Abstract
Inverted papilloma (IP) is a proliferative lesion of the epithelium lining the sinonasal tract. Although IP often recurs after surgical excision and is sometimes associated with squamous cell carcinoma of the sinonasal cavity (SNSCC), its presumed neoplastic nature and putative role as a precursor to squamous cell carcinoma have not been confirmed at the molecular genetic level. We analyzed the pattern of X chromosome inactivation in IPs from nine female patients. Inactivation of a single allele is seen in monoclonal proliferations and may be indicative of a neoplastic process. We also analyzed 28 IPs and 6 concurrent SNSCCs for loss of heterozygosity (LOH) on chromosomal arms 3p, 9p21, 11q13, 13q11, and 17p13. Losses at these loci occur frequently during neoplastic transformation of the upper respiratory tract and can be detected in squamous cell carcinomas and the progenitor lesions from which they arise. X chromosome analysis was informative in four of the nine IPs. All four lesions demonstrated a monoclonal pattern of inactivation. LOH was not detected in any nondysplastic areas from the 28 IPs, but LOH at one or more chromosomal loci was present in all six of the concurrent SNSCCs. We conclude that IPs are monoclonal proliferations, yet they do not fit the profile of a prototypic precursor lesion. Unlike squamous epithelial dysplasia, IPs do not routinely harbor several of the key genetic alterations that are associated with malignant transformation of the upper respiratory tract.
Inverted papilloma (IP) is a distinctive proliferative lesion of the epithelium lining the sinonasal tract. Clinically, IP typically presents as a large polypoid mass unilaterally involving contiguous regions of the sinonasal tract. Its light microscopic appearance is dominated by the expansive downward (ie, inverted) growth of epithelial cells into the underlying submucosa. Although not a common lesion of the sinonasal tract, it is clinically challenging due to its propensity to recur and its association with malignancy. Approximately one-half of IPs recur after surgical excision, and about 5 to15% arise in association with a synchronous or metachronous squamous cell carcinoma of the sinonasal tract (SNSCC). 1,2 Conventional light microscopic examination is not very useful in predicting recurrence or malignancy.
Although the morphological features and clinical behavior of IP have been well described, its pathogenesis is poorly understood. Uncertainties regarding the nature and classification of IP are reflected in the plethora of synonyms used to designate this lesion. At one extreme, the term “papillary hypertrophic sinusitis” 3 conveys the notion that IP is a manifestation of chronic inflammation of the sinonasal tract. At the other extreme, the terms “papillary respiratory epithelial carcinoma” 4 and “cylindrical cell carcinoma” 5 suggest that IP is, at the very least, premalignant.
Molecular genetic approaches have now made it possible to document the presence and progression of human neoplasia. In the upper respiratory tract, for example, the precursor lesion of squamous cell carcinoma is now recognized as a clonal expansion of epithelial cells that invariably harbor specific genetic alterations. In a previous study, we found that the majority of squamous epithelial dysplasias harbor minimal losses at chromosomal loci 3p, 9p, 11q, 13q, and 17p. 6 The accumulation of these genetic alterations, in turn, drives the progression toward malignancy. 6,7 To better understand the nature of IP, we first tried to discern whether IP represents a monoclonal proliferation. We then attempted to determine whether IP, like a prototypic precursor lesion, accumulates key genetic alterations that are important in malignant transformation of the upper respiratory tract.
Materials and Methods
Cases
Twenty-eight cases of IP were retrieved from the surgical pathology files of The Johns Hopkins Hospital. In six of the cases, the IP was associated with a SNSCC (Figure 1) ▶ . In all cases, tissues had been fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin. All available slides were examined, and at least one representative tissue block was selected for DNA isolation.
Figure 1.
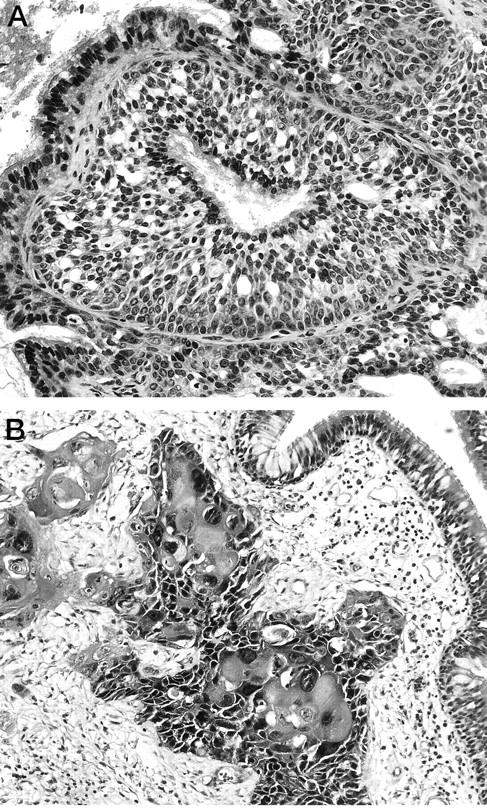
An example of a nondysplastic inverted papilloma (A) with an associated squamous cell carcinoma (B).
DNA Extraction
Microdissection was performed so that IP and normal nonepithelial tissue could be separately analyzed for X chromosome inactivation and LOH. In six cases, a distinct component of SNSCC was also present, and these carcinomas were separately microdissected and analyzed. Twenty-five sequential 14-μm sections were placed on a glass slide, and each component was individually microdissected using a dissecting microscope and polymerase chain reaction (PCR) clean technique. The samples were placed in xylenes overnight, pelleted in 70% ethanol, dried, incubated in sodium dodecyl sulfate/proteinase K at 58°C for 72 hours, and then subjected to phenol-chloroform extraction and ethanol precipitation as previously described. 8
Detection of X Chromosome Inactivation
We used X chromosome inactivation to determine the clonal status of IPs removed from nine female patients. In females, the inactivation of an X chromosome occurs randomly during early embryogenesis, remains stable throughout the lifetime of the cell, and is passed on to all daughter cells. 9 The human androgen receptor assay is a PCR-based method that utilizes a highly variable region of trinucleotide repeats in the first exon of the androgen receptor gene, with a number of CpG restriction sites located close to the repeats. 10 X chromosome inactivation is manifested by CpG island methylation. After digestion with a methyl-sensitive restriction enzyme and amplification using PCR, it is possible to distinguish between the active and inactive alleles in an informative (heterozygous) female. For a given tissue sample, nonselective amplification of both alleles indicates a mixed population of cells. Conversely, selective amplification of a single allele indicates a uniform cell population derived from a single clone.
X chromosome inactivation analysis was performed as previously described. 11 Briefly, extracted DNA from normal and lesional tissue was first subjected to HhaI digestion. Subsequently, the DNA fragments were PCR-amplified using primers for the androgen receptor located on the X chromosome. The primers included both a polymorphic repeat region and an HhaI digestion site.
Detection of Loss of Heterozygosity (LOH)
We examined the status of chromosomal arms 3p, 9p21, 11q13, 13q11, and 17p13 using nine microsatellite markers including D3S1067 and D3S1007 (chromosomal arm 3p); IFN-α, D9S736, and D9S171 (chromosomal arm 9p21); D11S873 and PYGM (chromosomal arm 11q13); D13S170 (chromosomal arm 13q21), and TP-53 (chromosomal arm 17p13). These chromosomal loci were selected because they are lost frequently (>40%) in head and neck squamous cell carcinoma (HNSCC), 7,12 and they contain important genetic determinants for the development of HNSCC. For example, 9p21 contains the p16 (MTS1) gene, a cyclin/cdk inhibitor involved in cell cycle regulation, and the p19ARF gene, a second putative tumor suppressor gene involved in the regulation of p53. 13,14 The chromosomal band 11q13 includes the bcl-1/int-2 locus, an amplicon carrying one of the few oncogenes implicated in HNSCC, cyclin D1. 15 Allelic imbalance in this region may represent amplification of cyclin D1. The p53 gene is found on chromosomal arm 17p13, which also corresponds to an area of frequent LOH in head and neck squamous cell carcinoma. 16 Chromosomal arm 3p contains at least three putative tumor suppressor loci. 17 Although the 13q21 chromosomal band contains an area with frequent LOH near the Rb locus, the pertinent loss may actually involve a novel tumor suppressor gene. 18 Importantly, these alterations are also frequently detected in the early precursor lesions of HNSCC.
Microsatellite markers were obtained from Research Genetics (Huntsville, AL). Before amplification, 50 ng of one primer from each pair was end labeled with [γ-32P]ATP (20 MCi; Amersham, Arlington Heights, IL) and T4 kinase (New England Biolabs, Beverly, MA) in a total volume of 50μl. PCR reactions were carried out in a total volume of 12.5 μl containing 10 ng of genomic DNA, 0.2 ng of labeled primer, and 15 ng of each unlabeled primer. The PCR buffer included 16.6 mmol/L ammonium sulfate, 67 mmol/L Tris (pH 8.8), 6.7 mmol/L magnesium chloride, 10 mmol/L β-mercaptoethanol, 1% dimethyl sulfoxide to which were added 1.5 mmol/L deoxynucleotide triphosphates and 1.0 units of Taq DNA polymerase (Boehringer Mannheim, Indianapolis, IN). PCR amplifications of each primer set were performed for 30 to 35 cycles consisting of denaturation at 95°C for 30 seconds, annealing at 50–60°C for 60 seconds, and extension at 70°C for 60 seconds as described. 12 One-third of the PCR product was separated on 8% urea-formamide-polyacrylamide gels and exposed to film from 4 to 48 hours. For informative cases, allelic loss (or allelic imbalance in the case of the 11q13 locus) was scored if one allele was >60% decreased in tumor DNA when compared to the same allele in normal control DNA.
Results
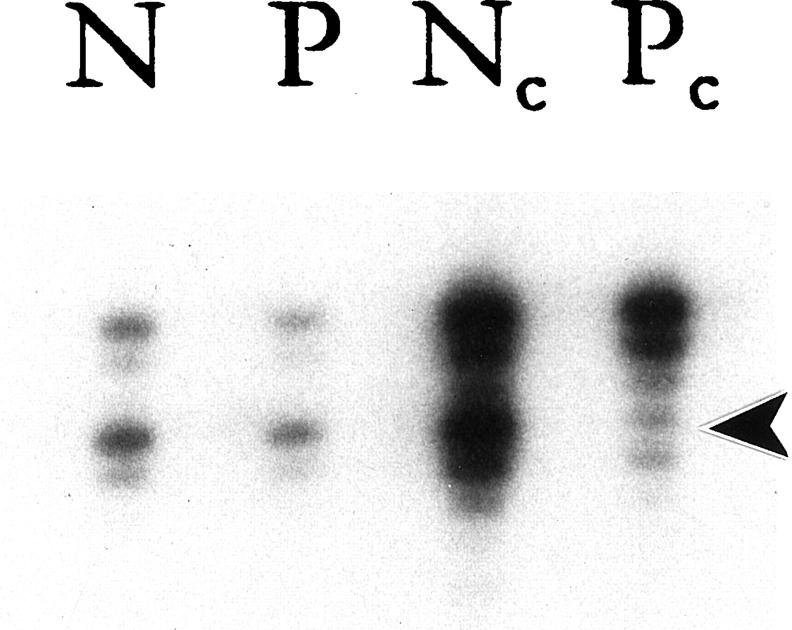
Nine of the 27 IPs were from female patients. Of these nine cases, four were informative at the androgen receptor gene locus. All four demonstrated a significant reduction in the intensity of a single allele (Figure 2) ▶ . This pattern of X chromosome inactivation supports the monoclonal nature of IPs. One of these 4 cases was associated with a synchronous infiltrating SNSCC. Although the IP and the SNSCC both showed a monoclonal pattern of X chromosome inactivation, the inactivated allele was not the same. For this particular case, the IP and the SNSCC appeared to arise from different clones.
Figure 2.
Representative example of X chromosome inactivation at the androgen receptor locus. DNA samples from normal tissues (N) and papilloma (P) were subjected to a mock reaction (rows 1 and 2) or digested with HhaI (rows 3 and 4, c = cut) followed by PCR amplification. After digestion with HhaI, the normal specimen revealed no change in relative intensity of the two alleles, a normal polyclonal pattern. Conversely, the papilloma displayed a significant reduction in the intensity of the lower allele (arrowhead) as compared to the upper allele, a typical monoclonal pattern.
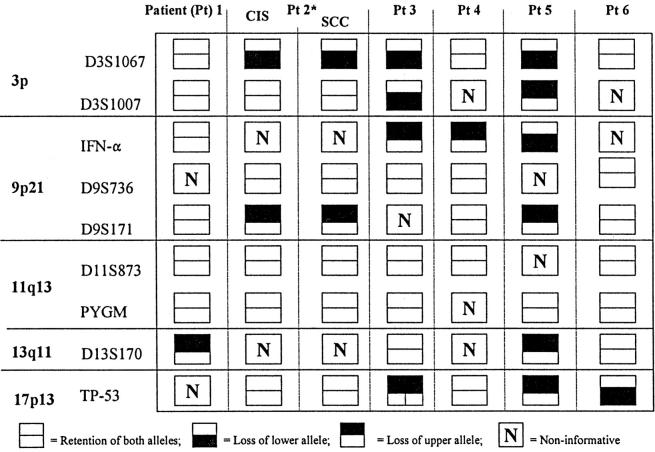
All of six of the synchronous SNSCCs demonstrated LOH in at least one chromosomal locus tested (Table 1 ▶ , Figure 3 ▶ ). The number of chromosomal arms demonstrating LOH in these carcinomas ranged from one to four. Case 2 demonstrated in situ and infiltrating squamous cell carcinoma, and both components showed the same pattern of LOH (Table 1 ▶ and Figure 3A ▶ ). In striking contrast, LOH was not detected in any of the 28 IPs, including the six cases associated with a synchronous SNSCC.
Table 1.
Loss of Heterozygosity in Squamous Cell Carcinomas Associated with Inverted Papillomas
*Patient 2 had carcinoma in situ (CIS) within an inverted papilloma, and a component of infiltrating squamous cell carcinoma (SCC)
Figure 3.
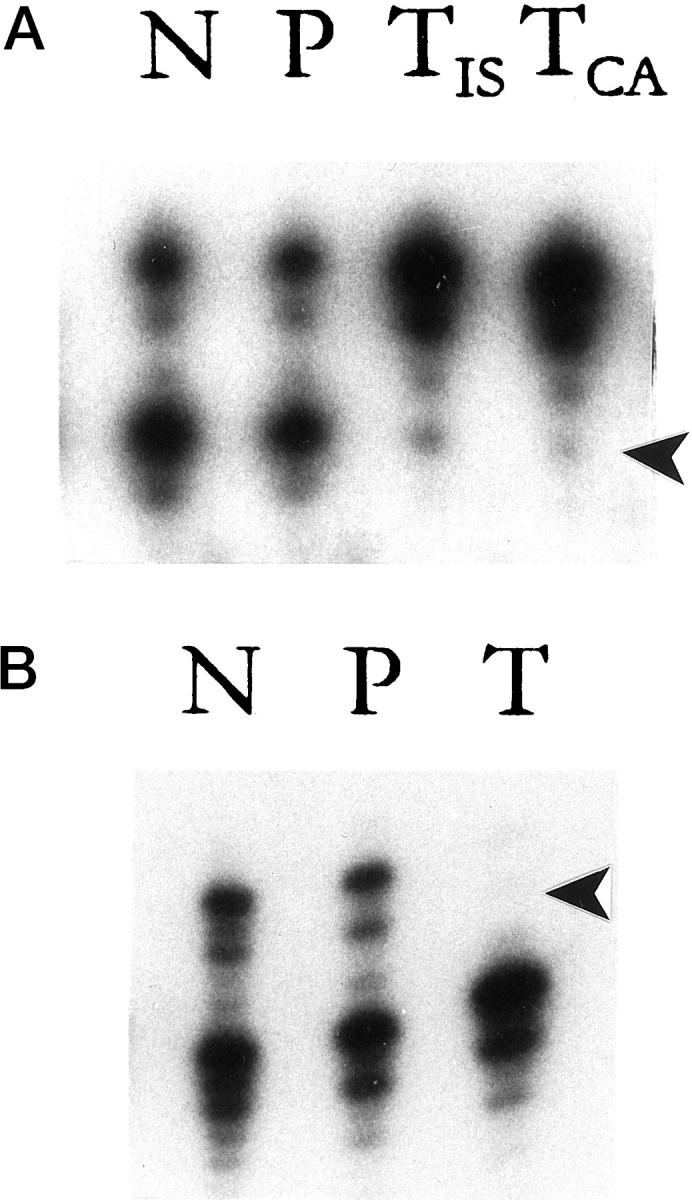
Analysis of allelic status in paired normal tissue (N), papilloma (P), and squamous cell carcinoma (TIS, in situ carcinoma; TCA, invasive carcinoma) in two representative patients with synchronous sinonasal squamous cell carcinomas. The selected markers are from chromosome 3 (marker D3S1067; A) and chromosome 9 (marker D9S171; B). In Patient 2 (A), both alleles are retained in nondysplastic areas of the papilloma, but the lower allele is lost in areas of in situ and invasive squamous cell carcinoma. In Patient 5 (B), both alleles are retained in the papilloma, but the upper allele is lost in the invasive squamous cell carcinoma. Arrowhead indicates the lost allele.
Discussion
The true nature of IPs has been subject to various interpretations. Some observers have considered them a manifestation of chronic sinusitis, 3 whereas others have regarded them as premalignant. 4,5 Accordingly, treatment recommendations have historically varied from simple excisions to radical cancer resections. 1 The current consensus view lies somewhere between these extremes. Based on its well-documented capacity to spread, destroy, and recur, IP is generally acknowledged as a neoplasm and managed by aggressive, albeit nonmutilating, excision to ensure complete removal. 19,20 The precise relationship of IP to SNSCC is less certain. Although a subset of IP is associated with carcinomas, it is unknown whether this association is coincidental, indirect (eg, independent lesions that share common etiologies), or direct (eg, stages along a proliferation-dysplasia-carcinoma continuum).
Using a molecular genetic approach, we attempted to confirm the clonal nature of IP and clarify its status as a putative precursor lesion of SNSCC. X chromosome inactivation is an effective method for distinguishing monoclonal from polyclonal proliferations. 11 In support of the view that IPs are neoplasms, we found that IPs arise uniformly from a single progenitor cell. The propensity of IPs to recur locally after surgical excision likely reflects the process of intramucosal clonal spread, extension to the surgical margin, and subsequent re-expansion of the residual neoplastic clone.
Just because a lesion is monoclonal does not necessarily mean that it will consistently progress to a malignant neoplasm. The cysts of polycystic renal disease, for example, are lined by monoclonal proliferations of epithelial cells, but these cells rarely develop into renal cell carcinomas. 21 According to current models of tumorigenesis, true precursor lesions are regarded as clonal cellular proliferations that invariably harbor alterations of critical oncogenes and tumor suppressor genes. As these genetic alterations accumulate, the lesion passes through various stages of histological progression, ultimately culminating in a carcinoma. In the well-established precursor lesions of head and neck squamous cell carcinoma (ie, squamous epithelial dysplasia), these genetic alterations can be detected in the earliest stages of tumor progression, even before the morphological features of dysplasia are well developed. 6,22 Because of their entwined anatomical proximity, several investigators have speculated that IP is a requisite progenitor lesion that directly gives rise to SNSCC. Although our results confirm that IP is indeed monoclonal, they do not support this notion that IP is necessarily premalignant. In one case where an IP and its synchronous SNSCC could be compared for patterns of X chromosome inactivation, the two neoplasms arose from entirely different clones. Although this one case does not discount the well-documented observation that IP sometimes harbor areas of dysplasia and carcinoma in situ, it does raise the possibility that the IP and synchronous or metachronous SNSCC can sometimes arise as independent lesions.
The benign nature of IPs was further demonstrated by the striking absence of key genetic alterations that occur commonly and early during progression toward HNSCC. None of the nondysplastic IPs, including six associated with squamous cell carcinoma, demonstrated LOH at 3p, 9p, 17p, 11q, or 13q. In another study addressing the molecular alterations of IPs, 23 p53 gene mutations were observed in areas of dysplasia and invasive carcinoma but not in nondysplastic IPs. Thus, IPs are unlike recognized classic precursor lesions, which accumulate key genetic alterations. 24,25 Indeed, they are quite different from squamous epithelial dysplasia, a well-recognized progenitor of HNSCC, where specific genetic alterations are present in the earliest stages of tumor progression, even before the morphological features of dysplasia are well developed. 6,22
Unlike squamous cell carcinomas involving other sites of the head and neck, those arising in the sinonasal tract are not strongly associated with smoking activity. Instead, epidemiological studies have implicated occupational exposure, rather than tobacco exposure, as a major determinant for cancer development, 26 and molecular genetic studies have pointed to human papilloma virus as an important etiological factor. 23,27 Conceivably, tumor progression in the sinonasal tract may involve genetic alterations that are not routinely present in smoking-related cancers. However, when we evaluated the six synchronous SNSCCs for LOH at 3p, 9p, 17p, 11q, and 13q, we found that their genetic profile was very similar to squamous cell carcinomas arising elsewhere in the upper respiratory tract. 12 In all six SNSCCs, LOH involved at least one of the chromosomal arms tested, and most tumors had demonstrated LOH at multiple loci. Etiological differences aside, the development of squamous cell carcinoma of the upper respiratory tract appears to involve similar genetic pathways for tumors arising within and outside of the sinonasal tract.
We have confirmed that IPs are monoclonal proliferations, consistent with common patterns of behavior including the propensity to spread, destroy, and recur following incomplete removal. Although monoclonal, they are not necessarily premalignant. IPs do not fit the profile of a prototypic precursor lesion in that they do not harbor critical genetic alterations that are known to drive malignant transformation of the upper respiratory tract. In some instances, concurrent IPs and SNSCC may even arise from entirely different progenitor cells.
Footnotes
Address reprint requests to Dr. William H. Westra, Department of Pathology, Meyer 7–181, 600 North Wolfe Street, Baltimore, Maryland 21287. E-mail: wwestra@jhmi.edu.
Supported by grant R01 DE012588-01 from the National Institute of Dental and Craniofacial Research.
References
- 1.Hyams VJ: Papillomas of the nasal cavity and paranasal sinuses: a clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol 1971, 80:192-206 [DOI] [PubMed] [Google Scholar]
- 2.Lawson W, Ho BT, Shaari CM, Biller HF: Inverted papilloma: a report of 112 cases. Laryngoscope 1995, 105:282-288 [DOI] [PubMed] [Google Scholar]
- 3.Lehman RH: Papillary sinusitis. Ann Otol 1949, 58:507. [PubMed] [Google Scholar]
- 4.Willis RA: Pathology of Tumors. 1960:pp 360-356 Butterworth and Company, London
- 5.Ormerod FC: Cylindrical-cell carcinoma of the nose. Acta Otolaryngol 1952 (suppl), 100:84–97 [DOI] [PubMed]
- 6.Califano J, van der Riet P, Westra WH, Nawroz H, Clayman G, Piantadosi S, Corio R, Lee D, Greenberg B, Koch W, Sidransky D: Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res 1996, 56:2488-2492 [PubMed] [Google Scholar]
- 7.Califano J, Westra WH, Meininger G, Corio R, Koch WM, Sidransky D: Genetic progression and clonal relationship of recurrent premalignant head and neck lesions. Clin Cancer Res 1999, 57:1862-1867 [PubMed] [Google Scholar]
- 8.van der Riet P, Karp D, Farmer E, Wei Q, Grossman L, Tokino K, Ruppert JM, Sidransky D: Progression of basal cell carcinoma through loss of chromosome 9p and inactivation of a single p53 allele. Cancer Res 1994, 54:25-27 [PubMed] [Google Scholar]
- 9.Lyon MF: Evolution of X-chromosome inactivation in mammals. Nature 1974, 250:651-653 [DOI] [PubMed] [Google Scholar]
- 10.Mashal RD, Lester SC, Sklar J: Clonal analysis by study of X chromosome inactivation in formalin-fixed paraffin-embedded tissue (erratum published in Cancer Res 1994, 54: 1873). Cancer Res 1993, 53:4676-4679 [PubMed] [Google Scholar]
- 11.Bedi GC, Westra WH, Gabrielson E, Koch W, Sidransky D: Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res 1996, 56:2484-2487 [PubMed] [Google Scholar]
- 12.Nawroz H, van der Riet P, Hruban RH, Koch W, Ruppert JM, Sidransky D: Allelotype of head and neck squamous cell carcinoma. Cancer Res 1994, 54:1152-1155 [PubMed] [Google Scholar]
- 13.Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA: Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368:753-756 [DOI] [PubMed] [Google Scholar]
- 14.Sherr CJ, Roberts JM: Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 1995, 9:1149-1163 [DOI] [PubMed] [Google Scholar]
- 15.Callender T, El Naggar AK, Lee MS, Frankenthaler R, Luna MA, Batsakis JG: PRAD-1 (CCND1)/cyclin D1 oncogene amplification in primary head and neck squamous cell carcinoma. Cancer 1994, 74:152-158 [DOI] [PubMed] [Google Scholar]
- 16.Soder AI, Hopman AH, Ramaekers FC, Conradt C, Bosch FX: Distinct nonrandom patterns of chromosomal aberrations in the progression of squamous cell carcinomas of the head and neck. Cancer Res 1995, 55:5030-5037 [PubMed] [Google Scholar]
- 17.Maestro R, Gasparotto D, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M: Three discrete regions of deletion at 3p in head and neck cancers. Cancer Res 1993, 53:5775-5779 [PubMed] [Google Scholar]
- 18.Yoo GH, Xu HJ, Brennan JA, Westra W, Hruban RH, Koch W, Benedict WF, Sidransky D: Infrequent inactivation of the retinoblastoma gene despite frequent loss of chromosome 13q in head and neck squamous cell carcinoma. Cancer Res 1994, 54:4603-4606 [PubMed] [Google Scholar]
- 19.Myers EN, Schramm VLJ, Barnes ELJ: Management of inverted papilloma of the nose and paranasal sinuses. Laryngoscope 1981, 91:2071-2084 [DOI] [PubMed] [Google Scholar]
- 20.Myers EN, Fernau JL, Johnson JT, Tabet JC, Barnes EL: Management of inverted papilloma. Laryngoscope 1990, 100:481-490 [DOI] [PubMed] [Google Scholar]
- 21.Qian F, Watnick TJ, Onuchic LF, Germino GG: The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 1996, 87:979-987 [DOI] [PubMed] [Google Scholar]
- 22.Westra WH, Sidransky D: Phenotypic and genotypic dysparity in premalignant lesions: of calm water and crocodiles. J Natl Cancer Inst 1998, 90:1500-1501 [DOI] [PubMed] [Google Scholar]
- 23.Caruana SM, Zwiebel N, Cocker R, McCormick SA, Eberle RC, Lazarus P: p53 alteration and human papilloma virus infection in paranasal sinus cancer. Cancer 1997, 79:1320-1328 [PubMed] [Google Scholar]
- 24.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL: Genetic alterations during colorectal tumor development. N Engl J Med 1988, 319:525-532 [DOI] [PubMed] [Google Scholar]
- 25.Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 [DOI] [PubMed] [Google Scholar]
- 26.Cann CI, Fried MP, Rothman KJ: Epidemiology of squamous cell cancer of the head and neck. Otolaryngol Clin North Am 1985, 18:367-388 [PubMed] [Google Scholar]
- 27.Furuta Y, Shinohara T, Sano K, Nagashima K, Inoue K, Tanaka K, Inuyama Y: Molecular pathologic study of human papillomavirus infection in inverted papilloma and squamous cell carcinoma of the nasal cavities and paranasal sinuses. Laryngoscope 1991, 101:79-85 [DOI] [PubMed] [Google Scholar]