Abstract
Laminins are a large family of heterotrimeric basement membrane molecules that mediate crucial cell functions such as adhesion, proliferation, migration, and differentiation. Up to now, three distinct laminins have been identified in the normal human small intestinal epithelium. Laminin-1 (α1β1γ1) and laminin-5 (α3β3γ2) are mainly expressed at the base of villus cells, whereas laminin-2 (α2β1γ1) is restricted to the bottom of the crypts. The expression of these molecules has not yet been studied in Crohn’s disease (CD), but it could be altered, in light of the important changes occurring in the architecture of the crypt-villus axis under the active state of the disease. To test this hypothesis, the expression of laminin α1, α2, and α3 subunits was analyzed in control, inflamed, and corresponding uninflamed CD small intestinal specimens by indirect immunofluorescence and reverse transcriptase-polymerase chain reaction. Surprisingly, α1 and α3 remained strongly expressed by all villus cells, whereas α2, normally expressed in the bottom of the crypts in control and uninflamed CD specimens, was lacking in inflamed CD specimens. However, this loss of α2 expression was associated with a significant up-regulation of both α1 and α3 expression in the crypts of inflamed CD specimens. A significant up-regulation of the α1 subunit was also observed in the crypts of uninflamed CD specimens. At the transcript levels, α1 was found significantly higher in inflamed than uninflamed CD specimens. Taken together, these observations identify important alterations in laminin expression in the small intestine with CD and suggest that compositional changes in the epithelial basement membrane may play a role in this disease.
Crohn’s disease is a chronic inflammatory bowel condition with a relatively high incidence among Caucasians and with a peak age of onset of between 15 and 25 years. 1,2 Whereas the disease may affect any segment of the digestive tract, it most frequently involves the small intestine (small intestine only, 30%; ileum and cecum, 40%). 2,3 Pathological features of Crohn’s disease include transmural and granulomatous inflammation, fibrosis, alteration of the crypt-villus architecture, and epithelial cell injury. 1,2,4
The etiology of the disease remains incompletely understood but it is becoming more evident that interacting environmental and genetic factors are both of great importance. 2,5 Another concept that has begun to receive considerable attention pertaining to intestinal inflammation is the involvement of essentially all cellular and acellular components of the mucosa, including immune cells and epithelial, mesenchymal, and endothelial cells, as well as the extracellular matrix (ECM), and that the dysfunction of any component of this intricate system may lead to a disruption in communication resulting in pathological inflammation. 5
The importance of ECM molecules in the regulation of inflammation, including the recruitment and state of activation of leukocytes, has been clearly demonstrated in various systems. 6-10 However, the information is still extremely limited for intestinal inflammation. 11 Indeed, in contrast to the normal human intestine where the expression, location, and possible function of most ECM molecules, namely laminins, type IV collagens, fibronectins, and tenascin, as well as their receptors in the integrin family, have been determined, 12,13 almost nothing is known about ECM composition and function in inflammatory bowel diseases. 14
As a first step in investigating this question, we have chosen to focus on laminins. Laminins represent a major component of all basement membranes both quantitatively and functionally. 15 The members of this multigene family have been shown to mediate several cellular activities, namely the promotion of adhesion, migration, growth, and tissue-specific gene expression, depending on the laminin and cell type studied. 13,15,16 In the human small intestine, three distinct laminins have been identified: laminin-1 (α1β1γ1) and laminin-5 (α3β3γ2), which are mainly expressed at the base of villus cells, and laminin-2 (α2β1γ1), which is restricted to the bottom of the crypts. In this study, the expression and distribution of the three α chains specific to these laminins have been analyzed in controls as well as in inflamed and uninflamed mucosa from patients with Crohn’s disease, by indirect immunofluorescence and reverse transcriptase (RT)-polymerase chain reaction (PCR). Our observations identify major alterations in the pattern of laminin expression in crypts of the small intestine with Crohn’s disease and therefore support the hypothesis that ECM molecules can participate in the chronic intestinal inflammation cascade.
Materials and Methods
Tissues
Eleven specimens of distal ileum with Crohn’s disease were available for the study. For each patient (five males and six females; ages 22–40), samples from both inflamed and uninflamed (resection margin) areas were obtained, and the diagnosis was confirmed by a pathologist. Three of these patients received no treatment before surgery. Six control specimens obtained from the nondiseased part (at least 10 cm distant from lesions) of resected ileum for pathologies other than Crohn’s disease (bowel obstruction, primary lymphoma, or tumor) were also included in the study. All tissues were processed within 1 hour after surgery. The project was in accordance with a protocol approved by the Institutional Human Research Review Committee of the Université de Sherbrooke for the use of human material. The preparation and embedding of tissues for cryosectioning were performed as described previously. 17,18
Indirect Immunofluorescence
Cryosections, 2–3 μm thick, were cut on a Jung Frigocut 2800N cryostat (Leica Canada, Saint-Laurent, Canada) and fixed in methanol (10 minutes, −20°C) for the detection of α1 (4C7 19 ) and α2 (5H2 and 2G9 19,20 ) or in freshly prepared 2% paraformaldehyde in phosphate-buffered saline (60 minutes, 4°C) for the detection of α3 (BM2 21 ). The secondary antibody was Cy2-conjugated goat anti-mouse IgG (Amersham-Pharmacia, Baie d’Urff, Canada). Sections were stained with Evan’s blue (0.01% in phosphate-buffered saline), mounted in glycerol:phosphate-buffered saline (9:1) containing 0.1% paraphenylene diamine, and viewed with a Reichert Polyvar 2 microscope (Leica Canada) equipped for epifluorescence. In all cases, no immunofluorescent staining was observed when primary antibodies were replaced by mouse nonimmune serum.
RNA Extraction and RT-PCR
Total RNAs from the six available series of paired, inflamed and uninflamed CD specimens were prepared by Clontech’s Atlas Total RNA Isolation protocol (Clontech, Palo Alto, CA) and stored in RNasecure (Ambion, Austin, TX) at −80°C. First, strand complementary DNA synthesis with Superscript II (Life Technologies, Inc., Burlington, Canada) was performed on 5 μg total RNA by using oligo(dT) 12-18 (Amersham-Pharmacia) as primer. Conditions for amplification of laminin α1 and α2 chains and S14, used as endogenous control, have been described previously. 22,23 For laminin α3 chain, we used the sense primer 5′-GGACCTCAACGTCGGTCA-3′ and the antisense primer 5′-CAGGGATCCTCAGTGTCGTC-3′, which amplified a 209-bp amplicon at nucleotides 4814–5023 of laminin α3. 24 Single-stranded complementary DNA was amplified for 30 cycles of denaturation (1 minute at 94°C) and annealing/extension (1 minute at 53°C and 1 minute at 72°C) in a thermal cycler (Perkin-Elmer DNA thermal cycler, Model 480, Foster City, CA) in the presence of 250 μmol/L deoxyribonucleoside triphosphates and 2.5 U of Taq polymerase (Qiagen, Mississauga, Canada).
Results
Distribution of Laminin α Chains along the Crypt-Villus Axis
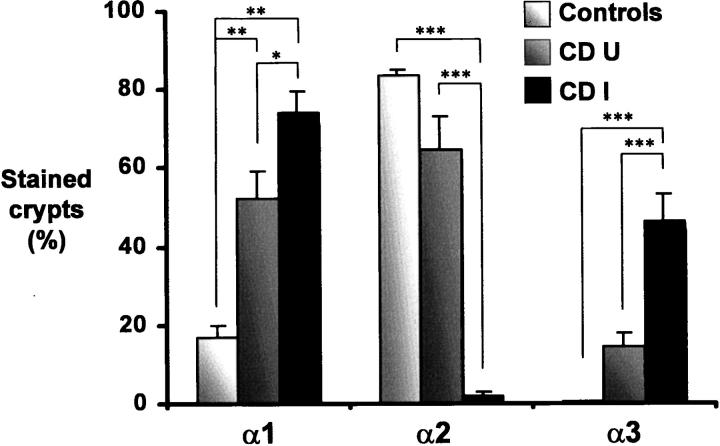
The expression of the α1, α2, and α3 chains of laminin and their distribution at the epithelial basement membrane along the crypt-villus axis were determined by indirect immunofluorescence with chain-specific monoclonal antibodies. In control specimens not affected by CD, the α1 chain was uniformly detected in the villus, but it was below the detection level in most crypts (Figure 1a) ▶ as also noted for the α3 chain, whereas the α2 chain was found to be confined to the lower half of the crypts as previously observed. 18,25 In uninflamed CD specimens (resection margins), the normal histology of the ileal mucosa was relatively well preserved (Figure 1, b–e) ▶ , and the distribution of the α2 and α3 chains was comparable to controls, α2 being restricted to the bottom of the crypts (Figure 1d) ▶ and α3 to the villi (Figure 1e) ▶ . However, in addition to its normal distribution in the villus, the α1 chain was consistently found also in a large proportion of the crypts, in most studied specimens (10 of 11), according to a staining pattern ranging from the lower half of the glands (Figure 1b) ▶ to the entire gland (Figure 1c) ▶ . In inflamed CD specimens, the villi were short and wide, whereas the crypts were irregular and generally exhibited a larger diameter (Figure 1, f–h) ▶ . The α1 and α3 chains were found in the basement membrane of all villi and also in a large proportion of crypts (Figure 1, f and h) ▶ . However, the α2 chain was not detected in these specimens (Figure 1g) ▶ . As summarized in Figure 2, a ▶ significant up-regulation of the α1 chain was observed in the crypts of both uninflamed and inflamed CD specimens, whereas alterations in the other laminin α chains were restricted to the crypts of inflamed CD specimens, the α3 chain being significantly increased and the α2 chain being mostly undetectable (Figure 2) ▶ .
Figure 1.
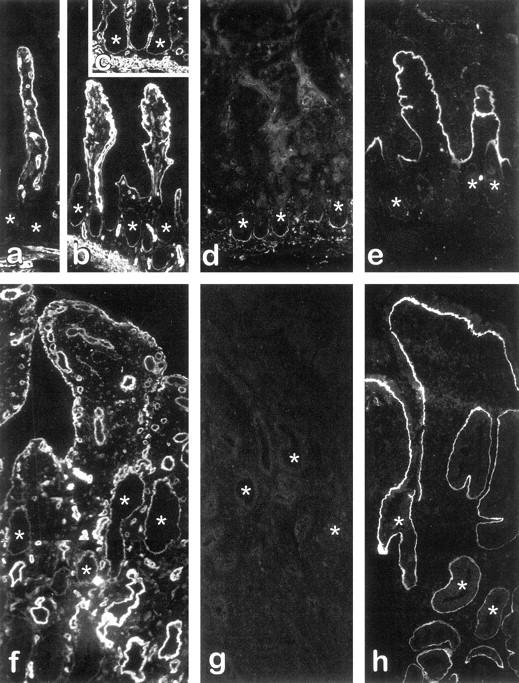
Expression and distribution of laminins in the small intestine from patients with CD. Indirect immunofluorescence micrographs from representative fields of cryosections from control specimens (a) as well as uninflamed (b–e) and inflamed (f–h) paired regions of CD specimens stained for the detection of laminin α1 (a–c, f), α2 (d, g), and α3 (e, h) chains. The α1 chain was found to be expressed in the basement membrane of villus cells under all conditions (a–c, f) as well as in crypts (asterisks) of uninflamed (b, c) and inflamed (f) CD specimens, but not in crypts of control specimens (a). The α2 chain remained normally expressed in uninflamed CD specimens, being restricted to the lower half of the crypts (d), whereas it was consistently undetectable in most inflamed CD specimens (g). The α3 chain was detected only in the basement membrane of the villus in uninflamed tissues (e), whereas it was found to stain both villus and crypt in their inflamed counterparts (h). Original magnification, ×128. Asterisks denote crypts.
Figure 2.
Expression of laminins in the crypts of control and CD small intestine. Proportions (in percentages) of crypts expressing laminin α1,2, and 3 chains were determined by indirect immunofluorescent staining in control as well as in paired uninflamed (CDU) and inflamed (CDI) CD small intestinal sections by counting a minimum of 100 crypts on each section. Partially stained crypts (see Figure 1b ▶ as an example of minimally labeled crypts) were scored as positive. Six distinct specimens were used as controls, whereas 11 paired specimens were analyzed in the CD group. Asterisks over bars indicate a statistical difference: *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Expression of Laminin α Chain Transcripts in CD
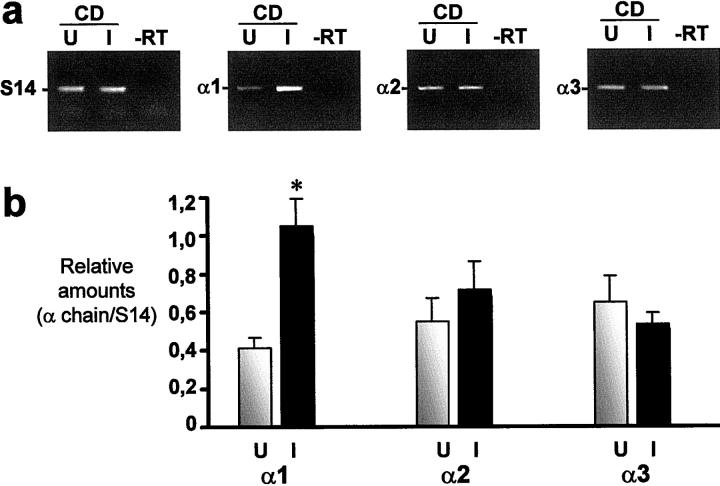
Laminin α chain expression in CD was further investigated at the transcript level by RT-PCR on 6 of the 11 specimens studied by indirect immunofluorescence. As shown in Figure 3a ▶ , the three laminin α chains were found to be expressed in all CD specimens, including α2. Furthermore, the relative amount of α1 mRNA was found to be significantly higher in inflamed than uninflamed CD specimens, although the α2 and α3 transcript levels remained comparable, statistically (Figure 3b) ▶ .
Figure 3.
Expression of laminin α chain transcripts in small intestine from patients with CD. a: Representative RT-PCR analysis of α1, α2, and α3 chain mRNA in uninflamed (U) and corresponding inflamed (I) CD specimens. -RT, RT omitted. S14 transcript was determined to ensure complementary DNA integrity and to compare amounts of starting RNA material in the various samples. b: Amounts of α1, α2, and α3 transcripts in paired uninflamed (U) and inflamed (I) specimens (n = 6) were determined relative to S14. Statistical differences between U and I were noted for α1 (asterisk: P < 0.05).
Discussion
These results showing alterations of laminin expression in the CD small-intestinal mucosa represent clear evidence that ECM molecules can be involved in chronic intestinal inflammation, as postulated recently. 5 Our data pointed out three particular phenomena occurring in the inflamed and uninflamed CD small intestine, respectively. First, there is a major reorganization of the basement membrane of the crypts in inflamed intestinal segments, which consists of the disappearance of laminin-2 and its replacement by laminin-1 and laminin-5, as indicated by the distribution patterns of their respective α chain expression. Indeed, the α1 and γ1 chains, which are shared by both laminin-1 and -2, have been found constitutively expressed along the crypt-villus axis in the human small intestine. 18,26,27 A switch from laminin α2 to α1 chain expression would thus result in the expression of laminin-1 instead of laminin-2. Alternatively, because 4C7 (which is the only antibody available for immunodetection of α1) may also recognize the α5 laminin chain, 28 the expression of laminin-10 (α5β1γ1) in the human small intestine remains a possibility. The expression of heterotrimeric α3β3γ2 laminin-5 in inflamed CD crypts is supported by the extracellular deposition of the α3 chain in the basement membrane (see Figure 1 ▶ h), as well as of β3 and γ2 chains (data not shown). However, because the α3 chain can also complex with β1, β2, and γ1, it cannot be excluded, at this time, that a proportion of α3 is expressed as laminin-6 (α3β1γ1) and/or laminin-7 (α3β2γ1). 29
The second particular feature observed in the small intestine of CD patients is the expression of the laminin α1 chain in the crypts of uninflamed segments, a phenomenon rarely observed in control specimens from non-CD patients. 18,26 It is interesting that, in most of these specimens, the crypt staining for laminin-1 invariably included the bottom of the glands (see Figure 1,b and c ▶ ), the site of laminin-2 expression. This situation is reminiscent of that of the fetus, in which laminin-1 and -2 are coexpressed in the developing crypts. 27
A third feature of potential pathological interest is the up-regulation in the expression of the α1 transcript in the small-intestinal mucosa of inflamed CD specimens. Increased production of ECM molecules in inflammatory bowel diseases has been previously observed for type III collagen 30 and tenascin, 31 a phenomenon that seems to be related to an increased proliferation of various cellular elements of the lamina propria, such as smooth muscle cells and fibroblasts. 4,11 The higher expression of the α1 chain in the inflamed CD mucosa observed herein appears consistent with these previous observations because α1, in contrast to α2 and α3, is also expressed by nonepithelial cellular elements of the intestinal mucosa, namely smooth muscle cells and blood vessels. 18,25
Taken together, these observations raise two questions. (a) What is the mechanism of alteration of laminin expression in chronic inflammation? (b) What are the functional consequences of laminin redistribution along the crypt-villus axis? For the first question, cytokines and growth factors are likely to play a central role. Indeed, as recently reviewed, alteration in the profile of numerous cytokines, as well as growth factors, is one of the hallmarks of intestinal inflammation. 11 As demonstrated with transforming growth factor β, 32 these molecules can affect gene expression in various cell types including epithelial cells and subepithelial myofibroblasts, both responsible for various laminin chain expression. 23 The presence of elevated levels of matrix metalloproteinases in regions of inflamed mucosa in Crohn’s disease may also contribute to compositional changes in the basement membrane. 33 This latter possibility should be considered for the laminin α2 chain, which disappears at the protein level as shown by immunofluorescence, while its transcript remains normally expressed. Cytokines could also be responsible for the higher expression of laminin-1 in the crypts of histologically normal CD mucosa, because the production of some, such as tumor necrosis factor-α and interleukin-1β and -6, also appears to be abnormally elevated in uninvolved CD mucosal biopsies. 34
To the second question pertaining to the functional consequences of alterations in laminin expression, it may be hypothesized that replacement of laminin-2 by laminin-1 and -5 in crypts of inflamed CD specimens, as well as coexpression of laminin-1 and -2 in the crypts of uninflamed CD specimens, is of functional relevance, based on the evidence that laminin-1 and -2 exhibit a distinct ability to modulate intestinal cell-specific gene expression in vitro. 35 However, laminins mediate their effects through membrane receptors, namely integrins. Many of the laminin-binding integrins have been found to be expressed in the normal small intestine, including α2β1, α3β1, α7Bβ1, and α6β4, 17,22,36 which can bind differentially to distinct laminins and mediate specific cell functions. 13,16 Further work will thus be required to better delineate, at the cellular level, the functional implications of laminin expression alteration.
Acknowledgments
The authors thank Dr. R. Burgeson, Cutaneous Biology Research Center, Harvard Medical School, Boston, MA, and E. Engvall, The Burnham Institute, La Jolla, CA, for their kindness in providing the antibodies, Dr. M. Lessard from the Department of Pathology (Université de Sherbrooke) for his cooperation in providing the specimens, and Dr. P. H. Vachon for critical reading of the manuscript.
Footnotes
Address reprint requests to Jean-François Beaulieu, Département d’anatomie et de biologie cellulaire, Faculté de médecine, Université de Sherbrooke, Sherbrooke, Qué., Canada J1H 5N4. E-mail: jf.beaul@courrier. usherb.ca.
Supported by Grants MT-11289 and GR-15186 from the Medical Research Council of Canada and grants from the Fonds pour la Formation des Chercheurs et l’Aide la Recherche.
References
- 1.Chadwick VS: Etiology of chronic ulcerative colitis and Crohn’s disease. Phillips SF Pemberton JH Shorter RG eds. The Large Intestine: Physiology, Pathophysiology, and Disease. 1991, :pp 445-463 Raven Press, New York [Google Scholar]
- 2.Stenson WF: Inflammatory bowel disease. ed 2 Yamada T eds. Textbook of Gastroenterology, 1995, :pp 1748-1806 JB Lippincott, Philadelphia [Google Scholar]
- 3.Janowitz HD, Mauer K: Crohn’s disease. Gitnick G eds. Inflammatory Bowel Disease. 1991, :pp 101-112 Igaku-Shoin, New York [Google Scholar]
- 4.Schattenfroh S, Bartels M, Nagel E: Early morphological changes in Crohn’s disease. Acta Anat 1994, 149:237-246 [PubMed] [Google Scholar]
- 5.Fiocchi C: Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998, 115:182-205 [DOI] [PubMed] [Google Scholar]
- 6.Heino J: Integrin-type extracellular matrix receptors in cancer and inflammation. Ann Med 1993, 25:335-342 [DOI] [PubMed] [Google Scholar]
- 7.Pakianathan DR: Extracellular matrix proteins and leukocyte function. J Leukocyte Biol 1995, 57:699-702 [DOI] [PubMed] [Google Scholar]
- 8.Sheppard D: Epithelial integrins. BioEssays 1996, 18:655-660 [DOI] [PubMed] [Google Scholar]
- 9.Raghow R: The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J 1994, 8:823-831 [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Shaw S: Lymphocyte interactions with extracellular matrix. FASEB J 1991, 5:2292-2299 [DOI] [PubMed] [Google Scholar]
- 11.Fiocchi C: Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol 1997, 273:G769-G775 [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu J-F: Expression of extracellular matrix components and integrins in relationship to intestinal epithelial cell differentiation in vivo. Prog Histochem Cytochem 1997, 31:1-76 [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu J-F: Integrins and human intestinal cell functions. Front Biosci 1999, 4:d310-d321 [DOI] [PubMed] [Google Scholar]
- 14.Bouatrouss Y, Poisson J, Beaulieu J-F: Studying the basement membrane. Preedy VR Watson RR eds. Methods in Disease: Investigating the Gastrointestinal Tract. 1998, :pp 191-200 Greenwich Medical Media, London [Google Scholar]
- 15.Timpl R, Brown J: Supramolecular assembly of basement membranes. BioEssays 1996, 18:123-132 [DOI] [PubMed] [Google Scholar]
- 16.Mercurio AM: Laminin receptors: achieving specificity through cooperation. Trends Cell Biol 1995, 5:419-423 [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu J-F: Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci 1992, 102:427-436 [DOI] [PubMed] [Google Scholar]
- 18.Beaulieu J-F, Vachon PH: Reciprocal expression of laminin A-chain isoforms along the crypt-villus axis in the human small intestine. Gastroenterology 1994, 106:829-839 [DOI] [PubMed] [Google Scholar]
- 19.Engvall E, Earwicker D, Haaparanta T, Ruoslahti E, Sane JR: Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul 1990, 1:731-740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leivo I, Engvall E: Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve, and muscle development. Proc Natl Acad Sci USA 1988, 85:1544-1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rousselle P, Lunstrum GP, Keene DR, Burgeson RE: Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol 1991, 114:567-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basora N, Vachon PH, Herring-Gillam FE, Perreault N, Beaulieu J-F: Relation between integrin α7Bβ1 expression in human intestinal cells and enterocytic differentiation. Gastroenterology 1997, 113:1510-1521 [DOI] [PubMed] [Google Scholar]
- 23.Perreault N, Herring-Gillam FE, Desloges N, Belanger I, Pageot L-P, Beaulieu J-F: Epithelial versus mesenchymal contribution to the extracellular matrix in the human intestine. Biochem Biophys Res Commun 1998, 248:121–126 [DOI] [PubMed]
- 24.Ryan MC, Tizard R, VanDevanter DR, Carter WG: Cloning of the lamA3 gene encoding the α3 chain of the adhesive ligand epiligrin: expression in wound repair. J Biol Chem 1994, 269:22779-22787 [PubMed] [Google Scholar]
- 25.Leivo I, Tani T, Laitinen L, Bruns R, Kivilaakso E, Lehto VP, Burgeson RE, Virtanen I: Anchoring complex components laminin-5 and type VII collagen in the intestine: association with migration and differentiating enterocytes. J Histochem Cytochem 1996, 44:1267-1277 [DOI] [PubMed] [Google Scholar]
- 26.Simon-Assmann P, Duclos B, Orian-Rousseau V, Arnold C, Mathelin C, Engvall E, Kedinger M: Differential expression of laminin isoforms and α6-β4 integrins subunits in the developing human and mouse intestine. Dev Dyn 1994, 201:71-85 [DOI] [PubMed] [Google Scholar]
- 27.Perreault N, Vachon PH, Beaulieu J-F: Appearance and distribution of laminin A chain isoforms and integrin α2, α3, α6, β1 and β4 subunits in the developing human small intestinal mucosa. Anat Rec 1995, 242:242-250 [DOI] [PubMed] [Google Scholar]
- 28.Tiger CE, Champliaud MF, Pedrosa-Domello F, Thornell E, Ekblom P, Gullberg D: Presence of laminin α5 chain and lack of laminin α1 chain during human muscle development and in muscular dystrophies. J Biol Chem 1997, 272:28590-28595 [DOI] [PubMed] [Google Scholar]
- 29.Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, Timpl R, Tryggvason K, Yamada Y, Yurchenco PD: A new nomenclature for the laminins. Matrix Biol 1994, 14:209-211 [DOI] [PubMed] [Google Scholar]
- 30.Stallmach A, Schupman D, Riese HH, Matthes H, Rieken EO: Increased collagen type III synthesis by fibroblasts isolated from strictures of patients with Crohn’s disease. Gastroenterology 1992, 102:1920-1929 [DOI] [PubMed] [Google Scholar]
- 31.Riedl S, Kadmon M, Tandara A, Hinz U, Moller P, Herfarth C, Faissner A: Mucosal tenascin C content in inflammatory and neoplastic diseases of the large bowel. Dis Colon Rectum 1998, 41:86-92 [DOI] [PubMed] [Google Scholar]
- 32.Babyatsky MW, Rossiter G, Podolsky DK: Expression of transforming growth factor α and β in colonic mucosa in inflammatory bowel disease. Gastroenterology 1996, 110:975-984 [DOI] [PubMed] [Google Scholar]
- 33.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth: Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. J Clin Pathol 1994, 47:113–116 [DOI] [PMC free article] [PubMed]
- 34.Reimund J-M, Wittersheim C, Dumont S, Muller CD, Kenney JS, Baumann R, Poindron P, Duclos B: Increased production of tumor necrosis factor-α, interleukin-1β, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn’s disease. Gut 1996, 39:684-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vachon PH, Beaulieu J-F: Extracellular heterotrimeric laminin promotes differentiation in human enterocytes. Am J Physiol 1995, 268:G857-G867 [DOI] [PubMed] [Google Scholar]
- 36.Basora N, Herring-Gillam FE, Boudreau F, Perreault N, Pageot L-P, Simoneau M, Bouatrouss Y, Beaulieu J-F: Expression of functionally distinct variants of the β4A integrin subunit variant in relation to the differentiated state in human intestinal cells. J Biol Chem 1999, 274:29819-29825 [DOI] [PubMed] [Google Scholar]