coadministration with thalidomide, exerted any significant effects on the growth kinetics of embryoid bodies, indicating that thalidomide may act specifically on vasculogenesis and angiogenesis in embryoid bodies (Figure 5) ▶ .
Figure 3.
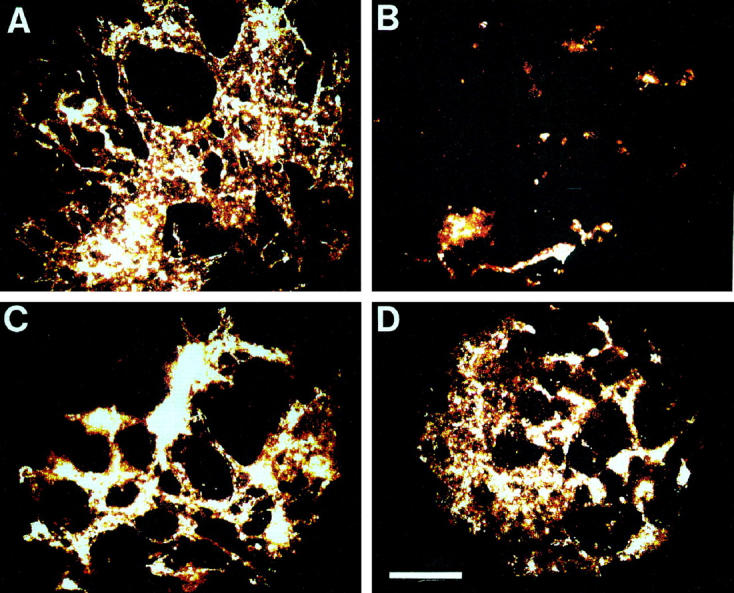
Effect of the hydroxyl radical scavengers mannitol (10 mmol/L) and 2-mercaptoethanol (300 μmol/L) on the anti-angiogenic effects exerted by thalidomide. A: Control sample, treated with the solvent DMF: B: Thalidomide (100 μg/ml) treated sample. C: Sample treated with mannitol (10 mmol/L) and thalidomide (100 μg/ml). D: Sample treated with 2-mercaptoethanol (300 μmol/L) and thalidomide (100 μg/ml). The capillary areas of representative embryoid bodies are shown. Scale bar, 200 μm.
Figure 4.
Quantitative analysis of the capillary areas in embryoid bodies treated with thalidomide (100 μg/ml), thalidomide in the presence of the hydroxyl radical scavengers mannitol (10 mmol/L) and 2-mercaptoethanol (300 μmol/L), and mannitol (10 mmol/L) and 2-mercaptoethanol (300 μmol/L) in the absence of thalidomide. Shown is representative of three independent experiments. Significance (P < 0.05) is indicated by an asterisk.
Figure 5.
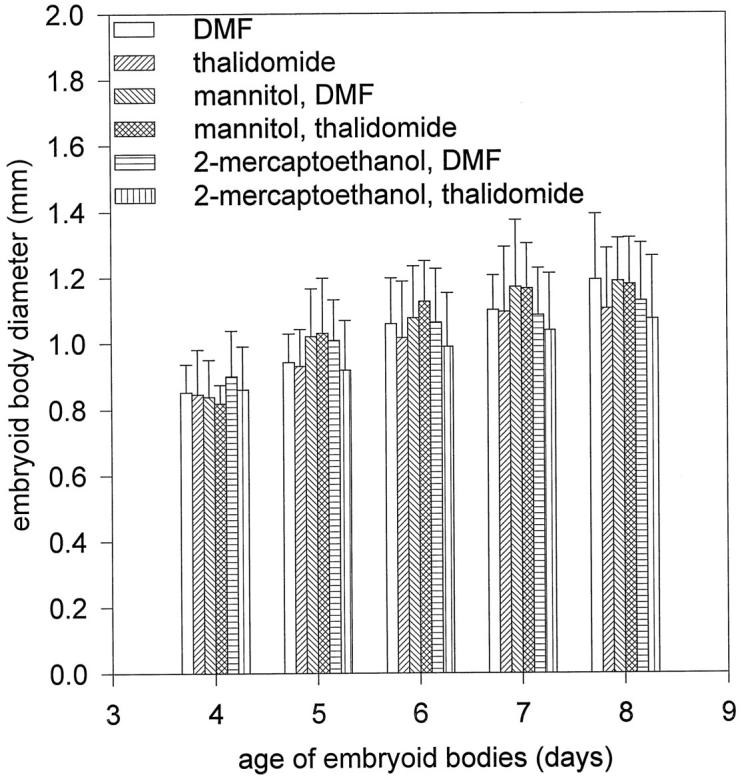
Growth kinetics of embryoid bodies during the incubation time (day 3 to day 8 of development) with thalidomide and hydroxyl radical scavengers. Embryoid bodies were treated with DMF (the solvent for thalidomide), thalidomide (100 μg/ml), mannitol (10 mmol/L) + DMF, mannitol (10 mmol/L) + thalidomide (100 μg/ml), 2-mercaptoethanol (300 μmol/L) + DMF, thalidomide (100 μg/ml) + 2-mercaptoethanol (300 μmol/L).
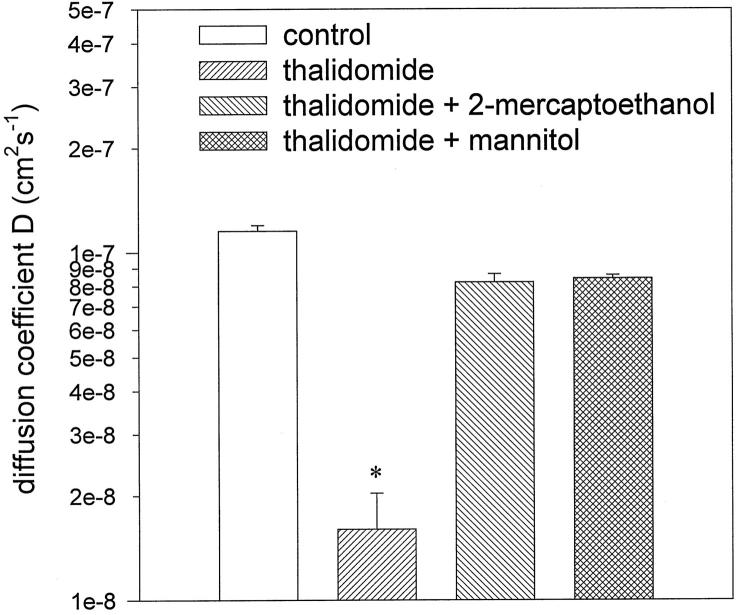
We have previously shown that the capillary structures differentiated in embryoid bodies improve the diffusion properties of the tissue, ie, in the vascularized tissue the diffusion coefficient D of the cell-permeable fluorescent anthracycline doxorubicin is significantly increased as compared to an unvascularized tissue. 9 To evaluate whether thalidomide treatment impaired diffusion, embryoid bodies treated from day 3 to day 8 of cell culture were incubated with doxorubicin and the diffusion coefficient of this compound was evaluated as described previously. Because the effect of thalidomide on doxorubicin diffusion should be reversed in the presence of hydroxyl radical scavenger, parallel experiments were performed using embryoid bodies treated with thalidomide in the presence of either mannitol or 2-mercaptoethanol. Figure 6 ▶ demonstrates that the diffusion coefficient D amounted to 9.12 · 10−8 ± 1.5 · 10−8 cm 2 s−1 in the control (treated only with the solvent DMF). Thalidomide significantly reduced D to 1.37 · 10−8 ± 0.19 · 10−8 cm 2 s−1, which indicates that inhibition of capillary formation impaired the diffusion properties of the tissue. In the presence of mannitol and 2-mercaptoethanol the inhibitory effect of thalidomide on the tissue diffusion properties was significantly reversed. D amounted to 7.5 · 10−8 ± 1.7 · 10−8 cm 2 s−1 for mannitol and and 6.5 · 10−8 ± 1.7 · 10−8 cm 2 s−1 for 2-mercaptoethanol, which was not significantly different from control (n = 4 embryoid bodies for each experimental condition in three independent experiments). From these data we concluded that the anti-angiogenic effects of thalidomide could be reversed by hydroxyl radical scavengers. Hydroxyl radical scavengers restored not only the extension of the capillary area but also the functionality of the capillaries developed in embryoid bodies.
Figure 6.
Diffusion coefficients D for doxorubicin in 8-day old embryoid bodies treated either with thalidomide (100 μg/ml) or thalidomide in the presence of the hydroxyl radical scavengers 2-mercaptoethanol (300 μmol/L) and mannitol (10 mmol/L). The control sample was treated with DMF, the solvent for thalidomide. Shown is a representative of three independent experiments which yielded comparable results. Significance (P < 0.05) is indicated by an asterisk.
Discussion
The present study reports for the first time on the molecular mechanisms of the anti-angiogenic effects exerted by the sedative drug thalidomide. Thalidomide’s anti-angiogenic action was tested in embryoid bodies, ie, an embryonic tissue that differentiates cell types of ectodermal, mesodermal, and endodermal origin and recapitulates several steps of postimplantation murine development. Using this embryonic tissue we could optimally mimic the physiological situation in an intact embryo, thereby achieving greater relevance for the investigation of the embryopathic effects of this compound. A further advantage of a complex tissue over endothelial cell cultures is the fact that xenobiotic substances have to be metabolized before they exert their toxic effects. This seems also to be the case for thalidomide, which has been shown to be effective in the rabbit and mouse corneal micropocket assay where the drug is administered orally or intraperitoneally. 5,6 However, thalidomide did not exert any anti-angiogenic effect in either the chorioallantoic membrane assay or in endothelial cells in culture, where presumably no metabolic activation of the drug occurs. 5 Recently it has been shown in a rat aorta model and in human aortic endothelial cells that thalidomide inhibited microvessel formation only in the presence of microsomes, whereas in the absence of microsomes thalidomide had no effects on microvessel formation. 18 This supports the notion that a metabolite of thalidomide is responsible for its anti-angiogenic effects.
In the present study thalidomide in a concentration of 100 μg/ml significantly inhibited blood vessel development in embryoid bodies, when the compound was administered during the period of vasculogenesis and angiogenesis which has been previously shown to occur between day 4 and day 8 of embryoid body development. 9 However, 10 μg/ml of the compound did not exert significant effects on the formation of capillary structures. The relatively high concentrations of thalidomide that were necessary to achieve the anti-angiogenic effect may be due to species-dependent differences in the susceptibility toward thalidomide teratogenicity. This assumption is underscored by the recent observations of Parman and colleagues 8 of a lack of DNA oxidation by thalidomide in mice. Furthermore, previous studies have demonstrated an absence of susceptibility of rodents to orally administered concentrations of thalidomide that are teratogenic in humans. 19,20
The inhibition of blood vessel formation had the consequence of impaired diffusion properties of the tissue as evaluated by determination of the diffusion constant D of the fluorescent anthracycline doxorubicin using the recently developed optical probe technique. 13,14 The impaired diffusion properties of embryonic tissue as a consequence of limited blood supply may result in the limb defects seen in children after thalidomide treatment of their pregnant mothers. It has been previously mentioned that the limb bud is a particularly vulnerable target to a teratogen that inhibited endothelial cell function. 5 Besides its effect on endothelial cell differentiation, thalidomide did not exert any overall toxicity in the embryoid body assay, in that embryoid body growth was not impaired after thalidomide treatment. From this we concluded that the antiproliferative effect of thalidomide appeared to be restricted to endothelial cells. The low toxicity of thalidomide has been known for long time and was one of the reasons to recommend this drug for the treatment of indisposition of pregnant women. 4
The experiments of the present study demonstrate that thalidomide exerts its anti-angiogenic properties via the generation of ROS. Interestingly, prolonged incubation (24 hours) with 100 μg/ml thalidomide and PGA did not result in an accumulation of ROS, as compared to an incubation for 4 hours, indicating the short lifetimes of ROS and the effective antioxidant defense systems present in embryoid bodies. Furthermore, PGA did not form ROS more efficiently than thalidomide, excluding the possibility that ROS are generated exclusively by hydrolysis products of thalidomide. A slight but not significant increase in ROS was achieved after a 24-hour incubation with 10μg/ml thalidomide and PGA, indicating that lower concentrations of the compounds may also be bioactive.
The toxicity of ROS on endothelial cells has been extensively investigated in recent years, because oxidative damage of blood vessels is a frequent event during reoxygenation after hypoxia. Moreover, endothelial cell injury is observed during acute inflammatory processes when activated neutrophils generate reactive oxygen metabolites within the target cell. 21 Because embryoid bodies constitutively generate ROS by an enzyme closely related to the respiratory burst nicotinamide adenine dinucleotide phosphate (NADPH) oxidase of neutrophils (Sauer H, Rahimi G, Hescheler J, Wartenburg M, unpublished observations), it can be ruled out that low levels of ROS per se impair vasculogenesis and angiogenesis in the embryoid body model.
Our data indicate that the reactive oxygen intermediate generated by thalidomide is the hydroxyl radical. Mannitol and 2-mercaptoethanol, which specifically scavenge hydroxyl radicals, abolished the increased DCF fluorescence observed after thalidomide treatment. Likewise, the anti-angiogenic effect of thalidomide was absent, and capillary formation apparently not different from the untreated control was observed when thalidomide was administered in the presence of hydroxyl radical scavengers. This was further corroborated by diffusion studies, which demonstrated that in the presence of hydroxyl radical scavengers the diffusion properties of the embryoid body tissue were restored to levels not significantly different from control. From these data we concluded that hydroxyl radical scavengers reestablished both the extension of the capillary network differentiated in embryoid bodies and the functionality of the capillaries.
ROS formation, including hydroxyl radicals, by thalidomide has been recently demonstrated and was counteracted by antioxidants and inhibitors of lipoxygenases and prostaglandin H synthase, 22 indicating that these peroxidases may bioactivate the compound. Highly reactive hydroxyl radicals may induce DNA oxidation in either endothelial cell precursors or endothelial cells during vasculogenesis and angiogenesis in the embryo. This notion is supported by recent experiments that demonstrate that phenytoin and structurally related drugs including thalidomide initiated the oxidation of DNA in embryonic tissues. 8,22 Phenytoin has been previously demonstrated to cause phalangeal hypoplasia in rabbit fetuses by a mechanism that is preceded by ischemia and vascular disruption. 23,24
Acknowledgments
This work is part of the M.D. thesis of J. Günther.
Footnotes
Address reprint requests to Dr. Maria Wartenberg, Department of Neurophysiology, Robert-Koch-Strasse 39, D-50931 Cologne, Germany. E-mail: hs@physiologie.uni-koeln.de.
References
- 1.McBride WG: Thalidomide and congenital abnormalities. Lancet 1961, 2:1358 [Google Scholar]
- 2.Lenz W: Thalidomide and congential abnormalities Lancet 1962, 1:45
- 3.Saphir A: Angiogenesis: the unifying concept in cancer? J Natl Cancer Inst 1997, 89:1480-1481 [PubMed] [Google Scholar]
- 4.Stephens TD: Proposed mechanisms of action in thalidomide embryopathy. Teratology 1988, 38:229-239 [DOI] [PubMed] [Google Scholar]
- 5.D’Amato RJ, Loughnan MS, Flynn E, Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 1994, 91:4082-4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenyon BM, Browne F, D’Amato RJ: Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res 1997, 64:971-978 [DOI] [PubMed] [Google Scholar]
- 7.Wells PG, Kim PM, Laposa RR, Nicol CJ, Parman T, Winn L: Oxidative damage in chemical teratogenesis. Mutat Res 1997, 396:65-78 [DOI] [PubMed] [Google Scholar]
- 8.Parman T, Wiley MJ, Wells PG: Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med 1999, 5:582-585 [DOI] [PubMed] [Google Scholar]
- 9.Wartenberg M, Günther J, Hescheler J, Sauer H: The embryoid body as a novel in vitro assay system for antiangiogenic agents. Lab Invest 1998, 78:1301-1314 [PubMed] [Google Scholar]
- 10.Vittet D, Prandini M-H, Berthier R, Schwetzer A, Martin-Sisteron H, Uzan G, Dejana E: Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood 1996, 88:3424-3431 [PubMed] [Google Scholar]
- 11.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T: Vasculogenesis and angiogenesis in embryonic stem-cell-derived embryoid bodies. Development 1988, 102:471-478 [DOI] [PubMed] [Google Scholar]
- 12.Robertson E, Bradley A, Kuehn M, Evans M: Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature 1986, 323:445-448 [DOI] [PubMed] [Google Scholar]
- 13.Wartenberg M, Hescheler J, Acker H, Diedershagen H, Sauer H: Doxorubicin distribution in multicellular prostate cancer spheroids evaluated by confocal laser scanning microscopy and the ‘optical probe technique’. Cytometry 1998, 31:137-145 [DOI] [PubMed] [Google Scholar]
- 14.Wartenberg M, Acker H: Quantitative recording of vitality patterns in living multicellular spheroids by confocal microscopy. Micron 1995, 26:395-404 [DOI] [PubMed] [Google Scholar]
- 15.Frenkel K, Gleichauf C: Hydrogen peroxide formation by cells treated with a tumor promoter. Free Radic Res Commun 1991, 12–13 part 2:783-794 [DOI] [PubMed] [Google Scholar]
- 16.Tsai LY, Lee KT, Liu TZ: Evidence for accelerated generation of hydroxyl radicals in experimental obstructive jaundice of rats. Free Radic Biol Med 1998, 24:732-737 [DOI] [PubMed] [Google Scholar]
- 17.Hiramoto K, Ojima N, Kikugawa K: Conversion of nitroxide radicals by phenolic and thiol antioxidants. Free Radic Res 1997, 27:45-53 [DOI] [PubMed] [Google Scholar]
- 18.Bauer KS, Dixon SC, Figg WD: Inhibition of angiogenesis by thalidomide requires metabolic activation, which is species-dependent. Biochem Pharmacol 1998, 55:1827-1834 [DOI] [PubMed] [Google Scholar]
- 19.Szabo KT, Steelman RL: Effects of maternal thalidomide treatment on pregnancy, fetal development, and mortality of the offspring in random-bred mice. Am J Vet Res 1967, 28:1823-1828 [PubMed] [Google Scholar]
- 20.Scott WJ, Fradkin R, Wilson JG: Non-confirmation of thalidomide induced teratogenesis in rats and mice. Teratology 1970, 5:33-36 [DOI] [PubMed] [Google Scholar]
- 21.Varani J, Ward PA: Mechanisms of endothelial cell injury in acute inflammation. Shock 1994, 2:311-319 [DOI] [PubMed] [Google Scholar]
- 22.Winn LM, Wells PG: Evidence for embryonic prostaglandin H synthase-catalyzed bioactivation and reactive oxygen species-mediated oxidation of cellular macromolecules in phenytoin and benzo[a]pyrene teratogenesis. Free Radic Biol Med 1997, 22:607-621 [DOI] [PubMed] [Google Scholar]
- 23.Danielson MK, Danielsson BR, Marchner H, Lundin M, Rundqvist E, Reiland S: Histopathological and hemodynamic studies supporting hypoxia and vascular disruption as explanation to phenytoin teratogenicity. Teratology 1992, 46:485-497 [DOI] [PubMed] [Google Scholar]
- 24.Danielsson BR, Danielson MK, Tomson T: Phenytoin causes phalangeal hypoplasia in the rabbit fetus at clinically relevant free plasma concentrations. Teratology 1995, 52:252-259 [DOI] [PubMed] [Google Scholar]