Abstract
Given the findings that (1) systemic opioid antinociception varies by estrous stage in females, and (2) the magnitude of sex differences in opioid antinociception is negatively correlated with opioid agonist efficacy, we hypothesized that sex differences in the function of the descending pain modulatory system are likely influenced by estrous stage in females and by the number of available opioid receptors therein. The present study tested these hypotheses by (1) comparing antinociception produced by morphine microinjection to the ventral periaqueductal gray (vPAG) in females at different stages of the estrous cycle, and (2) examining systemic morphine antinociception in males vs. females under conditions of reduced vPAG mu opioid receptor availability. When estrous stage of females was not controlled for (Experiment 1), there was no significant sex difference in tail withdrawal antinociception following morphine microinjection (0.3-10 μg), although morphine was more potent in males than females in producing immobility. Experiment 2 showed that intra-vPAG morphine produced less antinociception and immobility in estrus than in diestrus females; that is, only estrus females' response to morphine was lower than that of males. Experiment 3 showed that microinjection of the irreversible mu opioid antagonist β-funaltrexamine (β-FNA) into the vPAG shifted the systemic morphine dose-effect curve farther to the right in females than in males. That is, a reduction in available vPAG mu opioid receptors had a greater impact on opioid antinociception in females than in males, suggesting that females have fewer vPAG mu opioid receptors than males. Overall, these data suggest that ovarian hormones and PAG mu opioid receptor density contribute to sex differences in antinociception produced by morphine.
Keywords: Analgesia, Gender, Locomotor Activity, Opioids
1. Introduction
Sex differences in opioid antinociception have been observed in mice [7, 38], rats [2, 9-11], monkeys [40] and humans [19-21]. In animal models, mu opioid agonists have been shown to be more potent or efficacious in males than in females [2, 10, 11, 38], although this is not always the case [e.g., 38]. One possible explanation for sex differences in opioid antinociception is sex differences in the structure or function of the descending pain modulatory system, including the midbrain periaqueductal gray (PAG).
Opioid administration into the PAG in the rat [24, 25, 31, 34, 53] and cat [41], as well as electrical stimulation of the PAG in humans [23] produces antinociception. This antinociception can be attenuated by concurrent administration of an opioid antagonist such as naloxone [1, 17, 26], indicating that antinociception evoked from the PAG is opioid receptor-mediated. Moreover, the PAG appears to be a critical structure for the antinociception produced by systemic opioids because selective inactivation of the PAG attenuates this antinociception [33, 44, 57]. It has been suggested that the caudal ventral PAG (vPAG, including the dorsal raphe nucleus) is the region most sensitive to mu opioid receptor-mediated antinociception [56].
The prominent role of the PAG in opioid antinociception suggests that sex differences in its structure or function contribute to sex differences in antinociception produced by mu agonists. In fact, sex differences in the anatomical and functional organization of the descending pain modulatory pathway have been reported recently. Female rats have more PAG to rostral ventromedial medulla (RVM) output neurons than do males, and persistent pain activates more output neurons in males than in females [35]. Furthermore, mu opioid receptor expression in vPAG is two-fold higher in male than in female rats [55]. Sex differences in antinociception have been observed after opioid administration to the vPAG [31, 53, 55] and RVM [6]; however, whereas several studies have shown that supraspinal administration of morphine or DAMGO produces greater antinociception in males [6, 28, 29, 31], other studies have reported greater antinociception in females [53] or no sex difference [27, 29]. Disagreement among such studies may result from differences in the type/intensity of the nociceptive stimulus, efficacy of the mu agonist, use of awake vs. anesthetized animals, estrous phase of females, and genotype (strain/vendor) of the rodent [11, 12, 38]. In regard to estrous phase, previous studies of the antinociceptive effects of systemically administered mu agonists show that female rats in estrus typically are less sensitive than females in other stages [15, 50, 51]. Furthermore, Bodnar and colleagues demonstrated in 1989 that morphine administered into the lateral ventricles produced less antinoception in female rats in estrus than those in proestrus or diestrus [28]; subsequent studies conducted in the same laboratory consistently reported less antinociception in estrus females compared to males when mu agonists were administered to the vPAG [30-32], suggesting that the estrous phase effect occurs within vPAG.
The objective of the present study was to determine whether sex differences in morphine antinociception mediated by the caudal vPAG depend on estrous stage in females and mu opioid receptor availability. To test the hypothesis that sex differences in morphine antinociception depend on estrous stage in females, we first compared males and females without regard to estrous stage (Experiment 1), and then directly compared the behavioral responses to intra-vPAG morphine in estrus vs. diestrus females (Experiment 2). Given that microinjection of morphine into the vPAG produces both antinociception and immobility [39], both of these behaviors were assessed. The lower density of mu opioid receptors in female compared to male rats suggests that reducing mu opioid receptor availability will produce a greater inhibition of opioid antinociception in female than male rats. This hypothesis was tested by measuring systemic morphine antinociception following microinjection of the irreversible mu receptor-selective antagonist β-FNA into the vPAG (Experiment 3).
2. Materials and methods
2.1 Subjects
Age-matched 3- to 6-month old male (375–500 g) and female (240–300 g) Sprague-Dawley rats (bred in-house from Taconic Farms stock, Germantown, NY) were used. Female rats were randomly selected rather than pre-screened for estrous cycle length or regularity. Rats were housed in same-sex pairs, males and females in separate rooms maintained at 21.5 ± 1.0°C on a 12:12 hr light:dark cycle (lights on at 0700 hr). Except during testing, rats received food and water ad libitum. Testing was conducted between approximately 1200 and 1500 hr.
2.2 Drugs
Morphine sulfate and β-FNA HCl (National Institute on Drug Abuse, Bethesda, MD) were dissolved in physiological saline (0.9% NaCl).
2.3 Apparatus
The tail withdrawal procedure utilized a 5-gal water bath (Precision Scientifics, Chicago, IL) set at 52.0 ± 0.5°C. Rats were restrained in Plexiglas tubes with an opening at one end (IITC Inc., Los Angeles, CA), which allowed the tail to hang freely. Locomotor activity was measured by placing rats into clear Plexiglas cages (40 × 20 × 43 cm), which were then placed within a photobeam apparatus (Opto-varimex, Columbus Instruments, Columbus, OH). Fifteen photobeams spaced 2.5 cm apart and 8 cm above the cage floor spanned the width of the cage. Microinjections were made using a 5-μl syringe placed into an infusion pump (Harvard Apparatus PHD 2000, Holliston, MA).
2.4 Procedure
2.4.1 Surgery
Rats received penicillin G i.m. approximately 30 min before surgery (11,000 units/kg). Rats were then anesthetized with Equithesin (24.3 mg/kg pentobarbital and 106.3 mg/kg chloral hydrate), and given 0.5 ml lidocaine (10.0 mg/ml) i.d. as a local anesthetic. Rats were mounted on a stereotaxic apparatus and implanted with a 22-gauge guide cannula aimed at the caudal vPAG; the cannula was anchored with 3 or 4 stainless steel screws and cranioplastic cement. Cannulae were placed only on the right side for uniformity. Coordinates from the intersection of the midline and interaural sutures were (in mm): Males: AP = 0.0; L = −2.2; V = −4.9; Females: AP = −0.5; L = −1.9; V = −4.6 [42]. For all rats, the incisor bar was set at −3.0 mm and the lateral arm of the stereotaxic apparatus was angled 12° toward the sagittal suture. The guide cannula was capped with a 28-gauge dummy cannula. Rats were given at least 5 days to recover before testing began. Prior to testing, rats were habituated to the microinjection technique by bringing the rat into the testing environment and inserting the injection cannula into the guide cannula. This procedure also helped to reduce direct mechanical activation of cells by the injection cannula, which projected 2 mm beyond the guide cannula [39].
2.4.2 Vaginal Cytology
Vaginal smears were obtained from female rats immediately after testing (Experiments 1 and 3), or before and after testing (Experiment 2). After drying, smears were stained with Giemsa (Sigma Chemical Co., St. Louis, MO). Proestrus was identified as a predominance of nucleated epithelial cells, estrus as a predominance of cornified epithelial cells, diestrus-1 as the presence of leukocytes and scattered nucleated and/or cornified epithelial cells, and diestrus-2 as the relative lack of any cells [18]. Rats that were judged to be between proestrus and estrus were categorized as estrus, based on hormonal and reproductive behavioral similarity of this phase to estrus.
2.4.3 Behavioral Testing
Experiment 1
Rats (N=30/sex) were microinjected with morphine (0.3, 1.0, 3.0 or 10.0 μg/0.5 μl) or sterile saline (0.5 μl) into the vPAG. The injection took 30 s, and the injection cannula was left in place for an additional 30 s to reduce backflow up the guide cannula. The pump was run post-injection to verify free flow of fluid from the injection cannula. Doses were administered in pseudo-random order, with rats receiving up to 5 microinjections/tests, spaced at least 4 days apart.
Rats were tested on the 52°C warm water tail withdrawal assay 5 min after the injection. The distal 5 cm of the tail was immersed into the water and latency to withdraw at least 4 cm of the tail was measured with a stopwatch. If a rat did not respond within 20 sec its tail was removed from the water bath and the maximal score of 20 sec was recorded. Rats were tested on the tail withdrawal assay again at 15, 30, 60, 90 and 120 min post-injection. Additionally, beginning at 16 min post-injection, rats were placed into locomotor chambers and activity was quantified as the number of photobeam breaks occurring within 5 min.
Experiment 2
Experimentally naïve females (N=50) were implanted with a guide cannula aimed at the vPAG as in Experiment 1. Beginning at least one week after surgery, vaginal smears were taken daily (approximately 11 a.m.) until rats were found to be in estrus or diestrus-1. Sterile saline (0.5 μl) or morphine (1.0 μg/0.5 μl) was then microinjected, and antinociception and locomotor activity were measured as described above. When possible, females were tested in estrus-diestrus pairs, so that the amount of handling between the two groups was approximately equal. Each rat was tested only once.
Experiment 3
Experimentally naïve rats (N=30/sex) were implanted with a guide cannula as in Experiment 1. Sterile saline (0.5 μl) or 1 μg/0.5 μl β-FNA was microinjected to the vPAG. Twenty-three and a half hr later, rats were tested on the tail withdrawal assay to obtain a baseline latency. Then morphine was injected s.c., cumulatively in quarter-log unit increments until a complete dose-effect curve was obtained. For example, 3.2 mg/kg morphine was injected, and the rat was tested on the tail withdrawal assay 30 min later. Then 2.4 mg/kg morphine (total 5.6 mg/kg) was injected, and rats were re-tested 30 min later. Then 4.4 mg/kg morphine (total 10 mg/kg) was injected, and rats were tested 30 min later, etc., until the response cutoff of 20 sec was reached.
2.4.4 Histology
Rats were overdosed with Equithesin. Then 0.5 μl of cresyl violet stain was infused into the microinjection site and brains were removed and stored in 10% formalin. Each brain was sliced via vibratome to a thickness of approximately 60 μm, mounted on a gelatinized slide, stained using haematoxylin and eosin, and viewed under a microscope to assess cannula placement.
2.5 Data Analysis
In each experiment, data from rats that had cannula placements outside of vPAG (defined as ventrolateral PAG and dorsal raphe nucleus), and data from rats that were tested with two doses or fewer (Experiment 1) before the cannula became occluded, were not included in the statistical analyses. In Experiment 1, each individual time-effect curve was transformed to an Area-Under-the-Curve (AUC) value using the trapezoidal rule. Because each rat was not tested on all doses, a more conservative between-subjects (Dose & Sex) Analysis of Variance (ANOVA) was used to analyze AUC values and locomotor scores (number of photobeam breaks). In Experiment 2, AUC values and locomotor scores were analyzed via ANOVA, with Dose and Estrous Stage as between-subjects factors. Data were included only from rats that were confirmed to be in either diestrus-1 or estrus before and after testing. In Experiment 3, tail withdrawal latency data were converted to Percent Maximum Possible Effect (%MPE): [(Drug Latency − Control Latency) ÷ (Cutoff Latency (20 sec) − Control Latency)] × 100. Using at least one data point above and one data point below the 50% MPE, an ED50 value was calculated for each rat's morphine dose-effect curve via linear interpolation. ED50 values were then compared using ANOVA, with Antagonist Dose and Sex as between-subjects factors. T-tests with Bonferroni adjustments were used for post-hoc comparisons.
3. Results
3.1 Morphine's Antinociceptive Effects
Antinociceptive effects were examined first as a function of vlPAG or DRN (or other) cannula placement. Figure 1 shows individual placements, mapped according to magnitude of effect. There were no clear differences in antinociception between rats with vlPAG vs. DRN cannula placements; thus, data from these two areas were combined for further analysis of sex differences.
Figure 1.
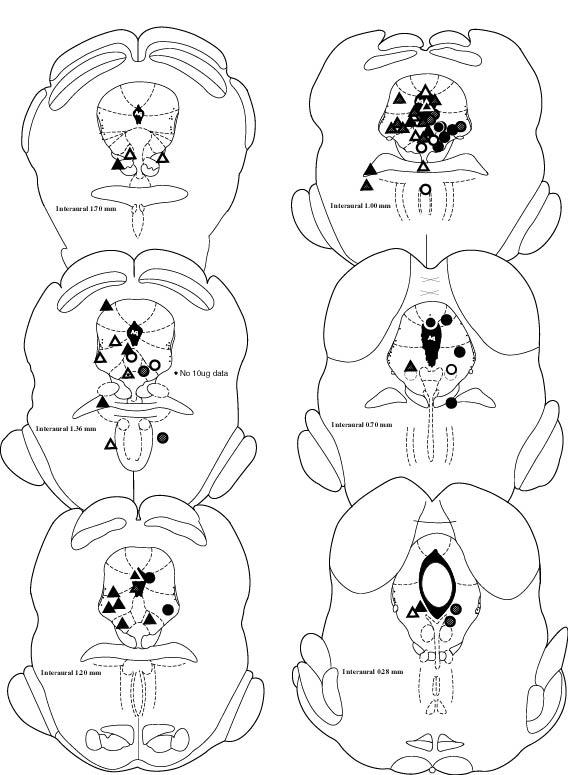
Cannula placements, Experiment 1 (morphine microinjections). Symbols are coded by the maximal antinociceptive effect observed across all doses of morphine in the 52°C tail withdrawal test. Open circles/triangles: male/female rats that showed <33% MPE at ≥ four time points. Striped circles/triangles: male/female rats that showed 33% to 66% MPE at ≥ two time points. Filled circles/triangles: male/female rats that showed >66% MPE at ≥ two time points. For clarity, female placements are marked primarily on the left and male placements are marked on the right side of the brain maps (actual cannula placements were all on the right). Not shown are placements for 3 males (all in vlPAG) and 6 females (5 in vlPAG, 1 in DRN) that were only tested with saline and 0.3 μg morphine.
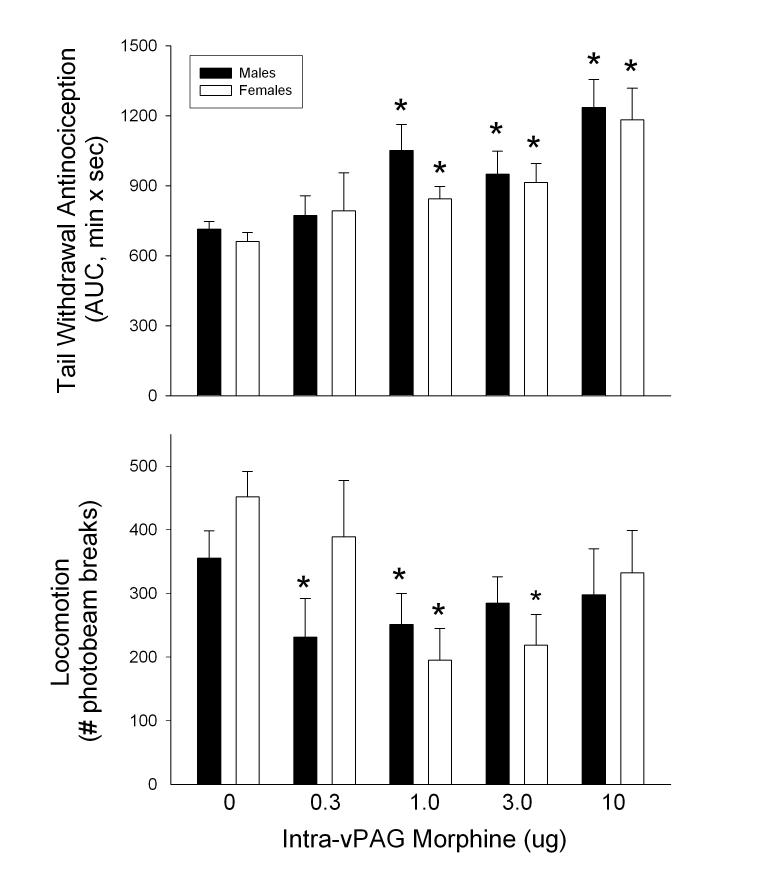
Analysis of combined data showed that there were no significant sex differences in control (saline) tail withdrawal latencies: averaged across the time course, latencies were 6.07 ± 0.42 sec in males and 5.37 ± 0.37 sec in females (t(44)=1.67, p=0.10). There was also no significant sex difference in the time course of antinociception after microinjections of morphine to vPAG (data not shown); latency data were therefore transformed to AUC values. Figure 2 (top panel) shows a dose-dependent increase in tail withdrawal antinociception in both sexes following microinjection of morphine into the vPAG (Dose: F(4,171) = 13.67, p < 0.001). Post-hoc analysis showed that all doses of morphine except 0.3 μg significantly increased AUC values compared to saline controls (1.0 μg: p = 0.002; 3.0 μg: p = 0.003; 10.0 μg: p < 0.001). There were no significant sex differences in morphine's antinociceptive effect.
Figure 2.
Antinociceptive and locomotor effects of morphine microinjected into vPAG in male and female rats. Top panel: Effect of intra-vPAG morphine on the 52°C warm water tail withdrawal test measured 5-120 min post-injection. Bottom panel: Effect of intra-vPAG morphine on spontaneous locomotor activity measured 16-21 min post-injection. Each bar is the mean + S.E.M. of 17 males or 20 females, except at 0.30 μg, where n=11 males and 13 females. *significantly different from same-sex, saline (0) control group, p<0.05.
3.2 Morphine's Locomotor Effects
Figure 2 (bottom panel) shows locomotor activity measured 16-21 min post-injection in rats with vPAG cannula placements. There was no significant difference in locomotion between males and females following saline administration, although females tended to be slightly more active. Morphine significantly decreased locomotor activity (increased immobility) in both sexes (Dose: F(4,165) = 4.99, p = 0.001); post-hoc analysis showed that locomotor activity at the 1.0 μg (p < 0.001) and 3.0 μg (p = 0.003) doses was significantly decreased compared to the saline control condition. There were no significant sex differences in maximal morphine-induced immobility, however, the lowest dose of morphine, 0.30 μg, significantly decreased locomotor activity in males (t(29)=2.07, p = 0.048) but not females. Thus, morphine was more potent but equally efficacious in males compared to females. Interestingly, morphine produced a U-shaped dose-effect curve in both sexes, with greater decreases in locomotor activity at the lower than at the higher doses. Examination of individual data revealed that, primarily at 10 μg morphine, several rats showed large increases in locomotor activity compared to their own control score (up to approximately 400% of control), while most other rats showed a ≥ 60% decrease. Hyperlocomotion (>150% of control) occurred in 6 males (4 vlPAG, 2 DRN) and 3 females (all vlPAG), primarily at 10 μg, which increased the overall means at this dose.
Influence of Estrous Stage on Behavioral Effects of Intra-vPAG Morphine
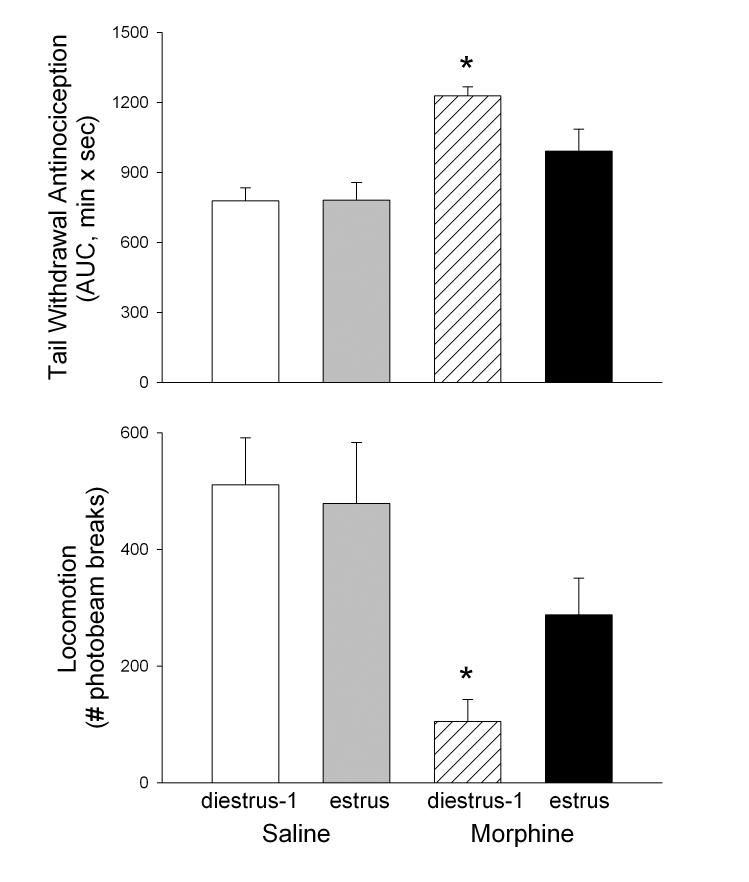
In Experiment 1, approximately 10% of females were in proestrus, with 7% in estrus, 48% in diestrus-1, and 33% in diestrus-2 (2% were inadvertently not sampled) when assessed immediately after nociceptive testing. Because there were unequal or inadequate numbers of females in each stage at each dose, the influence of estrous stage on morphine's behavioral effects could not be examined with this dataset. Thus, Experiment 2 was conducted to determine whether the antinociceptive effect of morphine microinjected into the vPAG differs when females are in estrus vs. diestrus-1 phases (the phases we have found to be most different when testing systemic morphine antinociception [48]). Figure 3 shows that morphine produced antinociception (top panel, F(1,29)=9.42, p=0.005) and immobility (bottom panel, F(1,29)=22.41, p<0.001), but these effects were statistically significant only in females that were tested during diestrus-1 (antinociception: p=0.007; immobility: p=0.001). That is, rats tested during estrus were relatively resistant to the antinociceptive effects of morphine administered to the vPAG.
Figure 3.
Antinociceptive and locomotor effects of 1.0 μg morphine microinjected to vPAG in female rats tested in either diestrus-1 or estrus (based on vaginal cytology). Top panel: Effect of intra-vPAG saline or morphine on the 52°C warm water tail withdrawal test measured 5-120 min post-injection. Bottom panel: Effect of intra-vPAG saline or morphine on spontaneous locomotor activity measured 16-21 min post-injection. Each bar is the mean + S.E.M. of 6-7 (saline) or 9-11 (1.0 μg morphine) females. *significantly different from saline-injected females in same estrous stage, p<0.05.
3.4 Site-specific Antagonism of Systemic Morphine
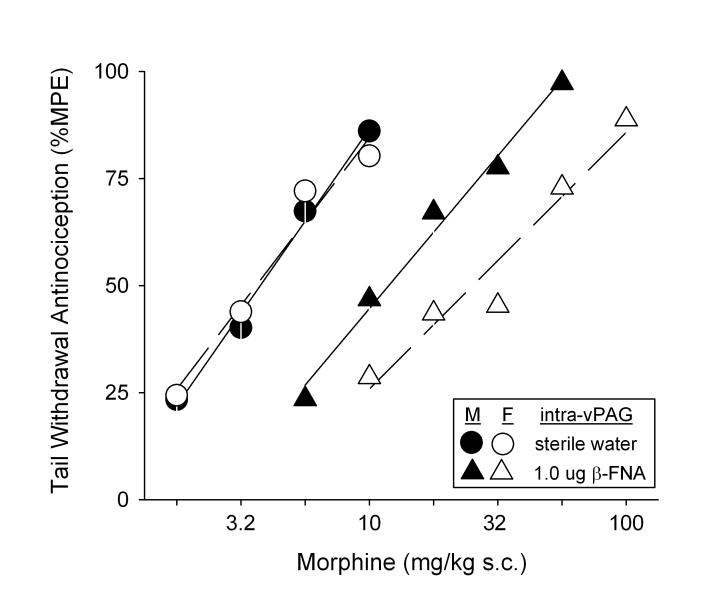
Figure 4 shows antagonism of systemic morphine-induced antinociception by intra-vPAG β-FNA (Experiment 3). In control rats (those receiving intra-site saline), s.c. morphine was only slightly more potent in males than females: ED50 = 6.1 ± 1.5 mg/kg vs. 7.9 ± 3.7 mg/kg, respectively. In rats pre-treated with intra-vPAG β-FNA (1 μg), the morphine dose-effect curves were ≥ ½ log unit to the right of the control curves: ED50 values were significantly higher in β-FNA-treated rats compared to controls (Antagonist Dose: F(1,38) = 12.02, p = 0.001). There was a sex difference in the degree to which β-FNA blocked morphine's antinociceptive effect: ED50 values in β-FNA-treated females were significantly higher than those in β-FNA-treated males: 42.6 ± 10.2 mg/kg vs. 17.0 ± 3.3 mg/kg, respectively (t(14.4) = − 2.50, p =0.025). In this experiment, 4% of females were in proestrus immediately after testing (1 of 10 females in the control group), with approximately 17% in estrus (2 of 10 controls, 2 of 13 β-FNA-treated), 70% in diestrus-1 (5 of 10 controls, 11 of 13 β-FNA-treated), and 9% in diestrus-2 (2 of 10 controls). Thus, similar to Experiment 1, most females in Experiment 3 were in diestrus-1 at the time of testing.
Figure 4.
Antagonism of systemic (s.c.) morphine antinociception by the irreversible, mu opioid receptor-selective antagonist β-FNA microinjected to vPAG in male (M) vs. female (F) rats. β-FNA (1.0 μg) was administered 24 hr before testing with morphine. Each point is the mean ± S.E.M. of 9 males or 10 females (control groups (“sterile water”)), or 10 males or 13 females (β-FNA groups).
4. Discussion
The present study examined sex differences in morphine-induced antinociception and immobility mediated by the vPAG. In Experiment 1, females' estrous stage was not selected (and most females were in diestrus-1 or -2 at the time of testing), and there was no significant sex difference in tail withdrawal antinociception after intra-vPAG microinjection of morphine. Morphine was, however, more potent in males than in females in producing immobility. Experiment 2 showed that morphine's behavioral effects were greater in diestrus-1 than estrus females, suggesting that the lack of sex differences in Experiment 1 may be due to the predominance of diestrus females in that experiment. Experiment 3 showed that decreasing the availability of mu opioid receptors in the vPAG via β-FNA microinjection decreased the potency of systemic morphine to a significantly greater extent in females compared to males. Because most females in Experiment 3 were in diestrus-1 – a stage at which females are very similar to males in their morphine sensitivity – this result suggests that mu opioid receptor density is probably lower even in diestrus females compared to males. Thus, both estrous stage and reduced mu opioid receptor availability in females appear to contribute to sex differences in morphine's behavioral effects mediated by the vPAG.
In rodent models of antinociception, systemically administered mu opioid agonists are often reported to be more potent in males than in females [2, 10, 11, 38], although this is not always the case [e.g., 38]. Brain morphine levels have been reported to be higher in male than in female rats [14] and mice [7] after systemic injection of morphine, but other studies report no sex differences in plasma or brain morphine levels in rats despite large sex differences in morphine's behavioral effects [8, 10]. The persistence of sex differences in antinociception when opioids are administered i.c.v. [28, 29], directly into the RVM [6], or directly into the PAG [31, 53] also argues against a purely pharmacokinetic explanation. Thus, sex differences in opioid antinociception are probably caused by one or more pharmacodynamic factors, such as sexually dimorphic brain loci at which opioids act.
Site-specific opioid administration to areas of the descending pain modulatory system has been examined by several groups. Boyer and colleagues [6] reported greater tail withdrawal antinociception in male than female rats after intra-RVM morphine microinjection (1-10 μg). Similarly, Krzanowska and Bodnar [31] reported that at doses of 2.5 and 5.0 μg morphine administered to vPAG, male rats had significantly longer tail-flick latencies and higher jump thresholds compared to estrus female rats. Recently, Murphy and colleagues [55] also showed that intra-vPAG morphine was more potent in male than female rats against persistent inflammatory pain. In contrast, another previous study [27] as well as the present one shows no significant sex difference in antinociception after intra-PAG morphine microinjection, and Tershner and colleagues [53] reported that in anesthetized rats, DAMGO microinjected into vPAG was more potent in females compared to males. The present study suggests that the extent to which sex differences in opioid antinociception mediated by the PAG are observed depend on estrous stage and availability of mu opioid receptors at the time of testing.
Female rats have been shown to be least sensitive to the antinociceptive effects of systemically administered mu opioid agonists approximately 24 hr after plasma gonadal steroids peak [3, 5, 45, 50, 51], that is, during vaginal estrus. Experiment 2 of the present study suggests that the same is true when morphine is administered directly into the vPAG. Bodnar and colleagues report similar findings [47]. Thus, it is likely that sex differences were not observed in Experiment 1 because only 7% of females were in estrus during testing. Two of the previous studies in which females were significantly less sensitive than males to site-specific morphine also had more estrus females than in Experiment 1 of the present study: Bodnar and colleagues [30-32] tested cycling female rats only during estrus, and in the Boyer et al. [6] study, approximately 39% of females were in estrus, with 44% in diestrus-1 or -2 on the day of testing (J. Boyer, personal communication). Estrous stage of female rats was not reported in the Tershner et al. [53] or Kanarek et al. [27] studies. If females in these studies were predominantly in diestrus at the time of testing, the present results suggest that opioid antinociception would not be significantly greater in males than females. One additional factor that may be important is the use of anesthesia. We recently observed that an anesthetic dose of pentobarbital enhanced morphine antinociception to a significantly greater extent in female compared to male rats [13] – thus it is possible that the observation of greater DAMGO antinociception in females in the Tershner et al. [53] study also reflects the fact that rats were tested while anesthetized.
Previous studies have shown that mu opioid agonists microinjected into the PAG [30, 39] and RVM [6] affect locomotor activity in rats. In the present study morphine was more potent in its locomotor depressant effect in male compared to female rats, as reported previously for site-specific and systemic mu opioid agonists [6, 30, 49]. As with antinociception, sex differences were probably limited by the fact that females in Experiment 1 were predominantly in diestrus at the time of testing; Experiment 2 suggests that morphine-induced immobility is attenuated in estrus relative to diestrus females. The hyperactivity that was also observed in some rats (resulting in a U-shaped dose-effect curve) has been reported previously after morphine microinjection to dorsal and lateral regions of the PAG in rats [39, 46], but it cannot be determined from these data whether that behavioral effect differs by sex or estrous stage.
Experiment 3 showed that 1.0 μg β-FNA microinjected unilaterally to the vPAG shifted the systemic morphine dose-effect curve significantly to the right in both sexes. A previous study in male rats showed that naloxone methobromide microinjected to the PAG attenuated antinociception induced by a single s.c. dose of morphine [37]. It also has been shown that small doses of various opioid antagonists microinjected to the PAG can attenuate antinociception induced by β-endorphin or morphine microinjected to the PAG [48, 54] or amygdala [42] in male rats. The antagonism experiment in the present study confirms that activation of mu opioid receptors in the vPAG plays an important role in antinociception produced by systemic morphine in both male and female rats.
The relatively greater antagonism observed in females compared to males suggests that females – even those in diestrus – have a smaller reserve of functional mu opioid receptors than do males. This finding corroborates the recent report of reduced mu opioid receptor expression in the vPAG of female compared to male rats [55]. The greater antagonism observed in females cannot be explained by a preponderance of estrus females in the β-FNA-treated group relative to the saline-treated group; in fact there were more diestrus females in the β-FNA-treated group than in the control group, which would tend to decrease rather than increase the difference between the two female groups. We previously observed a similar sex difference in antagonism of s.c. morphine-induced hotplate antinociception by i.c.v. β-FNA: 5-10 μg i.c.v. β-FNA shifted the morphine dose-effect curve significantly farther to the right in females than in males, and a very high dose of β-FNA, 40 μg, completely flattened the morphine dose-effect curve in females but not in males [16]. Thus, it appears that females in any stage of the estrous cycle may have a smaller mu opioid receptor reserve than do males; however, this difference may not be great enough to result in significant sex differences in opioid antinociception with relatively high efficacy agonists like morphine, unless females are tested in estrus or a significant fraction of mu receptors are made unavailable (in this case, via irreversible antagonist treatment). Previous studies comparing opioid agonists that vary in efficacy also support the hypothesis that differential opioid receptor density underlies sex differences in opioid antinociception. Picker and colleagues have demonstrated that sex differences in opioid antinociception increase in magnitude as the efficacy of the agonist decreases [4, 11, 52]. Because low to intermediate efficacy agonists must activate more receptors than high efficacy agonists to produce antinociception, the functional significance of small sex differences in opioid receptor density would be magnified when testing lower efficacy agonists.
It is possible that the mechanism underlying decreased opioid sensitivity in estrus females also relates to supraspinal opioid receptor availability. Although it is not yet known whether PAG mu opioid receptor density fluctuates in females across the estrous cycle, this has been demonstrated in other brain areas such as hypothalamus [21, 36]. Therefore, perhaps any factor that decreases supraspinal opioid receptor availability (e.g., estrus or an irreversible antagonist), or increases opioid receptor “demand” for producing an antinociceptive effect (e.g., lower efficacy agonists or greater intensity noxious stimuli) may increase the magnitude of observed sex differences.
In summary, the present findings confirm that the midbrain PAG is important in morphine-induced antinociception and immobility in male and female rats. Sex differences in intra-vPAG morphine's behavioral effects were minimal when females were not selected for estrous stage, perhaps because estrus females are less sensitive to morphine than diestrus females, and there were few estrus females in Experiment 1. Greater antagonism of morphine's effects by the irreversible mu opioid antagonist β-FNA microinjected to the vPAG in females compared to males suggests that differences in mu opioid receptor density in the vPAG contribute to sex differences in morphine antinociception.
Acknowledgements
Supported by NIDA grant DA 10284 (R.M.C.) and minority supplement to DA 10284 (S.A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akil H, Mayer DJ, Liebeskind JC. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976;191:961–2. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- 2.Baamonde AI, Hidalgo A, Andres-Trelles F. Sex-related differences in the effects of morphine and stress on visceral pain. Neuropharmacology. 1989;28:967–70. doi: 10.1016/0028-3908(89)90197-4. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee P, Chatterjee TK, Ghosh JJ. Ovarian steroids and modulation of morphine-induced analgesia and catalepsy in female rats. Eur J Pharmacol. 1983;96:291–4. doi: 10.1016/0014-2999(83)90319-9. [DOI] [PubMed] [Google Scholar]
- 4.Barrett AC, Cook CD, Terner JM, Roach EL, Syvanthong C, Picker MJ. Sex and rat strain determine sensitivity to kappa opioid-induced antinociception. Psychopharmacology. 2002;160:170–181. doi: 10.1007/s00213-001-0949-2. [DOI] [PubMed] [Google Scholar]
- 5.Berglund LA, Simpkins JW. Alterations in brain opiate receptor mechanisms on proestrous afternoon. Neuroendocrinology. 1988;48:394–400. doi: 10.1159/000125040. [DOI] [PubMed] [Google Scholar]
- 6.Boyer JS, Morgan MM, Craft RM. Microinjection of morphine into the rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Res. 1998;796:315–8. doi: 10.1016/s0006-8993(98)00353-9. [DOI] [PubMed] [Google Scholar]
- 7.Candido J, Lutfy K, Billings B, Sierra V, Duttaroy A, Inturrisi CE, Yoburn BC. Effect of adrenal and sex hormones on opioid analgesia and opioid receptor regulation. Pharmacol Biochem Behav. 1992;42:685–92. doi: 10.1016/0091-3057(92)90015-8. [DOI] [PubMed] [Google Scholar]
- 8.Cicero TJ, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharmacol Biochem Behav. 2000;65:91–6. doi: 10.1016/s0091-3057(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 9.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–73. [PubMed] [Google Scholar]
- 10.Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine's antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–44. [PubMed] [Google Scholar]
- 11.Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the rat mu opioid receptor. Psychopharmacology. 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- 12.Craft RM. Sex differences in opioid analgesia: “From mouse to man”. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Craft RM, Leitl MD. Potentiation of morphine antinociception by pentobarbital in female vs. male rats. Pain. 2006;121:115–125. doi: 10.1016/j.pain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Craft RM, Kalivas PW, Stratmann JA. Sex differences in discriminative stimulus effects of morphine in the rat. Behav Pharm. 1996;7:764–778. [PubMed] [Google Scholar]
- 15.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Craft RM, Tseng AH, McNiel DM, Furness MS, Rice KC. Receptor-selective antagonism of opioid antinociception in female vs. male rats. Behav Pharm. 2001;12:591–602. doi: 10.1097/00008877-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Dickenson AH, Oliveras JL, Besson JM. Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res. 1979;170:95–111. doi: 10.1016/0006-8993(79)90943-0. [DOI] [PubMed] [Google Scholar]
- 18.Freeman M. The ovarian cycle in the rat. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press; New York: 1988. pp. 1893–1928. [Google Scholar]
- 19.Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, Levine JD. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci Lett. 1996;205:207–9. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- 20.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–50. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 21.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–45. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 22.Hammer R. Mu-opiate receptor binding in the medial preoptic area is cyclical and sexually dimorphic. Brain Res. 1990;515:187–192. doi: 10.1016/0006-8993(90)90595-3. [DOI] [PubMed] [Google Scholar]
- 23.Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–6. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- 24.Jacquet YF, Lajtha A. Paradoxical effects after microinjection of morphine in the periaqueductal gray matter in the rat. Science. 1974;185:1055–7. doi: 10.1126/science.185.4156.1055. [DOI] [PubMed] [Google Scholar]
- 25.Jensen TS, Yaksh TL. Comparison of the antinociceptive action of mu and delta opioid receptor ligands in the periaqueductal gray matter, medial and paramedial ventral medulla in the rat as studied by the microinjection technique. Brain Res. 1986;372:301–12. doi: 10.1016/0006-8993(86)91138-8. [DOI] [PubMed] [Google Scholar]
- 26.Jensen TS, Yaksh TL. Examination of spinal monoamine receptors through which brainstem opiate-sensitive systems act in the rat. Brain Res. 1986;363:114–27. doi: 10.1016/0006-8993(86)90663-3. [DOI] [PubMed] [Google Scholar]
- 27.Kanarek RB, Mandillo S, Wiatr C. Chronic sucrose intake augments antinociception induced by injections of mu but not kappa opioid receptor agonists into the periaqueductal gray matter in male and female rats. Brain Res. 2001;920:97–105. doi: 10.1016/s0006-8993(01)03039-6. [DOI] [PubMed] [Google Scholar]
- 28.Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34:119–27. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- 29.Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- 30.Krzanowska E, Bodnar RJ. Sex differences in locomotor activity following beta- endorphin in the ventrolateral periaqueductal gray. Physiol Behav. 2000;68:595–8. doi: 10.1016/s0031-9384(99)00242-5. [DOI] [PubMed] [Google Scholar]
- 31.Krzanowska EK, Bodnar RJ. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–30. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- 32.Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- 33.Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolaterial periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Lewis VA, Gebhart GF. Evaluation of the periaqueductal central gray (PAG) as a morphine- specific locus of action and examination of morphine-induced and stimulation- produced analgesia at coincident PAG loci. Brain Res. 1977;124:283–303. doi: 10.1016/0006-8993(77)90886-1. [DOI] [PubMed] [Google Scholar]
- 35.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maggi R, Limonta P, Dondi D, Piva F. Modulation of the binding characteristics of hypothalamic mu opioid receptors in rats by gonadal steroids. J Steroid Biochem Molec Biol. 1991;40:113–121. doi: 10.1016/0960-0760(91)90174-4. [DOI] [PubMed] [Google Scholar]
- 37.Manning BH, Franklin KB. Morphine analgesia in the formalin test: reversal by microinjection of quaternary naloxone into the posterior hypothalamic area or periaqueductal gray. Behav Brain Res. 1998;92:97–102. doi: 10.1016/s0166-4328(97)00130-7. [DOI] [PubMed] [Google Scholar]
- 38.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–89. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 39.Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–66. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- 40.Negus SS, Mello NK. Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther. 1999;290:1132–40. [PubMed] [Google Scholar]
- 41.Oliveras JL, Besson JM, Guilbaud G, Liebeskind JC. Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Exp Brain Res. 1974;20:32–44. doi: 10.1007/BF00239016. [DOI] [PubMed] [Google Scholar]
- 42.Pavlovic ZW, Cooper ML, Bodnar RJ. Opioid antagonists in the periaqueductal gray inhibit morphine and beta- endorphin analgesia elicited from the amygdala of rats. Brain Res. 1996;741:13–26. doi: 10.1016/s0006-8993(96)00880-3. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Vol. 2. Academic Press; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 44.Randich A, Thurston CL, Ludwig PS, Robertson JD, Tasmussen C. Intravenous morphine-induced activation of vagal afferents: Peripheral, spinal, and CNS substrates mediating inhibition of spinal nociception and cardiovascular responses. J Neurophys. 1992;68:1027–1045. doi: 10.1152/jn.1992.68.4.1027. [DOI] [PubMed] [Google Scholar]
- 45.Ryan SM, Maier SF. The estrous cycle and estrogen modulate stress-induced analgesia. Behav Neurosci. 1988;102:371–80. doi: 10.1037//0735-7044.102.3.371. [DOI] [PubMed] [Google Scholar]
- 46.Sandner G, Schmitt P, Karli P. Mapping of jumping, rearing, squealing and switch-off behaviors elicited by periaqueductal gray stimulation in the rat. Physiol Behav. 1987;39:333–9. doi: 10.1016/0031-9384(87)90231-9. [DOI] [PubMed] [Google Scholar]
- 47.Shane R, Bernal SY, Rozengurtel S, Bodnar RJ. Estrus phase differences in female rats in morphine antinociception elicited from the ventrolateral periaqueductal gray. Int J Neurosci. doi: 10.1080/00207450600910259. in press. [DOI] [PubMed] [Google Scholar]
- 48.Smith DJ, Robertson B, Monroe PJ, Taylor DA, Leedham JA, Cabral JD. Opioid receptors mediating antinociception from beta-endorphin and morphine in the periaqueductal gray. Neuropharmacology. 1992;31:1137–50. doi: 10.1016/0028-3908(92)90010-m. [DOI] [PubMed] [Google Scholar]
- 49.Stewart J, Rodaros D. The effects of gonadal hormones on the development and expression of the stimulant effects of morphine in male and female rats. Behav Brain Res. 1999;102:89–98. doi: 10.1016/s0166-4328(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 50.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terner JM, Lomas LM, Picker MJ. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005;6:372–383. doi: 10.1016/j.jpain.2005.01.354. [DOI] [PubMed] [Google Scholar]
- 52.Terner JM, Lomas LM, Smith ES, Barrett AC, Picker MJ. Pharmacogenetic analysis of sex differences in opioid antinociception in rats. Pain. 2003;106:381–391. doi: 10.1016/j.pain.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Tershner SA, Mitchell JM, Fields HL. Brainstem pain modulating circuitry is sexually dimorphic with respect to mu and kappa opioid receptor function. Pain. 2000;85:153–9. doi: 10.1016/s0304-3959(99)00257-2. [DOI] [PubMed] [Google Scholar]
- 54.Tseng LF, Tang R. Different mechanisms mediate beta-endorphin- and morphine- induced inhibition of the tail-flick response in rats. J Pharmacol Exp Ther. 1990;252:546–51. [PubMed] [Google Scholar]
- 55.Wang X, Traub RJ, Murphy AZ. Sexually dimorphic mu opioid receptor expression in the midbrain periaqueductal gray may contribute to the observed sex differences in intra-periaqueductal gray morphine in a persistent pain model. Neuroscience. (submitted) [Google Scholar]
- 56.Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- 57.Zambotti F, Zonta N, Parenti M, Tommasi R, Vicentini L, Conci F, Mantegazza P. Periaqueductal gray matter involvement in the muscimol-induced decrease of morphine antinociception. Naunyn-Sch Arch Pharmacol. 1982;318:368–369. doi: 10.1007/BF00501180. [DOI] [PubMed] [Google Scholar]