Abstract
Human skin reconstructs are three-dimensional in vitro models consisting of epidermal keratinocytes plated onto fibroblast-contracted collagen gels. Cells in skin reconstructs more closely recapitulate the in situ phenotype than do cells in monolayer culture. Normal melanocytes in skin reconstructs remained singly distributed at the basement membrane which separated the epidermis from the dermis. Cell lines derived from biologically early primary melanomas of the radial growth phase proliferated in the epidermis and the basement membrane was left intact. Growth and migration of the radial growth phase melanoma cells in the dermal reconstruct and tumorigenicity in vivo were only observed when cells were transduced with the basic fibroblast growth factor gene, a major autocrine growth stimulator for melanomas. Primary melanoma cell lines representing the more advanced stage vertical growth phase invaded the dermis in reconstructs and only an irregular basement membrane was formed. Metastatic melanoma cells rapidly proliferated and aggressively invaded deep into the dermis, with each cell line showing typical invasion and growth characteristics. Our results demonstrate that the growth patterns of melanoma cells in skin reconstructs closely correspond to those in situ and that basic fibroblast growth factor is critical for progression.
Human skin reconstructs consist of artificial skin constructed from isolated cutaneous cell populations. 1 The reconstructs comprise a stratified, terminally differentiated epidermal compartment and a dermal compartment consisting of fibroblasts embedded in collagen. 2-4 The coculture of epidermal and dermal cells in a physiological context simulates human skin, and has been used as an alternative to animal testing for studies of skin barrier function, 5 skin irritation, 6,7 wound healing, 8 and ultraviolet light-induced damage. 9,10 Recently, skin reconstructs have been used for treatment of burns 11 and other wounds. 12,13 Most studies with human skin reconstructs have focused on keratinocytes, whereas melanocytes have received little attention. In normal human skin, melanocytes are aligned at the basement membrane, separating the epidermis from dermis, and communicate through dendritic extensions with multiple keratinocytes to form an epidermal melanin unit.
Methods for the isolation and culture of human melanocytes 14,15 are now routine and have been useful in studying melanocyte function. Such studies indicate the role of cell-cell and cell-matrix interactions in the regulation of melanocytic phenotype and function. 10,16,17 In monolayer culture, human melanocytes proliferate, display a bi- or tripolar morphology, and express melanoma-associated antigens. 18 However, in cocultures with keratinocytes, melanocytes more closely resemble the phenotype seen in situ, maintaining a constant ratio with keratinocytes, exhibiting a multidendritic morphology, and expressing no melanoma-associated antigens. 19-22 Melanocytes in coculture are functional, and melanosomes from melanocytes have been successfully transferred to keratinocytes in a skin equivalent model. 23,24 More recently, several skin reconstruction models have been used to investigate the biological properties of normal human melanocytes. 9,10,25-27
Tissue culture of melanoma cells also induces a different phenotype due to the two-dimensional growth conditions, and the biological properties of cultured melanoma cells only partially resemble those in situ. 28 Cultured cells from radial growth phase (RGP) melanoma have characteristics of both malignant and non-malignant cells: they are immortal but do not grow anchorage independently in soft agar nor are they tumorigenic in mice. 29 In addition, RGP cells require exogenous growth factors 30 for continuous growth in culture due to limited autoproduction of mitogens. In a typical RGP lesion, melanoma cells predominantly reside in the epidermis, with little invasion into the dermis. RGP primary melanomas are considered metastasis-incompetent, ie, they do not invade lymphatics and capillaries. Melanoma cells from the biologically advanced vertical growth phase (VGP) of primary lesions have an infinite lifespan 31 they proliferate independently of exogenous growth factors, with the exception of insulin, 32 because they produce a variety of growth factors. 33 Of these, basic fibroblast growth factor (bFGF) appears to be the most important, because inhibition of bFGF or its receptor in melanoma cells with antisense oligodeoxynucleotides inhibits their growth. 34-37
In this study, we analyzed the growth patterns of melanoma cells representing different stages of tumor progression. As a model, we used human skin reconstructs with normal melanocytes, RGP and VGP primary and metastatic melanomas, which were all incorporated into the epidermis to simulate the physiological context. We found distinct growth patterns that mirrored the lesions from which the cells were originally derived, as well as a remarkable stability in the biological phenotype of melanoma cells over time in culture. With melanoma progression, there was an aberrant formation of the basement membrane and the ability of VGP and metastatic melanoma cells to invade the dermis and proliferate there. RGP melanomas survived in the dermal in vitro environment and were tumorigenic in vivo only when cells were transduced to overexpress the bFGF gene.
Materials and Methods
Tissue Culture
Keratinocytes, fibroblasts, and melanocytes were isolated from neonatal foreskins (obtained from Cooperative Human Tissue Network, Philadelphia, PA) after routine circumcision, and cultured as described. 21 All reagents were obtained from Sigma Chemical Co. (St. Louis, MO) except for recombinant human epidermal growth factor (EGF) and bovine pituitary extract (BPE), which were obtained from Gibco-BRL (Grand Island, NY). Keratinocyte cultures were maintained in keratinocyte growth medium (KGM) composed of MCDB 153 medium supplemented with amino acids, 38 0.1 mmol/L ethanolamine, 0.1 mmol/L O-phosphorylethanolamine, 5 × 10−7 mol/L hydrocortisone, 5 μg/ml insulin, 5 ng/ml recombinant human EGF, and 100 μg/ml BPE. Keratinocytes at passage 2–5 were used for skin reconstructs.
Human primary (RGP: WM35; VGP: WM793 and WM115) and metastatic melanoma (WM852) cells were isolated and cultured as described. 29-31 Sbcl2 RGP-like cells were a gift of Dr. B. Giovanelli (Stehlin Foundation for Cancer Research, St. Joseph Hospital, Houston, TX). The highly aggressive melanoma cell lines 451Lu and 1205Lu were selected from lung metastatic lesions in mice after subcutaneous (s.c.) injection of WM164 and WM793 melanoma cells, respectively. 39,40 Melanoma cells were maintained in melanocyte growth medium in the absence of EGF, phorbol ester, and BPE. All cell lines were used between 50 and 100 passages in culture except Sbcl2 cells, which were at more than 200 passages. All cultures were tested twice a year for absence of mycoplasma contamination.
In Vitro Reconstruction of Human Skin and Melanoma
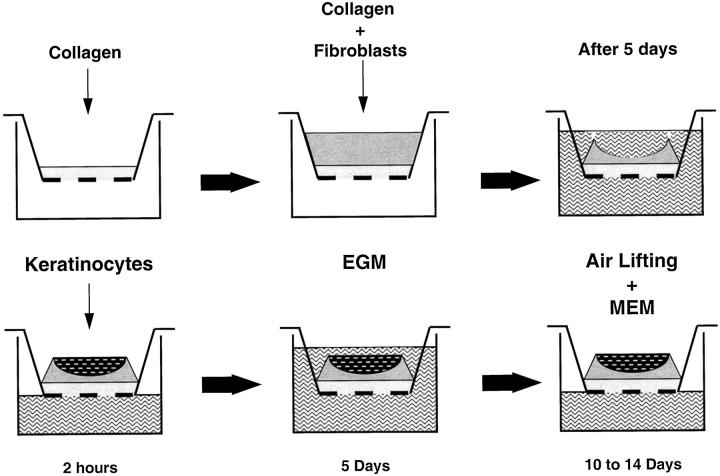
Reconstructs were generated using described techniques and media formulations 3,30,41 with modifications. For dermal reconstruction, 1 ml of a cell-free buffered collagen solution consisting of rat tail collagen, type I (Collaborative Biomedical, Bedford, MA), at a final concentration of 1.35 mg/ml in DMEM with 10% FCS was added to tissue culture inserts (Transwell, Costar, Cambridge, MA) in 6-well plates (Figure 1) ▶ . This precoated acellular layer was then overlaid with 3 ml of fibroblast-containing collagen (1.125 × 105/ml). After a 5-day incubation at 37°C, fibroblasts had contracted the collagen gel, which formed a concave central area for subsequent seeding of epidermal cells. For epidermal reconstruction, the mature dermal reconstructs were rinsed and equilibrated with 2 ml of epidermal growth medium (EGM) as described. 41 Minor modifications included the addition of 2% dialyzed FCS and the omission of linoleic acid. After 1 hour, EGM was removed, and the surface of the dermal reconstructs was allowed to dry. Neonatal foreskin keratinocytes (1.5 × 105/reconstruct, in a total volume of 50 μl) were seeded onto the concave center of the dermal reconstructs and incubated at 37°C for 2 hours to allow attachment of the seeded cells. Composite cultures were then submerged by adding 3 ml of EGM outside and 2 ml inside of the insert. Seeded keratinocytes were allowed to attach and proliferate. After 4 to 6 days of submerged culture, with regular feedings every 2 days, skin reconstructs were lifted to the air-liquid interface and medium was switched to maintenance medium 41 with similar modifications described for EGM above. After 10 to 14 days of air exposure, skin reconstructs were harvested. Skin reconstructs were fixed overnight with 4% paraformaldehyde, dehydrated, and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E). For incorporation of melanocytes or melanomas, cells were seeded together with keratinocytes onto dermal reconstructs at a 1:5 ratio of melanocytic cells to keratinocytes. Culture conditions were the same as for reconstructs containing keratinocytes alone. Human melanoma cell lines derived from RGP (WM35, Sbcl2) and VGP primary (WM793, WM115) and metastatic melanomas (451Lu, 1205Lu, WM852) were tested in the reconstructs. All experiments were performed in duplicate. For testing tumorigenicity of Sbcl2 cells, 5 × 10 6 cells were injected s.c. into 5 SCID mice per group. Tumor growth was monitored for 4 weeks. Paraffin sections of reconstructs and tumor tissues were stained immunohistochemically with antibodies detecting type IV collagen, laminin, Ki67 proliferation marker, or S100 melanocytic markers all using standard methods. Apoptosis in paraffin sections was evaluated using an in situ apoptosis detection kit (ApopTag, Oncor, Gaithersburg, MD).
Figure 1.
Schematic of in vitro reconstruction of human skin. Collagen is layered into inserts followed by a layer of collagen with fibroblasts. After 5 days, collagen is constricted by the fibroblasts. Keratinocytes with melanocytes/melanoma cells are added into the developing nest, grown submerged in epidermal growth medium (EGM) for 5 days, followed by air lifting and medium change to maintenance medium (MEM).
Adenoviral Vectors
The adenoviral vector carrying the lacZ reporter gene (LacZ/Ad5) was obtained from the Vector Core, University of Pennsylvania (Philadelphia, PA). The adenoviral vector carrying the bFGF gene (bFGF/Ad5) has been described in detail elsewhere. 42 bFGF/Ad5 induces in normal melanocytes, at 20 plaque forming units (PFU)/cell, at 48 hours, a biologically active 18-kd protein that is found predominantly in the cytoplasm but that can also be found in the culture supernatant. Through the use of adenoviral vectors expressing β galactosidase or green fluorescent protein we showed that, in preliminary experiments, 100% of melanoma cells were transduced after 48 hours when infected at 20 PFU/cell. Melanocytes and melanoma cells transduced with bFGF/Ad5 express the protein in vitro and in vivo. 42
Results
In Vitro Reconstruction of Human Skin
Human skin reconstructs resembled the architecture of skin in situ (Figure 2) ▶ . The dermal equivalent contained extracellular matrix material (collagen) with interspersed fibroblasts. The fibroblasts were single, bipolar, and horizontally oriented, and migrated into the acellular collagen gel, forming a homogeneously structured dermis. The epidermal equivalent comprised proliferating basal cells and sequentially differentiated stratified cell layers (Figure 2A) ▶ . The basal cells were vertically oriented, well-organized and cuboidal to columnar, containing oval-shaped nuclei. Numerous layers of progressively flattening keratinocytes with less prominent nuclei constituted the stratum spinosum. A stratum granulosum, approximately 3 layers thick, formed above the stratum spinosum. A stratum corneum consisting of flattened, anucleate corneocytes developed on top. Compared to human skin, skin reconstructs lacked rete ridges. When melanocytes were mixed with keratinocytes, they settled at the interface between dermis and epidermis (Figure 2, B and C) ▶ , where they remained singly with a multidendritic morphology throughout the life span of the reconstructs of 28 to 35 days.
Figure 2.
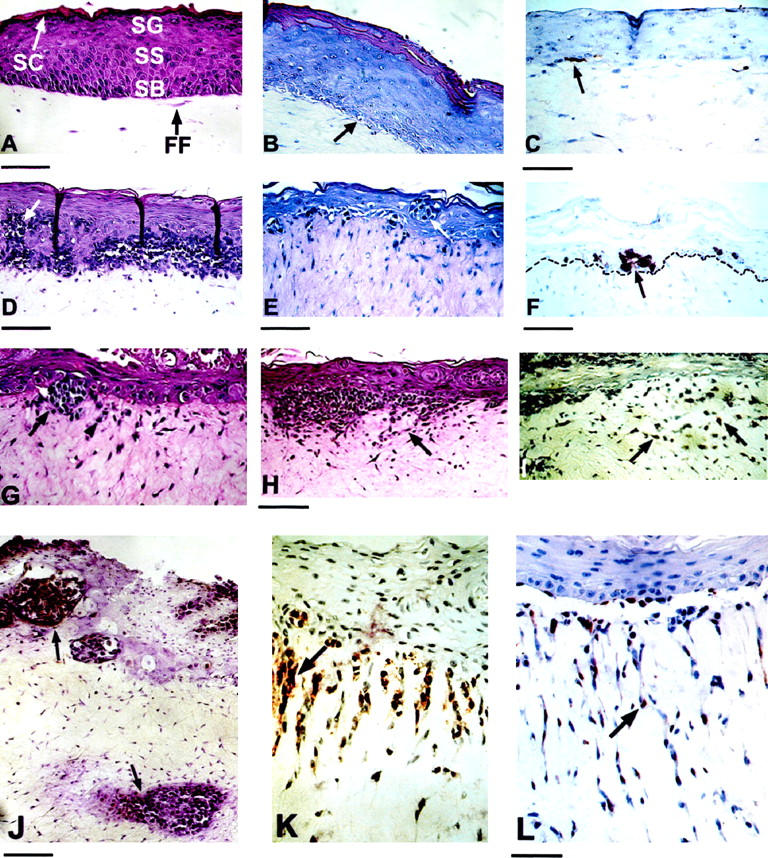
Characterization of human skin reconstructs. Reconstructs containing no melanocytic cells (A), normal melanocytes (B and C), or RGP (D-F), VGP (G-I), and metastatic (J-L) melanoma cells. A: H&E staining of reconstructs containing keratinocytes as the only cellular component in the epidermis. Keratinocytes form the typical epidermal structure, consisting of proliferating basal cells and sequentially differentiated stratified cell layers. SB, stratum basale; SS, stratum spinosum; SG, stratum granulosum; SC, stratum corneum; FF, fibroblast embedded in collagen. Bar, 17.2 μm. B: H&E staining of skin reconstruct with fibroblasts, keratinocytes, and melanocytes. Bar, 9.9 μm. Melanocytes (arrow) are singly located within the stratum basale and maintain a ratio of 1:5 to 1:10 with basal keratinocytes. C: Staining of melanocyte (arrow) for S-100 protein. Bar, 14.85 μm. D: H&E staining of melanoma reconstructs containing RGP melanoma cells Sbcl2. Melanoma cell nests within the epidermis and band-like melanoma cell aggregates at the epidermal-dermal junction. Arrowhead indicates apoptotic bodies in melanoma cells. Bar, 23.7 μm. E: H&E staining of RGP melanoma cells WM35, which form nests in the epidermis (arrow). Bar, 16.95 μm. F: Staining of WM35 melanoma cells for S-100. The broken line highlights the basement membrane. G: H&E staining of VGP melanoma WM793 in skin reconstructs. Arrow indicates melanoma cell cluster at the epidermal-dermal junction. Arrowhead shows individual melanoma cells infiltrating the dermis. Bar, 34.4 μm. H: H&E staining of reconstruct with VGP melanoma WM115 cells. Arrow indicates a melanoma cells infiltrating the dermis. Bar, 12.25 μm. I: Staining of WM793 for S-100 expression. Numerous melanoma cells infiltrate the dermis (arrows). Bar, 17.1 μm. J: S-100 staining of reconstructs of metastatic melanoma 451Lu cells. Melanoma cells form clusters (arrows) within the epidermis, at the epidermal-dermal junction, and in the dermis. Bar, 11.25 μm. K: S-100 staining for metastatic 1205Lu cells. Arrow indicates vertical invasive growth of melanoma cell strands deep into the dermis. Bar, 11.25 μm. L: Staining of metastatic WM852 reconstructs. Arrow shows aggressive vertical invasive growth of individual melanoma cells deep into the dermis. Bar, 11.1 μm.
Reconstruction of Primary and Metastatic Melanomas
To study the biological properties of melanoma cells from different stages of progression, we incorporated seven human melanoma cell lines derived from RGP and VGP primary and metastatic melanomas into skin reconstructs. RGP melanoma cells Sbcl2 formed nests within the epidermis and band-like tumor cell aggregates at the epidermal-dermal junction (Figure 2D) ▶ . RGP primary melanoma cells, WM35 (Figure 2E) ▶ , were disposed as individual cells and as small nests within the epidermis and at the epidermal-dermal junction. Staining for S-100 protein (Figure 2F) ▶ demonstrated the lack of competence for invasive growth into the dermis. VGP primary melanoma cells WM793 (Figure 2G) ▶ and WM115 (Figure 2H) ▶ formed nests and clusters at the epidermal-dermal junction, and exhibited invasive growth into the dermis as demonstrated by staining for S-100 protein (Figure 2I) ▶ . Whereas WM793 VGP melanoma cells invaded the dermis in clusters with only a few individual cells preceding the cluster, WM115 cell invaded the dermis more individually.
When metastatic melanoma cells were incorporated into skin reconstructs, they displayed rapid proliferation and aggressive invasive growth deep into the dermis. As shown in Figure 2J ▶ , 451Lu metastatic melanoma cells formed tumor cell clusters at the epidermal-dermal junction, and exhibited growth of tumor cell nests in the dermis. Figure 2K ▶ reflects that 1205Lu metastatic cells were disposed as tumor cell nests at the epidermal-dermal junction and showed vertical invasive growth of tumor cell strands deep into the dermis. WM852 metastatic cells (Figure 2L) ▶ also displayed rapid vertical growth of individual tumor cells deep into the dermis.
Basement Membrane Development
Staining for type IV collagen (a component of the lamina densa) of normal human skin reconstructs revealed linear deposition along the epidermal-dermal junction (Figure 3A) ▶ , indicating that in a physiological context, keratinocytes and fibroblasts synthesize and deposit basement membrane proteins in vitro. Laminin was also deposited in the basement membrane zone, with diffuse and weak staining in the epidermis (not shown). When RGP primary melanoma WM35 were seeded together with keratinocytes, they produced collagen type IV within the epidermal nests (Figure 3, B and C) ▶ . At the same time, a seemingly intact basement membrane was formed. Seeding of VGP primary melanoma cells WM115 together with keratinocytes resulted in epidermal and dermal growth (Figure 3, D and E) ▶ . The basement membrane was irregular and collagen type IV was scattered throughout the lesion. When metastatic melanomas were seeded together with keratinocytes, collagen type IV distribution was similar to VGP melanomas within and surrounding tumor cell nests.
Figure 3.
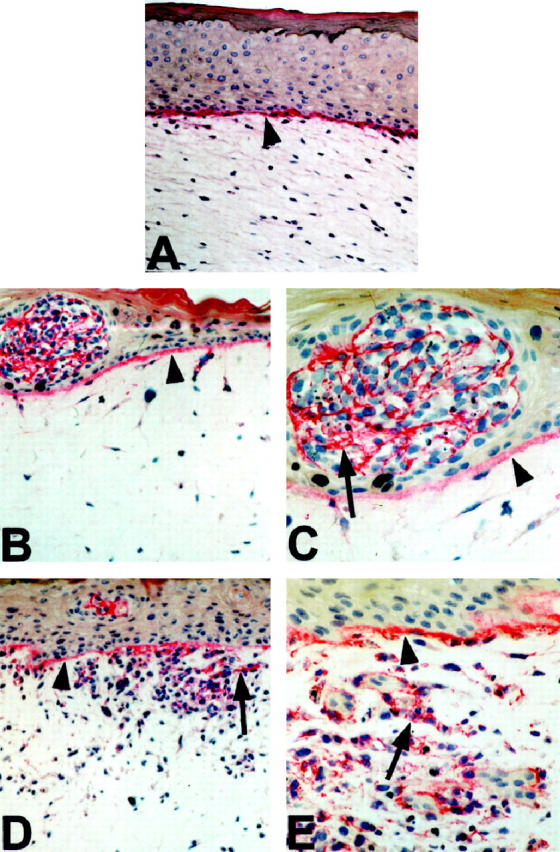
Presence of collagen type IV in reconstructs containing melanocytic cells at different stages of tumor progression. A: Normal skin reconstructs. Linear deposition of collagen type IV (arrowhead) in the basement membrane zone at the dermo-epidermal junction. Magnification, ×50. B: Deposits of collagen type IV with an epidermal nest of RGP melanoma WM35. Arrowhead indicates the basement membrane. Magnification, ×50. C: Same reconstruct as B. Magnification, ×100. D: Collagen deposits surrounding VGP melanoma WM115 (arrow), which have invaded the basement membrane (arrowhead). Magnification, ×50. E: Same reconstruct as D. Magnification, ×100.
bFGF Production Supports RGP Primary Melanomas in Dermis and in Vivo
RGP melanomas either cannot penetrate the basement membrane for migration into the dermis (see Figure 2, D ▶ -F), or on entering the dermis they undergo apoptosis. 4 Because bFGF is a major growth and survival factor for melanocytes and melanomas, we determined whether bFGF could induce proliferation of RGP melanoma cells in the dermis in the absence of an overlaying keratinocyte layer. Sbcl2 transduced with the control vector LacZ/Ad5 and then embedded together with fibroblasts remained singly distributed in the dermal matrix (Figure 4A) ▶ , and the cells did not proliferate (Figure 4C) ▶ . Parental Sbcl2 cells or cells transduced with control do not produce bFGF as determined by immunostaining and Western blotting. 42 On the other hand, when the melanoma cells were transduced with the bFGF gene before incorporation into reconstructs, they expressed bFGF and formed small nests within the matrix (Figure 4B) ▶ . The bFGF-overexpressing Sbcl2 cells also migrated outside of the dermis and adhered to its surface, where they proliferated and formed nests (Figure 4D) ▶ . The Sbcl2 cells transduced with bFGF were also tumorigenic when injected s.c. into SCID mice, whereas LacZ transduced control cells were not. The control Sbcl2 cells all died within 4 days after injection and the injected cells were no longer detectable. On the other hand, bFGF-transduced cells developed into palpable lesions within 10 days. After 14 days a tumor nodule had formed (Figure 4E) ▶ with numerous cells expressing the proliferation marker Ki67 (Figure 4F) ▶ . Due to the transient nature of adenovirus-induced gene expression, tumors regressed after 3 to 4 weeks.
Figure 4.
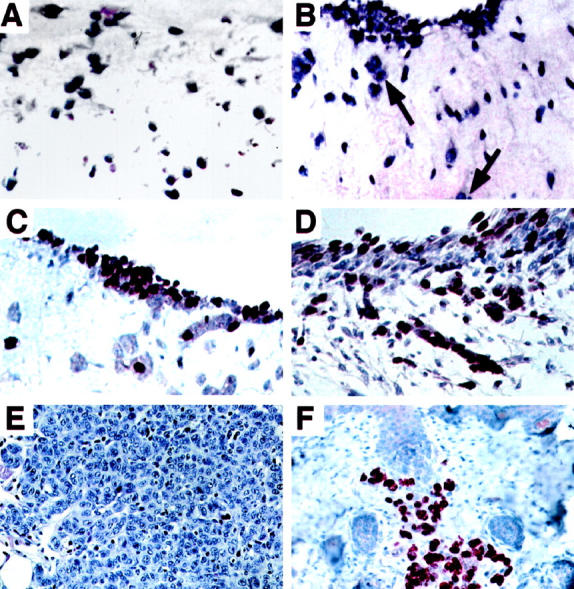
Survival, growth, and migration of RGP melanoma cells Sbcl2 in dermal equivalent after transduction with bFGF gene. A: Sbcl2 melanoma cells transduced with lacZ control gene and embedded together with fibroblasts in dermis were stained with hematoxylin and eosin. Magnification, ×50. B: Sbcl2 cells transduced with bFGF and mixed with fibroblasts in collagen form small clusters in the collagen and migrate out of the matrix for nest formation (arrows). Magnification, ×50. C: Sbcl2 cells transduced with LacZ control vector and stained for proliferation marker Ki67. Magnification, ×50. D: Sbcl2 cells transduced with bFGF, stained for Ki67. Section was counterstained with Mayer’s hematoxylin. Magnification, ×50. E: H & E stain of tumor from SCID mouse injected s.c. 4 days earlier with bFGF-transduced Sbcl2 cells of section. Magnification, ×40. F: Section of bFGF-transduced Sbcl2 cells stained after 14 days for Ki67 proliferation marker. Magnification, ×40.
Discussion
The clinical and histological features of melanoma development and progression have been well described, 43 and a sequence of steps has been proposed: common acquired nevi, dysplastic nevi, RGP and VGP primary melanomas, and metastatic melanomas. Due to the easy accessibility of the lesions and recent advances in culture techniques, cell lines from various stages of tumor progression are available in culture and have been extensively characterized. 44 The biological properties of cultured melanocytic cells resemble those in vivo, but typical characteristics of cultured cells such as growth factor requirements, anchorage-independent growth, growth factor production and infinite life span cannot be directly related to in vivo growth. The antigenic profile of VGP primary and metastatic melanoma cells is comparable between cultured cells and those in situ but the profile of melanocytes and RGP cells is very different. 21,22,45 Cultured tumor cells also acquire chromosomal abnormalities such as mutations or deletions in the p16 gene, 46 which could make it difficult to interpret results obtained with cell lines. Our model represents a new tool for the in vitro characterization of cell lines from different stages of progression that mimics biological properties in vivo better than previous attributes. In skin reconstructs consisting of artificial dermis and epidermis, melanocytic cells from different stages of progression show remarkable consistency in their growth and migration patterns, as would be expected from observations in normal skin and patients with melanoma. Normal melanocytes home to the basement membrane, where they are placed singly within the basal keratinocyte without apparent proliferation. 23,24 The reconstructs confirm clinical observations that RGP primary melanomas proliferate predominantly in the epidermis, whereas VGP melanomas grow invasively into the dermis. Metastatic melanomas aggressively invade both epidermis and dermis. Within each group, cell lines show individual growth patterns, confirming the unique nature of each lesion and the derived cell line. The reconstruction of melanoma also confirms our previous in vivo studies, in which melanoma cells proliferated orthotopically in human skin grafted to immunodeficient mice with characteristics similar to those in patients. 40 Thus, despite long-term culture of at least 50 subcultures melanoma cell lines remained stable in their biological phenotype when grown in vitro or in vivo in a cutaneous environment that is similar to that in patients.
Melanocytes adhere to keratinocytes through E-cadherin. 47 This close interaction allows keratinocytes to regulate expression of cell surface antigens on melanocytes and to control cell growth. 21,22 Melanoma cells do not express E-cadherin 48 and thus grow independently of keratinocytes. Although RGP melanomas grow separated from the basement membrane in the upper layers of the epidermis, they remain dependent on keratinocytes. When RGP melanoma cells are transduced with the β3 subunit of the αvβ3 vitronectin receptor, they grow invasively into the dermis 4 and are tumorigenic in immunodeficient mice. It remains unknown which genes are activated to trigger the increased growth and invasiveness after overexpression of an adhesion receptor.
The present study suggests that RGP primary melanoma cells require activation of the gene bFGF for survival, proliferation, and migration into the dermis. bFGF is apparently the most important growth factor in melanoma. 28 bFGF has autocrine growth stimulatory functions because cells cannot survive in vitro and in vivo without it. 17,34-37 RGP melanomas do not express bFGF protein when cell extracts are tested by Western blotting, although RNA can be detected after 30 cycles by reverse transcriptase-polymerase chain reaction. 49 The low level production of bFGF by RGP melanomas is apparently insufficient for survival of those RGP melanoma cells that begin to enter the dermis from the epidermis. In the epidermis, keratinocytes provide the necessary bFGF for melanocytes 17 and apparently also for RGP cells. bFGF appears to stimulate the expression of an enzyme(s) that degrades collagen I which is tightly constricted by the embedded fibroblasts. The up-regulation of metalloproteinases for collagens by bFGF has been reported. 50
Reconstructs displayed the basement membrane that separates the epidermis and dermis. Apparently both keratinocytes and fibroblasts contribute to its formation in normal skin reconstructs. 51 In RGP melanoma reconstructs, fibroblasts and keratinocytes can still form an intact basement membrane, but the melanoma cells begin to synthesize their own collagen IV. The synthesis of collagen type IV and laminin by melanomas has also been demonstrated in patients’ lesions. 52 VGP melanomas traversing into the dermis no longer allow the formation of an intact basement membrane. Instead, collagen type IV appears to be randomly distributed throughout the lesion. It is not clear whether this reflects increased degradation or decreased production of the collagen type IV. However melanoma cells producing their own collagen type IV are unlikely to increase the production of degradative enzymes. It is possible that the malignant cells transmit signals to fibroblasts in the stroma to produce less collagen. Further studies are needed to determine the cross-talk between normal and malignant cells for the production of proteolytic enzymes and their activators. The human skin reconstruct model should be ideally suited to investigate the relative contribution of individual genes for invasion. Molecular engineering of each normal skin constituent and of the tumor cells will allow a better dissection of each step of invasion.
Footnotes
Address reprint requests to Dr. Meenhard Herlyn, The Wistar Institute, 3601 Spruce Street, Philadelphia, PA 19104. E-mail: herlynm@wistar.upenn.edu.
Supported in part by Natinal Institutes of Health grants CA47159, CA25874, CA76674, and CA10815 and by NASA Grant NAG 9–832.
References
- 1.Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T: A living tissue formed in vitro and accepted as a full thickness skin equivalent. Science 1981, 211:1042-1054 [DOI] [PubMed] [Google Scholar]
- 2.Bilbo PR, Nolte CJM, Oleson MA, Mason VS, Parenteau NL: Skin in complex culture: the transition from ‘culture’ phenotype to organotypic phenotype. J Toxicol-Cutaneous Ocular Toxicol 1993, 12:183–196
- 3.Chen C-S, Lyons-Giordano B, Lazarus GS, Jensen PJ: Differential expression of plasminogen activators and their inhibitors in an organotypic skin co-culture system. J Cell Sci 1993, 106:45-53 [DOI] [PubMed] [Google Scholar]
- 4.Hsu M-Y, Shih D-T, Meier FE, Van Belle P, Hsu J-Y, Elder DE, Buck CA, Herlyn M: Adenoviral gene transfer of β3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. Am J Pathol 1998, 153:1435-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonetti O, Hoogstrate JA, Bialik W, Kempenaar JA, Schrijvers AHGJ, Bodde HE, Ponec M: Visualisation of diffusion pathways across the stratum corneum of native and in vitro reconstructed epidermis by confocal laser scanning microscopy. Arch Dermatol Res 1995, 287:465-473 [DOI] [PubMed] [Google Scholar]
- 6.Kuroyanagi Y, Shiraishi A, Tanaka M, Kageyama H, Ootage N, Shioya N: Cytotoxicity tests for antimicrobial agents using cultured skin substitutes fixed at interface of air and culture medium. J Biomater Sci Polym Ed 1996, 7:1005-1015 [DOI] [PubMed] [Google Scholar]
- 7.Ponec M, Kempenaar J: The use of human skin recombinants as an in vitro model for testing the irritation potential of cutaneous irritants. Skin Pharmacol 1995, 8:49-59 [DOI] [PubMed] [Google Scholar]
- 8.Jansson K, Kratz G, Haegerstrand A: Characterization of a new in vitro model for studies of reepithelization in human partial thickness wounds. In Vitro Cell Dev Biol Anim 1996, 32:534-540 [DOI] [PubMed] [Google Scholar]
- 9.Archambault M, Yaar M, Gilchrest BA: Keratinocytes and fibroblasts in a human skin equivalent model enhance melanocyte survival and melanin synthesis after ultraviolet irradiation. J Invest Dermatol 1995, 104:859-867 [DOI] [PubMed] [Google Scholar]
- 10.Nakazawa K, Nakazawa H, Sahuc F, Lepavec A, Collombel C, Damour O: Pigmented human skin equivalent: new method of reconstitution by grafting an epithelial sheet onto a non-contractile dermal equivalent. Pigment Cell Res 1997, 10:382-390 [DOI] [PubMed] [Google Scholar]
- 11.Rennekampff HO, Kiessig V, Hansbrough JF: Current concepts in the development of cultured skin replacements. J Surg Res 1996, 62:288-295 [DOI] [PubMed] [Google Scholar]
- 12.Limat A, Mauri D, Hunziker T: Successful treatment of chronic leg ulcers with epidermal equivalents generated from cultured autologous outer root sheath cells. J Invest Dermatol 1996, 107:128-135 [DOI] [PubMed] [Google Scholar]
- 13.Sabolinski ML, Alvarez O, Auletta M, Mulder G, Parenteau NL: Cultured skin as a “smart material” for healing wounds: experience in venous ulcers. Biomaterials 1996, 17:311-320 [DOI] [PubMed] [Google Scholar]
- 14.Eisinger M, Marko O: Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and choleratoxin. Proc Natl Acad Sci USA 1982, 79:2018-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu M-Y, Herlyn M: Cultivation of normal human epidermal melanocytes. Jones GE eds. Human Cell Culture Protocols. 1996, :pp 9-20 NJ, Humana Press, Totowa [DOI] [PubMed] [Google Scholar]
- 16.Herlyn M, Shih I-M: Interactions of melanocytes and melanoma cells with the micro-environment. Pigment Cell Res 1994, 7:81-88 [DOI] [PubMed] [Google Scholar]
- 17.Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott G, Moellmann G, McGuire J: Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol 1988, 107:1611-1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herlyn M, Rodeck U, Mancianti ML, Cardillo FM, Lang A, Ross AH, Jambrosic J, Koprowski H: Expression of melanoma-associated antigens in rapidly dividing human melanocytes in culture. Cancer Res 1987, 47:3057-3061 [PubMed] [Google Scholar]
- 19.DeLuca M, D’Anna F, Bondanza S, Franzi AT, Cancedda R: Human epithelial cells induce human melanocyte growth in vitro but only skin keratinocytes regulate its proper differentiation in the absence of dermis. J Cell Biol 1988, 107:1919-1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott GA, Haake AR: Keratinocytes regulate melanocyte number in human fetal and neonatal skin equivalents. J Invest Dermatol 1991, 97:776-781 [DOI] [PubMed] [Google Scholar]
- 21.Valyi-Nagy IT, Hirka G, Jensen PJ, Shih IM, Juhasz I, Herlyn M: Undifferentiated keratinocytes control growth, morphology, and antigen expression of normal melanocytes through cell-cell contact. Lab Invest 1993, 69:152-158 [PubMed] [Google Scholar]
- 22.Shih I-M, Elder DE, Hsu M-Y, Herlyn M: Regulation of Mel-CAM/Muc18 expression on melanocytic cells of different stages of tumor progression by normal keratinocytes. Am J Pathol 1994, 145:837-845 [PMC free article] [PubMed] [Google Scholar]
- 23.Topol BM, Haines HB, Dubertret L, Bell E: Transfer of melanosomes in a skin equivalent model in vitro. J Invest Dermatol 1986, 87:642-647 [DOI] [PubMed] [Google Scholar]
- 24.Valyi-Nagy IT, Murphy GF, Mancianti ML, Whitaker D, Herlyn M: Phenotypes and interactions of human melanocytes and keratinocytes in an epidermal reconstruction model. Lab Invest 1990, 62:314-324 [PubMed] [Google Scholar]
- 25.Besson S, Surleve Bazeille JE, Pain C, Donatien P, Taieb A: Ex vivo study of skin phototypes. J Invest Dermatol 1996, 107:684–688 [DOI] [PubMed]
- 26.Todd C, Hewitt SD, Kempenaav J, Noz K, Thody A, Ponec M: Co-culture of human melanocytes and keratinocytes in a skin equivalent model: effect of ultraviolet radiation. Arch Dermatol Res 1993, 285:455-459 [DOI] [PubMed] [Google Scholar]
- 27.Haake AR, Scott GA: Physiologic distribution and differentiation of melanocytes in human fetal and neonatal skin equivalents. J Invest Dermatol 1991, 96:71-77 [DOI] [PubMed] [Google Scholar]
- 28.Herlyn M: Molecular and Cellular Biology of Melanoma. RG Landes Co., Austin, TX, 1993
- 29.Satyamoorthy K, Dejesus E, Linnenbach A, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M: Melanoma cell lines from different stages of progression and their biological and molecular analysis. Melanoma Res 1997, 7:S35-S42 [PubMed] [Google Scholar]
- 30.Hsu M-Y, Elder DE, Herlyn M: The Wistar melanoma (WM) cell lines. Human Cell Culture, vol 3, Solid Cancers. Edited by JRW Masters, B Palsson. Norwell, MA, Kluwer Academic Publishers, 1999, pp 259–274
- 31.Herlyn M, Thurin J, Balaban G, Bennicelli JL, Herlyn D, Elder DE, Bondi E, Gueny D, Nowell P, Clark WH: Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res 1985, 45:5670-5676 [PubMed] [Google Scholar]
- 32.Rodeck U, Herlyn M, Menssen HD, Furlanetto RW, Koprowski H: Metastatic but not primary melanoma cells grow in vitro independently of exogenous growth factors. Int J Cancer 1987, 40:687-690 [DOI] [PubMed] [Google Scholar]
- 33.Rodeck U: Growth factor independence and growth regulatory pathways in human melanoma development. Cancer Met Rev 1993, 12:219-226 [DOI] [PubMed] [Google Scholar]
- 34.Becker D, Meier CB, Herlyn M: Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J 1989, 8:3685-3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Becker D: Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med 1997, 3:887-893 [DOI] [PubMed] [Google Scholar]
- 36.Becker D, Johnson DE, Lee PL, Rodeck U, Herlyn M: Inhibition of the FGF receptor 1 (FGFR-1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene 1992, 7:2303-2313 [PubMed] [Google Scholar]
- 37.Halaban R, Kwon BS, Ghosh S, Delli-Bovi P, Baird A: Fibroblast growth factor as an autocrine growth factor for melanomas. Oncogene Res 1988, 3:177-186 [PubMed] [Google Scholar]
- 38.Pittelkow MR, Scott RE: New techniques in the in vitro culture of human skin keratinocytes and perspectives of their use for grafting of patients with extensive burns. Mayo Clin Proc 1986, 61:771-777 [DOI] [PubMed] [Google Scholar]
- 39.Herlyn D, Adachi K, Koprowski H, Herlyn M: Experimental model of human melanoma metastasis. Melanoma Research: Genetics, Growth Factors, Metastases and Antigens. Series on Cancer Treatment and Research. Edited by K Nathanson. Norwell, MA, Kluwer Academic Publishers, 1991, pp 105–118 [DOI] [PubMed]
- 40.Juhasz I, Albelda SM, Elder DE, Murphy GF, Adachi K, Herlyn D, Valyi-Nagy IT, Herlyn M: Growth and invasion of human melanomas in human skin grafted to immunodeficient mice. Am J Pathol 1993, 143:528-537 [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C-S, Lavker RM, Rodeck U, Risse B, Jensen PJ: Use of a serum-free epidermal culture model to show deleterious effects of epidermal growth factor on morphogenesis and differentiation. J Invest Dermatol 1995, 104:107-112 [DOI] [PubMed] [Google Scholar]
- 42.Nesbit M, Nesbit HKE, Bennet J, Andl T, Hsu M-Y, DeJesus E, McBrian E, Gupta AR, Eck SL, Herlyn M: Basic fibroblast growth factor induces a transformed phenotype in normal human melanocytes. Oncogene 1999, in press [DOI] [PubMed]
- 43.Clark WH, Jr: Human cutaneous malignant melanoma as a model for cancer. Cancer Met Rev 1991, 10:83-88 [DOI] [PubMed] [Google Scholar]
- 44.Meier F, Satyamoorthy K, Nesbit M, Hsu M-Y, Schitteck B, Garbe C, Herlyn M: Molecular events in melanoma development and progression. Frontiers in Bioscience 1998, 3:1005-1010 [DOI] [PubMed] [Google Scholar]
- 45.Mancianti ML, Gyšrfi T, Shih I-M, Valyi-Nagy I, Levengood G, Menssen H-D, Halpern AC, Elder DE, Herlyn M: Growth regulation of cultured human nevus cells. J Invest Dermatol 1993, 100:281S−287S [DOI] [PubMed]
- 46.Guldberg P, Kirkin AF, Gornbaek K, thor Straten P, Ahrenkiel V, Zeuthen J: Complete scanning of the CDK4 gene by denaturing gradient gel electrophoresis: a novel missense mutation but low overall frequency of mutations in sporadic metastatic malignant melanoma. Int J Cancer 1997, 72:780–783 [DOI] [PubMed]
- 47.Tang A, Eller MS, Hara M, Yaur M, Hirohashi S, Gilchrest BA: E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci 1994, 107:983-992 [DOI] [PubMed] [Google Scholar]
- 48.Hsu M-Y, Wheelock MJ, Johnson KR, Herlyn M: Shifts in cadherin profiles between human normal melanocytes and melanomas. J Invest Dermatol Symp Proc 1996, 1:188-194 [PubMed] [Google Scholar]
- 49.Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M: Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer 1994, 56:853-857 [DOI] [PubMed] [Google Scholar]
- 50.Buckley-Sturrock A, Woodward SC, Senior RM, Griffin GL, Klagsbrun M, Davidson JM: Differential stimulation of collagenase and chemotactic activity in fibroblasts derived from rat wound repair tissue and human skin by growth factors. J Cell Physiol 1989, 138:70-78 [DOI] [PubMed] [Google Scholar]
- 51.Smola H, Stark HJ, Thieketer G, Mirancea N, Krieg T, Fusenig NE: Dynamics of basement membrane formation by keratinocyte-fibroblast interactions in organotypic skin culture. Exp Cell Res 1998, 239:399-410 [DOI] [PubMed] [Google Scholar]
- 52.Natali PG, Nicotra MR, DiFilippo F, Bigotto A: Expression of fibronectin, fibronectin isoforms and integrin receptors in melanocytic lesions. Br J Cancer 1995, 71:1243-1247 [DOI] [PMC free article] [PubMed] [Google Scholar]