Abstract
Short-term elevation of circulating glucocorticosteroids (GCs) in vertebrates facilitates the adoption of a distinct emergency life history state, which allows individuals to cope with perturbations and recover homeostasis at the expense of temporarily suppressing nonessential activities. Although GC responses are viewed as a major evolutionary mechanism to maximize fitness through stress management, phenotypic variability exists within animal populations, and it remains unclear whether interindividual differences in stress physiology can explain variance in unequivocal components of fitness. We show that the magnitude of the adrenocortical response to a standardized perturbation during development is negatively related to survival and recruitment in a wild population of long lived birds. Our results provide empirical evidence for a link between stress response, not exposure to stressors, and fitness in a vertebrate under natural conditions. Recent studies suggest that variability in the adrenocortical response to stress may be maintained if high and low GC responders represent alternative coping strategies, with differential adaptive value depending on environmental conditions. Increased fitness among low GC responders, having a proactive personality, is predicted under elevated population density and availability of food resources, conditions that characterize our study population.
Keywords: animal personality, corticosterone, glucocorticosteroids, reproduction, survival
Exposure to environmental perturbations constitutes a major selective force in natural populations. Animals have evolved behavioral and physiological strategies to avoid the deleterious effects of stressors, and among vertebrates, the adrenocortical response is one of the most conserved physiological mechanisms aimed at this end (1–3). In response to modifying factors (e.g., decreased food resources, predation, harsh weather), vertebrates activate the hypothalamous–pituitary–adrenal axis, which triggers a rapid release of glucocorticosteroids (GCs) from the adrenal glands into the bloodstream (4). Elevations of circulating GCs, in turn, redirect individuals into a distinct emergency life history state (3) with changes in physiology and behavior (e.g., increased gluconeogenesis and mobilization of fat stores, suppressed territorial and reproductive behavior, irruptive migration; refs. 2 and 4), aimed at coping with the perturbation and recovering homeostasis at the expense of temporarily suppressing nonessential activities. The quantification of circulating GC titers has become a useful tool in psychology, animal husbandry, and conservation biology because elevations of plasma levels constitute a physiological marker of exposure to stress (e.g., refs. 5–7), and the latter has deleterious effects on fitness. However, even within animal populations exposed to constant environments there is a strong interindividual variability in the adrenocortical response to standardized stressors (8–10), and it remains unknown whether such natural variability exerts an impact on fitness. Only recently, studies in fish, birds, and mammals, including humans, suggest that interindividual differences in stress-coping responses are key attributes defining personality types (10–14), and variability in natural populations is maintained through different payoffs on adaptive capacity and vulnerability to disease. We tested whether individual variation in the GC response to stress early in life has long-term consequences to unequivocal components of fitness: survival and reproduction. As a study model we used the European white stork (Ciconia ciconia), a large water bird that shows deferred breeding and a maximum life span of >33 years under natural conditions (15). In June 2000 we assessed the physiological response to a standardized perturbation in 35 wild nestlings hatched in a colony near Seville, Spain. Using the capture and restraint protocol (16), we collected a blood sample within the first minute after capture (baseline sample) and a second blood sample after 45 min of restraint (stress-induced sample). The concentration of circulating corticosterone was determined in the blood samples, and during the next 5 years (2001–2005) we monitored survival and recruitment through intensive and extensive field surveys of banded birds on a very large scale.
Results and Discussion
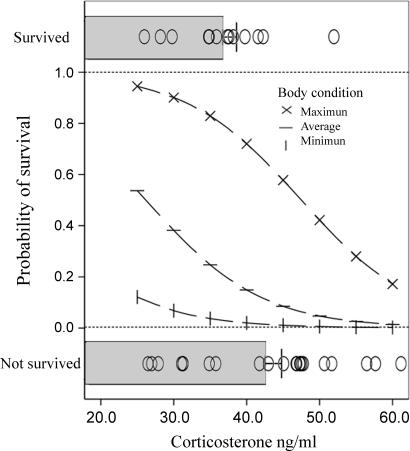
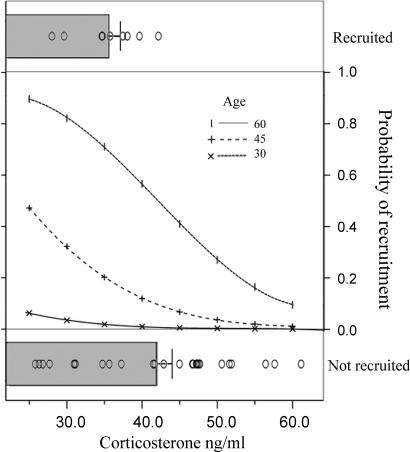
From 6,723 field observations of individuals fitted with large plastic alpha-numeric bands, we gathered positive resightings on 13 (37%) experimental birds. Survival probability was negatively related to stress-induced corticosterone levels (χ2 = 5.59, P < 0.05; Fig. 1), positively related to body condition (χ2 = 5.01, P < 0.05) and age at sampling (χ2 = 5.53, P < 0.05) and no other significant effect was found (Table 1). Although increased mortality among high GC responders may suggest a potential for natural selection to operate on stress physiology, there is a general agreement that this force exerts its effects by maximizing reproduction of the adapted individuals, even at the expense of health and survival (17). For this reason, we also analyzed the probability of recruitment of white storks into the breeding population. Of the 35 nestlings subjected to experimental assessment of stress response, 9 birds (26%) were recruited as breeders. The probability of recruitment was negatively related to stress-induced GC levels (χ2 = 5.50, P < 0.05) and positively related to nestling age at blood sampling (χ2 = 4.39, P < 0.05) with no other significant effect found (Table 1 and Fig. 2).
Fig. 1.
Probability of survival to adulthood as a function of stress-induced corticosterone and body condition during development. (Top and Bottom) The actual recorded levels of stress-induced corticosterone in individuals that survived and did not survive, respectively (shaded bars indicate mean ± SEM corticosterone, and open dots represent individual values). (Middle) The predicted survival function according to the statistical model. The model predictions have been generated for a range of body condition estimates (maximum, minimum, and average condition scores according to study sample) to illustrate the simultaneous effects of both stress-induced response and body condition on survival probability. The probability of survival decreases as stress-induced corticosterone levels increase; however, for a given level of corticosterone, the survival probability is higher when the bird is in good condition.
Table 1.
Results from the generalized linear models assessing the probability of survival and recruitment as a function of nestling traits
| Dependent variable | Predictor | Estimate | SEM | χ2 | P |
|---|---|---|---|---|---|
| Survival* | Intercept | −6.5038 | 4.8762 | 1.78 | 0.1823 |
| Baseline corticosterone, ng·ml−1 | — | — | 0.67 | 0.4115 | |
| Stress-induced corticosterone, ng·ml−1 | −0.1260 | 0.0631 | 5.59 | 0.0181 | |
| Age | 0.2155 | 0.1125 | 5.53 | 0.0187 | |
| Body condition | 25.3771 | 13.4716 | 5.01 | 0.0252 | |
| Brood size | — | — | 3.39 | 0.0656 | |
| Blood lead levels, μg·liter−1 | — | — | 3.51 | 0.0608 | |
| Sex | — | — | 0.19 | 0.6628 | |
| Recruitment† | Intercept | −4.2608 | 4.1365 | 1.06 | 0.3030 |
| Baseline corticosterone, ng·ml−1 | — | — | 0.07 | 0.7891 | |
| Stress-induced corticosterone, ng·ml−1 | −0.1262 | 0.0627 | 5.50 | 0.0191 | |
| Age | 0.1623 | 0.0895 | 4.39 | 0.0361 | |
| Body condition | — | — | 1.63 | 0.2012 | |
| Brood size | — | — | 1.97 | 0.1606 | |
| Blood lead levels, μg·liter−1 | — | — | 1.18 | 0.2775 | |
| Sex | — | — | 1.17 | 0.2796 |
*Binomial distribution of errors and logit link function. Explained deviance: 25.03%.
†Binomial distribution of errors and logit link function. Explained deviance: 19.30%.
Fig. 2.
Probability of recruitment into the breeding population as a function of stress-induced corticosterone and nestling age (in days) at sampling. (Top and Bottom) The actual recorded levels of stress-induced corticosterone in recruited and nonrecruited individuals, respectively (shaded bars indicate mean ± SEM corticosterone, and open dots represent individual values). (Middle) The predicted probability of recruitment according to the statistical model. The model predictions have been generated for a range of ages to illustrate the simultaneous effects of both stress-induced response and nestling age on recruitment probability.
Our study provides empirical evidence of a link between the physiological response to stress early in life and both reproduction and survival in a vertebrate and clearly indicates superior long-term fitness among phenotypes with reduced GC stress responses. Recent studies in wild populations of mammals, birds, and reptiles have reported associations between baseline (18) or chronic (6, 7) GC levels and survival. However, this association is ultimately confounded by differential exposure to stressors among individuals, affecting circulating GC levels, or relates to short-term survival (7). In contrast, our results relate long-term survival and recruitment to the nature of an individual's physiological response to a standardized source of stress. Because baseline corticosterone had no effect on stork fitness (Table 1), and it was unrelated to experimentally induced GC titers (Pearson r = 0.128, P = 0.47), our findings suggest a link between fitness and stress physiology rather than exposure to stressors. With regard to the effects of nestling age on survival and recruitment reported here, our assessment of developmental response to stress took place during 2 consecutive days; therefore, nestling age reflects variability in hatching date within the stork colony. Laying date has an almost ubiquitous association with parental quality in seasonal breeding birds, with good-quality individuals breeding earlier in the season (19). Older, earlier-hatched nestlings may thus be the offspring of high-quality breeders, showing enhanced survival and recruitment as a result of better genes, better parental care, or both. The positive effect of mass residuals on survival is consistent with an intuitive notion that the physical condition of nestlings is also a major determinant on individual fitness, possibly linked to parental quality and/or timing of breeding (20). With regard to the negative association between adrenocortical function and both survival and recruitment, there is evidence that the behavioral and physiological response to stress is consistent over time (8, 10, 12). High GC responders may trigger more often, or more robust, emergency responses to other sorts of perturbations, not only during development but also later in life. In the long term, frequent activation of the hypothalamous–pituitary–adrenal axis may lead to chronic exposure to elevated GC levels, with deleterious consequences on growth and maturity, immune and reproductive function, brain function, and cognitive abilities (2, 4, 21, 22), all of which may affect survival and reproduction. Our results are consistent with studies conducted on laboratory rats establishing a positive association between GC stress response and mortality risk (10). However, the latter animal model (Sprague–Dawley rats, Rattus norvegicus) was artificially selected to develop spontaneous tumors in adulthood (23), and it remained unclear whether the association between the stress response and fitness was applicable to vertebrates outside of a controlled laboratory setting, and more important, whether other critical components of fitness such as reproduction could trade off the proposed benefits of a reduced GC response on longevity.
Although phenotypic plasticity may account for the reported variability in GC response, genetic selection and inbreeding also provide evidence for a strong genetic component to adrenocortical function (9, 12, 24, 25). The GC response to stress, through its effects on fitness, is expected to be a trait subjected to strong natural selection, but natural variability will occur if divergent selective forces vary among populations or within a population over time. Research on humans (11) and other animals (12–14) suggests that variability in adrenocortical responses represents alternative strategies with associated payoffs on adaptive capacity and vulnerability to disease that change according to environmental conditions. Behavior under conditions of mild stress shows consistent patterns in all vertebrates: exploratory activity, boldness, and aggressiveness covary in the same way and shape distinct behavioral strategies also referred to as personalities or coping styles (12, 13). In fact, the adrenocortical response to stress and other neuroendocrine traits seems to be causally linked, possibly through pleiotropy, gene linkage, or coselection (26), to these behavioral strategies (10, 12, 13, 24, 25). Proactive individuals (also called bold, aggressive, hawk personalities or fast explorers; refs. 12, 13, 24, and 25) typically show reduced GC elevations, but high activation of the sympathetic axis that facilitates the “fight or flight” response to stress. On the other hand, reactive individuals (also called shy, cooperative, dove personalities or slow explorers) are characterized by high GC secretion but low sympathetic activation in response to stress that facilitate the “freeze and hide” coping strategy (10, 12, 13, 24, 25). The success of proactive vs. reactive coping strategies is postulated to vary as a function of population density and predictability of food resources, with proactive individuals being more successful when density is high and food is stable and abundant (13, 25, 27, 28). Interestingly, our study colony of white storks has experienced a pronounced growth during the last 25 years, and with ≈400 nests is currently the largest Spanish colony (29), and surely one of the largest and most dense in the world. In addition, the unique environment surrounding the colony (i.e., marshes of Doñana National Park) offers high predictability and abundance of food resources. Our results, therefore, concur with a conceptual framework that predicts selection of low GC responders, proactive phenotypes under similar environmental scenarios (13, 27, 28), and provides a parsimonious evolutionary explanation for the reported link between fitness and adrenocortical function.
Materials and Methods
Assessment of the Adrenocortical Response to Stress and Traits of Nestlings.
We used the “capture and restraint paradigm” (16) to assess baseline GCs (blood samples collected within the first minute after capture) and stress-induced adrenocortical response (blood samples collected 45 min after capture) through determination of circulating corticosterone levels. Nestling storks respond to short-term human handling by activating their hypothalamous–pituitary–adrenal axis (30). Our criterion for selecting this estimate of stress-induced response after 45 min of restraint was based on previous work reporting that the corticosterone response to handling tends to peak and reach a plateau between 30 and 45 min in stork nestlings ≈30 days of age or older (31). To avoid the effects of differential exposure to uncontrolled sources of environmental perturbation, we sampled birds from a single breeding colony (Dehesa de Abajo, marshes of Doñana, southwest Spain), during a 2-day period, and between 8 and 11:00 a.m. to minimize potential diel effects. After accessing a nest, storks were immediately brought down to the ground for serial blood sampling. Blood samples were kept in ice coolers until centrifuged (7,000 × g, 10 min) the same day of capture, and plasma was frozen and stored at −80°C for further determination of corticosterone levels. Plasma corticosterone was determined through RIA in duplicate tubes following standard methods as described (30–32). Antiserum and purified corticosterone for the standards were purchased from Sigma–Aldrich (Oakville, Canada); [3H]corticosterone was purchased from New England Nuclear (Woodbridge, Canada). Corticosterone measurements were performed on reconstituted organic ethyl ether extracts of the plasma samples; extraction efficiency was >90%, average assay precision was 0.084 ng·ml−1, the minimum detection limit of the assay was 0.10 ng·ml−1, and intraassay and interassay coefficients of variation were <8%. The study colony was located in the vicinity of an area affected by a mine spill containing lead in 1998 (33). Because lead has previously been shown to affect circulating glucocorticoids (34), we also determined blood levels of lead in the collected samples following methodology as described (34) and with the aim of controlling for potential effects on survival and recruitment (see below). All of the birds showed blood lead levels <200 μg/liter, the threshold value reported to cause sublethal effects in birds (35). Before returning the birds to their nests, they were banded with plastic-coded leg bands that allowed identification in the field, and their wing chord and body mass were measured. Because male and female white storks look alike, we resorted to molecular sexing of the birds (36). Nestling age, which ranged from 29 to 59 days old, was estimated according to a regression equation of age on wing chord (37). A body condition index was calculated as the individual's residual value from a regression of log10 body mass on log10 wing chord. To avoid unnecessary suffering or pain to study subjects we followed protocols in concert with Spanish laws and prioritized ethical considerations over scientific goals.
Estimation of Survival and Recruitment Probabilities.
During the period 2001–2005, we gathered information on survival and recruitment through intensive and extensive field surveys of banded birds both at the local, national, and international scales. Our focal colony has been intensively monitored during the last two decades. Annual monitoring included accurate nest counts, breeding outcome, and marking of most fledglings with plastic-coded leg bands easily readable at distance by using spotting scopes, to determine survival, recruitment, and dispersal of individuals (see, e.g., ref. 38). The same intensive monitoring was conducted within a radius of ≈40 km, covering the marshes and surroundings of Doñana National Park, this whole study area holding ≈2,000 nests of white storks in 2004 (29). Field surveys to monitor nests and identify bands of marked birds were conducted 5 days a week, from mid-February to late July, covering all of the colonies and the main feeding areas for the species. During the period 2001–2005 we resighted 1,078 birds previously born in our study area. Additionally, we consulted the national banding and resighting data bank at Estación Biológica de Doñana (Seville, Spain), which compiles information on >20,000 white storks banded across Spain and further resighted elsewhere worldwide. This process yielded information on 5,645 additional birds resighted in the same period and would allow detection of long-distance dispersers. The frequency of birds dispersing from our large study area seems negligible; only one of the 5,645 resightings obtained across Spain and neighboring countries in Europe and Africa corresponded to a stork born in Doñana (i.e., only 1 of 1,079 birds born in Doñana and later resighted elsewhere), which moved <100 km for its first breeding attempt. This frequency yields a probability of dispersal away from the study area <0.093% (i.e., 1/1,079). Considering that this estimation is very conservative (because it does not take into account individuals that died and were never resighted elsewhere after banding), we may expect that <1 individual (i.e., 0.032 individuals) of the 35 birds comprising our experimental sample actually dispersed away from the study site. This probability is rather negligible to have any effect on the reported results and conclusions. Average age of first breeding in our population is 3.37 ± 0.9 years (n = 361; J.L.T. and R. Jovani, unpublished data), which is similar to that found in other populations [e.g., 3.4 years (39)], and thus the 5-year period of monitoring after the adrenocortical assessment seems reasonable for detecting most recruits. In fact, only 2.2% of birds recruited at 6 years old.
Modeling survival through capture–mark–recapture techniques requires large sample sizes (18), which are difficult to obtain through work such as ours. We thus relied on the analysis of return rates (e.g., ref. 40), assuming that postfledging mortality occurred in individuals not resighted during the 5-year period after blood sampling, and that nonrecruited birds died before reaching first-breeding age. These seem to be reasonable assumptions, given the population traits and the intensive monitoring effort described above. Generalized linear models (GLMs) were used (40, 41) for analyzing the probability of survival and probability of recruitment in relation to baseline and stress-induced corticosterone, age, sex, brood size, body condition, and blood levels of lead. For this kind of binary data (fledglings surviving or recruited = 1, and fledglings not surviving or not being recruited = 0), a binomial error and a logistic link function are adequate, resulting in a GLM that approaches a logistic regression (42). Each explanatory variable was tested for significance following standard backwards procedures, where the nonsignificant factors and covariates are sequentially removed from the saturated model. The result is the most adequate model for explaining the variability in the response variable, where only the significant explanatory variables are retained.
Acknowledgments
We thank F. Hiraldo, R. Jovani, J. M. Terrero, F. J. Vilches, M. C. Quintero, A. Román, J. C. Núñez, S. Cabezas, G. García, D. Serrano, A. Tamayo, J. Ramírez, J. L. Arroyo, C. Sánchez, B. Jiménez, R. Tobar, C. Alonso, R. Álvarez, N. Pastor, R. Rodríguez, J. A. Godoy, the Equipo de Seguimiento de Procesos Naturales, and the Oficina de Anillamiento de la Estación Biológica de Doñana for help and the hundreds of voluntary ornithologists that annually monitor banded white storks across Spain. The Consejería de Medio Ambiente-Junta de Andalucía and the Ayuntamiento de Puebla del Río provided permits to work at the stork colony. J.B. was supported by the Programa Integrado de Inserción Profesional of the Spanish Ministerio de Educación y Ciencia/European Community, and R.B. was supported by a predoctoral Formación de Profesorado Universitario fellowship from the Spanish Ministerio de Educación y Cultura. Financial support was provided by Research Project B0S2002-00857 of the Spanish Ministerio de Ciencia y Tecnología (to J.L.T.), the Junta de Andalucía (to J.L.T.), and a Natural Sciences and Engineering Research Council grant (to G.R.B.).
Abbreviation
- GC
glucocorticosteroid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct submission.
References
- 1.Romero M. Trends Ecol Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM, Romero LM, Munck AU. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 3.Wingfield JC, Maney DL, Breuner CW, Honey PK, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Am Zool. 1998;38:191–206. [Google Scholar]
- 4.Wingfield JC, Kitaysky AS. Int Comp Biol. 2002;42:600–609. doi: 10.1093/icb/42.3.600. [DOI] [PubMed] [Google Scholar]
- 5.Wikelski M, Cooke SJ. Trends Ecol Evol. 2006;21:38–46. doi: 10.1016/j.tree.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Romero LM, Wikelski M. Proc Natl Acad Sci USA. 2001;98:7366–7370. doi: 10.1073/pnas.131091498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabezas S, Blas J, Marchant TA, Moreno S. Horm Behav. 2007;51:313–320. doi: 10.1016/j.yhbeh.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Cockrem JF, Silverin B. Gen Comp Endocrinol. 2002;125:197–206. doi: 10.1006/gcen.2001.7750. [DOI] [PubMed] [Google Scholar]
- 9.Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. J Evol Biol. 2006;19:343. doi: 10.1111/j.1420-9101.2005.01034.x. [DOI] [PubMed] [Google Scholar]
- 10.Cavigelli SA, McClintock MK. Proc Natl Acad Sci USA. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson DS. Philos Trans R Soc London B. 1998;353:199–205. [Google Scholar]
- 12.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen GC, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 13.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Wilson DS, Clark AB, Coleman K, Dearstyne T. Trends Ecol Evol. 1994;9:442–446. doi: 10.1016/0169-5347(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 15.del Hoyo J, Elliot A, Sargatal J. Handbook of the Birds of the World. Barcelona: Lynx Edicions; 1994. [Google Scholar]
- 16.Wingfield JC. In: Perspectives in Comparative Endocrinology. Davey KG, Peter RE, Tober SS, editors. Ottawa: National Research Council of Canada; 1994. pp. 520–528. [Google Scholar]
- 17.Nesse RM. Med Health Care Philos. 2001;4:37–47. doi: 10.1023/a:1009938513897. [DOI] [PubMed] [Google Scholar]
- 18.Brown CR, Brown MB, Raouf SA, Smith LC, Wingfield JC. Ecology. 2005;86:1034–1046. [Google Scholar]
- 19.Forslund P, Pärt T. Trends Ecol Evol. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. [DOI] [PubMed] [Google Scholar]
- 20.Naef-Daenzer B, Widmer F, Nuber M. J Anim Ecol. 2001;70:730–738. [Google Scholar]
- 21.Kitaysky AS, Kitaiskaia EV, Piatt JF, Wingfield JC. Horm Behav. 2003;43:140–149. doi: 10.1016/s0018-506x(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 22.Sapolsky RM. Science. 1996;273:749. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 23.Lohrke H, Hesse B, Goerttler K. Z Versuchstierk. 1982;24:225–230. [PubMed] [Google Scholar]
- 24.Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM. J Neuroendocr. 2003;15:256–267. doi: 10.1046/j.1365-2826.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- 25.Carere C, Groothuis TGG, Mostl E, Daan S, Koolhaas JM. Horm Behav. 2003;43:540–548. doi: 10.1016/s0018-506x(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 26.Price T, Langen T. Trends Ecol Evol. 1992;7:307–310. doi: 10.1016/0169-5347(92)90229-5. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti C, Drent PJ. Anim Behav. 2000;60:131–140. doi: 10.1006/anbe.2000.1443. [DOI] [PubMed] [Google Scholar]
- 28.Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. Proc R Soc London Ser B. 2004;271:847–852. doi: 10.1098/rspb.2004.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina B, Del Moral JC. La Cigüeña Blanca en España: VI Censo Internacional. Madrid: SEO/BirdLife; 2005. [Google Scholar]
- 30.Blas J, Baos R, Bortolotti GR, Marchant T, Hiraldo F. Func Ecol. 2005;19:315–322. [Google Scholar]
- 31.Blas J, Baos R, Bortolotti GR, Marchant TA, Hiraldo F. Gen Comp Endocrinol. 2006;148:172–180. doi: 10.1016/j.ygcen.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Blas J, Pérez-Rodríguez L, Bortolotti G, Viñuela J, Marchant T. Proc Natl Acad Sci USA. 2006;103:18633–18637. doi: 10.1073/pnas.0609189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimalt JO, Ferrer M, Macpherson E. Sci Total Environ. 1999;242:3–11. doi: 10.1016/s0048-9697(99)00372-1. [DOI] [PubMed] [Google Scholar]
- 34.Baos R, Blas J, Bortolotti GR, Marchant TA, Hiraldo F. Environ Health Perspect. 2006;114:1497–1501. doi: 10.1289/ehp.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franson J. In: Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations. Beyer NW, Heinz GH, Redmon-Norwood AW, editors. Boca Raton, FL: Lewis; 1996. pp. 265–279. [Google Scholar]
- 36.Ellegren H. Proc R Soc London Ser B. 1996;263:1635–1641. doi: 10.1098/rspb.1996.0239. [DOI] [PubMed] [Google Scholar]
- 37.Negro JJ, Tella JL, Blanco G, Forero MG, Garrido-Fernandez J. Physiol Biochem Zool. 2000;73:97–101. doi: 10.1086/316724. [DOI] [PubMed] [Google Scholar]
- 38.Jovani R, Tella JL. Ecography. 2004;27:611–618. [Google Scholar]
- 39.Barbraud C, Barbraud J-C, Barbraud M. Ibis. 1999;141:469–479. [Google Scholar]
- 40.Tella JL, Bortolotti GR, Dawson RD, Forero MG. Proc R Soc London Ser B. 2000;267:891–895. doi: 10.1098/rspb.2000.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crawley MJ. Methods in Ecology. In: Lawton JH, Likens GE, editors. GLIM for Ecologists. Cambridge, UK: Blackwell; 1993. [Google Scholar]
- 42.McCullagh P, Nelder JA. Generalized Linear Models. 2nd Ed. New York: Chapman & Hall; 1989. [Google Scholar]